Abstract
Purpose of Review
This review describes the novel category of wearable ECG monitors and identifies where patients, healthcare providers, and device manufacturers should focus efforts to maximize the clinical benefit of these devices.
Recent Findings
Notable wearable ECG monitors include the AliveCor Kardia devices, Apple Watch Series 4, and several others. The most common use case is monitoring for atrial fibrillation. The available evidence validates the ability of the Kardia devices and Apple Watch to distinguish atrial fibrillation from sinus rhythm. Key questions for manufacturers include how to calibrate each device’s algorithms and streamline workflows for healthcare providers.
Summary
Wearable ECG monitors are currently most useful to detect atrial fibrillation. Further study is needed to demonstrate whether wearable ECG monitors improve patient outcomes, and to expand their use into other indications. Device manufacturers and healthcare providers must work together to establish new workflows to process and act on wearable ECG data.
Keywords: Atrial fibrillation, Stroke, Wearables, Electrocardiogram, Apple Watch, AliveCor
Introduction: the Promise and the Hype
“We always overestimate the change that will occur in the next two years and underestimate the change that will occur in the next ten.”– Bill Gates [1]
In recent years, consumer technology and medicine have been on an inevitable collision course, perennially promising to revolutionize healthcare but always falling just short. Over time, our smart devices have acquired more sensors and increasingly powerful processors, electronic health records have digitized vast amounts of health data, and digital workflows have spread to the hospital and clinic. Now that smart devices can be continuously worn, medicine and high technology appear destined to finally converge.
Cardiovascular medicine is a particularly attractive target for consumer technology giants. Apple, Alphabet (formerly Google), Amazon, and Microsoft, to name a few notable companies, each have projects aimed at heart health. This is not a coincidence. Heart disease in its various forms is prevalent, morbid, and measurable in ways well suited to our digital age. After all, electrocardiography (ECG) has been used for over a century and photoplethysmography (PPG) is standard in both clinical and consumer contexts. What is new is that ECG technology is now being built into wearable devices capable of making a diagnosis even before a physician is involved. Health data is increasingly being collected and interpreted outside of the healthcare enterprise, heralding the decentralization of medicine from the hospital and clinic to the open world in which patients live.
However, like all technologies, wearable ECG monitors are subject to the cycle of hype and disillusionment that Bill Gates described over two decades ago. This review aims to describe novel consumer-targeted wearable ECG monitors, scrutinize the promises made to consumers, and pinpoint gaps that must be addressed to establish their clinical utility. We will point out areas where key stakeholders—patients, healthcare providers, and device manufacturers—should focus attention to prepare themselves for the future of cardiac rhythm monitoring.
The Landscape
Only a handful of wearable devices record ECG tracings. The most prominent of these are the Apple Watch Series 4 and the Kardia devices made by AliveCor, including the KardiaBand watch strap and the smartphone attachment KardiaMobile [2–4]. QardioCore and Hexoskin are chest-worn devices capable of recording ECG tracings [5, 6]. Digital health companies Withings and Verily (a subsidiary of Alphabet) have also announced upcoming smartwatches with ECG technology [7, 8]. These devices and several others are listed in Table 1 and will be the focus of this review.
Table 1.
Description of wearable ECG devices
| Device | Company | Features | Price | Form factor | FDA clearance status and indication | Notes |
|---|---|---|---|---|---|---|
| Apple Watch Series 4 | Apple | Continuous HR monitoring Opportunistic AF detection On-demand 1-lead ECG with AF vs sinus rhythm classification |
Starts at $399 | Watch | ECG App: cleared to create, record, store, transfer, and display single channel ECG, and to determine presence of AF or sinus rhythm Heart rhythm notifications: cleared to analyze pulse rate data to identify irregular heart rhythms suggestive of AF and notify the user |
Requires Apple iPhone Heart rhythm notification feature utilizes PPG alone and is not cleared for use in patients with known AF ECG app is cleared for use in patients with AF but not other arrhythmias |
| KardiaMobile | AliveCor | On-demand 1-lead ECG with AF vs sinus rhythm classification | $99 with optional $9.99/month Premium service | Smartphone attachment | Cleared to create, record, store, transfer, and display single channel ECG, and to determine presence of AF or sinus rhythm | Works with iOS or Android devices |
| KardiaMobile 6L | AliveCor | On-demand 6-lead ECG with AF vs sinus rhythm classification | $149 with optional $9.99/month Premium service | Smartphone attachment | Cleared to create, record, store, transfer, and display six channel ECG, and to determine presence of AF, sinus rhythm, tachycardia, and bradycardia via KardiaAI platform | Works with iOS or Android devices |
| KardiaBand | AliveCor | On-demand 1-lead ECG with AF vs sinus rhythm classification | $99 for band Requires $9.99/month Premium service |
Watch strap | Cleared to create, record, store, transfer, and display single channel ECG, and to determine presence of AF or sinus rhythm | Works only with Apple Watch |
| QardioCore | Qardio | Continuous 1-lead ECG HRV calculation |
Not yet for sale in US pending FDA clearance | Chest strap | Pending | Requires an iOS device Requires a prescription |
| Hexoskin | Hexoskin | Continuous 3-lead ECG HRV calculation |
Starts at $399 | Athletic shirt | Unknown status | Works with iOS or Android devices |
| Amazfit Health Band | Huami | On-demand 1-lead ECG | n/a | Wrist band | Unknown status | Currently available in China |
| CALM | EMC Healthcare | Continuous 1-lead ECG | $260 | Chest strap or sticker | Unknown status | Works with iOS or Android devices Currently sold for research and athletic use |
| Heartbit | Heartbit | Continuous 3-lead ECG | n/a | Athletic Shirt | Pending | |
| Move ECG | Withings | On-demand 1-lead ECG | TBD, expected launch Spring 2019 | Watch | Pending | Works with iOS or Android devices |
| Study Watch | Verily | On-demand 1-lead ECG | n/a | Watch | Cleared to record, store, transfer and display single-channel ECG rhythms | Investigational device Requires a prescription Transfers waveforms to a server to be interpreted by the patient’s physician Does not include any analysis features |
Summary of features, prices, and form factors of consumer-targeted wearable ECG devices that are on the United States market or have been announced [2–20]
AF atrial fibrillation, ECG electrocardiogram, HR heart rate, HRV heart rate variability, PPG photoplethysmography, FDA Food and Drug Administration
Key Questions
Figure 1 depicts the steps by which consumer-targeted wearable ECG monitors can improve patient outcomes. Within this framework, there are six important questions to be asked of any wearable ECG monitor:
What job does the device accomplish for the consumer/patient?
What physical form does the device take?
What are the diagnostic test characteristics of the device and its algorithms?
How will the device integrate into the workflows of healthcare providers?
Does the device improve patient outcomes, and does it do so in a cost-effective manner?
What legal, financial and social obstacles might affect the device’s adoption?
Fig. 1.
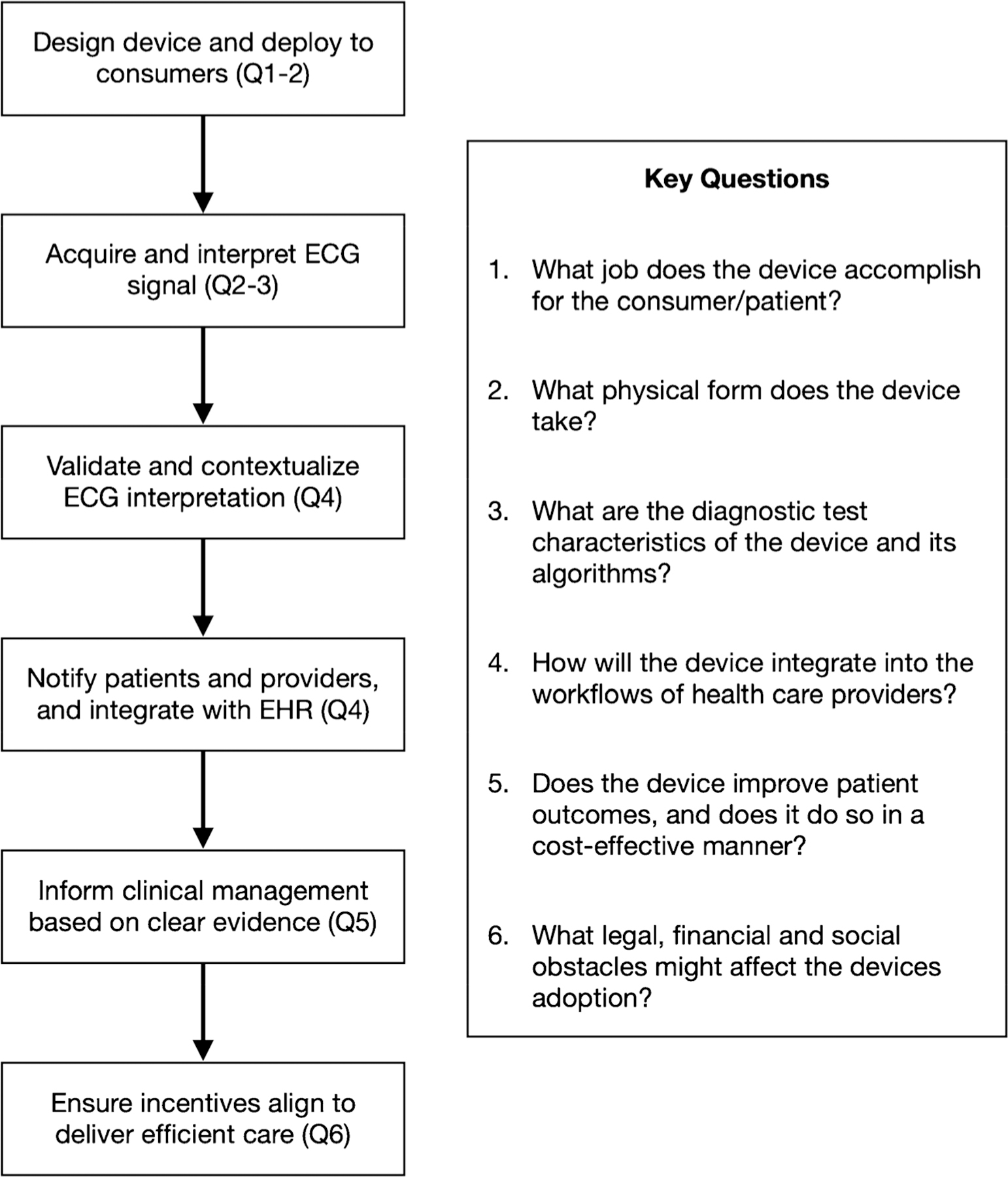
A framework describing the steps by which wearable ECG monitors can improve patient outcomes, and associated key questions
We will examine each of these questions in turn, with examples drawn from various wearable ECG monitors.
Jobs-to-be-done
The first question that any new technology must address is: what purpose does it serve? In medical terminology: what is the indication? In The Innovator’s Dilemma, Clayton Christensen defines innovation as the direction of a new technology toward a “job-to-be-done,” the task a potential customer is trying to accomplish [21]. For example, if my job-to-be-done is to communicate with a friend, the technologies I could use include pen and paper, a telegraph, a telephone, or text messaging. While the technology varies, my end goal is the same.
What is the job-to-be-done by wearable ECG monitors? The answer often depends on whether you ask the patient or their healthcare provider. Although a patient’s goal might be to maximize their general health, their provider may have a distinct and more specific goal, for example, monitoring for atrial fibrillation (AF).
Review of marketing websites for wearable ECG monitors reflects the dual nature of the job-to-be-done. The most concrete function advertised for these devices is detection of AF. The KardiaMobile, KardiaBand, Apple Watch Series 4, and Withings Move ECG are all marketed for this purpose [2–4, 7]. The Apple Watch additionally detects tachycardia or bradycardia, with user-adjustable thresholds [22]. However, both AliveCor and Apple also make a more general promise to consumers that their devices offer “peace of mind,” with Apple labeling their device a “guardian” [2, 4].
Some manufacturers of other devices employ less specific marketing for products, without mention of AF or any other specific cardiac condition. For example, the QardioCore claims that it “track[s] your complete heart health” and uses ECG tracings for “deeper heart health insights” [5]. The Hexoskin emphasizes the number of sensors it contains, which is marketed to “healthies” and researchers [6]. Neither of these companies describe which specific heart conditions their devices monitor. Marketing for the Amazfit Health Band includes this exceedingly broad claim: “From irregular heart beat (arrhythmias) to weakness in the heart (cardiomyopathy), any risk or danger can be instantly detected with the Health Band” [9].
While the Kardia devices and Apple Watch are FDA cleared to discriminate AF from sinus rhythm, the QardioCore, Hexoskin, and Amazfit Health Band are not cleared for any indication [10–13]. It is important to note the difference between FDA “clearance” and “approval.” FDA pre-market “approval” is a higher standard typically required for devices that pose more than minimal risk of harm to patients, and requires submission of clinical data that demonstrate the safety and effectiveness of the device [23]. On the other hand, devices with lower risk of harm can be “cleared” by demonstrating substantial equivalence to a similar device already on the market, or via a “De Novo” request that demonstrates acceptable safety parameters if no comparable devices exist [23]. The existing wearable ECG monitors evaluated by the FDA fall into the moderate-risk category, requiring only FDA clearance.
Among the devices that monitor for AF, and even between features of a single device, there can be different indications and target audiences. Although they are available to anyone for purchase, marketing for the Kardia devices tends to focus on patients with a known diagnosis of AF. The AliveCor product pages highlight testimonials such as: “If you are suffering from AFib… you should get this” and “My cardiologist loves the reports it produces” [3, 4]. In contrast, Apple markets the Apple Watch as a screening device for those without known arrhythmia. In fact the PPG-based irregular rhythm notification algorithm is not FDA cleared for use in patients with AF [14, 22]. The Apple Watch’s ECG app is a separate feature that is cleared for use to supplement rhythm discrimination of AF from normal sinus rhythm, and is explicitly not recommended for patients with other known arrhythmias [13].
When purchasing a wearable ECG device, patients should expect it to serve a defined purpose beyond generic promises of promoting cardiac health. While Apple, AliveCor, and Withings market their wearable ECG monitors to the narrow indication for which they have sought FDA clearance—namely detecting AF—patients ought to be skeptical of other manufacturers’ unsubstantiated claims of promoting general “heart health.” They should expect manufacturers to communicate a device’s purpose clearly in their marketing, without making promises that they cannot keep. Moreover, current marketing does not acknowledge inconsistent recommendations in practice guidelines surrounding screening for AF using electrocardiographic monitors [24, 25].
AF screening is a natural first application for wearable ECG monitors given the condition’s prevalence and ease of diagnosis, and the possibility of it first presenting with a potentially preventable stroke. However, healthcare providers should work with manufacturers to identify new indications for wearable ECG monitors that are relevant to clinical practice. For example, in addition to screening asymptomatic consumers, wearable ECG monitors could become a longer-term, less invasive alternative to conventional event monitors or implantable loop recorders to screen for AF in patients with cryptogenic stroke.
Looking beyond AF, wearable ECG monitors could screen for other asymptomatic or paroxysmal arrhythmias, detect pre-excitation patterns, or identify a long QT interval in a patient who might otherwise first present with sudden cardiac death. In patients who have undergone cardioversion or ablation, they could be used to monitor for recurrent arrhythmia. Wearable ECG monitors could track the QT interval in patients starting antiarrhythmic drugs, avoiding admission to a telemetry unit. Indeed, some of these uses for wearable ECG monitors have been studied [26, 27].
In addition to electrophysiologic indications, new machine learning techniques are being developed to use ECG data to derive insight into other conditions. Buzzwords such as “heart rate variability” are already used in marketing for wearable ECG monitors [5, 6, 9], and though they do not yet have clear clinical utility, heart rate variability and other parameters are being studied in many cardiac and non-cardiac diseases. The amount of useful data provided by wearable ECG monitors will likely only grow as they become more widespread and well-studied.
Form Factors
The form of a wearable ECG monitor influences the contexts in which it can be used and the ECG leads it employs, in turn affecting how well it serves its function. For example, compare two products made by AliveCor: the smartphone-attached KardiaMobile and the wrist-worn KardiaBand. While both provide on-demand ECG functionality, the KardiaBand allows for continuous heart rate monitoring via PPG, prompting the user to obtain an ECG if an abnormality is detected [3, 4], making it better suited to continuous rhythm monitoring.
However, wrist-worn devices are best suited to measuring ECG lead I, which is not the ideal lead to detect P waves, potentially affecting the device’s ability to discriminate AF from sinus rhythm. The KardiaMobile, in comparison, has a pair of electrodes that can easily be positioned to measure virtually any vector. AliveCor has also announced a new device, KardiaMobile 6L, that contains a third electrode to allow for measurement of a 6-lead ECG [15].
Other devices, including the QardioCore, Hexoskin, CALM, and Heartbit, are larger and worn across the chest or as a shirt, allowing for measurement of different or multiple leads, but also making them more cumbersome to wear [5, 6, 16–18]. Although each form factor has its advantages, the unobtrusive nature of the wrist-based wearable and its potential for continuous rhythm monitoring when paired with PPG may make it more attractive for patients and therefore more useful to providers.
Algorithms
While ECG technology has existed for over a century, it has always required interpretation by a knowledgeable expert. As cardiac rhythm assessment shifts away from the healthcare enterprise to become more consumer-directed, a major challenge for device manufacturers is creating the algorithms that interpret raw ECG data for the end-consumer. These algorithms are diagnostic tests governed by all the same parameters—sensitivity, specificity, positive- and negative-predictive values—as any other diagnostic test.
The question for device manufacturers is how to tune these algorithms. Just as clinical medicine has screening and confirmatory tests that detect the same disease with different test characteristics, different consumer products with different jobs-to-be-done must adopt different algorithms based on the purpose they serve. Furthermore, clinicians must account for the characteristics of these algorithms—and disease prevalence in the population being tested—when interpreting their output.
Take the Apple Watch Series 4 as an example. The Apple Watch is a mass-market device whose audience has a low pre-test probability of AF, as evidenced by the Apple Heart Study, in which only 6% of participants were 65 or older [28]. Therefore, a more specific algorithm is desirable to limit false positive results, thereby reducing patient anxiety and burden to the healthcare system. Although this will leave some consumers with AF undiagnosed, they are no worse off than if they never purchased the device. In fact, a preference for specificity over sensitivity is seen in Apple’s FDA request for De Novo classification, which sets a target of 92% specificity and 90% sensitivity for their device’s AF detection algorithm [13].
Conversely, a device for patients with known AF may prioritize sensitivity over specificity, given higher pre-test probability. This population is more likely to tolerate false positives to detect more episodes of AF, since recurrence could change management. The AliveCor devices have been shown to have higher sensitivity than specificity across multiple studies, as summarized in Table 2 [29, 30, 31•, 32–34].
Table 2.
Published evidence validating AF detection algorithms
| Device Study | Sensitivity* | Specificity* | Kappa | Population | Reference standard | Notes |
|---|---|---|---|---|---|---|
| KardiaMobile | ||||||
| Lau et al. (2013) | 98% | 97% | 0.92 | Patients with and without AF | Cardiologist interpretation of 12-lead ECG | |
| Lowres et al. (2014) | 98.5% | 91.4% | Not reported | Opportunistic sample of patients age ≥ 65 in community pharmacies, excluding those with paced rhythm |
Cardiologist interpretation of KardiaMobile ECG ± 12-lead ECG | |
| Desteghe et al. (2016) | 78.9% (geriatric ward) 54.5% (cardiology ward) |
97.9% (geriatric ward) 97.5% (cardiology ward) |
0.80 (geriatric ward) 0.57 (cardiology ward) |
Hospitalized patients on geriatric or cardiology wards, excluding PPM/ICD patients | Consensus interpretation of 12- or 6-lead ECG by two EPs | Includes unreadable recordings |
| Chan et al. (2017) | 66.7% | 99.6% | Not reported | Patients ≥ 65 with HTN or DM at an outpatient clinic, excluding PPM/ICD patients | Interpretation of AliveCor ECG by two cardiologists | |
| Brasier et al. (2018) | 99.6% | 97.8% | Not reported | Hospitalized patients without PPM/ICD | Interpretation of KardiaMobile ECG by at least two cardiologists | Excluded unclassifiable recordings |
| Koshy et al. (2018) | 77% (unclassified marked incorrect) 100% (unclassified excluded) |
76% (unclassified marked incorrect) 95% (unclassified excluded) |
0.53 (unclassified marked incorrect) 0.86 (unclassified excluded) |
Consecutive patients undergoing electrical cardioversion for AF/AFL | Cardiologist interpretation of 12-lead ECG | |
| William et al. (2018) | 96.6% | 94.1% | 0.89 | Consecutive patients with AF admitted for antiarrhythmic drug initiation | EP interpretation of 12-lead ECG | Excluded unclassifiable recordings |
| KardiaBand | ||||||
| Bumgarner et al. (2018) | 93% | 84% | 0.77 | Consecutive patients with AF presenting for cardioversion | EP interpretation of 12-lead ECG | Excluded unclassifiable recordings |
| Wasserlauf et al. (2019) | 97.5% (by episode lasting ≥1 h) 97.7% (by duration, i.e. 1 h blocks) 100% (by patient during time worn) |
n/a | n/a | 24 patients with implantable cardiac monitors and history of paroxysmal AF | Implantable cardiac monitor rhythm determination | Demonstrated PPV of 39.9%, though likely underestimated due to false positives on implantable cardiac monitors |
| Apple Watch | ||||||
| FDA De Novo classification request | 98.3% | 99.6% | Not reported | Patients with known AF or no known cardiac rhythm abnormalities | Interpretation of 12-lead ECG by three cardiologists | Excludes unclassifiable recordings |
Data displayed for the validation of AF ECG detection algorithms in the KardiaMobile, KardiaBand, and Apple Watch devices [13, 29, 30, 31•, 32–36, 37•, 38]
Sensitivity and specificity refer to performance of the AF detection algorithm compared with the specified reference standard
AF atrial fibrillation, AFL atrial flutter, DM diabetes mellitus, ECG electrocardiogram, EP electrophysiologist, FDA Food and Drug Administration, HR heart rate, HRV heart rate variability, HTN hypertension, ICD implantable cardioverter/defibrillator, PPG photoplethysmography, PPM permanent pacemaker, PPV positive predictive value
The algorithms used in wearable ECG monitors need not be static. If algorithms could dynamically adjust based on a patient’s pre-test probability of AF. For example, a device could estimate pre-test probability by calculating a patient’s CHARGE-AF score [39] and use a higher sensitivity algorithm for patients with higher scores. Electronic health record (EHR) integration could also offer manufacturers feedback on whether a diagnosis was confirmed, allowing for better training of artificial intelligence (AI) algorithms.
Recently the first AI-powered diagnostic test requiring no human input was cleared by the FDA [40]. In the future, wearable ECG monitors employing algorithms of near-perfect sensitivity and specificity may become a new gold standard, rendering the distinction between screening and monitoring algorithms irrelevant and obviating the need for validation testing. Nevertheless, when deployed at scale even slightly imperfect algorithms will surface disease that would otherwise have gone undetected.
Workflows
The implementation of EHRs has shown that digitization without improvements to workflows can impede rather than facilitate patient care. Therefore, to be clinically effective, new medical technologies require careful attention to provider workflows.
At a minimum, a wearable ECG monitor must provide clinicians with the output of its algorithms (e.g., AF or sinus rhythm) and the corresponding rhythm strip. However, the device’s clinical utility could be improved by providing relevant context, as is already done in some EHR-based clinical decision support tools that are being studied to improve outcomes in atrial fibrillation [41]. For example, a device that is integrated with an EHR system could use the patient’s CHARGE-AF score [39] and the device’s sensitivity and specificity to calculate the positive predictive value and posterior probability of AF given a positive result. It could further provide the patient’s CHA2DS2-VASc [42] and HAS-BLED [43] scores to help providers decide about anticoagulation. The device could quantify the patient’s AF burden and use accelerometer data to correlate episodes of AF with decreased activity tolerance. Indeed, companies such as Google, Fitbit, and Apple are consolidating data stored in traditional EHRs onto their respective platforms, putting them in prime position to integrate EHR and wearable ECG data [44–47].
The largest obstacle wearable ECG monitors face is how they will integrate into existing healthcare infrastructure. One promised benefit of wearable ECG monitors is that they can present outpatient rhythm data to clinicians in near real-time before a patient becomes symptomatic. In their current form, the KardiaBand, KardiaMobile, and Apple Watch largely rely on the consumer to bring abnormal results to the attention of their provider. In doing so they leverage existing healthcare workflows, rather than redefining them. However, the current infrastructure of healthcare would be entirely overwhelmed by the volume of notifications that wearable ECG monitors are likely to generate. This is a critical problem that will need to be jointly addressed by manufacturers, providers, and other stakeholders within the healthcare enterprise.
Some manufacturers have attempted to address provider workflows by creating remote patient monitoring systems that integrate with their devices. AliveCor’s KardiaPro platform aims to be the clinician’s dashboard to monitor patients using their devices [48]. Qardio offers a similar service called QardioMD that integrates with the company’s connected ECG, blood pressure, and weight tracking products [49]. These remote patient monitoring systems represent a departure from the traditional patient care workflow from being patient-initiated and office- or hospital-based to one that is continuous and not bound to healthcare settings. While they pose a risk of fragmenting provider workflow by compartmentalizing patient data, if properly integrated with existing EHR systems, they can become powerful, streamlined tools for providers to manage arrhythmias in outpatients.
To adapt the healthcare enterprise to the forthcoming world of high-volume notifications and remote data collection, manufacturers must collaborate with providers and healthcare administrators to identify how remote patient monitoring systems can integrate into new clinical workflows. This requires not only development of intuitive and easy-to-use software but also the creation of new infrastructure to efficiently receive, integrate, contextualize, and respond to data created by wearable ECG devices. Consumer- and provider-oriented decision support tools that prompt individuals and their providers to take specific action based on findings may be important, but uncertainty exists about the optimal format of such tools and the amount of arrhythmia warranting evaluation. An alternative standard for high-throughput management could be modeled on existing systems such as anticoagulation or pacemaker/defibrillator clinics, which utilize specialized support staff and remote patient management to semi-algorithmically process and respond to large volumes of patient data.
In designing a “wearables clinic” and its accompanying software, there are several important principles to consider. First, real-time notifications to providers must be used for only the most important, immediately actionable information to minimize the risk of alarm fatigue and limit the burden to the system. For example, detection of new AF in a patient requiring anticoagulation may merit a notification whereas recurrent AF in an anticoagulated patient would not. Second, historic ECG data must be easily interpretable in an overview format, with the option to view more granular detail, down to individual tracings, on demand. Third, robust workflows for basic management of common findings must be developed. For example, patients found by their device to have new AF should seamlessly have the diagnosis validated, have guideline-indicated evaluations and tests coordinated, and receive education and a prescription for anticoagulation as appropriate. Finally, there must be an easily accessible human face to the clinic, to contextualize the findings of wearable ECG monitors for patients and provide education and reassurance when needed.
Evidence
Providers and their patients should judge wearable ECG monitors by the evidence that proves their benefit. This evidence comes in three types: validation (does the device accomplish what it claims to do?), outcomes (does it help patients?), and cost-effectiveness (is it worth it?).
Most data on wearable ECG monitors are validation studies testing algorithm performance in distinguishing AF from sinus rhythm (see Table 2). The AliveCor KardiaMobile (previously the AliveCor Heart Monitor) has been on the market the longest and correspondingly has been studied more than other monitors. In 2013, Lau et al. published the first validation study of the AliveCor AF detection algorithm, which found a sensitivity of 98% and specificity of 97% [29]. Several subsequent studies found similar results, as long as “unclassifiable” tracings were excluded [30, 31•, 33, 34]. However, performance suffered if “unclassifiable” tracings were included, with Desteghe et al. reporting sensitivity as low as 36.8% in one sub-population of cardiac patients [32]. Notably, Koshy et al. found that rhythm assessment by the AliveCor device supplemented by physician review of unclassifiable tracings performed better than either alone in most cases [31•], highlighting the importance of reader interpretive skill in maximizing effectiveness and minimizing misdiagnosis when implementing wearable ECG technology [50, 51].
Other devices have more limited data, with only two published peer-reviewed studies of the KardiaBand and none of the Apple Watch’s ECG app, though the clinical data submitted to the FDA as part of Apple’s De Novo classification request is publicly available [13, 35, 36, 37•]. Apple first introduced AF detection to the Apple Watch via PPG, and launched the Apple Heart Study which demonstrated a positive predictive value of 84% [28, 52]. Similarly, the eHeart study used Apple Watch PPG data to detect AF with 98% sensitivity and 90.2% specificity [53]. With the release of its new ECG app, Apple also announced the HEARTLINE study, which will examine whether the combination of the PPG-based screening and the ECG app can diagnose AF and, more importantly, prevent stroke [54].
Most published studies merely validate use of wearable ECG monitors, and few have examined their effect on patient outcomes or cost-effectiveness on a large scale. REHEARSE-AF found that the KardiaMobile detects more AF than routine care, but the study was not powered to detect a difference in clinical outcomes [55••]. REHEARSE-AF and several other studies have examined the cost-effectiveness of AF screening, and have estimated that screening is cost-effective for preventing stroke [32, 34, 51, 55••]. The ongoing iHEART study is investigating the impact of the KardiaMobile along with behavior altering messaging on patient outcomes, including recurrent AF and quality of life scores [56].
Wearable ECG monitors, particularly the KardiaMobile, have also been studied for indications other than AF detection. The KardiaMobile’s measurement of the QTc interval was shown to be comparable to a standard ECG [27]. The ST LEUIS pilot study suggested that the KardiaMobile can be used to diagnose cardiac ischemia using a 12-lead ECG equivalent (with each lead taken serially) [57]. While not conducted with a wearable device, a recent study sponsored by AliveCor used deep learning to screen for hyperkalemia (albeit with poor specificity) using two ECG leads, implying that this could be also done with Kardia devices [58].
The current evidence for wearable ECG monitors largely comprises validation studies of AF detection algorithms, primarily for the Kardia devices. However, the true potential of wearable ECG monitors lies in their ability not just to detect arrhythmia but to prevent complications such as stroke while being more convenient and less invasive than conventional rhythm monitoring. Patients and providers should await the results of upcoming studies, including iHEART and HEARTLINE, that test whether wearable ECG monitors improve patient outcomes before making any conclusions about the beneficial effects of the devices on patient health.
Obstacles to Adoption
In developing wearable ECG monitors, device manufacturers must account for the financial, legal, and social frameworks that determine how medicine is practiced. For example, consider how providers are compensated for utilizing wearable ECG monitor data. In January 2018, the Center for Medicare and Medicaid Services changed its rules regarding a preexisting Current Procedural Terminology (CPT) code that allowed providers to bill for “Remote Patient Monitoring” of data, including ECG data [59]. Beginning in January 2019, three new CPT codes allow providers to bill for setup of remote patient monitoring devices, monthly review of their data, and any remote interaction with patients that ensues [59].
In their marketing of their remote patient monitoring systems, both AliveCor and Qardio emphasize how their software facilitates billing and increases practice revenue [48, 49]. As the surfeit of medical data drains ever more of providers’ precious time, manufacturers must consider how time spent on their devices is compensated, to ensure that worth-while advancements in patient care are not neglected because nobody is paid to use them.
The use of AI in wearable ECG monitors also raises potential legal and ethical concerns. Though it can power highly accurate algorithms, AI is a “black box” that is difficult to interrogate if it gives unexpected output. AI is also susceptible to racial, gender, and socioeconomic bias in training datasets, potentially perpetuating harm to underserved groups. Currently, the law shields device manufacturers from liability if harm is incurred by patients, as long as they warn the prescribing provider (a “learned intermediary”) of the potential harms of the device [60]. However, this model does not apply when devices make diagnoses without mediation by a provider, or when algorithms evolve beyond the understanding of any intermediary [61]. The challenges faced by AI are not unique to wearable ECG monitors, but will need to be overcome if the full potential of AI in medicine is to be realized.
Security and privacy are two other related domains in which wearable ECG monitors will be tested. Wearable ECG monitors contain some of an individual’s most sensitive data. Being portable and internet-connected, they are inherently less secure than the siloed, centralized devices that preceded them. The interface between wearable ECG monitors and the traditional EHR is particularly vulnerable, as consumers are often forced to transmit data to their providers via less secure means such as e-mail. Information security and privacy will be of utmost importance as sources of patient data proliferate and become decentralized.
Conclusions: the Road Ahead
The future of cardiac rhythm monitoring presents several opportunities and challenges for patients, healthcare providers, and device manufacturers alike. Wearable ECG monitors promise more convenient and pervasive rhythm monitoring for patients, with the goal of improving their health. However, patients should be wary of devices that promise to promote overall health without explaining how they do so. They should look for devices that fulfill a specific, tangible purpose, which in the current market is most likely to be monitoring for AF.
Healthcare providers should explain to their patients that the existing evidence largely validates the ability of wearable ECG monitors (specifically the Kardia devices and Apple Watch) to distinguish AF from sinus rhythm, but it has not yet demonstrated improvement in patient outcomes. Providers should also look out for upcoming studies, such as the HEARTLINE study, that aim to answer this very question.
When interpreting data from their patients’ devices, providers must understand and consider the sensitivity and specificity of the device’s algorithms, like they would for any other diagnostic test. They should also know that adding their own interpretive skill to the device’s is still necessary and can improve diagnostic yield.
In their turn, manufacturers must make device test characteristics easily available to providers, and provide them with supporting data that maximizes the device’s clinical impact. Manufacturers should streamline the experience of using their devices for providers as well as patients. They should work within the existing healthcare enterprise to make data collection, data review, EHR integration, and billing seamless, with actionable notifications that do not overwhelm clinicians. Finally, manufacturers must also be held accountable for device security, patient privacy, and any unforeseen negative social impact of their devices on underserved populations.
Wearable ECG monitors promise an alluring future to providers and patients alike, in which cardiac rhythm is monitored continuously and used as a window into a patient’s cardiac health. Although some elements of this future—especially regarding management of AF—are rapidly approaching, others are further off. The quotation from Bill Gates that opens this review is followed by a less frequently cited sentence: “We always overestimate the change that will occur in the next two years and underestimate the change that will occur in the next ten. Don’t let yourself be lulled into inaction” [1]. Device manufacturers, healthcare providers, patients, and other stakeholders within the healthcare system have much more work to be done before the promised future of cardiac rhythm monitoring becomes reality.
Acknowledgments
We are grateful to Daniel B. Kramer, MD, MPH, for critical review of the manuscript.
Funding Information David McManus is supported by NIH grants R01HL126911, R01HL137734, R01HL137794, R01HL13660, R01HL141434, and U54HL143541.
Steven A. Lubitz is supported by NIH grant 1R01HL139731 and American Heart Association 18SFRN34250007.
Eric Ding is supported by a grant from the National Heart, Lung, Blood Institute (Grant #5T32HL120823).
Footnotes
Conflict of Interest Mostafa A. Al-Alusi and Eric Ding declare that they have no conflict of interest.
David McManus has received research support from Apple Computer, Bristol-Myers Squibb, Boehringher-Ingelheim, Flexcon, Fitbit Heart Rhythm Society, Pfizer, Samsung, Philips Healthcare, Biotronik, and has received consultancy fees or honoraria from Bristol-Myers Squibb, Pfizer, Flexcon, Boston Biomedical Associates, Samsung, and Rose Consulting.
Steven A. Lubitz receives sponsored research support from Bristol Myers Squibb / Pfizer, Bayer AG, and Boehringer Ingelheim, and has consulted for Bristol Myers Squibb / Pfizer and Bayer AG.
Human and Animal Rights and Informed Consent This article does not contain any studies with human or animal subjects performed by any of the authors.
References
Papers of particular interest, published recently, have been highlighted as:
• Of importance
•• Of major importance
- 1.Gates B, Myhrvold N, Rinearson P. The road ahead: Viking Press; 1995. [Google Scholar]
- 2.Apple Watch Series 4 Health [Internet]. Apple; [cited 2019 May 2]. Available from: https://www.apple.com/apple-watch-series-4/health/. [Google Scholar]
- 3.KardiaBand [Internet]. AliveCor; [cited 2019 May 2]. Available from: https://store.alivecor.com/products/kardiaband. [Google Scholar]
- 4.KardiaMobile [Internet]. AliveCor; [cited 2019 May 2]. Available from: https://store.alivecor.com/products/kardiamobile. [Google Scholar]
- 5.QardioCore [Internet]. GetQardio; [cited 2019 May 2]. Available from: https://www.getqardio.com/qardiocore-wearable-ecg-ekg-monitor-iphone/. [Google Scholar]
- 6.Hexoskin [Internet]. Hexoskin; [cited 2019 May 2]. Available from: https://www.hexoskin.com. [Google Scholar]
- 7.Withings Move ECG [Internet]. Withings; [cited 2019 May 2]. Available from: https://www.withings.com/us/en/move-ecg?gclid=EAIaIQobChMI6-ju_7_94QIVg0wNCh1ghQKnEAAYASAAEgLzRfD_BwEgclsrc=aw.ds. [Google Scholar]
- 8.Introducing Verily Study Watch [Internet]. Verily Blog; 2017. [cited 2019 May 16]. Available from: https://blog.verily.com/2017/04/introducing-verily-study-watch.html. [Google Scholar]
- 9.New Health Band Takes ECG Tracking to Heart [Internet]. Amazfit Blog; 2017. [cited 2019 May 2]. Available from: https://us.amazfit. com/blog/health-band. [Google Scholar]
- 10.KardiaAI 510(k) Notification K181823 [Internet]. Food and Drug Administration; 2019. [cited 2019 Jun 15]. Available from: https://www.accessdata.fda.gov/cdrh_docs/pdf18/K181823.pdf. [Google Scholar]
- 11.Kardia Band System 510(k) Notification K171816 [Internet]. Food and Drug Administration; 2017. [cited 2019 Jun 15]. Available from: https://www.accessdata.fda.gov/cdrh_docs/pdf17/K171816.pdf. [Google Scholar]
- 12.AliveCor Heart Monitor 510(k) Premarket Notification K142743 [Internet]. Food and Drug Administration; 2014. [cited 2019 Jun 15]. Available from: https://www.accessdata.fda.gov/cdrh_docs/pdf14/K142743.pdf. [Google Scholar]
- 13.De Novo classification request for ECG App DEN180044 [Internet]. Food and Drug Administration; 2018. [cited 2019 May 27]. Available from: https://www.accessdata.fda.gov/cdrh_docs/reviews/DEN180044.pdf. [Google Scholar]
- 14.De Novo classification request for irregular rhythm notification feature DEN180042 [Internet]. Food and Drug Administration; 2018. [cited 2019 May 27]. Available from: https://www.accessdata.fda.gov/cdrh_docs/reviews/DEN180042.pdf. [Google Scholar]
- 15.KardiaMobile 6L [Internet]. AliveCor; [cited 2019 May 20]. Available from: https://www.alivecor.com/kardiamobile6l/. [Google Scholar]
- 16.CALM-M for healthcare [Internet]. CALM Health; [cited 2019 May 16]. Available from: https://www.calm-health.com/calm-healthcare/. [Google Scholar]
- 17.HeartBit - technology [Internet]. HeartBit; [cited 2019 May 16]. Available from: https://theheartbit.com/technology/. [Google Scholar]
- 18.Revolutionary solution for exercise ECG detection [Internet]. Heartbit; 2018. [cited 2019 Jun 15]. Available from: https://drive.google.com/drive/folders/1TtJqy593UTSGUXS7UFETNgrFh1SiXC4e. [Google Scholar]
- 19.Study Watch Traditional 510(k) Premarket Notification K182456 [Internet]. Food and Drug Administration; 2019. [cited 2019 May 26]. Available from: http://www.accessdata.fda.gov/cdrh_docs/pdf18/K182456.pdf. [Google Scholar]
- 20.Triangle System 510(k) Premarket notification K142743 [Internet]. Food and Drug Administration; 2019. [cited 2019 Jun 15]. Available from: https://www.accessdata.fda.gov/cdrh_docs/pdf18/K183319.pdf. [Google Scholar]
- 21.Christensen CM. The innovators dilemma: when new technologies cause great firms to fail: Harvard Business Review Press; 2013. [Google Scholar]
- 22.Heart rate notifications on your Apple Watch [Internet]. Apple Support; [cited 2019 May 2]. Available from: https://support.apple.com/en-us/HT208931. [Google Scholar]
- 23.Overview of device regulation [Internet]. US Food and Drug Administration; [cited 2019 Jun 28]. Available from: https://www.fda.gov/medical-devices/device-advice-comprehensive-regulatory-assistance/overview-device-regulation. [Google Scholar]
- 24.US Preventive Services Task Force, Curry SJ, Krist AH, Owens DK, Barry MJ, Caughey AB, et al. Screening for atrial fibrillation with electrocardiography: US Preventive Services Task Force recommendation statement. JAMA. 2018;320:478. [DOI] [PubMed] [Google Scholar]
- 25.Kirchhof P, Benussi S, Kotecha D, Ahlsson A, Atar D, Casadei B, et al. 2016 ESC guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Eur Heart J. 2016;37: 2893–962. [DOI] [PubMed] [Google Scholar]
- 26.Tarakji KG, Wazni OM, Callahan T, Kanj M, Hakim AH, Wolski K, et al. Using a novel wireless system for monitoring patients after the atrial fibrillation ablation procedure: the iTransmit study. Heart Rhythm. 2015;12:554–9. [DOI] [PubMed] [Google Scholar]
- 27.Chung EH, Guise KD. QTC intervals can be assessed with the AliveCor heart monitor in patients on dofetilide for atrial fibrillation. J Electrocardiol. 2015;48:8–9. [DOI] [PubMed] [Google Scholar]
- 28.Turakhia MP, Perez MV. Results of a large-scale, App-based study to identify atrial fibrillation using a smartwatch: the Apple Heart Study. Oral session presented at: American College of Cardiology Scientific Sessions 2019 2019 Mar 16–18 New Orleans, LA. [Google Scholar]
- 29.Lau JK, Lowres N, Neubeck L, Brieger DB, Sy RW, Galloway CD, et al. iPhone ECG application for community screening to detect silent atrial fibrillation: a novel technology to prevent stroke. Int J Cardiol. 2013;165:193–4. [DOI] [PubMed] [Google Scholar]
- 30.William AD, Kanbour M, Callahan T, Bhargava M, Varma N, Rickard J, et al. Assessing the accuracy of an automated atrial fibrillation detection algorithm using smartphone technology: the iREAD study. Heart Rhythm. 2018;15:1561–5. [DOI] [PubMed] [Google Scholar]
- 31.•.Koshy AN, Sajeev JK, Negishi K, Wong MC, Pham CB, Cooray SP, et al. Accuracy of blinded clinician interpretation of single-lead smartphone electrocardiograms and a proposed clinical workflow. Am Heart J. 2018;205:149–53This study compared the test characteristics of the AliveCor rhythm classification algorithm to interpretation of single-lead AliveCor tracings by primary care providers and electrophysiologists in a sample of 100 individuals undergoing cardioversion with gold-standard 12-lead ECGs. The study demonstrated that supplementing device algorithms with physician interpretation improves AF detection over either alone.
- 32.Desteghe L, Raymaekers Z, Lutin M, Vijgen J, Dilling-Boer D, Koopman P, et al. Performance of handheld electrocardiogram devices to detect atrial fibrillation in a cardiology and geriatric ward setting. Europace. 2016;19:29–39. [DOI] [PubMed] [Google Scholar]
- 33.Brasier N, Raichle CJ, Dörr M, Becke A, Nohturfft V, Weber S, et al. Detection of atrial fibrillation with a smartphone camera: first prospective, international, two-centre, clinical validation study (DETECT AF PRO). EP Europace. 2019;21:41–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lowres N, Neubeck L, Salkeld G, Krass I, McLachlan AJ, Redfern J, et al. Feasibility and cost-effectiveness of stroke prevention through community screening for atrial fibrillation using iPhone ECG in pharmacies. Thromb Haemost. 2014;111:1167–76. [DOI] [PubMed] [Google Scholar]
- 35.Using Apple Watch for arrhythmia detection December 2018 [Internet]. 2018. [cited 2019 May 16]. Available from: https://www.apple.com/healthcare/site/docs/Apple_Watch_Arrhythmia_Detection.pdf.
- 36.Bumgarner JM, Lambert CT, Hussein AA, Cantillon DJ, Baranowski B, Wolski K, et al. Smartwatch algorithm for automated detection of atrial fibrillation. J Am Coll Cardiol. 2018;71: 2381–8. [DOI] [PubMed] [Google Scholar]
- 37.•.Wasserlauf J, You C, Patel R, Valys A, Albert D, Passman R. Smartwatch performance for the detection and quantification of atrial fibrillation. Circulation: Arrhythmia and Electrophysiology [Internet]. 2019. [cited 2019 May 29];12 Available from: 10.1161/CIRCEP.118.006834This study compared the KardiaBand device to conventional implantable cardiac monitors in 24 patients with paroxysmal AF, finding that the KardiaBand is highly sensitive for detecting episodes of AF lasting more than 1 h. The results suggest that wearable devices may be useful for long-term non-invasive monitoring for AF.
- 38.Chan P-H, Wong C-K, Pun L, Wong Y-F, Wong MM-Y, Chu DW-S, et al. Head-to-head comparison of the AliveCor heart monitor and microlife WatchBP office AFIB for atrial fibrillation screening in a primary care setting. Circulation. 2017;135:110–2. [DOI] [PubMed] [Google Scholar]
- 39.Alonso A, Roetker NS, Soliman EZ, Chen LY, Greenland P, Heckbert SR. Prediction of atrial fibrillation in a racially diverse cohort: the Multi-Ethnic Study of Atherosclerosis (MESA). J Am Heart Assoc. 2016;5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.FDA permits marketing of artificial intelligence-based device to detect certain diabetes-related eye problems [Internet]. FDA Newsroom; 2018. Available from: https://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm604357.htm. [Google Scholar]
- 41.Kapoor A, Amroze A, Golden J, Crawford S, O’Day K, Elhag R, et al. SUPPORT-AF: piloting a multi-faceted, electronic medical record-based intervention to improve prescription of anticoagulation. J Am Heart Assoc [Internet]. 2018. [cited 2019 Jun 28];7 Available from: 10.1161/JAHA.118.009946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lip GYH, Nieuwlaat R, Pisters R, Lane DA, Crijns HJGM. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor-based approach. Chest. 2010;137:263–72. [DOI] [PubMed] [Google Scholar]
- 43.Pisters R, Lane DA, Nieuwlaat R, de Vos CB, Crijns HJGM, Lip GYH. A novel user-friendly score (HAS-BLED) to assess 1-year risk of major bleeding in patients with atrial fibrillation: the Euro Heart Survey. Chest. 2010;138:1093–100. [DOI] [PubMed] [Google Scholar]
- 44.Institutions that support health records on iPhone (beta) [Internet]. Apple Support; 2019. [cited 2019 May 16]. Available from: https://support.apple.com/en-us/HT208647. [Google Scholar]
- 45.VA to provide capability for veterans to access their VA health data on Apple iPhones [Internet]. U.S. Department of Veteran Affairs News Releases; 2019. [cited 2019 May 16]. Available from: https://www.va.gov/opa/pressrel/pressrelease.cfm?id=5199. [Google Scholar]
- 46.Fitbit and Google announce collaboration to accelerate innovation in digital health and wearables [Internet]. Fitbit Press Releases; 2018. [cited 2019 Jun 28]. Available from: https://investor.fitbit.com/press/press-releases/press-release-details/2018/Fitbit-and-Google-Announce-Collaboration-to-Accelerate-Innovation-in-Digital-Health-and-Wearables/default.aspx. [Google Scholar]
- 47.AMA, Google launch health care interoperability & innovation challenge [Internet]. AMA Press Releases; 2018. [cited 2019 Jun 28]. Available from: https://www.ama-assn.org/press-center/press-releases/ama-google-launch-health-care-interoperability-innovation-challenge. [Google Scholar]
- 48.KardiaPro [Internet]. AliveCor; [cited 2019 May 2]. Available from: https://clinicians.alivecor.com. [Google Scholar]
- 49.QardioMD [Internet]. GetQardio; [cited 2019 May 2]. Available from: https://www.getqardio.com/qardiomd-heart-health/. [Google Scholar]
- 50.Bogun F, Anh D, Kalahasty G, Wissner E, Bou Serhal C, Bazzi R, et al. Misdiagnosis of atrial fibrillation and its clinical consequences. Am J Med. 2004;117:636–42. [DOI] [PubMed] [Google Scholar]
- 51.Welton NJ, McAleenan A, Thom HH, Davies P, Hollingworth W, Higgins JP, et al. Screening strategies for atrial fibrillation: a systematic review and cost-effectiveness analysis. Health Technol Assess. 2017;21:1–236. [DOI] [PubMed] [Google Scholar]
- 52.Turakhia MP, Desai M, Hedlin H, Rajmane A, Talati N, Ferris T, et al. Rationale and design of a large-scale, app-based study to identify cardiac arrhythmias using a smartwatch: the Apple Heart Study. Am Heart J. 2019;207:66–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tison GH, Sanchez JM, Ballinger B, Singh A, Olgin JE, Pletcher MJ, et al. Passive detection of atrial fibrillation using a commercially available smartwatch. JAMA Cardiol. 2018;3:409–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.The HEARTLINE Study [Internet]. The HEARTLINE Study; 2019. [cited 2019 May 16]. Available from: https://www.heartline.com. [Google Scholar]
- 55.••.Halcox JPJ, Wareham K, Cardew A, Gilmore M, Barry JP, Phillips C, et al. Assessment of remote heart rhythm sampling using the AliveCor heart monitor to screen for atrial fibrillation: The REHEARSE-AF Study. Circulation. 2017;136:1784–94This randomized controlled trial compared screening with intermittent twice weekly handheld AliveCor Kardia ECGs for 12 months to usual care, and found that intermittent screening was more likely to detect incident AF in patients ≥ 65 years of age. It also estimated cost-effectiveness of such ambulatory screening, suggesting a cost per diagnosis of $10,780.
- 56.Hickey KT, Hauser NR, Valente LE, Riga TC, Frulla AP, Creber RM, Whang W, Garan H, Jia H, Sciacca RR, Wang DY A single-center randomized, controlled trial investigating the efficacy of a mHealth ECG technology intervention to improve the detection of atrial fibrillation: the iHEART study protocol. BMC Cardiovasc Disord [Internet]. 2016. [cited 2019 Mar 5];16 Available from: 10.1186/s12872-016-0327-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Muhlestein JB, Le V, Albert D, Moreno FL, Anderson JL, Yanowitz F, et al. Smartphone ECG for evaluation of STEMI: results of the ST LEUIS Pilot Study. J Electrocardiol. 2015;48:249–59. [DOI] [PubMed] [Google Scholar]
- 58.Galloway CD, Valys AV, Shreibati JB, Treiman DL, Petterson FL, Gundotra VP, et al. Development and validation of a deep-learning model to screen for hyperkalemia from the electrocardiogram. JAMA Cardiology [Internet]. 2019. [cited 2019 May 6]; Available from: 10.1001/jamacardio.2019.0640. [DOI] [PMC free article] [PubMed]
- 59.Medicare Program; Revisions to payment policies under the physician fee schedule and other revisions to part B for CY 2019; Medicare Shared Savings program Requirements; Quality Payment Program; Medicaid Promoting Interoperability Program; Quality Payment Program–extreme and uncontrollable circumstance policy for the 2019 MIPS payment year; provisions from the Medicare Shared Savings Program–accountable care organizations–pathways to success; and expanding the use of telehealth services for the treatment of opioid use disorder under the substance use-disorder prevention that promotes opioid recovery and treatment (SUPPORT) for patients and communities act [internet]. Centers for Medicare & Medicaid Services; 2018. November Available from: https://s3.amazonaws.com/public-inspection.federalregister.gov/2018-24170.pdf. [Google Scholar]
- 60.Thornton RG. The learned intermediary doctrine and its effects on prescribing physicians. Proc (Bayl Univ Med Cent). 2003;16:359–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sullivan HR, Schweikart SJ. Are current tort liability doctrines adequate for addressing injury caused by AI? AMA J Ethics. 2019;21:E160–6. [DOI] [PubMed] [Google Scholar]


