Highlights
-
•
Arterial spin labeling brain MRI measure deafness impact on cerebral perfusion.
-
•
Deafness in childhood modifies the temporal perfusion evolution across age.
-
•
Cochlear implant pronostics is bad in case of high CBF values in occipital regions.
-
•
Cochlear implantation before 4 years old is required.
Keywords: Deafness, Children, Hearing loss, Cochlear implant results, Oral intelligibility scores, MRI, Arterial spin labeling, Auditory cortex, Brain development
Abstract
Age at implantation is considered to be a major factor, influencing outcomes after pediatric cochlear implantation. In the absence of acoustic input, it has been proposed that cross-modal reorganization can be detrimental for adaptation to the new electrical input provided by a cochlear implant. Here, through a retrospective study, we aimed to investigate differences in cerebral blood flow (CBF) at rest prior to implantation in children with congenital deafness compared to normally hearing children. In addition, we looked at the putative link between pre-operative rest-CBF and the oral intelligibility scores at 12 months post-implantation. Finally, we observed the evolution of perfusion with age, within brain areas showing abnormal rest-CBF associated to deafness, in deaf children and in normally hearing children. In children older than 5 years old, results showed a significant bilateral hypoperfusion in temporal regions in deaf children, particularly in Heschl’s gyrus, and a significant hyperperfusion of occipital regions. Furthermore, in children older than 5 years old, whole brain voxel-by-voxel correlation analysis between pre-operative rest-CBF and oral intelligibility scores at 12 months post-implantation, showed significant negative correlation localized in the occipital regions: children who performed worse in the speech perception test one year after implantation were those presenting higher preoperative CBF values in these occipital regions. Finally, when comparing mean relative perfusion (extracted from the temporal regions found abnormal on whole-brain voxel-based analysis) across ages in patients and controls, we observed that the temporal perfusion evolution was significantly different in deaf children than in normally hearing children. Indeed, while temporal perfusion increased with age in normally hearing children, it remained stable in deaf children. We showed a critical period around 4 years old, where in the context of auditory deprivation, there is a lack of synaptic activity in auditory regions. These results support the benefits of early cochlear implantation to maximize the effectiveness of auditory rehabilitation and to avoid cross-modal reorganization.
1. Introduction
One in 1000 newborns are identified with severe to profound hearing loss at birth (Butcher et al., 2019). Approximately 80% of prelingual deafness is due to genetic factors (Marlin et al., 2005) with about a hundred identified gene abnormalities (Ideura et al., 2019, Bai et al., 2019). Phenotypic expression of these genetic mutations is variable, with a hearing loss ranging from mild to severe. Despite the use of hearing aid devices, persistent lack of audibility may require patients to develop skills other than using sounds to communicate, relying particularly on visual cues (lip reading, cued speech, sign language) or tactile cues. Cochlear implants (CI) have proven to be a useful treatment option for individuals with severe to profound hearing loss and are part of the French National Authority for Health management guidelines (HAS 2012, https://www.hassante.fr/upload/docs/application/pdf/fiche_bon_usage_implants_cochleaires.pdf). It has been recognized that early implantation provides children with the best outcomes (Simon et al., 2019). Cochlear implantation is not recommended as an option for children over 5 years old, unless hearing loss is acquired or progressive (Simon et al., 2019).
Improvement of auditory perception after CI was investigated by electrophysiological studies using the latency of the P1 cortical auditory evoked potential (Dorman et al., 2007). Results showed that if children experience <3 years of auditory deprivation before implantation, P1 latencies fall back into the normal range. Children who are implanted after the age of 7 years old, however, generally do not develop normal P1 latencies.
The development of other somatosensory modalities, such as lip reading (Finney et al., 2001), sign language (MacSweeney et al., 2008), vibrotactile stimulation (Cardon and Sharma, 2019), as well as the response to reinstated auditory input after cochlear implantation (Kang et al., 2004) in deaf patients has been investigated by functional neuroimaging studies over the last two decades. Results have shown differences in cerebral organization in adults with congenital deafness (Finney et al., 2001, MacSweeney et al., 2008). However, it is not clear whether these differences stem from the acquisition of non-verbal communication skills, from deafness, or from both. Few functional studies have focused on children candidates for cochlear implantation, mainly because of the challenges associated with brain imaging studies in children. In a study by Lee et al, brain metabolism in 33 deaf children (age = 6.3 ± 2.3 years, with<70 dB HL pure tone thresholds with their hearing aids) was examined preoperatively using FDG-PET and correlated with postoperative speech perception scores (Lee et al., 2007). Results showed that the degree of hypometabolism within the auditory cortex before implantation was positively correlated with oral intelligibility scores after implantation: patients obtaining higher scores were those presenting stronger hypometabolism before CI, suggesting that the auditory cortices would not have been recruited by other cortical functions and could respond effectively to the auditory input reinstated by cochlear implantation. The results from fMRI and rest metabolism studies (Finney et al., 2001, MacSweeney et al., 2008, Cardon and Sharma, 2019) indicate that deafness can lead to cross-modal plasticity: the auditory cortex became more responsive to non-auditory stimulation in deaf individuals (Benetti et al., 2017). Nevertheless, the question remains of whether reorganization of auditory cortex in deaf individuals is due to the effects of auditory deprivation (bottom-up regulation) or the later acquisition of sign language (top-down regulation) (Cardin et al., 2016).
Brain functional investigations using nuclear imaging methods remain limited in children for obvious methodological reasons. It is difficult to justify the use of radiotracers for imaging children before cochlear implantation. However, recent advances in neuroimaging allow to measure rest brain function using arterial spin labeling (ASL) MRI sequence. This technique uses magnetically labeled arterial blood water as a diffusible flow tracer. By labeling inflowing blood water proximal to the target imaging region, the perfusion signal is subsequently calculated by comparison with a separate image acquired using a control pulse without labeling blood flow to remove static background signal and control for magnetization transfer effects. Therefore, ASL-MRI non-invasively assesses brain perfusion and provides an absolute and quantifiable voxel-by-voxel measurement of rest cerebral blood flow (CBF) without the administration of contrast material or exposure to ionizing radiation, (Detre and Alsop, 1999), offering new opportunities to quantify cerebral blood flow at rest in neonates and children. We sought to investigate whole-brain cortical perfusion in deaf children compared to normally hearing controls in three age groups: younger than 2 years old; from 2 to 5 years old; and from 5 to 16 years old. In addition, in deaf children aged from 5 to 16 years old, we aimed to investigate a putative relationship between preoperative cortical perfusion and clinical outcome after cochlear implantation using oral intelligibility scores at 12 months post-implantation. Finally, we aimed to study the evolution of perfusion with age, within brain areas showing abnormal rest CBF associated with deafness, in deaf children and in normally hearing children. For that purpose, we performed a retrospective study in 79 deaf children and 86 normally hearing control having undergone an ASL-MRI.
2. Methods
2.1. Participants
This retrospective study included 79 children candidates for cochlear implantation between 2012 and 2018 at our tertiary referral center (Hôpital Necker-Enfants Malades). All patients underwent a brain and inner ear MRI as part of the routine preoperative work-up at our institution, to exclude central nervous system causes of hearing loss and to obtain a reference brain imaging. Inclusion criteria were the absence of neurological or psychiatric disorders, no cochlear implants at the time of MRI, a routine MRI protocol including ASL-MRI. The median age at time of MRI scan was 3.2 years (range 0.5 to 15.8 years).
The control group was composed of 86 normally hearing children with a median age at time of MRI scan of 2.9 years (range 0.5 to 13.0 years). Within this group, 33 children were over 5 years old and were recruited by advertising to undergo an MRI for research purposes only, without any premedication. The fifty-three children under 5 years old underwent a brain MRI for various non-neurological reasons during the time of the study (non-exhaustively including: arthrogryposis, facial cutaneous angioma or hemangioma or lymphangioma, morphological evaluation of upper-limit head circumference, sub-cutaneous cyst, feeding difficulties, abnormal eye-movements, benign nystagmus, benign eye-motion disorder, isolated strabism, neo-natal diabetes with no severe hypoglycemia) and presented strictly normal scans and no hearing loss verified by routine clinical follow up.
Participants were divided into 3 subgroups based on their age at time of MRI. A subgroup under 2 years of age was composed of 28 patients (median age = 0.9 years, range 0.5 to 2.0 years) and 33 controls (median age = 1.0 year, 0.5 to 2.0 years). A subgroup aged between 2 and 5 years of age was composed of 26 deaf patients (median age = 3.2 years, 2.1 to 4.6 years) and 20 controls (median age = 2.9 years, 2.0 to 5.0 years). A subgroup older than 5 years of age was composed of 25 deaf patients (median age = 9.0 years, 5.2 to 15.8 years) and 33 controls (median age = 10.1 years, 6.0 to 13.0 years).
All children, patients and controls, younger than 5 years of age received standard premedication before their MRI (pentobarbital, 5 mg / kg). The study design was approved by the local ethics committee, 61-NCK, 20190621163621.
2.2. Clinical description
All patients had bilateral hearing loss. In the studied population 60.8% had a symmetric bilateral hearing loss and 39.2% had an asymmetric hearing loss with a right predominance in 77.4% of the cases. Seventy percent (70%) of patients were implanted on the right ear, 26% were implanted on the left ear, 4% received bilateral cochlear implantation and one child had not been implanted yet. Concerning the etiology, 47% had a hearing loss (HL) of unknown etiology, 5% had congenital cytomegalovirus infection (cCMV), 18% had a connexin gene mutation, 26% had an inner ear malformation (two of which had SCL26A4 mutation), and 4% had an auditory neuropathy.
For children over 5 years old, speech perception was evaluated in free field, before and 12 months after cochlear implantation based on the mean intelligibility score obtained using open-set recognition of French Fournier disyllabic words (OSW) (for 5 out of 25 of deaf children, the OSW scores were unknown). Prior to CI, following hearing aid rehabilitation, oral communication was evaluated with a mean intelligibility score of 64.5 ± 32%. Twelve months after CI, mean intelligibility score was of 92 ± 11.5% (see Fig. 1). There was no correlation between age and intelligibility scores either before or after cochlear implantation (see Fig. 1). The hearing thresholds before cochlear implantation showed that patients had some auditory perception with hearing aids at low frequencies. Perceived frequencies were mainly low frequencies 250 Hz (36 dB HL) and high frequencies remained difficult to perceive (63 dB HL at 4 kHz). Cochlear implants significantly improved perception of frequencies between 500 Hz and 4 kHz in comparison to hearing aids (see Fig. 2), improving the audibility of long-term average speech spectra (Byrne et al., 1994). For 3 deaf children, perceived frequency values were unknown.
Fig. 1.
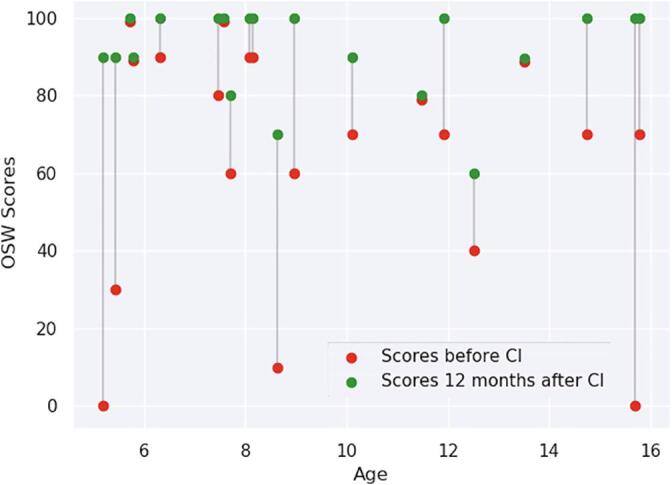
OSW scores before (red) and 12 months after cochlear implantation (green) for patients above 5 years old. After cochlear implants, mean intelligibility scores were statistically better (92 ± 11.5%) than before (64.5 ± 32%) (p < 0,001). There were no correlations between age and the intelligibility scores either before (β = −1.16 unit/year; t(18) = −0.539; p = 0.597) or after cochlear implantation (β = −0.08 unit/year; t(18) = −0.097; p = 0.924). (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Fig. 2.
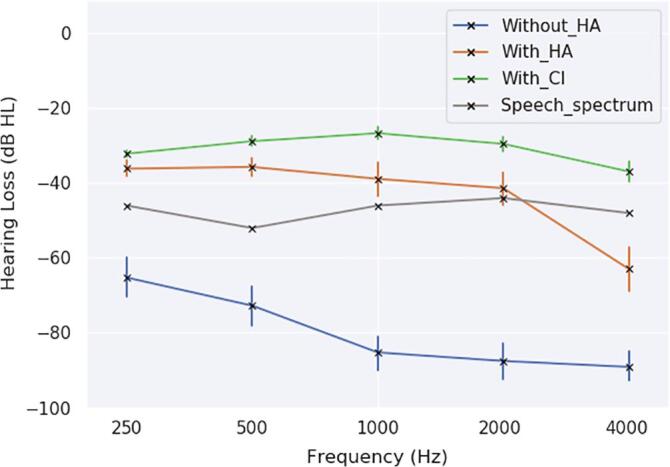
Mean auditory thresholds (dB HL), with error bars corresponding to the 68% confidence interval (standard error), for the group of deaf children older than 5 years old before cochlear implantation without hearing aids (HA) (blue), with hearing aids (orange) and after cochlear implantation (green) at different frequencies: 250 Hz, 500 Hz, 1000 Hz, 2000 Hz and 4000 Hz. The side with the best tonal audiometric thresholds has been reported on the figure. Speech auditory perception improved with the use of hearing devices. Although performance was similar with hearing aids and cochlear implant at the frequency of 250 Hz (p = 0,11), it was significantly better with the cochlear implant at frequencies of 500 Hz (p < 0,05), 1000 Hz (p < 0,05) and 2000 Hz (p < 0,05) and 4000 Hz (p < 0,001). The long term average speech spectrum energy is reported in gray: (250 Hz: −46 dB HL; 500 Hz: −52 dB HL; 1000 Hz: −46 dB HL; 2000 Hz: −44 dB HL; 4000 Hz: −48 dB HL). HL thresholds over the speech spectrum did not allow speech perception. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
2.3. Data acquisition
All images were acquired in the Department of Pediatric Radiology of Necker Children's Hospital (Paris, France) with a standard routine protocol including T1, T2 and ASL weighted sequences. Before June 2015, data from 33 of the 79 children candidates for cochlear implantation and 41 of the 86 controls were acquired on a 1.5 Tesla MRI scanner (Signa HDxt General Electric (GE) Medical System). After June 2015, data from 46 of the 79 children candidates for cochlear implantation and 45 of the 86 controls were acquired on a Discovery 3.0 Tesla MRI scanner (MR750 General Electric Medical System). We used normal-hearing earplugs for children, which attenuates the noise from 90 dB(A) to 60 dB(A).
On the 1.5 T scanner, 3D T1-weighted Fast Spoiled Gradient Echo (FSPGR) images were acquired with the following parameters: 240 axial slices; repetition time (TR) = 16.4 ms; echo time (TE) = 7.2 ms; resolution in plane = 0.47 × 0.47 mm; slice thickness = 1.2 mm; flip angle (FA) = 13°. ASL perfusion images were performed with a 3D pseudo continuous arterial spin labeling (3D pcASL) sequence using a fast spin echo acquisition with spiral filling of the K space (TR/TE = 4453/10.96 ms; 8 spiral arms × 512 sampling points; Freq = 512; post-labeling delay (PLD) = 1025 ms; FA = 155°; matrix size = 128 × 128; resolution in plane = 1.875 × 1.875 mm; slice thickness = 4 mm; field of view = 24 × 24 cm; bandwidth = 62.50; Imaging Options EDR, Fast, Spiral, Phase 8 Freq, NEX 3.00, Auto Shim On; 40 contiguous axial slices and duration time ≈ 4 min. Labeling duration is 1500 ms.
On the 3.0 T scanner, 3D T1-weighted FSPGR images were acquired with the following parameters: 156 axial slices; TR = 6.9 ms; TE = 3 ms; resolution in plane = 1 × 1 mm; slice thickness = 1 mm; FA = 12°. For most deaf patients, Cube T1 images were acquired with these parameters: 374 sagittal slices; TR = 500 ms; TE = 11.2 ms; resolution in plane = 0.47 × 0.47 mm; slice thickness = 1 mm; FA = 90°. ASL perfusion images were obtained using the same parameters as on the 1.5 T system.
2.4. Data preprocessing
For the data preprocessing, we used the quantification of the CBF and not only perfusion weighted images. The ASL sequence provided by General Electric Medical System included a proton-density image used for signal normalization during CBF quantification. CBF images are produced by the processing console automatically and we can measure the ASL quantitatively.
All T1-weighted and ASL-MRI images were first visually checked for major artifacts: we performed a two-step quality control, the first one by an expert radiologist right after acquisition (NB) and the second one by an expert image processing engineer (LF) before preprocessing to discard images with artifacts, such as motion, aliasing, ghosting, spikes, low signal to noise ratio. Then, all images were analyzed using SPM12 (http://www.fil.ion.ucl.ac.uk/spm, London, UK).
Native 3D T1-weighted FSPGR and Cube T1 images were segmented into gray matter, white matter and cerebrospinal fluid using the CAT12 toolbox (http://www.neuro.uni-jena.de/cat/) within SPM12 and deformation fields between native space and MNI space were obtained. The MNI template used for segmentation has been adapted according to the age of the given subject: a pediatric template was created with “Template-O-Matic” toolbox (http://dbm.neuro.uni-jena.de/software/tom/) using the NIH Pediatric MRI Data Repository (n = 404, age range 5–18 years) for children over 2 years old, and the Infant Brain Probability Templates (Altaye et al., 2008) for children under 2 years old.
For each child, the co-registration between the native CBF image and the native gray matter image was estimated. Then, the combination of the deformation fields and the estimated co-registration was applied to the CBF image to spatially normalize this image in the MNI space. The resulting image was checked for proper registration and smoothed with a Gaussian kernel of 10-mm full-width at half-maximum (FWHM). All spatially normalized images had the same resolution in plane (1.5 × 1.5 mm) and the same slice thickness (1.5 mm).
2.5. Statistical analysis
2.5.1. Whole-brain voxel-based analysis
For each subgroup, comparisons between deaf children and normally hearing controls were performed to investigate voxel-wise cortical perfusion differences using a two-sample t-test design within the framework of the general linear model (GLM) in SPM12. Age and MRI field strength were considered as confounding covariates. A proportional scaling of the CBF images was applied and set to a grand mean scaled value of 50 mL/dL/min to minimize inter-subject variability. Voxels included on an explicit binary mask were considered, corresponding to the cortical gray matter excluding the cerebellum.
2.5.2. Multiple regression analysis
An additional GLM analysis, using a multiple regression design, was applied to the smoothed CBF images spatially normalized in the subgroup of deaf children older than 5 years old, to investigate a putative relationship between preoperative cortical perfusion and clinical outcome after cochlear implantation. Oral intelligibility scores obtained 12 months after implantation were used as independent factors. Age and MRI field strength were considered as confounding covariates. Proportional scaling of the CBF images was also applied and set to a grand mean scaled value of 50 mL/dL/min in this analysis. All p-values were threshold at 0.05 and corrected for multiple comparisons with family-wise error correction (FWE).
2.5.3. Region-of-interest analysis
As a post-hoc analysis, a region of interest analysis was performed to investigate, in both groups, the evolution of rest CBF across age within temporal clusters identified in the whole-brain group comparison. Thus, the mean CBF from these clusters was extracted for all participants. The individual mean CBF values were normalized by the mean CBF measured within the cortical gray matter binary mask for each participant to obtain relative CBF. We used the residual values from the analysis as dependent variables, canceling out field strength effect using the ordinary least squares (OLS) model with the python stats models v0.9.0 module. P-values ≤ 0.05 were considered statistically significant.
3. Results
3.1. Whole-brain voxel-based comparisons
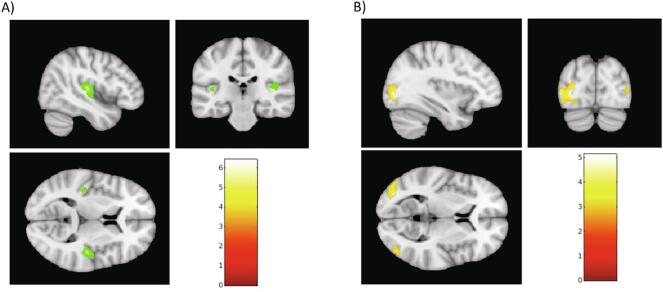
For children over 5 years old, whole-brain voxel-based comparison between rest CBF in deaf patients and normally hearing controls revealed significant bilateral decrease in rest CBF localized in the superior temporal and Heschl’s regions in patients (p ≤ 0.05, FWE correction) (see Fig. 3). This result appeared to be independent of the predominance of deafness, with no significant differences found between right-predominant and left-dominant deaf children. This result was independent of the side of the predominant hearing loss. No significant cluster was found using two-sample t-test comparison between right-predominant and left-predominant deaf children. Furthermore, no significant effect of the predominance of deafness was found on relative mean CBF from the Heschl ROI (ANOVA; F(2, 17) = 0.34; p = 0.718), with age and MRI field strength as confounding covariates. Decrease in rest CBF was more pronounced in the right hemisphere (t = 6.42; z(score) = 5.51; p(FWE-corr) < 0.001; MNI coordinates: x = 45, y = -21, z = 10) than in the left hemisphere (t = 5.19; z(score) = 4.65; p(FWE-corr) = 0.015; MNI coordinates: x = -42, y = -24, z = 8). In addition, a significant bilateral increase in rest CBF was observed in the left (t = 5.12; z(score) = 4.61; p(FWE-corr) = 0.018; MNI coordinates: x = –33, y = -82, z = 2) and right (t = 4.80; z(score) = 4.36; p(FWE-corr) = 0.048; MNI coordinates: x = 27, y = -72, z = 24) occipital regions.
Fig. 3.
Whole brain voxel-by-voxel SPM analyses comparing deaf children and normally-hearing children older than 5 years old. A) Significant rest-CBF decrease in temporal regions, more pronounced in the right hemisphere (t = 6.42; z(score) = 5.51; p(FWE-corr) < 0.001; MNI coordinates: x = 45, y = −21, z = 10) than in the left hemisphere (t = 5.19; z(score) = 4.65; p(FWE-corr) = 0.015; MNI coordinates: x = −42, y = −24, z = 8). B) Significant rest-CBF increase in left (t = 5.12; z(score) = 4.61; p(FWE-corr) = 0.018; MNI coordinates: x = −33, y = −82, z = 2) and right (t = 4.80; z(score) = 4.36; p(FWE-corr) = 0.048; MNI coordinates: x = 27, y = −72, z = 24) occipital regions. Images were selected for illustrative purposes.
There were no significant differences between groups for children between 2 and 5 years old and for children younger than 2 years old.
3.2. Multiple regression analysis
Clinical OSW scores were available for 20 out of 25 deaf children older than 5 years old. Whole-brain voxel-based multiple regression analysis showed a significant negative regression between rest-CBF before implantation and OSW scores at 12 months after implantation localized in left inferior and mid-occipital regions: deaf children who presented higher OSW scores 12 months after implantation were those who had lower rest-CBF values in these regions prior to implantation (t = 6.63; z(score) = 4.53; p(FWE-corr) = 0.036; MNI coordinates: x = –33, y = -81, z = -8). There was no significant positive regression with OSW scores at 12 months after implantation. No significant positive or negative regression were found between rest-CBF and clinical OSW scores before implantation.
3.3. Region-of-interest analysis
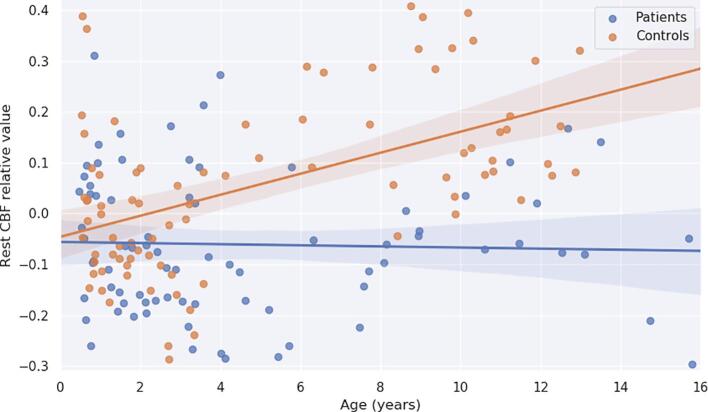
The evolution of rest-CBF across age within temporal regions was significantly different in both groups (t = 4.14; p = 0.0001) (see Fig. 4). While in normally hearing children rest-CBF increased with age (β = 0.0207 unit/year; t(161) = 5.67; p = 0.00001), in deaf children rest-CBF did not increase with age and remained stable over time (β = −0.0011 unit/year; t(161) = -0.29; p = 0.773).
Fig. 4.
Significant difference in age-related changes of the rest-CBF relative values in temporal regions for normally-hearing children (orange) and deaf children (blue) between 0 and 16 years (t = 4.09; p = 0.0001). The rest-CBF relative values are presented in arbitrary units. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
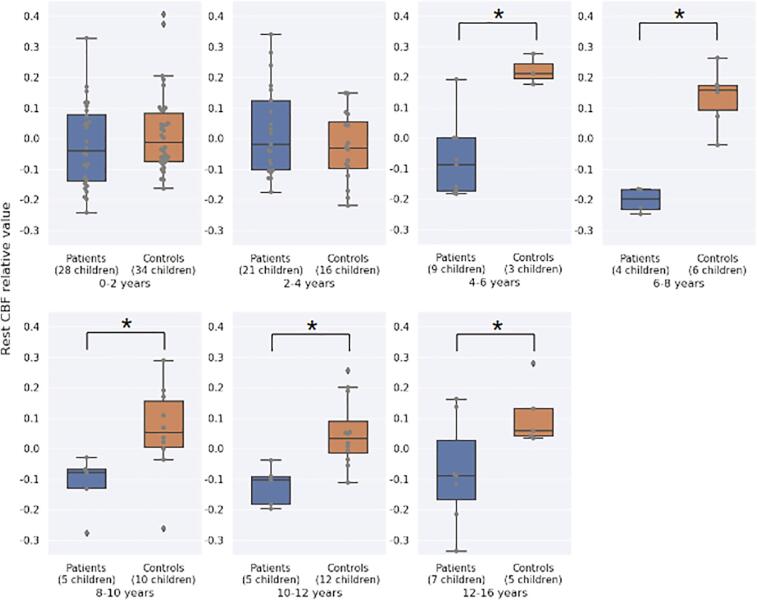
In addition, further analyses comparing mean rest-CBF relative values in these regions for specific age groups, showed no significant differences between deaf children and normally-hearing controls for 0–2 and 2–4 years old groups and a significant difference for the others age groups: 4–6, 6–8, 8–10, 10–12 and 12–16 years groups (Fig. 5).
Fig. 5.
Comparison of the mean rest-CBF relative values in temporal clusters identified in the whole-brain group comparison between normally-hearing children (orange) and deaf children (blue) for specific age groups. No significant differences for groups above 4 years: 0–2 years (t = 1.12; p = 0.266) and 2–4 years (t = −1.23; p = 0.226). However, we found significant differences for all groups after 4 years: 4–6 years (t = 5.82; p = 0.0003), 6–8 years (t = 7.46; p = 0.0001), 8–10 years (t = 2.74; p = 0.018), 10–12 years (t = 3.99; p = 0.002) and 12–16 years (t = 2.28, p = 0.047). (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
4. Discussion
To our knowledge, this is the first study to use 3D ASL-MRI at different ages and stages of brain development in infants and children as part of their preoperative work-up for cochlear implantation. It gives us insight on the brain reorganization secondary to deafness and subsequent hearing restoration from hearing aids and cochlear implants.
Our results showed that, in comparison to normally hearing children of the same age, deaf children above 5 years old present a significantly decreased cerebral blood flow at rest within temporal regions bilaterally. This decrease is more pronounced on the right side independently of the side of deafness if single-sided. In addition, a significant increased rest-CBF was also observed within occipital regions in deaf children. Importantly, even if conventional hearing devices were systematically used in patients, abnormal cortical blood flow at rest is still observed, suggesting that, even in the context of partial auditory stimulation, a functional reorganization occurs with the temporal cortex becoming less functional and the visual cortex becoming over functional. Comparable results have been reported by Coez et al. (2009) in a PET study involving adults with acquired hearing loss after auditory brainstem implant with a low average score of postoperative intelligibility. When compared to controls, patients presented higher rest PET CBF in bilateral occipital brain areas (left: x = −18, y = −86, z = 22; right: x = 14, y = −84, z = 26), and lower rest PET CBF in the left temporal areas (x = −58, y = −14, z = −2). The authors hypothesized that patients had developed and optimized their visual strategies to better perceive their environment.
Our results also showed that the degree of hyperperfusion within occipital regions regressed negatively with cochlear implant OSW scores in children older than 5 years old: deaf children who presented higher OSW scores obtained 12 months after CI were those who had lower rest CBF values in these regions prior to implantation. In normally hearing children, the role of the auditory cortical network in enabling processing of the acoustic cues required for language development is present very early in life and is quickly lost (Dehaene-Lambertz and Spelke, 2015). Profoundly deaf individuals will not be able to develop a full phonological inventory of their native language despite conventional hearing aids (McCandliss et al., 2002). Here, even though we show that deaf children seem to use the available and operational somato-sensory networks to establish ad-hoc communication (eg. visual networks if they have a deficient auditory cortical activity), we also show that over recruitment of this alternative network seems to disrupt rehabilitation of the function.
It is worth noticing that the OSW scores of most patients improved after cochlear implantation (Fig. 1), especially if the pre-OSW score was under 80%, which suggests that, despite previous partial auditory stimulation, cortical temporal areas are still capable of analyzing and processing the new incoming high-frequency phonemes that ensure further language development. As showed in Fig. 2, partial auditory stimulation prior to implantation reinforces postoperative speech performance. Even though postoperative ASL MR imaging is not feasible, our results lead us to suspect a change in brain activity at rest favoring auditory cortex over visual cortex. In a previous study comparing brain metabolism at rest using FDG-PET from post-lingually deaf adults to normally hearing controls before cochlear implant surgery, Han and colleagues described that bilateral temporal lobe hypometabolism (right: x = 54, y = -14, z = 6; left: x = −46, y = -24, z = 16) and bilateral occipital hypermetabolism (right: x = 18, y = −94, z = −4; left: x = −14, y = −94, z = −8) negatively correlated with speech outcomes at 1 year after implantation (Han et al., 2019). Here we corroborate these results in a pediatric population. Indeed, we describe not only a significantly decreased cerebral blood flow at rest within temporal regions bilaterally and significantly increased within left occipital regions, but we show that deaf children who undergo late cochlear implantation after hearing aid rehabilitation seem to develop sufficient language skills. These results reinforce the hypothesis that temporal regions remain relatively resistant to cross modal plasticity.
Our results showing differences in rest CBF across age within temporal regions between deaf and normally hearing children provides a better understanding of the developmental dynamics of deafness. Results from the whole brain group comparison showed no significant differences in rest CBF between deaf children and normally hearing children under 5 years old, suggesting that auditory deprivation does not immediately impact brain function at rest. Results showing the evolution of rest CBF across age within temporal regions indicate that functional changes, characterized by increase in rest CBF occur in these areas and continue progressively throughout childhood in normally hearing children. In deaf children, however, no rest CBF evolution was observed with age, suggesting a lack of synaptic activity in auditory cortex in the context of auditory deprivation. Importantly, the analysis performed in separated age groups showed that mean rest CBF within the Heschl’s gyri is comparable between deaf children and normally hearing controls between 0 and 4 years old, indicating equivalent brain neuronal activity. However, from this age on, significant differences are observed for all age groups (4–16 years old), which suggest that there might be a critical period around 4 years old where, in the context of auditory deprivation, typical brain maturation within the temporal cortices becomes significantly disturbed (Fig. 5). This could be associated with the fact that synapse formation begins after birth and increases rapidly in the early postnatal period and the synapses that are not activated by sensory stimulation are lost (Rakic et al., 1986). Interestingly, it has been shown that the time point for synapses loss differs across brain regions, occurring at about 2 years old in occipital areas, 4 years old in temporal areas and between 5 and 10 years old in the prefrontal cortex (Huttenlocher and Dabholkar, 1997). Previous electro-physiological studies using the P1-N1 complex of auditory evoked potentials corroborate this hypothesis, by describing a sensitive period for development of the primary auditory cortex in deaf children fitted with cochlear implants (Sharma et al., 2015): children implanted above age 3.5 years show considerable variability in P1 and N1 development, indicating poor clinical outcome, in contrast to the early implanted children.
Contrary to previous rest metabolism PET studies in deaf children, in our study all deaf children older than 5 years old included had benefited from early rehabilitation with conventional hearing aids. They had progressive deafness, meeting the eligibility criteria for late cochlear implants, including significant residual hearing (mean threshold of aided hearing was around 40 dB HL; Fig. 2) and spoken language skills. With hearing aids, all children presented functional verbal communication. Importantly, in the study of Lee et al. (2007), the best threshold of aided hearing was 70 dB HL, which might explain the negative correlation observed between age at implantation and oral intelligibility scores after cochlear implantation. In addition, even though some studies consider the impact of the “duration of auditory deprivation” (Han et al., 2019), in our study this parameter was not relevant since all children benefited from early screening and early rehabilitation by the use of hearing aids.
It is important to notice that rest CBF results presented in this study, obtained using ASL-MRI, strongly agree with FDG-PET results, indicating the robustness of these measures. The discrepancies in the neuroimaging literature in the field might be partially explained by heterogeneity of etiologies. Indeed, certain genetic causes of hearing loss can make any attempt at cochlear implant rehabilitation unsuccessful, as the neural system cannot withstand the additional current load (Delmaghani et al., 2015). In our sample, even though some patients had an identified genetic cause of deafness and despite the genetic screening recommendations, about 50% of etiologies unfortunately remained unknown. Interestingly, a preliminary study indicates that there were no notable differences between patients with connexin 26 gene mutations and patients with hearing loss from other genetic causes of unknown etiology (unpublished data). In a prospective study, we will address brain perfusion differences and cochlear implant benefit in sub-groups of different etiologies of genetic deafness. Another possible methodological limitation of this study was the use of pentobarbital in the group under 5 years old even if deaf patients and controls received the same premedication. Nevertheless, many recent studies reported no effects of pentobarbital on the regional distribution of CBF (Veselis et al., 2005, Carsin-Vu et al., 2018, Greicius et al., 2008). Moreover, other PET activation studies using the same sedation with pentobarbital found hyperperfusion in temporal regions (Boddaert et al., 2004) or hypoperfusion at rest when comparing groups of normally hearing, autistic versus non autistic children (Zilbovicius et al., 2000). DiFrancesco et al. (2013) performed a study on BOLD fMRI in infants under sedation: Comparing the Impact of Pentobarbital and Propofol on Auditory and Language Activation. They compared activation profiles in response to passive story-listening stimulation. Nembutal, had robust activation of primary and secondary sensory cortices. They concluded that clinical fMRI of sedated infants could be performed (DiFrancesco et al., 2013). We hypothesized that this limited effect of pentobarbital was similar using ASL-MRI. We also admit that the sequence parameters are not optimized for the different ages and field strengths across the study and therefore this may have influenced absolute measurement of CBF. Nevertheless, it could also be discussed as a limitation of our study that the scanner noise could have been cause an effect on perfusion. Although the scanner noise is reduced to 60 dB by earplugs it is possible that the noise partly cause a difference between hearing impaired children and hearing children especially since the sedation with pentobarbital may play a role. Pentobarbital may reduce the perception of the scanner noise also in hearing children and may lead to the lack of the difference between hearing impaired and hearing children.
OSW scores appeared to be of limited value to evaluate the effectiveness of CI, especially in the groups under 5 years old. Better outcome measures, such as listening effort including verbal response times to repeating back words (Coez et al., 2019), pupillometry (Steel et al., 2015, Aminihajibashi et al., 2019) or electrophysiology (Anderson et al., 2019, de Schonen et al., 2018) could improve the evaluation of cochlear implant effectiveness. Associated with these measures, rest-CBF investigations using ASL-MRI could help develop a useful prognostic tool to predict cochlear implant benefit.
5. Conclusion
This study provides insight into brain perfusion changes associated with deafness and subsequent auditory rehabilitation in children. A decrease rest-CBF in the auditory cortex is observed in children older than 5 years old and not in younger children, suggesting and motivating early cochlear implantation to maximize implant outcomes. These perfusion abnormalities seem to occur progressively and become significant around 4 years old, compared to typically hearing children. It would be interesting to undertake additional research in the early years of life in deaf children to fine-tune the analysis of early brain development and further determine critical periods in auditory brain development.
CRediT authorship contribution statement
Arnaud Coez: Conceptualization, Methodology, Investigation, Data curation, Writing - original draft, Writing - review & editing, Visualization, Project administration. Ludovic Fillon: Conceptualization, Methodology, Software, Validation, Formal analysis, Data curation, Writing - original draft, Writing - review & editing, Visualization. Ana Saitovitch: Validation, Writing - original draft, Writing - review & editing. Caroline Rutten: Writing - original draft, Writing - review & editing. Sandrine Marlin: Data curation, Formal analysis. Jennifer Boisgontier: Validation, Writing - original draft, Writing - review & editing, Visualization. Alice Vinçon-Leite: Investigation. Hervé Lemaitre: Software. David Grévent: Investigation. Charles-Joris Roux: Investigation. Volodia Dangouloff-Ros: Investigation. Raphaël levy: Investigation. Eric Bizaguet: Supervision. Isabelle Rouillon: Investigation. Eréa Noël Garabédian: Resources, Supervision. Françoise Denoyelle: Resources, Supervision. Monica Zilbovicius: Validation, Resources, Writing - original draft, Writing - review & editing, Supervision. Natalie Loundon: Investigation, Resources, Data curation, Writing - original draft, Writing - review & editing, Project administration. Nathalie Boddaert: Conceptualization, Methodology, Validation, Formal analysis, Resources, Data curation, Writing - original draft, Writing - review & editing, Visualization, Supervision, Project administration.
References
- Altaye M., Holland S.K., Wilke M., Gaser C. Infant brain probability templates for MRI segmentation and normalization. NeuroImage. 2008;43:721–730. doi: 10.1016/j.neuroimage.2008.07.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aminihajibashi S., Hagen T., Foldal M.D., Laeng B., Espeseth T. Individual differences in resting-state pupil size: evidence for association between working memory capacity and pupil size variability. Int. J. Psychophysiol. Off. J. Int. Organ. Psychophysiol. 2019;140:1–7. doi: 10.1016/j.ijpsycho.2019.03.007. [DOI] [PubMed] [Google Scholar]
- Anderson C.A., Wiggins I.M., Kitterick P.T., Hartley D.E.H. Pre-operative brain imaging using functional near-infrared spectroscopy helps predict cochlear implant outcome in deaf adults. J. Assoc. Res. Otolaryngol. JARO. 2019 doi: 10.1007/s10162-019-00729-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai X., Nian S., Feng L., Ruan Q., Luo X., Wu M., Yan Z. Identification of novel variants in MYO15A, OTOF, and RDX with hearing loss by next-generation sequencing. Mol. Genet. Genomic Med. 2019;7 doi: 10.1002/mgg3.808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benetti S., van Ackeren M.J., Rabini G., Zonca J., Foa V., Baruffaldi F., Rezk M., Pavani F., Rossion B., Collignon O. Functional selectivity for face processing in the temporal voice area of early deaf individuals. Proc. Natl. Acad. Sci. U. S. A. 2017;114:E6437–E6446. doi: 10.1073/pnas.1618287114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butcher E., Dezateux C., Cortina-Borja M., Knowles R.L. Prevalence of permanent childhood hearing loss detected at the universal newborn hearing screen: systematic review and meta-analysis. PLoS ONE. 2019;14 doi: 10.1371/journal.pone.0219600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boddaert N., Chabane N., Belin P., Bourgeois M., Royer V., Barthelemy C., Mouren-Simeoni M.-C., Philippe A., Brunelle F., Samson Y., Zilbovicius M. Perception of complex sounds in autism: abnormal auditory cortical processing in children. Am. J. Psychiatry. 2004;161:2117–2120. doi: 10.1176/appi.ajp.161.11.2117. [DOI] [PubMed] [Google Scholar]
- Byrne D., Dillon H., Tran K., Arlinger S., Wilbraham K., Cox R., Hagerman B., Hetu R., Kei J., Lui C., Kiessling J., Kotby M.N., Nasser N.H.A., El Kholy W.A.H., Nakanishi Y., Oyer H., Powell R., Stephens D., Meredith R., Sirimanna T., Tavartkiladze G., Frolenkov G.I., Westerman S., Ludvigsen C. An international comparison of long-term average speech spectra. J. Acoust. Soc. Am. 1994;96:2108–2120. doi: 10.1121/1.410152. [DOI] [Google Scholar]
- Cardin V., Smittenaar R.C., Orfanidou E., Rönnberg J., Capek C.M., Rudner M., Woll B. Differential activity in Heschl’s gyrus between deaf and hearing individuals is due to auditory deprivation rather than language modality. NeuroImage. 2016;124:96–106. doi: 10.1016/j.neuroimage.2015.08.073. [DOI] [PubMed] [Google Scholar]
- Cardon G., Sharma A. Somatosensory cross-modal reorganization in children with cochlear implants. Front. Neurosci. 2019;13:469. doi: 10.3389/fnins.2019.00469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carsin-Vu A., Corouge I., Commowick O., Bouzillé G., Barillot C., Ferré J.-C., Proisy M. Measurement of pediatric regional cerebral blood flow from 6 months to 15 years of age in a clinical population. Eur. J. Radiol. 2018;101:38–44. doi: 10.1016/j.ejrad.2018.02.003. [DOI] [PubMed] [Google Scholar]
- Coez A., Filippi R., Nahmani Y., Bischoff H., Legoff S., Ezine-Feller M., Bertaux A., Deraison S., Bizaguet E. Audiométrie vocale chronométrée: le temps de réaction pour répéter un mot, indicateur de l’effort attentionnel fourni. Cah. Audit. 2019;2:15–17. [Google Scholar]
- Coez A., Zilbovicius M., Ferrary E., Bouccara D., Mosnier I., Ambert-Dahan E., Kalamarides M., Bizaguet E., Syrota A., Samson Y., Sterkers O. Processing of voices in deafness rehabilitation by auditory brainstem implant. NeuroImage. 2009;47:1792–1796. doi: 10.1016/j.neuroimage.2009.05.053. [DOI] [PubMed] [Google Scholar]
- de Schonen S., Bertoncini J., Petroff N., Couloigner V., Van Den Abbeele T. Visual cortical activity before and after cochlear implantation: A follow up ERP prospective study in deaf children. Int. J. Psychophysiol. Off. J. Int. Organ. Psychophysiol. 2018;123:88–102. doi: 10.1016/j.ijpsycho.2017.10.009. [DOI] [PubMed] [Google Scholar]
- Dehaene-Lambertz G., Spelke E.S. The infancy of the human Brain. Neuron. 2015;88:93–109. doi: 10.1016/j.neuron.2015.09.026. [DOI] [PubMed] [Google Scholar]
- Delmaghani S., Defourny J., Aghaie A., Beurg M., Dulon D., Thelen N., Perfettini I., Zelles T., Aller M., Meyer A., Emptoz A., Giraudet F., Leibovici M., Dartevelle S., Soubigou G., Thiry M., Vizi E.S., Safieddine S., Hardelin J.-P., Avan P., Petit C. Hypervulnerability to sound exposure through impaired adaptive proliferation of peroxisomes. Cell. 2015;163:894–906. doi: 10.1016/j.cell.2015.10.023. [DOI] [PubMed] [Google Scholar]
- Detre J.A., Alsop D.C. Perfusion magnetic resonance imaging with continuous arterial spin labeling: methods and clinical applications in the central nervous system. Eur. J. Radiol. 1999;30:115–124. doi: 10.1016/s0720-048x(99)00050-9. [DOI] [PubMed] [Google Scholar]
- DiFrancesco M.W., Robertson S.A., Karunanayaka P., Holland S.K. BOLD fMRI in infants under sedation: Comparing the impact of pentobarbital and propofol on auditory and language activation. J. Magn. Reson. Imaging JMRI. 2013;38:1184–1195. doi: 10.1002/jmri.24082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorman M.F., Sharma A., Gilley P., Martin K., Roland P. Central auditory development: evidence from CAEP measurements in children fit with cochlear implants. J. Commun. Disord. 2007;40:284–294. doi: 10.1016/j.jcomdis.2007.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finney E.M., Fine I., Dobkins K.R. Visual stimuli activate auditory cortex in the deaf. Nat. Neurosci. 2001;4:1171–1173. doi: 10.1038/nn763. [DOI] [PubMed] [Google Scholar]
- Greicius M.D., Kiviniemi V., Tervonen O., Vainionpää V., Alahuhta S., Reiss A.L., Menon V. Persistent default-mode network connectivity during light sedation. Hum. Brain Mapp. 2008;29:839–847. doi: 10.1002/hbm.20537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han J.-H., Lee H.-J., Kang H., Oh S.-H., Lee D.S. Brain plasticity can predict the cochlear implant outcome in adult-onset deafness. Front. Hum. Neurosci. 2019;13:38. doi: 10.3389/fnhum.2019.00038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huttenlocher P.R., Dabholkar A.S. Regional differences in synaptogenesis in human cerebral cortex. J. Comp. Neurol. 1997;387:167–178. doi: 10.1002/(sici)1096-9861(19971020)387:2<167::aid-cne1>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- Ideura M., Nishio S.-Y., Moteki H., Takumi Y., Miyagawa M., Sato T., Kobayashi Y., Ohyama K., Oda K., Matsui T., Ito T., Suzumura H., Nagai K., Izumi S., Nishiyama N., Komori M., Kumakawa K., Takeda H., Kishimoto Y., Iwasaki S., Furutate S., Ishikawa K., Fujioka M., Nakanishi H., Nakayama J., Horie R., Ohta Y., Naito Y., Kakudo M., Sakaguchi H., Kataoka Y., Sugahara K., Hato N., Nakagawa T., Tsuchihashi N., Kanda Y., Kihara C., Tono T., Miyanohara I., Ganaha A., Usami S.-I. Comprehensive analysis of syndromic hearing loss patients in Japan. Sci. Rep. 2019;9:11976. doi: 10.1038/s41598-019-47141-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang E., Lee D.S., Kang H., Lee J.S., Oh S.H., Lee M.C., Kim C.S. Neural changes associated with speech learning in deaf children following cochlear implantation. NeuroImage. 2004;22:1173–1181. doi: 10.1016/j.neuroimage.2004.02.036. [DOI] [PubMed] [Google Scholar]
- Lee H.-J., Giraud A.-L., Kang E., Oh S.-H., Kang H., Kim C.-S., Lee D.S. Cortical activity at rest predicts cochlear implantation outcome. Cereb. Cortex N. Y. N. 2007;1991(17):909–917. doi: 10.1093/cercor/bhl001. [DOI] [PubMed] [Google Scholar]
- MacSweeney M., Capek C.M., Campbell R., Woll B. The signing brain: the neurobiology of sign language. Trends Cogn. Sci. 2008;12:432–440. doi: 10.1016/j.tics.2008.07.010. [DOI] [PubMed] [Google Scholar]
- Marlin S., Feldmann D., Blons H., Loundon N., Rouillon I., Albert S., Chauvin P., Garabédian E.-N., Couderc R., Odent S., Joannard A., Schmerber S., Delobel B., Leman J., Journel H., Catros H., Lemarechal C., Dollfus H., Eliot M.-M., Delaunoy J.-L., David A., Calais C., Drouin-Garraud V., Obstoy M.-F., Goizet C., Duriez F., Fellmann F., Hélias J., Vigneron J., Montaut B., Matin-Coignard D., Faivre L., Baumann C., Lewin P., Petit C., Denoyelle F. GJB2 and GJB6 mutations: genotypic and phenotypic correlations in a large cohort of hearing-impaired patients. Arch. Otolaryngol. Head Neck Surg. 2005;131:481–487. doi: 10.1001/archotol.131.6.481. [DOI] [PubMed] [Google Scholar]
- McCandliss B.D., Fiez J.A., Protopapas A., Conway M., McClelland J.L. Success and failure in teaching the [r]-[l] contrast to Japanese adults: tests of a Hebbian model of plasticity and stabilization in spoken language perception. Cogn. Affect. Behav. Neurosci. 2002;2:89–108. doi: 10.3758/cabn.2.2.89. [DOI] [PubMed] [Google Scholar]
- Rakic P., Bourgeois J.P., Eckenhoff M.F., Zecevic N., Goldman-Rakic P.S. Concurrent overproduction of synapses in diverse regions of the primate cerebral cortex. Science. 1986;232:232–235. doi: 10.1126/science.3952506. [DOI] [PubMed] [Google Scholar]
- Sharma A., Campbell J., Cardon G. Developmental and cross-modal plasticity in deafness: evidence from the P1 and N1 event related potentials in cochlear implanted children. Int. J. Psychophysiol. Off. J. Int. Organ. Psychophysiol. 2015;95:135–144. doi: 10.1016/j.ijpsycho.2014.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon F., Roman S., Truy E., Barone P., Belmin J., Blanchet C., Borel S., Charpiot A., Coez A., Deguine O., Farinetti A., Godey B., Lazard D., Marx M., Mosnier I., Nguyen Y., Teissier N., Virole B., Lescanne E., Loundon N. Guidelines (short version) of the French Society of Otorhinolaryngology (SFORL) on pediatric cochlear implant indications. Eur. Ann. Otorhinolaryngol. Head Neck Dis. 2019;136:385–391. doi: 10.1016/j.anorl.2019.05.018. [DOI] [PubMed] [Google Scholar]
- Steel M.M., Papsin B.C., Gordon K.A. Correction: binaural fusion and listening effort in children who use bilateral cochlear implants: a psychoacoustic and pupillometric study. PLoS ONE. 2015;10 doi: 10.1371/journal.pone.0141945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veselis R.A., Feshchenko V.A., Reinsel R.A., Beattie B., Akhurst T.J. Propofol and thiopental do not interfere with regional cerebral blood flow response at sedative concentrations. Anesthesiology. 2005;102:26–34. doi: 10.1097/00000542-200501000-00008. [DOI] [PubMed] [Google Scholar]
- Zilbovicius M., Boddaert N., Belin P., Poline J.B., Remy P., Mangin J.F., Thivard L., Barthélémy C., Samson Y. Temporal lobe dysfunction in childhood autism: a PET study. Positron emission tomography. Am. J. Psychiatry. 2000;157:1988–1993. doi: 10.1176/appi.ajp.157.12.1988. [DOI] [PubMed] [Google Scholar]