Abstract
Systematic studies on connective tissue disorders are scarce in sub-Saharan Africa. Our aim was to analyse the published clinical data on systemic sclerosis (SSc) in sub-Saharan Africa. A systematic review was carried out in accordance with the PRISMA guidelines. We screened the Embase, PubMed and African Health Sciences databases for literature published until March 2018. Searches produced 1210 publications. After abstract and full-text screenings, 91 publications were analysed, and epidemiological information and clinical features extracted. Publications were mostly publications case reports (36%), cross-sectional studies (26%) and case series (23%) and came predominantly from South Africa (45%), Nigeria (15%) and Senegal (14%). A total of 1884 patients were reported, 66% of patients came from South Africa. The patients were between 4 and 77 years old; 83% of patients were female. Overall, 72% had diffuse SSc. Raynaud´s phenomenon was reported in 78% and skin ulcerations in 42% of patients. Focal skin hypopigmentation was common and telangiectasia not frequent. Interstitial lung involvement was reported in 50%, pulmonary hypertension in 30%, heart involvement in 28% of patients. Oesophageal reflux was observed in 70% and dysphagia in 37% of patients. Antinuclear antibodies were positive in 65% of patients. Anti-centromere autoantibodies (9.2%) and RNA polymerase 3 antibodies (7.1%) were rare and anti-fibrillarin most frequent (16.5%). SSc presentations in sub-Saharan Africa differ from those reported in Europe and America by a frequent diffuse skin involvement, focal skin hypopigmentation and a high prevalence of anti-fibrillarin autoantibodies.
Keywords: Systemic sclerosis, sub-Saharan Africa, connective tissue disease, systematic review
Introduction
With substantial advances in the prevention and treatability of infections, the non-communicable diseases have now emerged as an important contributor to the global disease burden and become a significant driver of health care costs in low and middle-income countries. Among the non-communicable diseases, some musculoskeletal disorders such as systemic sclerosis (SSc), are characterized by large unmet medical needs and growing proportions of global morbidity and mortality.
Systemic sclerosis (SSc) is a chronic, autoimmune disease with a multisystem vasculopathy and an immense increase of fibrous tissue in affected organs. Even in high-income societies, SSc has a poor prognosis, as about 55% of affected patients die as a direct consequence of their disease [1]. In America, African descendants are known to have a particularly high SSc incidence and also a more severe disease course than Caucasians [2]. Various studies have identified differences in genetic disease associations between Caucasians and African Americans. Two seminal observations were however made in Africa itself, namely the first description of the prominent visceral involvement in SSc, coining the term ‘progressive systemic sclerosis’ [3] and the association of SSc with silica dust exposure [4].
Although the epidemiology, clinical phenotype and evolution of SSc organ involvement are intensively researched in economically advantaged countries, further robust data of SSc presentations in sub-Saharan Africa are largely lacking, prompting us to undertake a systematic review.
Methods
Information sources: we searched the Embase, PubMed, and African Health Sciences databases. Additionally, conference abstracts were retrieved by targeted searches of relevant websites, e.g. the African League against Rheumatism, the South African Rheumatism Arthritis Association, and the proceedings of the World Scleroderma Congresses. Reference lists of retrieved publications were also screened for publications not identified by the above search strategies. Search terms were defined (Table 1) including the corresponding Medical Subject Headings and Embase subject headings. A first search was carried out on August 31, 2017, and an update on March 29, 2018.
Table 1.
overview of the search terms used to retrieve publications
| Search terms |
|---|
| Scleroderma OR SSc OR systemic sclerosis OR CREST |
| AND |
| sub-Saharan Africa OR sub-Saharan Africa OR Central Africa OR Eastern Africa OR Western Africa OR Southern Africa OR Africa OR Djibouti OR Eritrea OR Ethiopia OR Somalia OR Somaliland OR Burundi OR Comoros OR Kenya OR Madagascar OR Malawi OR Mauritius OR Mozambique OR Rwanda OR Seychelles OR South Sudan OR Sudan OR Tanzania OR Zanzibar OR Uganda OR Zambia OR Zimbabwe OR Angola OR Cameroon OR Central African Republic OR Chad OR Congo OR Democratic Republic of the Congo OR DRC OR Zaire OR Equatorial Guinea OR Gabon OR Sao Tome OR Principe OR Botswana OR Lesotho OR Namibia OR South Africa OR Swaziland OR Benin OR Burkina Faso OR Cape Verde OR Cote d´Ivoire OR Ivory Coast OR Gambia OR Ghana OR Guinea OR Guinea Bissau OR Guinea-Bissau OR Liberia OR Mali OR Mauritania OR Niger OR Nigeria OR Senegal OR Sierra Leone OR Togo |
Study selection and data collection: two reviewers (VKJ and JE) independently screened the titles and abstracts. Patients were eligible for the analysis, if they were diagnosed with SSc by the reporting physician and resided within sub-Saharan Africa. Disagreement between the reviewers was resolved by consensus. In a second step, inclusion into this review was based on the full text of the remaining publications (JE), cross-checked by another author (VKJ). Data was extracted by one author (JE) and checked by another author (VKJ). In case of disagreement, a consensus was sought by discussion between authors.
Data items: ‘SSc patients’ were defined as patients diagnosed with SSc by the reporting physician and included if they were residing in sub-Saharan Africa. Information extracted included study methodology, patient demographics, and disease presentations. The prevalences of symptoms and organ involvement were calculated as percentages based on the denominator of the number of patients with available information, rather than the total number of patients included.
Current status of knowledge
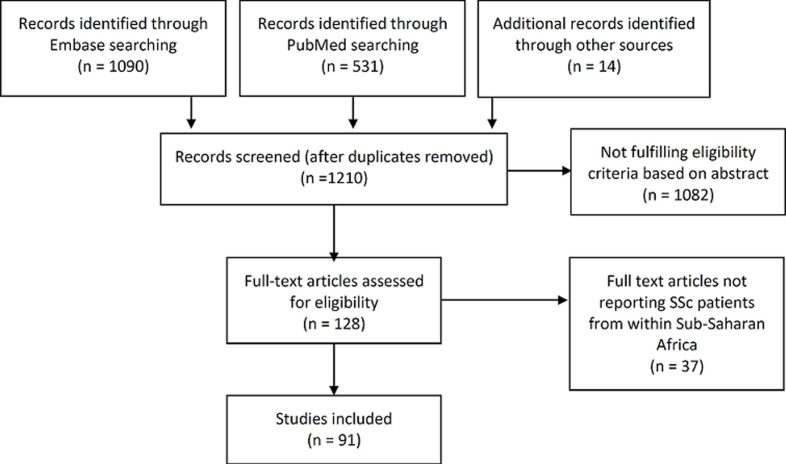
Study selection: we retrieved 1090 publications from Embase, 531 from PubMed and 14 from other sources (Figure 1). After removing duplicate publications, 1210 publications were left for screening. The selection process finally included 91 papers in this review (Figure 1).
Figure 1.
flow chart of paper collection and selection
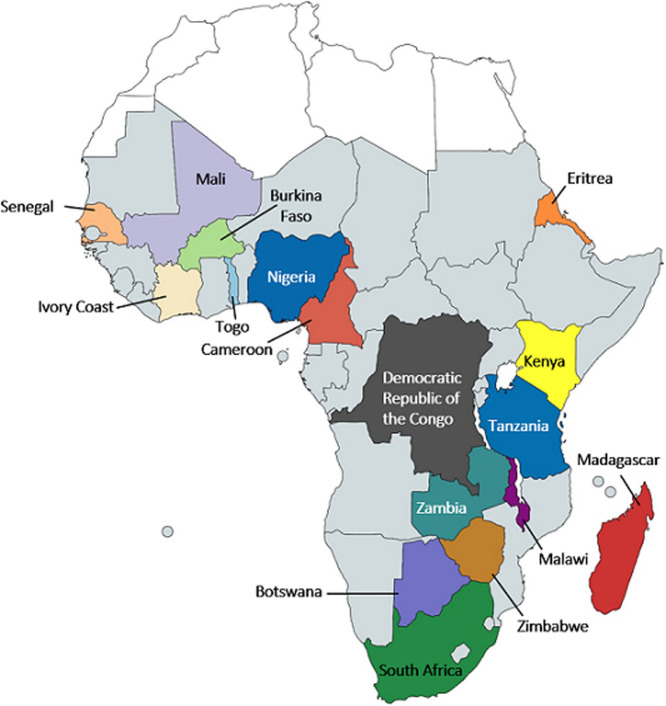
Study characteristics: the 91 publications were published between the years 1945 [3] and 2018 [5], described a total of 1884 SSc patients and were carried out in 17 of the 48 sub-Sahara African countries (Figure 2), predominantly in South Africa (41 publications [45]), Nigeria (14 publications [15]) and Senegal (13 publications [14]).
Figure 2.
origin of publications and patients
About a third of the publications [36%] were case reports [3,6-37], 23% were case series [4,38-57], 24 publications [26%] were cross-sectional studies [5,58-81], 11 [12%] were case control studies [82-92]. There was one longitudinal cohort study [93] and one blinded cross-over interventional study [94]. Most studies were published in English (72 [79%]) and the remainder in French (19 [21%]).
Patient and disease characteristics: in almost a third of the studies (28 [31%]), patients fulfilled the 1980 ACR or 2013 ACR/EULAR classification criteria [95,96]; two [7% of publications] studies used the Medsger and Masi criteria [97]. In the remaining 60 publications [67%] the authors failed to describe the classification criteria. With respect to patient numbers, the figures were slighty different: 914 patients [48% of all 1884 patients] fulfilled the ACR or ACR/EULAR classification criteria for SSc, and 71 patients [4%] were classified according to the Medsger and Masi SSc criteria. For the remaining 899 patients [48%] there was no disease definition.
The majority of patients [66%] was reported in South Africa. The country of residence of all SSc patients is shown in Figure 2. The sex ratio was 5.4:1, 981 patients [83%] were female and 200 [17%] male, of the remaining 703 patients´ sex was not reported. The patient age ranged between 4 and 77 years. We estimate the mean patient age as approximately 40 years, but were unable to calculate this figure precisely as several studies reported only age ranges. The mean age at disease onset was about 36 years and the mean disease duration approximately 4 years (range 1 month to 19 years).
Most patients [72%] had diffuse cutaneous SSc (575 patients), 224 patients [28%] had limited SSc (Table 2), only two patients had SSc sine scleroderma [36,54]. One South African study analysed SSc subsets systematically and with a meaningful number (174 patients) and found a similar proportion of diffuse cutaneous SSc [74%] [5].
Table 2.
clinical features of the 1884 SSc patients
| Manifestation | Patients with available information N [% of all SSc patients] | Positive N [% of patients with available information] |
|---|---|---|
| Raynaud´s phenomenon | 1085 [58] | 851 [78] |
| Ulcers | 727 [39] | 307 [42] |
| Diffuse cutaneous SSc | 801 [43] | 575 [72] |
| Limited cutaneous SSc | 224 [28] | |
| SSc sine scleroderma | 2 [0.2] | |
| Reduced Forced Vital Capacity | 184 [10] | 183 [99] |
| Interstitial lung disease* | 885 [47] | 440 [50] |
| Pulmonary hypertension | 576 [31] | 173 [30] |
| Estimated by echocardiography | 159 [92] | |
| Diagnosed by right heart catheterisation | 14 [8] | |
| Heart involvement | 524 [28] | 144 [28] |
| Renal crisis | 25 [1] | 9 [36] |
| Dysphagia | 417 [22] | 153 [37] |
| Reflux | 373 [20] | 261 [70] |
| Oesophageal dysfunction | 67 [4] | 42 [63] |
| Diarrhoea | 37 [2] | 9 [24] |
| Musculoskeletal involvement | ||
| ‘Arthritis’ | 631 [33] | 317 [50] |
| Autoimmune overlap conditions | ||
| Systemic lupus erythematosus overlap | 155 [8] | 18 [12] |
| Rheumatoid arthritis overlap | 159 [8] | 5 [3] |
| Sjögren´s syndrome overlap | 142 [8] | 9 [6] |
| Dermatomyositis overlap | 142 [8] | 10 [7] |
| Concomitant infections | ||
| Human Immunodeficiency Virus infection | 153 [8] | 9 [6] |
| Latent or active tuberculosis | 45 [2] | 22 [49] |
definition: radiographic evidence and/or reduced FVC in those with available information of at least one of the two items
Information on silica exposure was not available in 92% of study subjects, but was reported in 141 patients [8%] [4,45,48,49,91,92] which were mostly miners. Only 5 patients [0.3%] were reported to be free of silica exposure [14,21,50]. Two case control studies [91,92] suggested a substantially elevated SSc incidence in silica-exposed gold miners.
Raynaud´s phenomenon: RP was reported in 851 patients [78%]. In 11 patients there were symptoms that could be interpreted as RP, such as acrocyanosis in 3 patients [31,65] and digital ischaemia in 8 patients [17,52]. We had attributed these 11 patients to the 851 patients with RP. There was a wide geographical variation in RP prevalences. In a retrospective study conducted in Dakar, only 57% of 92 SSc patients had RP, [41] whereas in South Africa RP was reported in 92% of 153 patients, and in 94% of 34 patients [43,93].
Cutaneous manifestations: hypopigmented patchy skin lesions resembling vitiligo were reported in 7 of 13 SSc patients in a Togolese study [77]. A retrospective Senegalese study reported similar skin lesions in 70% of 92 patients [41]. Further reports of skin hypopigmentations with a ‘salt and pepper appearance’ were from Nigeria [8,46,54], Togo [51], and South Africa [36]. Papulous hypopigmentations were described as coexisting lichen sclerosus et atrophicus [18].
The extent of the skin fibrosis in terms of the mRSS [98] was only reported in two case reports [17,21] with scores of 18 and 23 and one case-control study in which the average mRSS was 24.7 [83]. The presence or absence of telangiectasia was documented in 321 patients [17%] of all patients]); in whom telangiectasia were present in 73 patients [23%]. In the publication with the most representative data, telangiectasia were present in only 19 of 151 patients [13%] [49].
Ulcers: as most patients were reported as having ‘cutaneous ulcerations’ [18,19,41,65] without providing the exact location, we were unable to assess ‘digital ulcers’. 307 patients [42%] had skin ulcers either at the time of inclusion in a study or during the follow-up observation. In 2 retrospective South African studies with greater detail, the frequencies of ever having had any ulceration were 71% (of 174 patients) and 44% (of 151 patients) [5,49].
Renal complications: renal involvement was documented in only 1% of all patients. Among the total of 25 patients in whom kidney function was reported, renal crisis was observed in 9 patients [36%], chronic kidney disease in 2 patients [8%], and proteinuria in 6 patients [24%]. Three retrospective South African studies found renal complications in 5 of 174 [3%] [5], 7 of 58 [12%] [45], and 1 of 52 SSc patients [2%] [86].
Interstitial lung disease (ILD): the definition of ILD was not specified in most publications. When we adjudicated all of radiographic evidence of lung fibrosis, restriction on lung function testing, or chronic cough and dyspnoea, the prevalence of ILD was 51% in 1039 SSc patients with available information on any of these items. Radiographic evidence of lung fibrosis was positive in 237 [44% of 534 patients with ILD]; in 128 [54%] by plain chest X-ray and in 109 [46%] patients by CT-scan. The presence or absence of ILD, if defined only as radiographic evidence of fibrosis, or an FVC below 80% of predicted, was reported in 885 [47% of all patients]. Among those, parenchymal lung fibrosis was observed in 440 [50%] of patients. A study from Kenya reported ILD by imaging or pulmonary function testing in 18 of 50 [36%] of SSc patients [44].
Spirometry results were reported in 184 of all SSc patients [6-8,10,13,14,17,19,24,26,27,29,38,39,41, 45,47-49,85], either as percentage of predicted, or as a dichotomized result (normal/abnormal). 183 of these patients featured a reduced FVC. In 110 patients, the FVC was reported as percentage of predicted; on average these patients showed a FVC of about 70% predicted.
Data on DLCO was sparse. In one South African study, the DLCO was impaired in 86% of 63 SSc patients [45]. More detailed information about the extent of DLCO decline was reported in another South African study that showed an average DLCO of 65% [49]. Pulmonary hypertension (PH): in 576 [31%] patients data on PH was recorded. 159 patients [30%] were deemed to have PH, mainly by echocardiographic pressure estimates [92%]; proof of PH by right heart catheterisation was provided in only 14 patients [8%]. In a Senegalese study on cardiovascular manifestations, PH was diagnosed in 5 of 29 SSc patients [17%] [59]. In Kenya PH was reported in 10 of 50 SSc patients [20%] [44]. In one retrospective South-African study, PH was diagnosed in 7 of 54 patients [13%] [45]. In 6 of these 7 patients, PH was accompanied by lung fibrosis. In a similar study from Dakar, Senegal, 12 out of 83 patients [14.5%] had PH [39]. Four of the 12 Senegalese PH patients had concomitant ILD.
Heart involvement: SSc-specific cardiac manifestations, including a reduced left ventricular ejection fraction, diastolic dysfunction, arrhythmias, pericardial effusion or where no obvious non-SSc cause of heart disease was evident, were reported in a total of 524 [28% of all patients], of whom 144 [28%] had heart involvement. Heart failure was reported in 27 [33%], arrhythmia in 33 [40%], pericarditis/pericardial effusion in 16 [20%] and myocardial infarction/damage in 6 [7%] of patients. In more detailed studies from Senegal, cardiac failure in terms of a depressed left ventricle ejection fraction was observed in 13 of 92 [14%] patients [41] and in a Kenyan study myocardial involvement in 11of 50 [22%] patients [44].
GIT involvement: GIT symptoms were addressed in almost half of all publications. Oesophageal involvement, including peptic lesions of the oesophagus, general oesophageal symptoms and heartburn, were the most frequent complaint and reported in 261 patients [70%] of 373 patients with available information. Heartburn as a symptom of reflux was explicitly reported as present in 113 patients and as absent in 1 patient. The prevalence of dysphagia was 37% (153 patients). Thus, dysphagia appears to represent the second most common GIT manifestation. The highest reported dysphagia prevalence was 66% in a study of 61 patients [45] and the lowest dysphagia prevalence was 8% in a study of 24 patients [48]; both studies were from South Africa.
Musculoskeletal manifestations: ‘arthritis’ was reported in 317 patients [50%] but the definition of arthritis was mostly not provided. Similarly, tendon friction rubs were reported to be present in 15 [0.3% of all SSc patients] patients. Muscle pain was reported to be present in 83 [39% of all SSc patients] and myositis in 58 patients [28]. Creatine kinase elevation was not reported.
Autoantibodies: antinuclear antibody (ANA) findings were reported in 1045 [46% of all patients]. Of these, 674 [65%] were ANA positive. Autoantibody specificities are shown in Table 3. Anti-fibrillarin autoantibodies were the most frequent SSc-specific ANA, followed by anti-topoisomerase 1 autoantibodies. RNA polymerase 3 and anti-centromere autoantibodies were infrequent; in one study of 73 black South African patients none of the patients had anti-centromere antibodies [42].
Table 3.
autoantibody specificity in 1145 patients with available autoantibody data
| Antibody specificity | ANA tested n | ANA positive n | % of antibody tested |
|---|---|---|---|
| ANA positive | 1045 | 674 | 64.5 |
| SSc-specific ANA | |||
| Topoisomerase 1 | 822 | 103 | 12.5 |
| Centromere | 382 | 35 | 9.2 |
| Fibrillarin (U3-RNP) | 85 | 14 | 16.5 |
| RNA polymerase 3 | 84 | 6 | 7.1 |
| Other ANA | |||
| PM-Scl | 84 | 2 | 2.4 |
| Jo-1 | 29 | 2 | 6.9 |
| U1-RNP | 335 | 84 | 25.1 |
| Sm | 55 | 5 | 9.1 |
| SSA (Ro) | 324 | 72 | 22.2 |
| SSB (La) | 309 | 30 | 9.7 |
| dsDNA | 122 | 4 | 3.3 |
| Antiphospholipid antibody specificities | |||
| Antiphospholipid | 41 | 26 | 63.4 |
| Anticardiolipin | 49 | 3 | 6.1 |
| Beta-2 glycoprotein 1 | 64 | 23 | 35.9 |
There was a high prevalence of antiphospholipid antibody specificities (anti-beta-2 glycoprotein-1, anti-phospholipid and anti-cardiolipin). These data were replicated in a Senegalese cross-sectional study that systematically analysed the association of anti-phospholipid antibodies with SSc, the lupus anticoagulant was found in 58% of patients [64]. The persistence of these autoantibodies at repeat testing was however not analysed and a clinical correlation with the antiphospholipid antibody syndrome not made [64].
Mortality: death was reported in 160 [9% of all SSc patients]; in most patients [83%] the cause of death was unknown. Pulmonary complications as the cause of death were reported in seven patients [25%]. Among patients with ILD, the mortality was high; 44.4% died as a consequence of ILD, followed by infection (in 22.2%) [49]. Heart involvement as a cause of death was described in ten patients [36%], and as a result of renal complications in five patients [18%]. Single cases of sudden death, cancer, meningitis, malabsorption, and septicaemia were also reported. In a South-African retrospective series, 20% of patients were known to have died; after a mean follow-up period of 45 months, 63% of deaths were directly attributed to SSc, mostly due to interstitial or vascular lung involvement or renal crisis [93].
Comorbidities: many SSc patients had medical comorbidities. Among the autoimmune diseases, reports mentioned systemic lupus erythematosus overlap in 18 patients [1% of all patients] [10,31,41,44,65] and dermatomyositis in 10 patients [0.5% of all patients] [13,39,41,44]. Further comorbidities were also reported rarely; Sjogren´s Syndrome in 9 patients [0.5% of all SSc patients] [41,44] and rheumatoid arthritis in 4 patients [0.2%] [41,44].
In terms of infections, ten of a total of 154 analysed SSc patients were HIV-seropositive [7%] [17,49]. There was no difference in HIV prevalence among patients with or without ILD [49]. Tuberculosis was diagnosed in 22 patients [13,29,38], of which 17 [77%] had latent tuberculosis [10,48,92] and the remaining 5 patients [23%] had active tuberculosis. Information about TB and HIV comorbidity was however not analysed in the vast majority of patients [98% and 92% respectively]. In one patient, ILD was complicated by concomitant aspergilloma [6].
SSc treatment: treatment mostly consisted of symptomatic medication; patients were often prescribed nonsteroidal anti-inflammatory drugs. Reflux was mostly medicated with proton pump blockers. The oldest study from 1945 reported SSc treatment with parathyroid hormone, vitamins and intravenous calcium [3]. Details on immunosuppressive treatment were provided predominantely in case reports, immunosuppressants were used in up to 80% of patients [44]. Two larger studies from Kenya and South Africa reported glucocorticosteroids as the immunosuppressant most frequently used (50% of patients and 62% respectively) [44,51]. Often relatively high glucocorticosteroid doses were administered (40-60mg per day) [10,53,57]. Methotrexate was used in 5% and 22.5% of patients [44,51]. Immunosuppressants less frequently administered were mycophenolate mofetil, intravenous cyclophosphamide, and azathioprine [44,49]. Three publications reported SSc treatment with hydroxychloroquine [40,44,60], the two case series studies among these reported hydroxychloroquine treatment in 10% [44], and 25% [40] of patients. Treatment with potential antifibrotic agents such as potassium-paraaminobenzoate [25] and D-penicillinamine [44,49,60] was also reported. A South African patient with renal crisis was treated with the angiotensin converting enzyme inhibitor captopril enabling cessation of haemodialysis [32]. Hematopoietic stem cell transplantation was not reported.
Discussion
This systematic review revealed that in sub-Saharan Africa, publications mostly come from South Africa, Nigeria and Senegal, potentially reflecting the gross domestic product and medical research budget of these nations [99,100]. More than half of the sub-Saharan countries had, however, no information on SSc. Nevertheless, research on SSc increased recently; about half of all reports were published in the last decade. Most patients [83%] were female. The sex ratio of 5.4:1 found in sub-Saharan Africa is well within the range reported in the large multinational database of the European Scleroderma Trial and research (EUSTAR) group (6.7:1), in other continents (3:1) and for African Americans (3.2:1) [2,101,102]. There is no indication that the sex ratio of SSc patients from sub-Saharan Africa differs significantly from that in other geographic regions. In this review, several studies reported a link between SSc and silica exposure [4,48,91,92]. Of our 1884 patients, only in 141 SSc patients a silicate exposure was noted. Regarding the interaction of silica dust and SSc unfortunately no statement can be made as we lack control groups.
More than 70% of patients in sub-Saharan SSc were reported to have diffuse cutaneous SSc. In the United States, diffuse SSc was reported in 60% of African Americans [103]. The high proportion of diffuse SSc in sub-Saharan Africans may in part be explained by economic factors situation and the organization of the health service in which patients with overt and extensive skin involvement are more likely to seek medical attention, while those with more subtle features do not, or are not referred for specialist care. Several papers [12,46,53,54,57] reported skin hypopigmentation frequently providing a ‘salt and pepper’ appearance of the skin. This feature is associated with anti-fibrillarin autoantibodies and seems to be much more than in the USA and Europe [104].
The overall prevalence of RP was 78% in our analysis, but the prevalence varied substantially across different sub-Saharan countries; the larger studies reported prevalences between 57% [41] and 96% [90] which is lower than the prevalence of 96% reported from EUSTAR [105]. Moreover, RP as the initial complaint of SSc occurred in only 16% of sub-Saharan patients as compared to 87% of the mostly Caucasians in EUSTAR [106]. This marked difference in RP prevalence might be due to a number of reasons. Firstly, differences in climatic conditions which modulate this vascular complication, with warmer climates in most sub-Saharan countries compared to European countries, is one explanation. An analysis of the EUSTAR database however did not show a significant relationship between prevalence of RP and ambient temperatures [101]. Studies in the US have not revealed differences in RP prevalence between African-Americans and other racial group [107]. Another possible reason might therefore be that RP symptoms are less likely to trigger a medical visit or are considered worth noting in low income countries. Finally, the difficulty of detecting the blue and red skin colour changes in darkly pigmented individuals may explain in part not only the low RP prevalence, but also the low prevalence of telangiectasia. In a recent South African study of cutaneous lupus, black patients were less likely to have the typical erythematous malar rash compared non-black patients [108].
Anti-topoisomerase 1 autoantibodies were detected in about one quarter of African American SSc patients [2,103]. The prevalence of anti-topoisomerase 1 autoantibodies in our study was somewhat lower, but the prevalence of the other ANA specificities similar with studies of African-Americans in which ANA were present in 87% of patients [103], anti-fibrillarin in 19% [2] and anticentromere in about 10% [103] and 7% [2] respectively.
With regards to the causes of death, about two thirds of all SSc patients appear to die from cardiorespiratory failure secondary to interstitial lung disease, primary or secondary PAH (Mohammed Tikly, personal communication). This systematic review could however not mirror these observations due to lack of detail and follow-up. Further research is clearly needed.
An interesting fact is that SSc in combination with HIV was reported very rarely. The combination with HIV was reported in 7% of patients with known HIV-status. It is unclear if SSc improves during the course of untreated HIV infection similar to the observation in some of the other connective tissue diseases, and if HIV-induced CD4-positive T-lymphocyte loss may account for the low prevalence of some SSc manifestations [109].
This review has systematically analysed considerable patient numbers in heterogeneous study designs. A likely bias result on the one hand from unbalances the various sub-Saharan countries from which reports are available, and differences in health care access in these countries on the other hand. It should also be noted that almost half of the patients were not formally reported to be classified as SSc according to international criteria particularly the recently formulated ACR/EULAR criteria which are more sensitive. Lastly but not least, it must be noted that not all observations can necessarily be attributed to black patients, as exemplified by South Africa, which has a high proportion of other ethnicities.
Conclusion
SSc is no rarity in sub-Saharan countries but its presentation differs from that in Caucasians. Diffuse skin involvement and focal skin hypopigmentation seem to appear more frequently. Prevalence of anti-fibrillarin autoantibodies seem to be higher likewise. More research must be undertaken to reduce bias and to elucidate the effects of tuberculosis and HIV-infection on SSc presentation and outcome
What is known about this topic
The presence of SSc has been described in a variety of sub-Saharan countries;
Robust data of SSc serology and organ manifestations in sub-Saharan Africa are lacking.
What this study adds
Analysis and overview of the published clinical data on systemic sclerosis (SSc) in sub-Saharan Africa.
Footnotes
Cite this article: Julian Nicolas Erzer et al. Systemic sclerosis in sub-Saharan Africa: a systematic review. Pan African Medical Journal. 2020;176(176). 10.11604/pamj.2020.37.176.22557
Competing interests
The authors declare no competing interests.
Authors' contributions
Study concept and design: Julian N. Erzer, Veronika K. Jaeger, Ulrich A. Walker. Acquisition of data: Julian N. Erzer, Veronika K. Jaeger. Analysis and interpretation of data: Julian N. Erzer, Veronika K. Jaeger, Ulrich A. Walker. Drafting manuscript: Julian N. Erzer, Veronika K. Jaeger, Ulrich A. Walker. Critical revision: Mohammed Tikly. All authors read and approved the final version of this manuscript and equally contributed to its content.
References
- 1.Tyndall A, Bannert B, Vonk M, Airò P, Cozzi F, Carreira PE, et al. Causes and risk factors for death in systemic sclerosis: a study from the EULAR scleroderma trials and research (EUSTAR) database. Ann Rheum Dis. 2010;69(10):1809–1815. doi: 10.1136/ard.2009.114264. [DOI] [PubMed] [Google Scholar]
- 2.Steen V, Domsic RT, Lucas M, Fertig N, Medsger TA. A clinical and serologic comparison of African American and Caucasian patients with systemic sclerosis. Arthritis Rheum. 2012;64(9):2986–2994. doi: 10.1002/art.34482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Goetz RH, Berne CB. The pathology of progressive systemic sclerosis (generalized scleroderma) with special reference to changes in the viscera. Clin Proc (Cape Town) 1945;4:337–392. [Google Scholar]
- 4.Erasmus LD. Scleroderma in goldminers on the Witwatersrand with particular reference to pulmonary manifestations. S Afr J Lab Clin Med. 1957;3(3):209–231. [PubMed] [Google Scholar]
- 5.Tikly M, Ickinger C, Dire Z. Causes and predictors of death in South Africans with systemic sclerosis. J Scleroderma Relat Disord. 2018;3:113–154. [Google Scholar]
- 6.Rakotoson JL, Vololontiana HMD, Raherison RE, Andrianasolo R, Rakotomizao JR, Randria MJD, et al. A rare case of huge aspergilloma developed within a lesion of pulmonary fibrosis secondary with a systemic scleroderma in an immunocompetent patient in Madagascar. Bull Soc Pathol Exot. 2011;104(5):325–328. doi: 10.1007/s13149-011-0141-9. [DOI] [PubMed] [Google Scholar]
- 7.Rakotoson JL, Vololontiana HMD, Raherison RE, Andrianasolo RL, Rakotomizao JR, Rakotoharivelo H, et al. Huge aspergilloma developed within a zone of scleroderma-related pulmonary fibrosis. Rev Pneumol Clin. 2012;68(1):31–35. doi: 10.1016/j.pneumo.2011.03.001. [DOI] [PubMed] [Google Scholar]
- 8.Ojo O, Omisore-Bakare M, Davidson B. A cosmetologist with systemic sclerosis. African J Rheumatol. 2017;5(1):38–41. [Google Scholar]
- 9.Ndiaye MB, Diao M, Bodian M, Kane AD, Mbaye A, Karma F, et al. A CREST syndrome revealed by a pulmonary arterial hypertension: Case report. Angeiologie. 2010;62(3):41–44. [Google Scholar]
- 10.Przybojewski JZ, Mynhardt JH, Van Der Walt JJ, Tiedt FAC. Cardiac involvement in mixed connective tissue disease. A fatal case of scleroderma combined with systemic lupus erythematosus. South African Med J. 1985;68(9):680–686. [PubMed] [Google Scholar]
- 11.Guillaume A, Herouin R, Loubieres R. Concerning two cases of generalized scleroderma in the African. Bord Med. 1978;11(2):101–105. [Google Scholar]
- 12.Nwafor CE, Alasia DD, Oyan BO. Congestive cardiac failure in a patient with systemic sclerosis: case report and literature review. Niger Heal J. 2015;15(4):161–169. [Google Scholar]
- 13.Allain T, Banda N, Van Gaalen F. Connective tissue disease in a Malawian man. Malawi Med J. 2010;22(1):31–33. doi: 10.4314/mmj.v22i1.55907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bergemann A, Tikly M. Cystic lung disease in systemic sclerosis: a case report with high resolution computed tomography findings. Rev Rhum Engl Ed. 1996;63(3):213–215. [PubMed] [Google Scholar]
- 15.Moorhouse S. Gastro-intestinal scleroderma. South African Med J. 1999;89(7):744. [PubMed] [Google Scholar]
- 16.Privat Y, Rousselot M, Faye I, Bellossi A. Generalized scleroderma in an African. Bull Soc Med Afr Noire Lang Fr. 1968;13(2):405–407. [PubMed] [Google Scholar]
- 17.Okong´o LO, Webb K, Scott C. HIV-associated juvenile systemic sclerosis: a case report. Semin Arthritis Rheum. 2015;44(4):411–416. doi: 10.1016/j.semarthrit.2014.08.005. [DOI] [PubMed] [Google Scholar]
- 18.Gordon W, Kahn LB, Dove J. Lichen sclerosus et atrophicus and scleroderma. S Afr Med J. 1972;46(7):160–163. [PubMed] [Google Scholar]
- 19.Holmgren G. Oesophageal stricture in scleroderma. Cent Afr J Med. 1970;16(10):239–241. [PubMed] [Google Scholar]
- 20.Bunn BK, van Zyl AW, Rahman L, van Heerden WF. Oral medicine case book 62: CREST syndrome. SADJ. 2014;69(7):324–325. [PubMed] [Google Scholar]
- 21.Benitha R, Modi M, Tikly M. Osteolysis of the cervical spine and mandible in systemic sclerosis: a case report with computed tomography and magnetic resonance imaging findings. Rheumatology. 2002;41(10):1198–1200. doi: 10.1093/rheumatology/41.10.1198. [DOI] [PubMed] [Google Scholar]
- 22.Olutola PS, Adelowo F. Osteolysis of the shaft of tubular bones in systemic scleroderma. Diagn Imaging Clin Med. 1985;54(6):322–325. [PubMed] [Google Scholar]
- 23.el-Amin AM. Peculiarities in dermatology. Cent Afr J Med. 1978;24(6):118–121. [PubMed] [Google Scholar]
- 24.Luiz DA, Moodley J, Naicker SN, Pudifin D. Pregnancy in scleroderma. A case report. S Afr Med J. 1986;69(10):642–643. [PubMed] [Google Scholar]
- 25.Silber W, Gitlin N. Progressive systemic sclerosis (diffuse scleroderma) treatment with Potaba--a preliminary report. S Afr Med J. 1965;39:453–454. [PubMed] [Google Scholar]
- 26.Ladipo GO. Progressive systemic sclerosis (scleroderma) First case report in a Nigerian, Dermatologica. 1976;153(3):196–201. [PubMed] [Google Scholar]
- 27.Jacyk WK. Progressive systemic sclerosis: a case report from Nigeria and review of African cases. J Trop Med Hyg. 1979;82(2):42–44. [PubMed] [Google Scholar]
- 28.Oguntona A, Adelowo O. Raynaud´s phenomenon in a black African woman. Niger Hosp Pract. 2008;2(1):7–9. [Google Scholar]
- 29.Chinniah K, Mody G. Recovery from severe dysphagia in systemic sclerosis-myositis overlap: a case report. Afr Health Sci. 2017;17(2):593–596. doi: 10.4314/ahs.v17i2.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Akintewe TA, Alabi GO. Scleroderma presenting with multiple keloids. Br Med J. 1985;291(6493):448–449. doi: 10.1136/bmj.291.6493.448-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dessein PH. Severe immune thrombocytopenia and the development of skin infarctions in a patient with an overlap syndrome. J Rheumatol. 1989;16(11):1494–1496. [PubMed] [Google Scholar]
- 32.Chapman PJ, Pascoe MD, Van Zyl-Smit R. Successful use of captopril in the treatment of “scleroderma renal crisis”. Clin Nephrol. 1986;26(2):106–108. [PubMed] [Google Scholar]
- 33.Hane AA, Ndir M, Badiane M, Ayad M, Kane A, Lamouche P, et al. Systemic scleroderma (apropos of a case with dominant pulmonary manifestations) Dakar Med. 1990;35(1):71–73. [PubMed] [Google Scholar]
- 34.Diouf A, Dieng MT, Diallo D, Moreira P, Diadhiou F. Systemic scleroderma and pregnancy: about one case. Contracept Fertil Sex. 1996;24(12):917–922. [PubMed] [Google Scholar]
- 35.Habtemichael A, Tesfamariam A, Tekie D. Systemic sclerosis presenting as CREST syndrome: a case report and review. J Eritrean Med Assoc. 2010;3(1):52–55. [Google Scholar]
- 36.De Villiers WJ, Jordaan HF, Bates W. Systemic sclerosis sine scleroderma presenting with vitiligo-like depigmentation and interstitial pulmonary fibrosis. Clin Exp Dermatol. 1992;17(2):127–131. doi: 10.1111/j.1365-2230.1992.tb00180.x. [DOI] [PubMed] [Google Scholar]
- 37.Ball M, Strobel M, Ndiaye B, Marchand JP. Unusual form of systemic scleroderma: Thibierge-Weissenbach or the “C.R.S.T. syndrome”. Dakar Med. 1981;26(3):309–314. [PubMed] [Google Scholar]
- 38.Diallo S, Dia D, Fall S, Kane A, Sane M, Thiam M, et al. Diffuse interstitial lung disease and connective tissue diseases: study of seven cases in Dakar. Dakar Med. 2008;53(3):255–259. [PubMed] [Google Scholar]
- 39.Diao M, Ndiaye MB, Kane A, Bodian M, Tchintchui NC, Mbaye A, et al. Pulmonary hypertension in scleroderma: about 12 cases. Pan Afr Med J. 2012;11:9. [PMC free article] [PubMed] [Google Scholar]
- 40.Seck SM, Dia D, Cisse MM, Gueye A, Diagne D, Fall S, et al. Pulmonary hypertension revealing systemic diseases: two Senegalese case reports. Dakar Med. 2007;52(3):175–179. [PubMed] [Google Scholar]
- 41.Dia D, Dieng MT, Sy TN, Diallo M, Fall S, Ndongo S, et al. Systemic scleroderma: 92 cases in Dakar. Dakar Med. 2003;48(2):82–86. [PubMed] [Google Scholar]
- 42.Pudifin DJ, Dinnematin H, Duursma J. Antinuclear antibodies in systemic sclerosis, Clinical and ethnic associations. South African Med J. 1991;80(9):438–440. [PubMed] [Google Scholar]
- 43.Dessein PH, Joffe BI, Metz RM, Al E. Autonomic dysfunction in systemic sclerosis: sympathetic overactivity and instability. Am J Med. 1992;93(2):143–150. doi: 10.1016/0002-9343(92)90043-b. [DOI] [PubMed] [Google Scholar]
- 44.Illovi C, Oyoo G. Characteristics of systemic sclerosis patients in Nairobi, Kenya?: a retrospective study. African J Rheumatol. 2013;1(1):23–27. [Google Scholar]
- 45.Tager RE, Tikly M. Clinical and laboratory manifestations of systemic sclerosis (scleroderma) in black South Africans. Rheumatology (Oxford) 1999;38(5):397–400. doi: 10.1093/rheumatology/38.5.397. [DOI] [PubMed] [Google Scholar]
- 46.Somorin AO, Mordi VI. Connective tissue disease in Nigeria with emphasis on scleroderma. Cent Afr J Med. 1980;26(3):59–63. [PubMed] [Google Scholar]
- 47.Dheda K, Lalloo UG, Cassim B, Mody GM. Experience with azathioprine in systemic sclerosis associated with interstitial lung disease. Clin Rheumatol. 2004;23(4):306–309. doi: 10.1007/s10067-004-0906-7. [DOI] [PubMed] [Google Scholar]
- 48.Cowie RL, Dansey RD. Features of systemic sclerosis (scleroderma) in South African goldminers. S Afr Med J. 1990;77(8):400–402. [PubMed] [Google Scholar]
- 49.Ashmore P, Tikly M, Wong M, Ickinger C. Interstitial lung disease in South Africans with systemic sclerosis. Rheumatol Int. 2017;0(0):1–6. doi: 10.1007/s00296-017-3893-0. [DOI] [PubMed] [Google Scholar]
- 50.Onadeko BO, Sofowora EO, Grillo IA. Investigation of Nigerians with diffuse radiographic pulmonary shadowing. Trans R Soc Trop Med Hyg. 1979;73(4):432–437. doi: 10.1016/0035-9203(79)90171-8. [DOI] [PubMed] [Google Scholar]
- 51.Akakpo AS, Teclessou JN, Mouhari-Touré A, Saka B, Matakloe H, Kakpovi K, et al. Les sclérodermies en milieu hospitalier à Lomé: étude de 50 cas. Med Sante Trop. 2017;27(4):446–448. doi: 10.1684/mst.2017.0701. [DOI] [PubMed] [Google Scholar]
- 52.Ickinger C, Tikly M. Macrovascular disease is associated with severe digital ischaemiain systemic sclerosis. Clin Rheumatol. 2013;32(2):S144–S144. [Google Scholar]
- 53.Bentley-Phillips CB, Geake TMS. Mixed connective tissue disease characterized by speckled epidermal nuclear IgG deposition in normal skin. Br J Dermatol. 1980;102(5):529–533. doi: 10.1111/j.1365-2133.1980.tb07650.x. [DOI] [PubMed] [Google Scholar]
- 54.Adelowo OO, Oguntona S. Scleroderma (systemic sclerosis) among Nigerians. Clin Rheumatol. 2009;28(9):1121–1125. doi: 10.1007/s10067-009-1191-2. [DOI] [PubMed] [Google Scholar]
- 55.Marik PE, Kark AL, Zambakides A. Scleroderma after silicone augmentation mammoplasty. A report of 2 cases. South African Med J. 1990;77(4):212–213. [PubMed] [Google Scholar]
- 56.Silber W, Orringer MB. Scleroderma reflux esophagitis. Surgery. 1982;91(5):606. [PubMed] [Google Scholar]
- 57.Cisse MM, Seck SM, Oumar DAK, Fall K, Lemrabott AT, Diallo M, et al. Scleroderma renal crisis in tropical region: two Senegalese cases. Pan Afr Med J. 2015;21:46. doi: 10.11604/pamj.2015.21.46.6344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ouédraogo D-D, Ntsiba H, Tiendrébéogo-Zabsonré J, Tiéno H, Bokossa LIF, Kaboré F, et al. Clinical spectrum of rheumatologic diseases in a department of rheumatology in Ouagadougou (Burkina Faso) Clin Rheumatol. 2014;33(3):385–389. doi: 10.1007/s10067-013-2455-4. [DOI] [PubMed] [Google Scholar]
- 59.Kane A, Ba SA, Barry F, Dieng MT, Sarr M, Diousse P, et al. Cardiovascular manifestations in systemic scleroderma. Presse Med. 1997;26(17):796–800. [PubMed] [Google Scholar]
- 60.Ouedraogo DD, Korsaga-Some N, Zabsonne Tiendrebeogo J, Tieno H, Kabore H, Niamba P, et al. Connective tissue diseases in hospital practice in Ouagadougou (Burkina Faso) Med Sante Trop. 2014;24(3):271–274. doi: 10.1684/mst.2014.0348. [DOI] [PubMed] [Google Scholar]
- 61.Mijiyawa M, Amanga K, Oniankitan OI, Pitche P, Tchangai-Walla K. Connective tissue diseases in the hospital outpatient service in Lome (Togo) La Rev Med interne. 1999;20(1):13–17. doi: 10.1016/s0248-8663(99)83004-5. [DOI] [PubMed] [Google Scholar]
- 62.Napo-Koura G, Pitche P, Tchangai-Walla K. Non-tumor dermatoses diagnosed in the pathologic anatomy laboratory of the Tokoin University hospital center, Lome (Togo) Sante. 1997;7(2):75–80. [PubMed] [Google Scholar]
- 63.Uyiekpen IE, Akinkugbe AO, Ayanlowo O, Omolayo OO. A nine year review of autoimmune connective tissue disorders presenting at a dermatology clinic in Nigeria. Clin Rheumatol. 2013;32(2):S143–S144. [Google Scholar]
- 64.Touré AO, Ly F, Sall A, Diatta A, Gadji M, Seck M, et al. Antiphospholipid antibodies and systemic scleroderma. Turkish J Hematol. 2013;30(1):32–36. doi: 10.4274/tjh.2012.0059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Diallo M, Niang SO, Kane A, Dieng MT, Ndiaye B. Antiphospholipid antibodies syndrome in dermatology in Dakar: 11 case report. Dakar Med. 2007;52(1):41–45. [PubMed] [Google Scholar]
- 66.Lutalo SK. Chronic inflammatory rheumatic diseases in black Zimbabweans. Ann Rheum Dis. 1985;44(2):121–125. doi: 10.1136/ard.44.2.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Malemba JJ, Mbuyi-Muamba JM. Clinical and epidemiological features of rheumatic diseases in patients attending the university hospital in Kinshasa. Clin Rheumatol. 2008;27(1):47–54. doi: 10.1007/s10067-007-0650-x. [DOI] [PubMed] [Google Scholar]
- 68.Hartshorne ST. Dermatological disorders in Johannesburg, South African. Clin Exp Dermatol. 2003;28(6):661–665. doi: 10.1046/j.1365-2230.2003.01417.x. [DOI] [PubMed] [Google Scholar]
- 69.Beltraminelli H, Kiprono S, Zuriel D, Swai B, Giabbani E, Grossmann H, et al. Dermatopathology in sub-Saharan Africa: a systematic 5-year analysis of all histopathological diagnoses from the Regional Dermatology Training Centre (RDTC) in Moshi, Tanzania. J Eur Acad Dermatology Venereol. 2015;29(7):1370–1375. doi: 10.1111/jdv.12877. [DOI] [PubMed] [Google Scholar]
- 70.Kodio B, Touré S, Pamanta IS, Eroumé T, Cissé IA. Evaluation of a year of consultation in Rheumatology Departmentat Teaching Hospital of Point G. Clin Rheumatol. 2013;32(2):S123–S123. [Google Scholar]
- 71.Noche CD, Kagmeni G, Dohvoma V, Bella AL, Mvogo CE, Singwe-Ngandeu M. Ophthalmic Manifestations in Chronic Inflammatory Rheumatic Diseases at a Referral Hospital of Yaounde, Cameroon. Ocul Immunol Inflamm. 2016:1–6. doi: 10.1080/09273948.2016.1212078. [DOI] [PubMed] [Google Scholar]
- 72.Ogunbiyi AO, Daramola OOM, Alese OO. Prevalence of skin diseases in Ibadan, Nigeria. Int J Dermatol. 2004;43(1):31–36. doi: 10.1111/j.1365-4632.2004.01967.x. [DOI] [PubMed] [Google Scholar]
- 73.Mercy E, Otike-Odibi B, Altraide D. Profile of autoimmune connective tissue disorders in the University of Port Harcourt Teaching Hospital, Port Harcourt, Nigeria. Niger Heal J. 2015;15(2):39–47. [Google Scholar]
- 74.Edrissy MEL, Aburaida KH, Ahmed AEH, Elhag OE, Ibrahim AS. Respiratory manifestations of connective tissue diseases in Sudanese patients. Int J Rheum Dis. 2012;15:116. [Google Scholar]
- 75.Tshiani A, Tshiefu K, Mbuyi-Muamba JM. Retrospective study of some rheumatic diseases observed at the University Hospital of Kinshasa, Zaire. Ann Soc Belg Med Trop (1920) 1980;60(2):223–226. [PubMed] [Google Scholar]
- 76.Micheletti RG, Steele KT, Kovarik CL. Robotic teledermatopathology from an African dermatology clinic. J Am Acad Dermatol. 2014;70(5):952–954. doi: 10.1016/j.jaad.2014.01.861. [DOI] [PubMed] [Google Scholar]
- 77.Pitche P, Amanga Y, Koumouvi K, Oniankitan O, Mijiyawa M, Tchangaï-Walla K. Scleroderma in a hospital setting in Togo. Med Trop (Mars) 1998;58(1):65–68. [PubMed] [Google Scholar]
- 78.Davis P, Stein M, Ley H, Johnston C. Serological profiles in the connective tissue diseases in Zimbabwean patients. Ann Rheum Dis. 1989;48(1):73–76. doi: 10.1136/ard.48.1.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Trollip S, Njobvu PD, McGill P, Chipeta J, Bucala R. Spectrum of connective tissue diseases at adult rheumatologyclinicis in Lusaka. Clin Rheumatol. 2013;32(2):S143–S143. [Google Scholar]
- 80.Olaosebikan BH, Adelowo OO, Animashaun BA, Akintayo RO. Spectrum of paediatric rheumatic diseases in Nigeria. Pediatr Rheumatol. 2017;15(1) doi: 10.1186/s12969-017-0139-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.O´Mahony D, Banach L, Mahapa D, Lancaster E, Linde G Van der, Williams B, et al. Teledermatology in a Rural Family Practice. South African Fam Pract. 2002;25(6):4–8. [Google Scholar]
- 82.Bernstein R, Prinsloo I, Zwi S, Andrew MJ, Dawson B, Jenkins T, et al. Chromosomal aberrations in occupation-associated progressive systemic sclerosis. South African Med J. 1980;58(6):235–237. [PubMed] [Google Scholar]
- 83.Frost J, Ramsay M, Mia R, Moosa L, Musenge E, Tikly M. Differential gene expression of MMP-1, TIMP-1 and HGF in clinically involved and uninvolved skin in South Africans with SSc. Rheumatol (United Kingdom) 2012;51(6):1049–1052. doi: 10.1093/rheumatology/ker367. [DOI] [PubMed] [Google Scholar]
- 84.Matatiele P, Tikly M, Tarr G, Gulumian M. DNA methylation similarities in genes of black South Africans with systemic lupus erythematosus and systemic sclerosis. J Biomed Sci. 2015;22(1):34. doi: 10.1186/s12929-015-0142-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Moodley YP, Lalloo UG. Exhaled nitric oxide is elevated in patients with progressive systemic sclerosis without interstitial lung disease. Chest. 2001;119(5):1449–1454. doi: 10.1378/chest.119.5.1449. [DOI] [PubMed] [Google Scholar]
- 86.Tikly M, Rands A, McHugh N, Wordsworth P, Welsh K. Human leukocyte antigen class II associations with systemic sclerosis in South Africans. Tissue Antigens. 2004;63(5):487–490. doi: 10.1111/j.0001-2815.2004.00199.x. [DOI] [PubMed] [Google Scholar]
- 87.Tikly M, Channa K, Theodorou P, Gulumian M. Lipid peroxidation and trace elements in systemic sclerosis. Clin Rheumatol. 2006;25(3):320–324. doi: 10.1007/s10067-005-0013-4. [DOI] [PubMed] [Google Scholar]
- 88.Tikly M, Frost J, Ramsay M, Marti Puig E, Rabionet R, Estivill X, et al. MicroRNAs targeting the wnt signalling pathway in black African patients with diffuse cutaneous systemic sclerosis. Arthritis Rheumatol. 2016;68:1081–1083. [Google Scholar]
- 89.Tikly M, Masoka X, Matiwane N, Gulumian M. Oxidative DNA damage in systemic sclerosis. Clin Exp Rheumatol. 2010;28(2):S180–S180. [Google Scholar]
- 90.Tikly M, Marshall SE, Haldar NA, Gulumian M, Wordsworth P, Welsh KI. Oxygen free radical scavenger enzyme polymorphisms in systemic sclerosis. Free Radic Biol Med. 2004;36(11):1403–1407. doi: 10.1016/j.freeradbiomed.2004.02.079. [DOI] [PubMed] [Google Scholar]
- 91.Sluis-Cremer GK, Hessel PA, Nizdo EH, Churchill AR, Zeiss EA. Silica, silicosis, and progressive systemic sclerosis. Br J Ind Med. 1985;42(12):838–843. doi: 10.1136/oem.42.12.838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Cowie RL. Silica-dust-exposed mine workers with scleroderma (systemic sclerosis) Chest. 1987;92(2):260–262. doi: 10.1378/chest.92.2.260. [DOI] [PubMed] [Google Scholar]
- 93.Tikly M, Jabaar N, Musenge E. Outcome and causes of death in systemic sclerosis-a South African experience. Clin Exp Rheumatol. 2010;28(2):S124–S124. [Google Scholar]
- 94.Silber W, Gitlin N. Progressive systemic sclerosis (diffuse scleroderma): a follow up report of treatment with Potaba. South African Med J. 1973;47(23):1001–1002. [PubMed] [Google Scholar]
- 95.Masi AT. Preliminary criteria for the classification of systemic sclerosis (scleroderma): subcommittee for scleroderma criteria of the American Rheumatism Association Diagnostic and Therapeutic Criteria Committee. Arthritis Rheum. 1980;23(5):581–90. doi: 10.1002/art.1780230510. [DOI] [PubMed] [Google Scholar]
- 96.Van Den Hoogen F, Khanna D, Fransen J, Johnson SR, Baron M, Tyndall A, et al. classification criteria for systemic sclerosis: an American college of rheumatology/European league against rheumatism collaborative initiative. Arthritis Rheum. 2013;65(11):2737–2747. doi: 10.1002/art.38098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Medsger TA, Masi AT. Epidemiology of Systemic Sclerosis (Scleroderma) Ann Intern Med. 1971;74(5):714–721. doi: 10.7326/0003-4819-74-5-714. [DOI] [PubMed] [Google Scholar]
- 98.Khanna D. Standardization of the modified Rodnan skin score for use in clinical trials of systemic sclerosis. J Scleroderma Relat Disord. 2017;2(1):11–18. doi: 10.5301/jsrd.5000231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.The World Bank. World development indicators: structure of output. 2017.
- 100.UNESCO Institute for Statistics. How much does your country invest in R&D. 2018. Accessed on 8 August 2018.
- 101.Walker UA, Tyndall A, Czirjak L, Denton CP, Farge D, Kowal-Bielecka O, et al. Geographic variation of disease manifestations in systemic sclerosis–a report from the EULAR Scleroderma Trials And Research (EUSTAR) group data base. Ann Rheum Dis. 2009;68(6):856–862. doi: 10.1136/ard.2008.091348. [DOI] [PubMed] [Google Scholar]
- 102.Chifflot H, Fautrel B, Sordet C, Chatelus E, Sibilia J. Incidence and prevalence of systemic sclerosis: a systematic literature review. Semin Arthritis Rheum. 2008;37(4):223–235. doi: 10.1016/j.semarthrit.2007.05.003. [DOI] [PubMed] [Google Scholar]
- 103.Mayes MD, Lacey J V, Beebe-Dimmer J, Gillespie BW, Cooper B, Laing TJ, et al. Prevalence, incidence, survival, and disease characteristics of systemic sclerosis in a large US population. Arthritis Rheum. 2003;48(8):2246–2255. doi: 10.1002/art.11073. [DOI] [PubMed] [Google Scholar]
- 104.Leroy V, Henrot P, Barnetche T, Cario M, Darrigade A-S, Manicki P, et al. Association of skin hyperpigmentation disorders with digital ulcers in systemic sclerosis: analysis of a cohort of 239 patients. J Am Acad Dermatol. 2019;80(2):478–484. doi: 10.1016/j.jaad.2018.07.033. [DOI] [PubMed] [Google Scholar]
- 105.Meier FMP, Frommer KW, Dinser R, Walker UA, Czirjak L, Denton CP, et al. Update on the profile of the EUSTAR cohort: an analysis of the EULAR scleroderma trials and research group database. Ann Rheum Dis. 2012;71(8):1355–1360. doi: 10.1136/annrheumdis-2011-200742. [DOI] [PubMed] [Google Scholar]
- 106.Jaeger VK, Wirz EG, Allanore Y, Rossbach P, Riemekasten G, Hachulla E, et al. Incidences and risk factors of organ manifestations in the early course of systemic sclerosis: a longitudinal EUSTAR study. PloS One. 2016;11(10):e0163894. doi: 10.1371/journal.pone.0163894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Gelber AC, Wigley FM, Stallings RY, Bone LR, Barker A V, Baylor I, et al. Symptoms of Raynaud´s phenomenon in an inner-city African-merican community: prevalence and self-reported cardiovascular comorbidity. J Clin Epidemiol. 1999;52(5):441–446. doi: 10.1016/s0895-4356(99)00015-3. [DOI] [PubMed] [Google Scholar]
- 108.Koch K, Tikly M. Spectrum of cutaneous lupus erythematosus in South Africans with systemic lupus erythematosus. Lupus. 2019;28(8):1021–1026. doi: 10.1177/0961203319856091. [DOI] [PubMed] [Google Scholar]
- 109.Walker UA, Tyndall A, Daikeler T. Rheumatic conditions in human immunodeficiency virus infection. Rheumatology. 2008;47(7):952–959. doi: 10.1093/rheumatology/ken132. [DOI] [PubMed] [Google Scholar]