Abstract
Cardiac neural crest (CNC) cells are pluripotent cells derived from the dorsal neural tube that migrate and contribute to the remodeling of pharyngeal arch arteries and septation of the cardiac outflow tract (OFT). Numerous molecular cascades regulate the induction, specification, delamination, and migration of the CNC. Extensive analyses of the CNC ranging from chick ablation models to molecular biology studies have explored the mechanisms of heart development and disease, particularly involving the OFT and aortic arch (AA) system. Recent studies focus more on reciprocal signaling between the CNC and cells originated from the second heart field (SHF), which are essential for the development of the OFT myocardium, providing new insights into the molecular mechanisms underlying congenital heart diseases (CHDs) and some human syndromes.
Congenital heart diseases (CHDs) result from abnormal morphogenesis of the embryonic cardiovascular system and usually involve defects in specific structural components of the developing heart and vessels. Decades of research in molecular embryology indicate that multiple distinct cell lineages give rise to the cardiovascular system. Neural crest cells are pluripotent cells and a subregion of the cranial neural crest that contributes to development of the third, fourth, and sixth pharyngeal arches and the cardiac outflow tract (OFT) is defined as the “cardiac neural crest” (CNC). Recently, a new population of myocardial precursor cells in the pharyngeal mesoderm that also contribute to the development of pharyngeal arches and the OFT was discovered and named as “second heart field” (SHF). This article summarizes the current knowledge about the molecular basis of CNC development and implication of the CNC in heart development and diseases with the underlying molecular mechanisms. Reciprocal signaling between the CNC and the SHF that is essential for development of the aortic arch (AA) system and OFT is also discussed along with the associated human diseases.
CNC: AN OVERVIEW
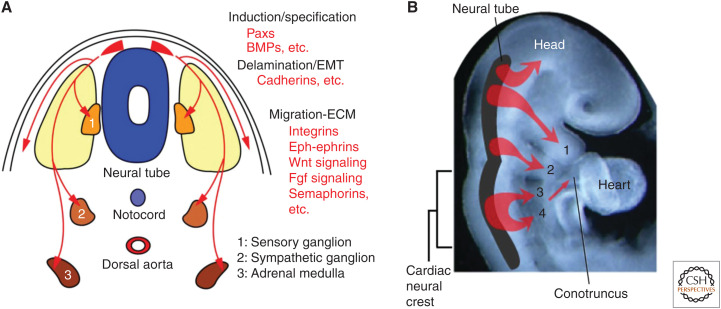
The CNC is a subpopulation of the neural crest (Kirby 2007; Yamagishi and Yamagishi 2014; Thattaliyath and Hutson 2016). Neural crest cells are multipotential cells that delaminate from the dorsal neural tube and migrate widely throughout the body as ectoderm-derived mesenchymal cells. Neural crest cells ultimately give rise to an enormous array of different cell types, tissues, and organs, including the peripheral and autonomic nervous systems, adrenal medulla, melanocytes, pharyngeal arches, and facial skeleton (Fig. 1). The neural crest can be axially divided into the cranial and trunk regions. Only the preotic cranial neural crest, but not trunk neural crest, differentiates into cartilage, bone, connective tissue, and smooth muscle, and contributes significantly to the development of the head and neck skeletal structures. The CNC is a unique subregion of the cranial neural crest in which a transitional region between the cranial and trunk neural crest, or between the otocyst and somite 3, migrates into the third, fourth, and sixth pharyngeal arches and the embryonic OFT, or conotruncus, and participates in development of the cardiovascular system as well as in the thymus, thyroid, and parathyroid gland (Fig. 1).
Figure 1.
Development of neural crest cells. (A) Transverse image of embryonic trunk, and (B) lateral view of rostral region of embryo around embryonic day 9–10 in mice that is equivalent to week 4–5 in humans are shown. Neural crest cells (shown in red) are multipotential cells that delaminate from the dorsal neural tube and migrate widely throughout the body as ectoderm-derived mesenchymal cells. Neural crest cells ultimately give rise to numerous different cell types, tissues, and organs, including the peripheral and autonomic nervous systems, adrenal medulla, melanocytes, pharyngeal arches, and facial skeleton. A subregion of the cranial neural crest cells originating between the otocyst and somite 3 is called the “cardiac neural crest cells” that migrates into the third, fourth, and sixth pharyngeal arches and the cardiac outflow tract (conotruncus). (BMPs) bone morphogenetic proteins, (EMT) epithelial-to-mesenchymal transition, (ECM) extracellular matrix.
The neural crest was initially discovered by Wilhelm His in 1868, and the CNC was first revealed to be essential for cardiovascular development by Kirby et al. (1983) using quail-chick chimeras and ablation models. Until recently, identification of neural crest–specific markers such as wingless-type MMTV integration site family, member 1 (Wnt1), paired box 3 (Pax3), acidic ribosomal phosphoprotein P0 (P0), and plexin-A2 (Plxna2) (Lee et al. 1997a; Jiang et al. 2000; Brown et al. 2001). Numerous studies using transgenic mice have facilitated lineage tracing of neural crest cells and tissue-specific mutation of targeted genes to explore the cellular and molecular mechanisms underlying heart development and the diseases associated with CNC. Importantly, CNC cells play central roles in development of the OFT and AA system.
HEART DEVELOPMENT AND DISEASES ASSOCIATED WITH CNC
Development of the OFT
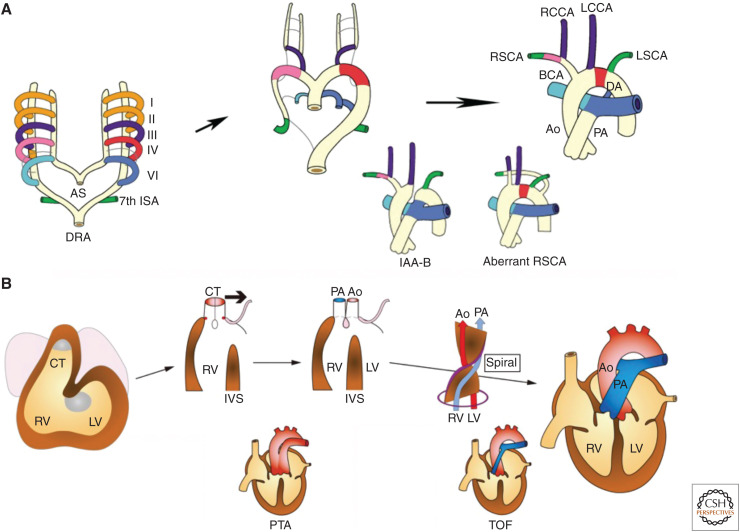
Around 4 weeks of gestation during the process of heart looping, the OFT or conotruncus shifts leftward, and the relation of the left ventricle to the aorta and the right ventricle to the pulmonary trunk is established (Yamagishi and Yamagishi 2014). Meanwhile, in the OFT, the conotruncal swellings or cushions form the conotruncal septum that divides a tubular structure into two great vessels, namely, the aorta and pulmonary trunk. Coalescence of conotruncal cushions occurs in a spiral fashion that accounts for the mature anatomical relationship of the aorta with the left ventricle and the pulmonary trunk with the right ventricle (Fig. 2). These proper connections are indispensable for establishing a separate systemic and pulmonary circulation after birth.
Figure 2.
Development and anomalies of the aortic arch (AA) system and the cardiac outflow tract (OFT). (A) Remodeling of bilaterally symmetric pharyngeal arch arteries (PAAs) into the AA system is shown in a color-coordinated fashion. Interrupted AA type B (IAA-B) results from abnormal regression of the left fourth PAA. Aberrant right subclavian artery (RSCA) results from abnormal regression of the right fourth PAA. (B) Developmental steps of the cardiac OFT, including leftward movement (bold arrow) of the conotruncus (CT) and septation of the CT into the aorta (Ao) and the pulmonary artery (PA) in a spiral fashion are shown. Persistent truncus arteriosus (PTA) resulting from a failure of septation of the CT. Tetralogy of Fallot (TOF) resulting from malalignment of the Ao to the left ventricle (LV). (I–VI) first to sixth PAA, (AS) aortic sac, (BCA) brachiocephalic artery, (DA) ductus arteriosus, (DRA) dorsal artery, (ISA) intersegmental artery, (IVS) interventricular septum, (LCCA) left common carotid artery, (LSCA) left subclavian artery, (RCCA) right common carotid artery, (RSCA) right subclavian artery, (RV) right ventricle.
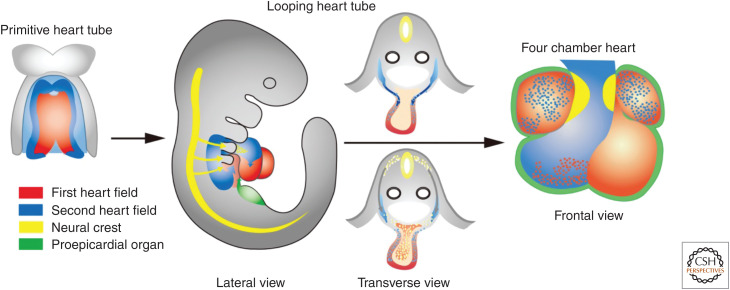
Decades of descriptive embryology including cell lineage tracing have improved our understanding of the developmental origins of the cardiovascular system (Srivastava 2006; Yamagishi et al. 2009; Kodo and Yamagishi 2011). In addition to progenitor cells derived from the CNC, those from the SHF play key roles in development of the OFT. SHF cells give rise to the OFT myocardium along with the subpulmonary conus, whereas CNC cells give rise to the OFT septum during development. In mammalian embryos, the SHF lies medially to the cardiac crescent, or the first heart field (FHF), and then behind the heart tube derived from the FHF, extending into the mesodermal layer of the pharyngeal arches (Fig. 3). The heart tube may predominantly provide a scaffold and on its rightward looping, SHF cells cross into the anterior and posterior of the heart tube, populating the OFT, future right ventricle, and atria. Addition of the SHF-derived myocardium to the OFT results in its elongation, which is necessary to allow the OFT to rotate and shorten sufficiently for correct alignment of the aorta and pulmonary trunk with their respective ventricles.
Figure 3.
Developmental origins of the outflow tract and other regions of the heart. The first heart field (red) gives rise to the primitive heart tube and eventually the left ventricle, the atria, and a part of the right ventricle. The second heart field (blue) gives rise to the outflow tract, the right ventricle and a part of the atria. The cardiac neural crest (yellow) gives rise to the outflow tract cushion that eventually forms the outflow tract septum. The proepicardial organ (green) gives rise to the epicardium.
When SHF cells give rise to the OFT, CNC cells migrate into the conotruncal cushions and form two condensed columns of cells, or a horseshoe-shaped septation complex. As the septation complex elongates in the aortic sac between the origins of the fourth and sixth pharyngeal arch arteries into the distal OFT, the common conotruncus is divided into the aorta and pulmonary trunk (Waldo et al. 1998, 1999; Keyte and Hutson 2012). Finally, the most proximal region of the OFT septum in the conus is formed like a zipper closing from the distal to proximal direction toward the ventricles with myocardialization in which myocardial cells invade into the cushions (van den Hoff et al. 1999).
Development of the AA System
The AA system originates from the pharyngeal arch arteries and is developed by their remodeling (Yamagishi and Yamagishi 2014). Pharyngeal arch arteries initially form as a bilaterally symmetrical series of arteries that connect the aortic sac to the paired dorsal aortas. During 4–5 weeks of gestation, the bilaterally symmetric pharyngeal arch arteries and the right and left dorsal aortae undergo remodeling that is a sequence of programmed asymmetrical expansion, regression, persistence, and change in the relative position of different vascular segments (Fig. 2). The first and second pharyngeal arch arteries almost completely regress except to form the maxillary and stapedial arteries, respectively. The third, fourth, and sixth pharyngeal arch arteries remodel and transform into the asymmetric great arteries, including the common carotid, a portion of AA, and the ductus arteriosus, whereas the fifth pharyngeal arch arteries completely regress before they fully develop.
The CNC cells migrate and target the third, fourth, and sixth pharyngeal arches that give rise to the AA system (Kirby et al. 1983). The CNC cells differentiate into the smooth muscle tunica media of the AA arteries, which is necessary for the persistence and repatterning of these arteries; however, they are not required for formation of these arteries (Bockman et al. 1987; Bergwerff et al. 1998).
CHD Involving the OFT and AA
OFT and AA defects involving the CHD occur due to abnormal development of the OFT and AA, which account for ∼30% of CHD, and usually require some intervention for patients during the first year of life (Hoffman and Kaplan 2002). Defects of CNC cells may lead to a variety of OFT and AA defects such as persistent truncus arteriosus (PTA), tetralogy of Fallot (TOF), and interrupted AA type B (IAA-B) (Kirby et al. 1983; Kirby 2007), which fail to establish a completely separated systemic and pulmonary circulation (Yamagishi and Yamagishi 2014). Although these CHDs have been commonly explained by the abnormal development of CNC cells since the first report on CNC, involvement of the SHF has been extensively investigated in recent times. The 22q11.2 deletion syndrome (22q11DS) is highly associated with OFT and AA defects. Basic and clinical research on 22q11DS has allowed us to explore the molecular basis underlying OFT and AA development and their defects as described later.
Persistent Truncus Arteriosus
PTA results from incomplete formation of the conotruncal septum. The pathogenesis of PTA is indicated by a key experimental ablation model as described later. If the vestiges of distal truncal septation develop, a short pulmonary trunk can be formed from which pulmonary arteries arise. A partial developmental failure of the distal truncal septum may result in an aorticopulmonary window. In contrast to PTA, semilunar valves are completely divided by the conal septum in the aorticopulmonary window.
Tetralogy of Fallot
TOF refers to the tetrad of overriding aorta, pulmonary stenosis, ventricular septal defect, and right ventricular hypertrophy. The anatomic spectrum of TOF is diverse, including TOF with pulmonary atresia and those with an absent pulmonary valve.
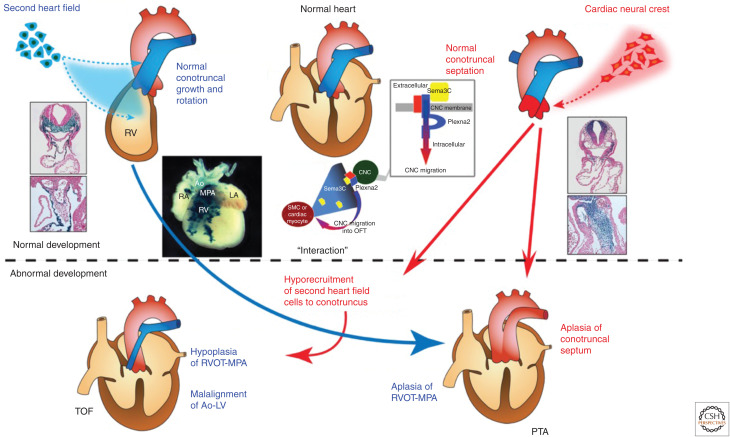
Although TOF is believed to result from malrotation of the OFT leading to misalignment of the outlet and trabecular septum, and consequent overriding of the aorta above the misaligned ventricular septum (Siwik et al. 2001), an alternative explanation is that hypoplasia or underdevelopment of the pulmonary infundibulum may also be responsible for the infundibular obstruction and malalignment of the outlet septum (Fig. 4; Maeda et al. 2006).
Figure 4.
Normal and abnormal development of the cardiac neural crest (CNC) and the second heart field (SHF) and their interaction implicated in the spectrum of outflow tract (OFT) defects. The SHF gives rise to the OFT myocardium and CNC gives rise to the OFT septum, respectively. Although the precise embryological mechanism remains uncertain, tetralogy of Fallot (TOF) is believed to result from malrotation of the OFT that leads to misalignment of the outlet and trabecular septum, and consequent overriding of the aorta (Ao) above the malaligned ventricular septum. Contribution of CNC is thought to be essential for proper rotation and septation of the OFT. Alternatively, hypoplasia and underdevelopment of the pulmonary infundibulum may also be responsible for the infundibular obstruction and malalignment of the outlet septum. Cre-mediated transgenic system in mice revealed that a subset of cells derived from the SHF contribute predominantly to the pulmonary infundibulum. Accordingly, developmental defects of the SHF may cause hypoplasia of the pulmonary infundibulum, resulting in TOF, and more severe decreased number or absence of this subset of cells may affect development and/or migration of CNC, resulting in persistent truncus arteriosus (PTA). This notion is consistent with the observation that OFT defects ranging from TOF to PTA are highly associated with 22q11DS. Recent studies suggested that reciprocal molecular signaling between SHF and CNC, such as semaphorin 3C (Sema3C) ligand expressed in the SHF and plexin A2 (Plexna2) and neuropillin-1 (Npn-1) receptors expressed in the CNC, was essential for correct navigation of CNC cells toward the SHF-derived OFT, eventually resulting in proper alignment and septation of the OFT. (RV) right ventricle, (RA) right atrium, (LA) left atrium, (MPA) main pulmonary artery, (RVOT) right ventricular outflow, (SMC) smooth muscle cell.
Interrupted Aortic Arch Type B
Most anomalies of the AA system result from regression of parts of the pharyngeal arch arteries and dorsal aortae that normally persist, and persistence of parts that normally regress. IAA-B results from abnormal regression of the left fourth pharyngeal arch artery.
Aberrant Right Subclavian Artery
Aberrant right subclavian artery occurs when the right fourth pharyngeal arch artery regresses abnormally. In this case, the right dorsal aorta cranial to the seventh intersegmental artery persists abnormally and forms the retroesophageal portion of the right subclavian artery. This vascular anomaly is highly associated with CHD involving the OFT.
The CNC Ablation Model
The neural crest ablation model has provided much of our knowledge about CNC function since its discovery. Ablation of the CNC before its migration basically leads to cardiovascular phenotypes including defective development of the OFT and abnormal patterning of the AA as primary CNC-related defects (Kirby et al. 1983; Porras and Brown 2008). It leads to failure of conotruncus partitioning, resulting primarily in PTA. In addition, noncardiovascular phenotypes include hypoplasia or aplasia of the thymus, parathyroid, and occasionally the thyroid gland, possibly due to a failure in interaction between the neural crest–derived mesenchyme and the pharyngeal pouch endoderm.
CNC ablation also results in altered SHF proliferation and abnormal myocardial function as secondary effects (Leatherbury et al. 1990; Farrell et al. 2001; Yelbuz et al. 2002; Waldo et al. 2005). Abnormal looping is the earliest defect seen after CNC ablation and can be observed before the CNC cells reach the OFT. Defective looping may be caused by failure of addition of the OFT myocardium to the heart tube from the anterior subpopulation of SHF cells. Thus, the looping defects observed after CNC ablation suggest that CNC cells are required for the normal deployment of SHF cells (Ward et al. 2005).
Other CNC Derivatives
CNC cells also contribute to the formation of some cardiovascular tissues other than the OFT and AA described above.
Cardiac Innervation and Conduction System
CNC cells give rise to the neurons and supporting cells of the cardiac ganglia for the entire parasympathetic innervation of the heart, whereas the sympathetic peripheral nerves are derived from the trunk neural crest (Kirby and Stewart 1983). Although the neural cell adhesion molecule, NCAM, is down-regulated during CNC migration, it is up-regulated as the cells aggregate in these ganglia (Thiery et al. 1982). Components of the cardiac conduction system including the bundle of His are largely innervated by CNC-derived cardiac ganglia, although the essential components of the cardiac conduction system are mainly myocardial in origin (Kirby et al. 1983; Miquerol et al. 2011). A large number of fibroblasts derived from the epicardium, endocardium, and CNC contribute to the mature conduction system, and CNC ablation leads to delayed maturation of the conduction system (Gurjarpadhye et al. 2007).
Semilunar Valves
There are two semilunar valves, namely, the aortic and pulmonary valve. Each valve has three cusps or leaflets that are remodeled from the cushion mesenchyme of OFT after its division. CNC cells are observed at the tip of the semilunar valve leaflets and contribute to their remodeling and maturation, although the leaflets mainly consist of endocardial-derived mesenchymal cells (Jiang et al. 2000; Jain et al. 2011; Phillips et al. 2013).
Coronary Vessels
The contribution of CNC to coronary vessels is still controversial. Coronary artery anomalies are frequently observed in the CNC ablation model (Hood and Rosenquist 1992); however, quail-chick chimeras of CNC show no contribution of the CNC to coronary arteries (Waldo et al. 1994). Recently, a murine cell lineage analysis showed that a few but significant Wnt1-expressing cells were present in the proximal coronary artery, and a quail-chick chimera analysis revealed that a population of preotic cranial neural crest cells, but not a population originally defined as CNC cells, contributed to the smooth muscle of the coronary arteries (Arima et al. 2012).
Interventricular Septum
CNC cells migrate into the most proximal OFT and may be involved in closure of the outflow part of the membranous interventricular septum (Waldo et al. 1998).
BASIC SCIENCE OF CNC
The numerous molecular cascades that regulate the induction, specification, delamination, and migration of the neural crest cells are described in the next section, although the complex mechanism underlying the multipotent nature of migratory neural crest cells is not fully understood.
Induction/Specification of CNC
The neural crest is induced at the dorsalmost tip of the neural tube when the bilateral neural folds grow up to develop the tube on the midline of embryos (Fig. 1; Basch et al. 2006; Murdoch et al. 2012). A member of the Pax transcription factor, Pax7, is expressed in the boundary between the neural and nonneural ectoderm as the earliest marker for neural crest induction. Another member, Pax3, promotes neural crest expansion before migration (Li et al. 1999; Conway et al. 2000; Epstein et al. 2000). Pax3 also plays a role in neural crest specification and migration by regulating its downstream targets such as the forkhead box transcription factor, FoxD3 (Dottori et al. 2001; Kos et al. 2001), and the secreted extracellular signaling molecule, Wnt1 (Dorsky et al. 1998; Fenby et al. 2008).
Bone morphogenetic proteins (BMPs) play multiple roles in development of the neural crest from specification to migration. BMP4 and BMP7 are secreted from the nonneural ectoderm and maintain Pax3 expression in the dorsal neural tube. It is still argued whether the neural crest is derived from the neural ectoderm or from the nonneural ectoderm (Selleck and Bronner-Fraser 1996; Weston et al. 2004). Increased BMP signaling in the surface ectoderm leads to neural crest specification at the border of neural and nonneural ectoderm (Liem et al. 1995). It also leads to up-regulation of cadherin 6b and cadherin7 in the premigratory neural crest, avoiding its exit from the neural tube.
Delamination
The neural crest cells, after induction and specification, delaminate from the neural tube and migrate segmentally along the pharyngeal arches and somites (Fig. 1). Because cadherins in the neural fold maintain the adherens junctions with the specified neural crest cells (Simard et al. 2005), they need a process of epithelial-to-mesenchymal transition (EMT) to delaminate from the neural tube. During EMT, they lose their cell–cell adhesion, change their cytoskeleton, and gain a motile phenotype as mesenchymal cells.
After the neural crest cells are specified, BMP4 and BMP7 are down-regulated in the nonneural ectoderm, leading to down-regulation of cadherins and inducing Snail2, which also represses cadherin6b and E-cadherin (Taneyhill et al. 2007). These signaling pathways allow EMT and delamination of the neural crest cells. Interestingly, cadherin6b and N-cadherin are down-regulated, but cadherin7 expression increases in the migrating neural crest cells, and overexpression of cadherin6b, N-cadherin, or cadherin7 prevents neural crest delamination (Nakagawa and Takeichi 1995, 1998). Precise regulation of the type and amount of cadherins may thus be essential for proper delamination of neural crest cells.
BMP4 and BMP7 also up-regulate the small GTPase RhoB (Liu and Jessell 1998). Expression of RhoB overlaps with Snail2 at delamination, but it is down-regulated in migrating neural crest cells (Del Barrio and Nieto 2004). RhoB may be required for neural crest cells to initiate directional migration because the Rho family of proteins is reported to promote detachment at the rear of migrating cells (Wheeler and Ridley 2004). Other than cadherins, fibulin is also expressed in premigratory neural crest cells, but not after their migration (Spence et al. 1992). Fibulin can interfere with migration-promoting proteins such as fibronectin and inhibit fibroblast migration (Twal et al. 2001).
As transcription factors, FoxD3 and Sox10 may be involved in delamination of the neural crest. Overexpression of FoxD3 and Sox10 results in increased and premature EMT/delamination of neural crest cells, respectively (Dottori et al. 2001; McKeown et al. 2005).
Migration
Once the neural crest cells delaminate from the neural tube, they begin to migrate as mesenchymal cells (Fig. 1). During their migration, they express the intermediate filament protein vimentin and a complex collection of integrins. They display filopodia, which are slender cytoplasmic projections that extend beyond the leading edge of lamellipodia (Mattila and Lappalainen 2008) and interact with the extracellular matrix (ECM). Integrins play numerous roles in cell signaling, cell shape, cell mobility, and the cell cycle. Especially for cranial neural crest cells, ECM proteins such as type I collagen and laminin with both integrin and nonintegrin receptors and integrins α5 and αv as well as fibronectin are important for migration (Duband et al. 1986; Coles et al. 2006; Strachan and Condic 2008; Milgrom-Hoffman et al. 2014; Turner et al. 2015). Integrin-like kinase facilitates their migration by reducing the expression of NCAM and promoting BMP signaling (Dai et al. 2013). In addition, neural crest cells express ECM-modulating factors, such as matrix metalloproteases (MMPs) and urokinase that are essential for remodeling the environment in their migratory pathway (Erickson and Isseroff 1989; Cai et al. 2000). The gap junction protein connexin 43 (Cx43), is expressed in migrating neural crest cells and is responsible for both directionality and motility in which an excess of Cx43 facilitates the migration (Sullivan and Lo 1995; Huang et al. 1998; Xu et al. 2006).
Some extrinsic ligand–receptor signaling is also responsible for neural crest migration. The Eph family of receptor tyrosine kinases binds membrane-anchored ephrins to control guidance (Davy et al. 2004). This Eph–ephrin signaling is likely to direct the migration of cranial neural crest cells from the neural tube to the pharyngeal arches (Davy and Soriano 2007; Mellott and Burke 2008). Migratory neural crest cells express numerous Eph receptors, and ligand ephrin is expressed adjacent to the migratory path of neural crest cells in which ephrin expression repels the migratory neural crest cells.
Semaphorins, a group of secreted ligands, are expressed along the migratory pathways of cranial/CNC cells in the pharynx and OFT. The semaphorin receptors plexin-A2, plexin-D1, and neuropilin-1 (Nrp1) are expressed in the migrating CNC cells. They have both antagonistic and positive effects on migration according to the ligand–receptor combination such that Sema6A and 6B ligands are expressed in the dorsal neural tube and lateral pharyngeal mesenchyme, whereas Sema3C ligand is expressed in the OFT in which Sema6A and 6B repel the neural crest cells and Sema3C attracts them (Serini et al. 2003; Toyofuku et al. 2008). These signaling pathways especially seem to promote guidance of neural crest cells away from the neural tube to their final target organs compared with Eph–ephrin signaling.
As a secreted extracellular signaling molecule, Wnt signaling plays a role in the induction as well as in the migration of the neural crest (Hamblet et al. 2002). Wnt1 is expressed in early migrating neural crest cells, and is turned off as the cells migrate away from the neural tube. Noncanonical Wnt5a is expressed in the pharyngeal mesoderm adjacent to migrating CNC cells and increases the calcium transients that can decrease cellular filopodia motility (Lohmann et al. 2005; Schleiffarth et al. 2007), resulting in slow migration of the CNC cells.
Also, a secreted extracellular signaling molecule, FGF8, is a key regulator of neural crest development. It is expressed in the pharyngeal ectoderm and endoderm adjacent to the CNC migratory pathway and is chemotactic for CNC cells (Hutson et al. 2006; Sato et al. 2011). In CNC ablation models, FGF8 signaling increases in the pharynx and the ablation phenotype can be rescued by reducing the level of FGF8. This signaling can be modulated by the ECM protein heparan sulfate (Zhang et al. 2015). Retinoic acid signaling through the retinoic acid receptor (RAR and RXR) heterodimer pathway is also associated with neural crest migration in the OFT as global knockout of these receptors results in PTA (Lee et al. 1997b; Jiang et al. 2002).
MOLECULAR MECHANISM UNDERLYING HEART DEVELOPMENT AND DISEASES ASSOCIATED WITH CNC
Molecular Mechanism of AA and OFT Development
Several signaling pathways are implicated in AA patterning. Mutations in the endothelin pathway (endothelin 1, ET1; endothelin receptor A, ETA; endothelin-converting enzyme 1, ECE-1) in mice display abnormal remodeling of pharyngeal arch arteries, resulting in anomalies of AA and the OFT despite normal CNC migration (Kurihara et al. 1995; Clouthier et al. 1998; Morishima et al. 2003). Mutations in transforming growth factor β (TGF-β) superfamily signaling, including BMP signaling intracellularly mediated by Smad proteins, lead to remodeling defects of pharyngeal arch arteries in mice (Molin et al. 2004; Nie et al. 2008). Overexpression of the TGF-β coreceptor endoglin, under the Wnt1 promoter, results in thickened and poorly organized vascular smooth muscle (Mancini et al. 2007). Conditional mutation of the transmembrane receptor, Notch2 under Pax3 promoter leads to decreased proliferation of CNC cells comprising the smooth muscle (Varadkar et al. 2008). Dominant-negative mutation of the MAML gene under the Pax3 promoter that blocks Notch signaling, leads to defective remodeling of pharyngeal arch arteries by inhibiting smooth muscle addition to these arteries (Manderfield et al. 2012). Knockdown of the transcription factor myocardin under Wnt1 or Pax3 promoter results in patent ductus arteriosus because of failure of CNC differentiation into smooth muscle (Huang et al. 2008).
Conditional mutation of N-cadherin under the Wnt1 promoter leads to PTA in mice. In these mice, CNC cells migrate normally into the OFT cushions, but remain rounded, leading to failure in OFT septation because of poor cell–cell contact (Luo et al. 2006). Conditional mutation of the TGF-β receptor Alk2 in the neural crest results in PTA as the CNC cells fail to enter the OFT cushions (Kaartinen et al. 2004). Overexpression of the TGF-β inhibitor Smad7 under the Wnt1 promoter before CNC delamination from the neural tube also leads to PTA, owing to reduced migration and increased cell death in the CNC (Tang et al. 2010).
Reciprocal Molecular Signaling for Interaction between CNC and SHF
OFT alignment and septation involve two distinct progenitor cell populations, the SHF and CNC, and, after CNC ablation, cell proliferation in the SHF is increased with elevated levels of FGF8 as mentioned above (Waldo et al. 2005; Hutson et al. 2006). Inhibition of Notch signaling within the SHF results in abnormal migration of CNC into the OFT (High et al. 2009). These observations suggest that FGF signaling from the CNC influences the SHF development, and vice versa, Notch signaling from the SHF modulates CNC behavior. Coordinated morphogenesis of the two progenitor cell populations is essential where they interact with each other and together form and divide the OFT properly.
Haploinsufficiency for the T-box transcription factor, Tbx1, which is a major genetic determinant of the 22q11DS, results in AA anomalies in mouse embryos (Lindsay et al. 2001). The proposed underlying mechanisms are the delayed initial growth and patterning of the fourth pharyngeal arch artery and abnormal CNC migration with poor smooth muscle differentiation. Homozygous mutation of Tbx1 in mice results in PTA. Delineation of the Tbx1 expression pattern sheds light on the molecular and cellular basis of normal and abnormal development of the OFT. Surprisingly, Tbx1 was not expressed in the CNC (Fig. 5; Garg et al. 2001; Yamagishi et al. 2003; Xu et al. 2004), but in the SHF, although the phenotype associated with the deletion of TBX1, or 22q11DS, closely resembled that of CNC ablation models. Tbx1 is preferentially expressed in the pharyngeal arches (mesodermal core and endodermal epithelium), the ventral half of the otic vesicle, and the head mesenchyme (Fig. 5). These results suggest that defects of CNC-derived tissues in 22q11DS may occur in a non-cell-autonomous fashion. A recent integrated study showed that proper spatiotemporal expression of Sema3C, regulated positively by Foxc1/Foxc2 and negatively by the Tbx1–Fgf8 cascade, is essential for the interaction between CNC and the SHF that correctly navigates CNC cells toward the SHF-derived OFT (Fig. 4; Plein et al. 2015; Kodo et al. 2009, 2017).
Figure 5.
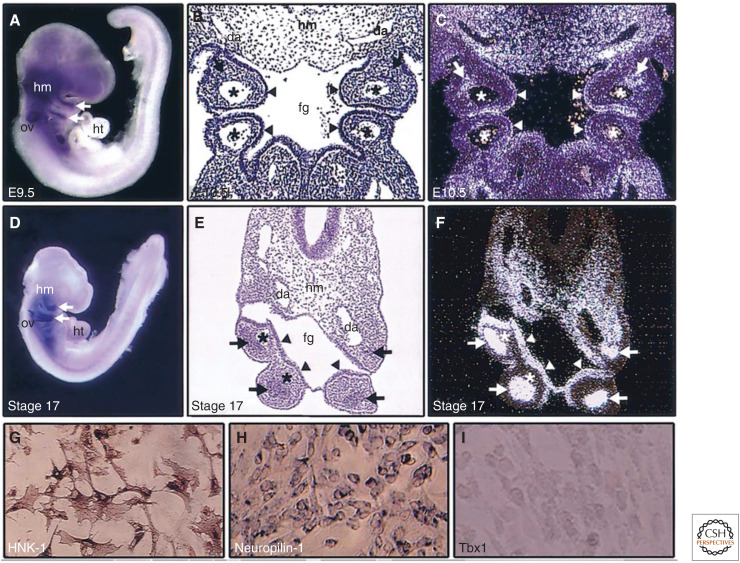
Expression patterns of Tbx1. Mouse (A–C) and chick (D–F) embryos and primary neural crest culture from chick embryos (G–I) are shown. RNA in situ hybridizations for whole-mount (A,D), coronal section (B,C), and transverse section (E,F) show Tbx1 expression (blue or white signals). In the pharyngeal arches, Tbx1 is expressed in mesodermal core (arrows) and endodermal epithelium (arrow heads), excluding the neural crest–derived mesenchyme surrounding the mesodermal core. Tbx1 is also expressed in head mesenchyme (hm) and otic vesicle (ov). B and E are bright field images of C and F, respectively. Asterisks indicate pharyngeal arch arteries. In chick primary neural crest culture cells, HNK-1 (G) and neuropilin-1 (H) are detected by immunocytochemistry and mRNA in situ hybridization, respectively, but Tbx1 is not detectable by mRNA in situ hybridization (I). (fg) foregut, (da) dorsal aorta, (ht) heart (from Yamagishi 2002, with permission).
CLINICAL IMPLICATIONS OF CNC
In humans, numerous diseases and syndromes are reported to be associated with the development of CNC. Among these, 22q11DS and CHARGE syndrome are described below. Because the CNC is a subpopulation of the cranial neural crest, defects in the early steps of neural crest development affect the CNC as well as the entire cranial neural crest, resulting in both craniofacial and cardiac phenotypes in these human syndromes.
22q11.2 Deletion Syndrome (22q11DS)
The 22q11DS is the most common genetic cause of a spectrum of AA and OFT defects with an incidence of one in 4000–5000 births (Scambler 2000; Yamagishi 2002). Most of these are sporadic in origin, whereas 10%–20% of the deletions are inherited as an autosomal dominant trait. The 22q11DS encompasses three distinct syndromes, namely, DiGeorge syndrome (DGS; OMIM#188400), velocardiofacial syndrome (VCFS; OMIM#192430), and conotruncal anomaly face syndrome (CAFS; OMIM#217095), also called “Takao syndrome.” Historically, DGS was originally characterized by CHD, hypoparathyroidism, and immune deficiency reported in 1965 from the field of immunology (DiGeorge 1965); VCFS was associated with cleft palate, CHD, a distinct facial appearance, and learning difficulties as reported in 1978 from the field of plastic surgery (Shprintzen et al. 1978); and CAFS or Takao syndrome was characterized by conotruncal CHD (OFT defects), a distinct facial appearance, and hypernasal voice in 1976 in Japanese patients from the field of pediatric cardiology (Kinouchi et al. 1976). In 1993, clinical genetic studies indicated that these syndromes have an overlapping phenotype and share a common heterozygous deletion of the 22q11.2 region (Driscoll et al. 1992; Scambler et al. 1992; Burn et al. 1993).
The structures primarily affected in patients with 22q11DS are derivatives of the embryonic pharyngeal arches and pouches and the cardiac OFT, which are contributed by the neural crest cells, suggesting that 22q11DS is a developmental field defect of the pharyngeal arch apparatus. The acronym “CATCH22 (cardiac defects, abnormal facies, thymic hypoplasia, cleft palate, hypocalcemia, and 22q11 deletions)” was proposed to aid in remembering the main features of the syndrome encompassing DGS, VCFS, and CAFS (Wilson et al. 1993). However, clinical use of this term may now be inappropriate because of the following reasons: (1) this term has a negative connotation indicating a situation in which it is impossible to do anything, originally from a novel entitled Catch-22 by Heller (1962); (2) the term “A” referring to “abnormal facies” is difficult to be accepted by patients and their family; and (3) the clinical spectrum associated with 22q11.2 deletion is much wider than was previously recognized as “CATCH” (Burn 1999).
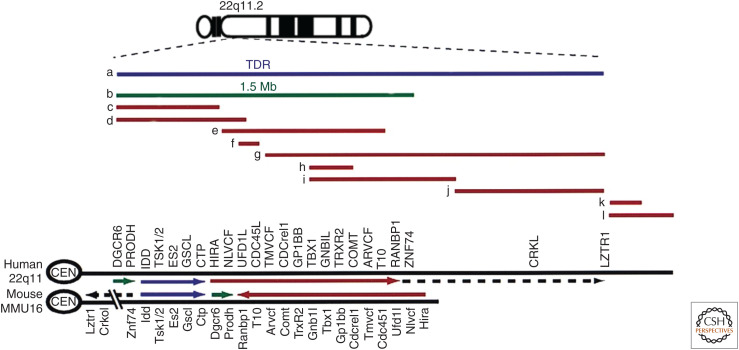
Despite the heterogeneous clinical presentations, remarkably homogenous deletions in the 22q11.2 region are present in patients with 22q11DS in which ∼90% of patients have a typical deletion of 3 Mb or 1.5 Mb, and only a few atypical deletions have been reported (Fig. 6; O'Donnell et al. 1997; McQuade et al. 1999; Yamagishi et al. 1999; Yamagishi and Srivastava 2003). Unequal recombination events between low copy repeat sequences flanking the typical 3-Mb or 1.5-Mb deletion region explain the recurrence of deletions with uniform size. On the contrary, the basis of phenotype variability of 22q11DS, which is even observed in familial cases including individuals with monozygotic twins (Yamagishi et al. 1998), remains to be elucidated. Suggested explanations include allelic variability, variable penetrance, and variable expressivity caused by environmental factors or stochastic events during fetal development.
Figure 6.
Genes and deletions in human chromosome 22q11.2 region and its syntenic region of mouse chromosome 16. Lines (a–l) show deleted regions of human patients. (a) 3 Mb typically deleted region (TDR); (b) smaller 1.5 Mb deletion; (c–l) atypical deletions. Gene names: DGCR6, DiGeorge critical region gene 6; PRODH, proline dehydrogenase; IDD, integral membrane protein deleted in DGS; TSK1/2, threonine/serine kinase-1/2; ES2, expressed sequence-2; GSCL, goosecoid-like; CTP, citrate transport protein; HIRA, histone regulatory A; NLVCF, nuclear localization signal protein in VCFS; UFD1L, ubiquitin fusion degradation 1-like; CDC45L, cell division cycle 45-like; TMVCF, transmembrane protein in VCFS; CDCrel1, cell division cycle-related-1; GP1BB, glycoprotein-1bb; TBX1, T-box protein-1; GNB1L, guanine nucleotide-binding protein b-1-like; TRXR2, thioredoxin reductase-2; COMT, catechol-O-methyltransferase; ARVCF, armadillo repeat gene in VCFS; T10, transcript-10; RANBP1, Ran-binding protein-1; ZNF74, zinc-finger protein 74; CRKL, v-crk virus oncogene-like; LZTR1, leucine-zipper transcriptional regulator-1 (adapted from Yamagishi 2002, with permission).
The gene encoding TBX1, located in the 3 Mb of the 22q11.2 critical region, is the best responsible gene of 22q11DS. A few single-point mutations in TBXl also recapitulate the phenotype of 22q11DS (Yagi et al. 2003). The cellular and molecular mechanism for heart development and disease involving CNC and Tbx1 is discussed above.
Approximately 75% of patients with 22q11DS have CHD. The type of CHD is characterized as OFT and AA defects including TOF (∼30%), IAA-B (∼15%), ventricular septal defect (∼15%), PTA (∼10%), and others (∼5%) (Goldmuntz et al. 1998). Alternatively, the 22q11.2 deletion is present in ∼60% of patients with IAA-B, ∼35% of patients with PTA, and ∼15% of patients with TOF. Specifically, it is detected in ∼55% of patients with TOF plus pulmonary atresia and major aortopulmonary collateral arteries (Maeda et al. 2000).
CHARGE Syndrome
CHARGE syndrome is an acronym characterized by the following defects: coloboma of the eye, heart defects, atresia of the nasal choanae, retarded growth and/or development, genital and/or urinal abnormalities, and ear anomalies (Sanlaville and Verloes 2007). A gene encoding a member of the chromodomain helicase DNA-binding proteins, CHD7, is the best responsible gene for this syndrome. Over 90% of patients with CHARGE syndrome have heterozygous mutations in CHD7 or semaphorin 3E (Bergman et al. 2011; Schulz et al. 2014). CHD7 regulates the class 3 semaphorins, including Sema3C, and genetically interacts with Tbx1 (Randall et al. 2009; Payne et al. 2015). CHD7 also represses p53 that may be associated with the phenotype of CHARGE syndrome including PTA (Van Nostrand et al. 2014). A mouse model of CHARGE syndrome, whirligig (whi), which has a heterozygous point mutation in the Chd7 gene, shows a phenotype similar to that of patients with CHARGE syndrome (Bosman et al. 2005). Homozygous whi/whi mice are embryonic lethal with misregulation of many genes involved in neural crest development and axon guidance, including semaphorins and ephrin receptors (Schulz et al. 2014).
CONCLUDING REMARKS
Since the discovery of the CNC, cellular and molecular mechanisms underlying heart development and diseases, especially involving the OFT and AA, have been explored extensively together with the discovery of numerous genes and a new cardiac progenitor population derived from the SHF. New technologies, such as single-cell analysis and next-generation sequencing, will provide more detailed findings in the near future. We need to clearly understand the nature of heart development and disease using these results and ultimately use our knowledge to improve the lives of children with CHD.
ACKNOWLEDGMENTS
The author thanks Keiko Uchida, Kazuki Kodo, Chihiro Yamagishi, and Eriko Nakamura for preparation of manuscript and figures. The author's work is supported by JSPS KAKENHI.
Footnotes
Editors: Benoit G. Bruneau and Paul R. Riley
Additional Perspectives on Heart Development and Disease available at www.cshperspectives.org
REFERENCES
- Arima Y, Miyagawa-Tomita S, Maeda K, Asai R, Seya D, Minoux M, Rijli FM, Nishiyama K, Kim KS, Uchijima Y, et al. 2012. Preotic neural crest cells contribute to coronary artery smooth muscle involving endothelin signalling. Nat Commun 3: 1267 10.1038/ncomms2258 [DOI] [PubMed] [Google Scholar]
- Basch ML, Bronner-Fraser M, García-Castro MI. 2006. Specification of the neural crest occurs during gastrulation and requires Pax7. Nature 441: 218–222. 10.1038/nature04684 [DOI] [PubMed] [Google Scholar]
- Bergman JE, Janssen N, Hoefsloot LH, Jongmans MC, Hofstra RM, van Ravenswaaij-Arts CM. 2011. CHD7 mutations and CHARGE syndrome: the clinical implications of an expanding phenotype. J Med Genet 48: 334–342. 10.1136/jmg.2010.087106 [DOI] [PubMed] [Google Scholar]
- Bergwerff M, Verberne ME, DeRuiter MC, Poelmann RE, Gittenberger-de Groot AC. 1998. Neural crest cell contribution to the developing circulatory system: implications for vascular morphology? Circ Res 82: 221–231. 10.1161/01.RES.82.2.221 [DOI] [PubMed] [Google Scholar]
- Bockman DE, Redmond ME, Waldo K, Davis H, Kirby ML. 1987. Effect of neural crest ablation on development of the heart and arch arteries in the chick. Am J Anat 180: 332–341. 10.1002/aja.1001800403 [DOI] [PubMed] [Google Scholar]
- Bosman EA, Penn AC, Ambrose JC, Kettleborough R, Stemple DL, Steel KP. 2005. Multiple mutations in mouse Chd7 provide models for CHARGE syndrome. Hum Molec Genet 14: 3463–3476. 10.1093/hmg/ddi375 [DOI] [PubMed] [Google Scholar]
- Brown CB, Feiner L, Lu MM, Li J, Ma X, Webber AL, Jia L, Raper JA, Epstein JA. 2001. PlexinA2 and semaphorin signaling during cardiac neural crest development. Development 128: 3071–3080. [DOI] [PubMed] [Google Scholar]
- Burn J. 1999. Closing time for CATCH22. J Med Genet 36: 737–738. 10.1136/jmg.36.10.737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burn J, Takao A, Wilson D, Cross I, Momma K, Wadey R, Scambler P, Goodship J. 1993. Conotruncal anomaly face syndrome is associated with a deletion within chromosome 22q11. J Med Genet 30: 822–824. 10.1136/jmg.30.10.822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai DH, Vollberg TM Sr, Hahn-Dantona E, Quigley JP, Brauer PR. 2000. MMP-2 expression during early avian cardiac and neural crest morphogenesis. Anat Rec 259: 168–179. [DOI] [PubMed] [Google Scholar]
- Clouthier DE, Hosoda K, Richardson JA, Williams SC, Yanagisawa H, Kuwaki T, Kumada M, Hammer RE, Yanagisawa M. 1998. Cranial and cardiac neural crest defects in endothelin-A receptor-deficient mice. Development 125: 813–824. [Google Scholar]
- Coles EG, Gammill LS, Miner JH, Bronner-Fraser M. 2006. Abnormalities in neural crest cell migration in laminin α5 mutant mice. Dev Biol 289: 218–228. 10.1016/j.ydbio.2005.10.031 [DOI] [PubMed] [Google Scholar]
- Conway SJ, Bundy J, Chen J, Dickman E, Rogers R, Will BM. 2000. Decreased neural crest stem cell expansion is responsible for the conotruncal heart defects within the Splotch (Sp2H)/Pax3 mouse mutant. Cardiovasc Res 47: 314–328. 10.1016/S0008-6363(00)00098-5 [DOI] [PubMed] [Google Scholar]
- Dai X, Jiang W, Zhang Q, Xu L, Geng P, Zhuang S, Petrich BG, Jiang C, Peng L, Bhattacharya S, et al. 2013. Requirement for integrin-linked kinase in neural crest migration and differentiation and outflow tract morphogenesis. BMC Biol 11: 107 10.1186/1741-7007-11-107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davy A, Soriano P. 2007. Ephrin-B2 forward signaling regulates somite patterning and neural crest cell development. Dev Biol 304: 182–193. 10.1016/j.ydbio.2006.12.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davy A, Aubin J, Soriano P. 2004. Ephrin-B1 forward and reverse signaling are required during mouse development. Genes Dev 18: 572–583. 10.1101/gad.1171704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Barrio MG, Nieto MA. 2004. Relative expression of Slug, RhoB, and HNK-1 in the cranial neural crest of the early chicken embryo. Dev Dyn 229: 136–139. 10.1002/dvdy.10456 [DOI] [PubMed] [Google Scholar]
- DiGeorge AM. 1965. Discussion on a new concept of the cellular basis of immunology. J Pediatr 67: 907. [Google Scholar]
- Dorsky RI, Moon RT, Raible DW. 1998. Control of neural crest cell fate by the Wnt signalling pathway. Nature 396: 370–373. 10.1038/24620 [DOI] [PubMed] [Google Scholar]
- Dottori M, Gross MK, Labosky P, Goulding M. 2001. The winged-helix transcription factor Foxd3 suppresses interneuron differentiation and promotes neural crest cell fate. Development 128: 4127–4138. [DOI] [PubMed] [Google Scholar]
- Driscoll DA, Budarf ML, Emanuel BS. 1992. A genetic etiology for DiGeorge syndrome: consistent deletions and microdeletions of 22q11. Am J Hum Genet 50: 924–933. [PMC free article] [PubMed] [Google Scholar]
- Duband JL, Rocher S, Yamada KM, Thiery JP. 1986. Interactions of migrating neural crest cells with fibronectin. Prog Clin Biol Res 226: 127–139. [PubMed] [Google Scholar]
- Epstein JA, Li J, Lang D, Chen F, Brown CB, Jin F, Lu MM, Thomas M, Liu E, Wessels A, et al. 2000. Migration of cardiac neural crest cells in Splotch embryos. Development 127: 1869–1878. [DOI] [PubMed] [Google Scholar]
- Erickson CA, Isseroff RR. 1989. Plasminogen activator activity is associated with neural crest cell motility in tissue culture. J Exp Zool 251: 123–133. 10.1002/jez.1402510203 [DOI] [PubMed] [Google Scholar]
- Farrell MJ, Burch JL, Wallis K, Rowley L, Kumiski D, Stadt H, Godt RE, Creazzo TL, Kirby ML. 2001. FGF-8 in the ventral pharynx alters development of myocardial calcium transients after neural crest ablation. J Clin Invest 107: 1509–1517. 10.1172/JCI9317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenby BT, Fotaki V, Mason JO. 2008. Pax3 regulates Wnt1 expression via a conserved binding site in the 5′ proximal promoter. Biochim Biophys Acta 1779: 115–121. 10.1016/j.bbagrm.2007.11.008 [DOI] [PubMed] [Google Scholar]
- Garg V, Yamagishi C, Hu T, Kathiriya IS, Yamagishi H, Srivastava D. 2001. Tbx1, a DiGeorge syndrome candidate gene, is regulated by sonic hedgehog during pharyngeal arch development. Dev Biol 235: 62–73. 10.1006/dbio.2001.0283 [DOI] [PubMed] [Google Scholar]
- Goldmuntz E, Clark BJ, Mitchell LE, Jawad AF, Cuneo BF, Reed L, McDonald-McGinn D, Chien P, Feuer J, Zackai EH, et al. 1998. Frequency of 22q11 deletions in patients with conotruncal defects. J Am Coll Cardiol 32: 492–498. 10.1016/S0735-1097(98)00259-9 [DOI] [PubMed] [Google Scholar]
- Gurjarpadhye A, Hewett KW, Justus C, Wen X, Stadt H, Kirby ML, Sedmera D, Gourdie RG. 2007. Cardiac neural crest ablation inhibits compaction and electrical function of conduction system bundles. Am J Physiol Heart Circ Physiol 292: H1291–H1300. 10.1152/ajpheart.01017.2006 [DOI] [PubMed] [Google Scholar]
- Hamblet NS, Lijam N, Ruiz-Lozano P, Wang J, Yang Y, Luo Z, Mei L, Chien KR, Sussman DJ, Wynshaw-Boris A. 2002. Dishevelled 2 is essential for cardiac outflow tract development, somite segmentation and neural tube closure. Development 129: 5827–5838. 10.1242/dev.00164 [DOI] [PubMed] [Google Scholar]
- Heller J. 1962. Catch-22. Jonathan Cape, London. [Google Scholar]
- High FA, Jain R, Stoller JZ, Antonucci NB, Lu MM, Loomes KM, Kaestner KH, Pear WS, Epstein JA. 2009. Murine Jagged1/Notch signaling in the second heart field orchestrates Fgf8 expression and tissue-tissue interactions during outflow tract development. J Clin Invest 119: 1986–1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman JI, Kaplan S. 2002. The incidence of congenital heart disease. J Am Coll Cardiol 39: 1890–1900. 10.1016/S0735-1097(02)01886-7 [DOI] [PubMed] [Google Scholar]
- Hood LC, Rosenquist TH. 1992. Coronary artery development in the chick: origin and deployment of smooth muscle cells, and the effects of neural crest ablation. Anat Rec 234: 291–300. 10.1002/ar.1092340215 [DOI] [PubMed] [Google Scholar]
- Huang GY, Wessels A, Smith BR, Linask KK, Ewart JL, Lo CW. 1998. Alteration in connexin 43 gap junction gene dosage impairs conotruncal heart development. Dev Biol 198: 32–44. 10.1006/dbio.1998.8891 [DOI] [PubMed] [Google Scholar]
- Huang J, Cheng L, Li J, Chen M, Zhou D, Lu MM, Proweller A, Epstein JA, Parmacek MS. 2008. Myocardin regulates expression of contractile genes in smooth muscle cells and is required for closure of the ductus arteriosus in mice. J Clin Invest 118: 515–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutson MR, Zhang P, Stadt HA, Sato AK, Li YX, Burch J, Creazzo TL, Kirby ML. 2006. Cardiac arterial pole alignment is sensitive to FGF8 signaling in the pharynx. Dev Biol 295: 486–497. 10.1016/j.ydbio.2006.02.052 [DOI] [PubMed] [Google Scholar]
- Jain R, Engleka KA, Rentschler SL, Manderfield LJ, Li L, Yuan L, Epstein JA. 2011. Cardiac neural crest orchestrates remodeling and functional maturation of mouse semilunar valves. J Clin Invest 121: 422–430. 10.1172/JCI44244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang X, Rowitch DH, Soriano P, McMahon AP, Sucov HM. 2000. Fate of the mammalian cardiac neural crest. Development 127: 1607–1616. [DOI] [PubMed] [Google Scholar]
- Jiang X, Choudhary B, Merki E, Chien KR, Maxson RE, Sucov HM. 2002. Normal fate and altered function of the cardiac neural crest cell lineage in retinoic acid receptor mutant embryos. Mech Dev 117: 115–122. 10.1016/S0925-4773(02)00206-X [DOI] [PubMed] [Google Scholar]
- Kaartinen V, Dudas M, Nagy A, Sridurongrit S, Lu MM, Epstein JA. 2004. Cardiac outflow tract defects in mice lacking ALK2 in neural crest cells. Development 131: 3481–3490. 10.1242/dev.01214 [DOI] [PubMed] [Google Scholar]
- Keyte A, Hutson MR. 2012. The neural crest in cardiac congenital anomalies. Differentiation 84: 25–40. 10.1016/j.diff.2012.04.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinouchi A, Mori K, Ando M, Takao A. 1976. Facial appearance of patients with conotruncal anomalies. Shonika (Pediatr Jpn) 17: 84. [Google Scholar]
- Kirby ML. 2007. Neural crest, great arteries and outflow septation. In Cardiac development (ed. Kirby ML), pp. 143–160. Oxford University Press, New York. [Google Scholar]
- Kirby ML, Stewart DE. 1983. Neural crest origin of cardiac ganglion cells in the chick embryo: identification and extirpation. Dev Biol 97: 433–443. 10.1016/0012-1606(83)90100-8 [DOI] [PubMed] [Google Scholar]
- Kirby ML, Gale TF, Stewart DE. 1983. Neural crest cells contribute to normal aorticopulmonary septation. Science 220: 1059–1061. 10.1126/science.6844926 [DOI] [PubMed] [Google Scholar]
- Kodo K, Yamagishi H. 2011. A decade of advances in the molecular embryology and genetics underlying congenital heart defects. Circ J 75: 2296–2304. 10.1253/circj.CJ-11-0636 [DOI] [PubMed] [Google Scholar]
- Kodo K, Nishizawa T, Furutani M, Arai S, Yamamura E, Joo K, Takahashi T, Matsuoka R, Yamagishi H. 2009. GATA6 mutations cause human cardiac outflow tract defects by disrupting semaphorin-plexin signaling. Proc Natl Acad Sci 106: 13933–13938. 10.1073/pnas.0904744106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kodo K, Shibata S, Miyagawa-Tomita S, Ong SG, Takahashi H, Kume T, Okano H, Matsuoka R, Yamagishi H. 2017. Regulation of Sema3c and the interaction between cardiac neural crest and second heart field during outflow tract development. Sci Rep 7: 6771 10.1038/s41598-017-06964-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kos R, Reedy MV, Johnson RL, Erickson CA. 2001. The winged-helix transcription factor FoxD3 is important for establishing the neural crest lineage and repressing melanogenesis in avian embryos. Development 128: 1467–1479. [DOI] [PubMed] [Google Scholar]
- Kurihara Y, Kurihara H, Oda H, Maemura K, Nagai R, Ishikawa T, Yazaki Y. 1995. Aortic arch malformations and ventricular septal defect in mice deficient in endothelin-1. J Clin Invest 96: 293–300. 10.1172/JCI118033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leatherbury L, Gauldin HE, Waldo K, Kirby ML. 1990. Microcinephotography of the developing heart in neural crest-ablated chick embryos. Circulation 81: 1047–1057. 10.1161/01.CIR.81.3.1047 [DOI] [PubMed] [Google Scholar]
- Lee M, Brennan A, Blanchard A, Zoidl G, Dong Z, Tabernero A, Zoidl C, Dent MA, Jessen KR, Mirsky R. 1997a. P0 is constitutively expressed in the rat neural crest and embryonic nerves and is negatively and positively regulated by axons to generate non-myelin-forming and myelin-forming Schwann cells, respectively. Mol Cell Neurosci 8: 336–350. 10.1006/mcne.1996.0589 [DOI] [PubMed] [Google Scholar]
- Lee RY, Luo J, Evans RM, Giguere V, Sucov HM. 1997b. Compartment-selective sensitivity of cardiovascular morphogenesis to combinations of retinoic acid receptor gene mutations. Circ Res 80: 757–764. 10.1161/01.RES.80.6.757 [DOI] [PubMed] [Google Scholar]
- Li J, Liu KC, Jin F, Lu MM, Epstein JA. 1999. Transgenic rescue of congenital heart disease and spina bifida in Splotch mice. Development 126: 2495–2503. [DOI] [PubMed] [Google Scholar]
- Liem KF Jr, Tremml G, Roelink H, Jessell TM. 1995. Dorsal differentiation of neural plate cells induced by BMP-mediated signals from epidermal ectoderm. Cell 82: 969–979. 10.1016/0092-8674(95)90276-7 [DOI] [PubMed] [Google Scholar]
- Lindsay EA, Vitelli F, Su H, Morishima M, Huynh T, Pramparo T, Jurecic V, Ogunrinu G, Sutherland HF, Scambler PJ, et al. 2001. Tbx1 haploinsufficieny in the DiGeorge syndrome region causes aortic arch defects in mice. Nature 410: 97–101. 10.1038/35065105 [DOI] [PubMed] [Google Scholar]
- Liu JP, Jessell TM. 1998. A role for rhoB in the delamination of neural crest cells from the dorsal neural tube. Development 125: 5055–5067. [DOI] [PubMed] [Google Scholar]
- Lohmann C, Finski A, Bonhoeffer T. 2005. Local calcium transients regulate the spontaneous motility of dendritic filopodia. Nat Neurosci 8: 305–312. 10.1038/nn1406 [DOI] [PubMed] [Google Scholar]
- Luo Y, High FA, Epstein JA, Radice GL. 2006. N-cadherin is required for neural crest remodeling of the cardiac outflow tract. Dev Biol 299: 517–528. 10.1016/j.ydbio.2006.09.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeda J, Yamagishi H, Matsuoka R, Ishihara J, Tokumura M, Fukushima H, Ueda H, Takahashi E, Yoshiba S, Kojima Y. 2000. Frequent association of 22q11.2 deletion with tetralogy of Fallot. Am J Med Genet 92: 269–272. [DOI] [PubMed] [Google Scholar]
- Maeda J, Yamagishi H, McAnally J, Yamagishi C, Srivastava D. 2006. Tbx1 is regulated by forkhead proteins in the secondary heart field. Dev Dyn 235: 701–710. 10.1002/dvdy.20686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mancini ML, Verdi JM, Conley BA, Nicola T, Spicer DB, Oxburgh LH, Vary CP. 2007. Endoglin is required for myogenic differentiation potential of neural crest stem cells. Dev Biol 308: 520–533. 10.1016/j.ydbio.2007.06.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manderfield LJ, High FA, Engleka KA, Liu F, Li L, Rentschler S, Epstein JA. 2012. Notch activation of Jagged1 contributes to the assembly of the arterial wall. Circulation 125: 314–323. 10.1161/CIRCULATIONAHA.111.047159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattila PK, Lappalainen P. 2008. Filopodia: molecular architecture and cellular functions. Nat Rev Mol Cell Biol 9: 446–454. 10.1038/nrm2406 [DOI] [PubMed] [Google Scholar]
- McKeown SJ, Lee VM, Bronner-Fraser M, Newgreen DF, Farlie PG. 2005. Sox10 overexpression induces neural crest-like cells from all dorsoventral levels of the neural tube but inhibits differentiation. Dev Dyn 233: 430–444. 10.1002/dvdy.20341 [DOI] [PubMed] [Google Scholar]
- McQuade L, Christodoulou J, Budarf M, Sachdev R, Wilson M, Emanuel B, Colley A. 1999. Patient with a 22q11.2 deletion with no overlap of the minimal DiGeorge syndrome critical region (MDGCR). Am J Med Genet 86: 27–33. [DOI] [PubMed] [Google Scholar]
- Mellott DO, Burke RD. 2008. Divergent roles for Eph and ephrin in avian cranial neural crest. BMC Dev Biol 8: 56 10.1186/1471-213X-8-56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milgrom-Hoffman M, Michailovici I, Ferrara N, Zelzer E, Tzahor E. 2014. Endothelial cells regulate neural crest and second heart field morphogenesis. Biol Open 3: 679–688. 10.1242/bio.20148078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miquerol L, Beyer S, Kelly RG. 2011. Establishment of the mouse ventricular conduction system. Cardiovasc Res 91: 232–242. 10.1093/cvr/cvr069 [DOI] [PubMed] [Google Scholar]
- Molin DG, Poelmann RE, DeRuiter MC, Azhar M, Doetschman T, Gittenberger-de Groot AC. 2004. Transforming growth factor β–SMAD2 signaling regulates aortic arch innervation and development. Circ Res 95: 1109–1117. 10.1161/01.RES.0000150047.16909.ab [DOI] [PubMed] [Google Scholar]
- Morishima M, Yanagisawa H, Yanagisawa M, Baldini A. 2003. Ece1 and Tbx1 define distinct pathways to aortic arch morphogenesis. Dev Dyn 228: 95–104. 10.1002/dvdy.10358 [DOI] [PubMed] [Google Scholar]
- Murdoch B, DelConte C, García-Castro MI. 2012. Pax7 lineage contributions to the mammalian neural crest. PLoS ONE 7: e41089 10.1371/journal.pone.0041089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagawa S, Takeichi M. 1995. Neural crest cell–cell adhesion controlled by sequential and subpopulation-specific expression of novel cadherins. Development 121: 1321–1332. [DOI] [PubMed] [Google Scholar]
- Nakagawa S, Takeichi M. 1998. Neural crest emigration from the neural tube depends on regulated cadherin expression. Development 125: 2963–2971. [DOI] [PubMed] [Google Scholar]
- Nie X, Deng CX, Wang Q, Jiao K. 2008. Disruption of Smad4 in neural crest cells leads to mid-gestation death with pharyngeal arch, craniofacial and cardiac defects. Dev Biol 316: 417–430. 10.1016/j.ydbio.2008.02.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Donnell H, McKeown C, Gould C, Morrow B, Scambler P. 1997. Detection of an atypical 22q11 deletion that has no overlap with the DiGeorge syndrome critical region. Am J Hum Genet 60: 1544–1548. 10.1086/523992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payne S, Burney MJ, McCue K, Popal N, Davidson SM, Anderson RH, Scambler PJ. 2015. A critical role for the chromatin remodeller CHD7 in anterior mesoderm during cardiovascular development. Dev Biol 405: 82–95. 10.1016/j.ydbio.2015.06.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips HM, Mahendran P, Singh E, Anderson RH, Chaudhry B, Henderson DJ. 2013. Neural crest cells are required for correct positioning of the developing outflow cushions and pattern the arterial valve leaets. Cardiovasc Res 99: 452–460. 10.1093/cvr/cvt132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plein A, Calmont A, Fantin A, Denti L, Anderson NA, Scambler PJ, Ruhrberg C. 2015. Neural crest-derived SEMA3C activates endothelial NRP1 for cardiac outflow tract septation. J Clin Invest 125: 2661–2676. 10.1172/JCI79668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porras D, Brown CB. 2008. Temporal–spatial ablation of neural crest in the mouse results in cardiovascular defects. Dev Dyn 237: 153–162. 10.1002/dvdy.21382 [DOI] [PubMed] [Google Scholar]
- Randall V, McCue K, Roberts C, Kyriakopoulou V, Beddow S, Barrett AN, Vitelli F, Prescott K, Shaw-Smith C, Devriendt K, et al. 2009. Great vessel development requires biallelic expression of Chd7 and Tbx1 in pharyngeal ectoderm in mice. J Clin Invest 119: 3301–3310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanlaville D, Verloes A. 2007. CHARGE syndrome: an update. Eur J Hum Genet 15: 389–399. 10.1038/sj.ejhg.5201778 [DOI] [PubMed] [Google Scholar]
- Sato A, Scholl AM, Kuhn EN, Stadt HA, Decker JR, Pegram K, Hutson MR, Kirby ML. 2011. FGF8 signaling is chemotactic for cardiac neural crest cells. Dev Biol 354: 18–30. 10.1016/j.ydbio.2011.03.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scambler PJ. 2000. The 22q11 deletion syndromes. Hum Mol Genet 9: 2421–2426. 10.1093/hmg/9.16.2421 [DOI] [PubMed] [Google Scholar]
- Scambler PJ, Kelly D, Lindsay E, Williamson R, Goldberg R, Shprintzen R, Wilson DI, Goodship JA, Cross IE, Burn J. 1992. Velo-cardio-facial syndrome associated with chromosome 22 deletions encompassing the DiGeorge locus. Lancet 339: 1138–1139. 10.1016/0140-6736(92)90734-K [DOI] [PubMed] [Google Scholar]
- Schleiffarth JR, Person AD, Martinsen BJ, Sukovich DJ, Neumann A, Baker CV, Lohr JL, Cornfield DN, Ekker SC, Petryk A. 2007. Wnt5a is required for cardiac outflow tract septation in mice. Pediatr Res 61: 386–391. 10.1203/pdr.0b013e3180323810 [DOI] [PubMed] [Google Scholar]
- Schulz Y, Wehner P, Opitz L, Salinas-Riester G, Bongers EMHF, van Ravenswaaij-Arts CMA, Wincent J, Schoumans J, Kohlhase J, Borchers A, et al. 2014. CHD7, the gene mutated in CHARGE syndrome, regulates genes involved in neural crest cell guidance. Hum Genet 133: 997–1009. 10.1007/s00439-014-1444-2 [DOI] [PubMed] [Google Scholar]
- Selleck MA, Bronner-Fraser M. 1996. The genesis of avian neural crest cells: a classic embryonic induction. Proc Natl Acad Sci 93: 9352–9357. 10.1073/pnas.93.18.9352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serini G, Valdembri D, Zanivan S, Morterra G, Burkhardt C, Caccavari F, Zammataro L, Primo L, Tamagnone L, Logan M, et al. 2003. Class 3 semaphorins control vascular morphogenesis by inhibiting integrin function. Nature 424: 391–397. 10.1038/nature01784 [DOI] [PubMed] [Google Scholar]
- Shprintzen RJ, GoldbergR B, Lewin ML, Sidoti EJ, Berkman MD, Argamaso RV, Young D. 1978. A new syndrome involving cleft palate, cardiac anomalies, typical facies, and learning disabilities: velo-cardio-facial syndrome. Cleft Palate J 15: 56–62. [PubMed] [Google Scholar]
- Simard A, Di Pietro E, Ryan AK. 2005. Gene expression pattern of Claudin-1 during chick embryogenesis. Gene Expr Patterns 5: 553–560. 10.1016/j.modgep.2004.10.009 [DOI] [PubMed] [Google Scholar]
- Siwik ES, Patel CR, Zahka KG. 2001. Tetralogy of Fallot. In Moss and Adams’ heart disease in infants, children, and adolescents including the fetus and young adult (ed. Allen HD, Gutgesell HP, Clark EB, Driscoll DJ), pp. 880–902. Lippincott Williams & Wilkins, Philadelphia. [Google Scholar]
- Spence SG, Argraves WS, Walters L, Hungerford JE, Little CD. 1992. Fibulin is localized at sites of epithelial–mesenchymal transitions in the early avian embryo. Dev Biol 151: 473–484. 10.1016/0012-1606(92)90186-K [DOI] [PubMed] [Google Scholar]
- Srivastava D. 2006. Making or breaking the heart: from lineage determination to morphogenesis. Cell 126: 1037–1048. 10.1016/j.cell.2006.09.003 [DOI] [PubMed] [Google Scholar]
- Strachan LR, Condic ML. 2008. Neural crest motility on fibronectin is regulated by integrin activation. Exp Cell Res 314: 441–452. 10.1016/j.yexcr.2007.10.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan R, Lo CW. 1995. Expression of a connexin 43/β-galactosidase fusion protein inhibits gap junctional communication in NIH3T3 cells. J Cell Biol 130: 419–429. 10.1083/jcb.130.2.419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taneyhill LA, Coles EG, Bronner-Fraser M. 2007. Snail2 directly represses cadherin6B during epithelial-to-mesenchymal transitions of the neural crest. Development 134: 1481–1490. 10.1242/dev.02834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang S, Snider P, Firulli AB, Conway SJ. 2010. Trigenic neural crest-restricted Smad7 over-expression results in congenital craniofacial and cardiovascular defects. Dev Biol 344: 233–247. 10.1016/j.ydbio.2010.05.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thattaliyath B, Hutson M. 2016. Neural crest. In Congenital heart diseases: the broken heart: clinical features, human genetics and molecular pathway (ed. Rickert-Sperling S, Kelly RG, Driscoll DJ), pp. 41–53. Springer, Wein, Germany. [Google Scholar]
- Thiery JP, Duband JL, Delouvée A. 1982. Pathways and mechanisms of avian trunk neural crest cell migration and localization. Dev Biol 93: 324–343. 10.1016/0012-1606(82)90121-X [DOI] [PubMed] [Google Scholar]
- Toyofuku T, Yoshida J, Sugimoto T, Yamamoto M, Makino N, Takamatsu H, Takegahara N, Suto F, Hori M, Fujisawa H, et al. 2008. Repulsive and attractive semaphorins cooperate to direct the navigation of cardiac neural crest cells. Dev Biol 321: 251–262. 10.1016/j.ydbio.2008.06.028 [DOI] [PubMed] [Google Scholar]
- Turner CJ, Badu-Nkansah K, Crowley D, van der Flier A, Hynes RO. 2015. α5 and αv integrins cooperate to regulate vascular smooth muscle and neural crest functions in vivo. Development 142: 797–808. 10.1242/dev.117572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Twal WO, Czirok A, Hegedus B, Knaak C, Chintalapudi MR, Okagawa H, Sugi Y, Argraves WS. 2001. Fibulin-1 suppression of fibronectin-regulated cell adhesion and motility. J Cell Sci 114: 4587–4598. [DOI] [PubMed] [Google Scholar]
- van den Hoff MJ, Moorman AF, Ruijter JM, Lamers WH, Bennington RW, Markwald RR, Wessels A. 1999. Myocardialization of the cardiac outflow tract. Dev Biol 212: 477–490. 10.1006/dbio.1999.9366 [DOI] [PubMed] [Google Scholar]
- Van Nostrand JL, Brady CA, Jung H, Fuentes DR, Kozak MM, Johnson TM, Lin CY, Lin CJ, Swiderski DL, Vogel H, et al. 2014. Inappropriate p53 activation during development induces features of CHARGE syndrome. Nature 514: 228–232. 10.1038/nature13585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varadkar P, Kraman M, Despres D, Ma G, Lozier J, McCright B. 2008. Notch2 is required for the proliferation of cardiac neural crest-derived smooth muscle cells. Dev Dyn 237: 1144–1152. 10.1002/dvdy.21502 [DOI] [PubMed] [Google Scholar]
- Waldo KL, Kumiski DH, Kirby ML. 1994. Association of the cardiac neural crest with development of the coronary arteries in the chick embryo. Anat Rec 239: 315–331. 10.1002/ar.1092390310 [DOI] [PubMed] [Google Scholar]
- Waldo K, Miyagawa-Tomita S, Kumiski D, Kirby ML. 1998. Cardiac neural crest cells provide new insight into septation of the cardiac outflow tract: aortic sac to ventricular septal closure. Dev Biol 196: 129–144. 10.1006/dbio.1998.8860 [DOI] [PubMed] [Google Scholar]
- Waldo KL, Lo CW, Kirby ML. 1999. Connexin 43 expression reflects neural crest patterns during cardiovascular development. Dev Biol 208: 307–323. 10.1006/dbio.1999.9219 [DOI] [PubMed] [Google Scholar]
- Waldo KL, Hutson MR, Stadt HA, Zdanowicz M, Zdanowicz J, Kirby ML. 2005. Cardiac neural crest is necessary for normal addition of the myocardium to the arterial pole from the secondary heart field. Dev Biol 281: 66–77. 10.1016/j.ydbio.2005.02.011 [DOI] [PubMed] [Google Scholar]
- Ward C, Stadt H, Hutson M, Kirby ML. 2005. Ablation of the secondary heart field leads to tetralogy of Fallot and pulmonary atresia. Dev Biol 284: 72–83. 10.1016/j.ydbio.2005.05.003 [DOI] [PubMed] [Google Scholar]
- Weston JA, Yoshida H, Robinson V, Nishikawa S, Fraser ST, Nishikawa S. 2004. Neural crest and the origin of ectomesenchyme: neural fold heterogeneity suggests an alternative hypothesis. Dev Dyn 229: 118–130. 10.1002/dvdy.10478 [DOI] [PubMed] [Google Scholar]
- Wheeler AP, Ridley AJ. 2004. Why three Rho proteins? RhoA, RhoB, RhoC, and cell motility. Exp Cell Res 301: 43–49. 10.1016/j.yexcr.2004.08.012 [DOI] [PubMed] [Google Scholar]
- Wilson DI, Burn J, Scambler P, Goodship J. 1993. DiGeorge syndrome: part of CATCH 22. J Med Genet 30: 852–856. 10.1136/jmg.30.10.852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu H, Morishima M, Wylie JN, Schwartz RJ, Bruneau BG, Lindsay EA, Baldini A. 2004. Tbx1 has a dual role in the morphogenesis of the cardiac outflow tract. Development 131: 3217–3227. 10.1242/dev.01174 [DOI] [PubMed] [Google Scholar]
- Xu X, Francis R, Wei CJ, Linask KL, Lo CW. 2006. Connexin 43-mediated modulation of polarized cell movement and the directional migration of cardiac neural crest cells. Development 133: 3629–3639. 10.1242/dev.02543 [DOI] [PubMed] [Google Scholar]
- Yagi H, Furutani Y, Hamada H, Sasaki T, Asakawa S, Minoshima S, Ichida F, Joo K, Kimura M, Imamura S, et al. 2003. Role of TBX1 in human del22q11.2 syndrome. Lancet 362: 1366–1373. 10.1016/S0140-6736(03)14632-6 [DOI] [PubMed] [Google Scholar]
- Yamagishi H. 2002. The 22q11.2 deletion syndrome. Keio J Med 51: 77–88. 10.2302/kjm.51.77 [DOI] [PubMed] [Google Scholar]
- Yamagishi H, Srivastava D. 2003. Unraveling the genetic and developmental mysteries of 22q11 deletion syndrome. Trends Mol Med 9: 383–389. 10.1016/S1471-4914(03)00141-2 [DOI] [PubMed] [Google Scholar]
- Yamagishi H, Yamagishi C. 2014. Embryology. In Cardiac CT and MR for adult congenital heart disease (ed. Saremi F), pp. 7–21. Springer, New York. [Google Scholar]
- Yamagishi H, Ishii C, Maeda J, Kojima Y, Matsuoka R, Kimura M, Takao A, Momma K, Matsuo N. 1998. Phenotypic discordance in monozygotic twins with 22q11.2 deletion. Am J Med Genet 78: 319–321. [DOI] [PubMed] [Google Scholar]
- Yamagishi H, Garg V, Matsuoka R, Thomas T, Srivastava D. 1999. A molecular pathway revealing a genetic basis for human cardiac and craniofacial defects. Science 283: 1158–1161. 10.1126/science.283.5405.1158 [DOI] [PubMed] [Google Scholar]
- Yamagishi H, Maeda J, Hu T, McAnally J, Conway SJ, Kume T, Meyers EN, Yamagishi C, Srivastava D. 2003. Tbx1 is regulated by tissue-specific forkhead proteins through a common Sonic hedgehog–responsive enhancer. Genes Dev 17: 269–281. 10.1101/gad.1048903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamagishi H, Maeda J, Uchida K, Tsuchihashi T, Nakazawa M, Aramaki M, Kodo K, Yamagishi C. 2009. Molecular embryology for an understanding of congenital heart diseases. Anat Sci Int 84: 88–94. 10.1007/s12565-009-0023-4 [DOI] [PubMed] [Google Scholar]
- Yelbuz TM, Waldo KL, Kumiski DH, Stadt HA, Wolfe RR, Leatherbury L, Kirby ML. 2002. Shortened outflow tract leads to altered cardiac looping after neural crest ablation. Circulation 106: 504–510. 10.1161/01.CIR.0000023044.44974.8A [DOI] [PubMed] [Google Scholar]
- Zhang R, Cao P, Yang Z, Wang Z, Wu JL, Chen Y, Pan Y. 2015. Heparan sulfate biosynthesis enzyme, Ext1, contributes to outflow tract development of mouse heart via modulation of FGF signaling. PLoS ONE 10: e0136518 10.1371/journal.pone.0136518 [DOI] [PMC free article] [PubMed] [Google Scholar]