Abstract
We estimated human immunodeficiency virus incidence and incidence rate ratios (IRRs) for black and Hispanic vs white populations in 6 cities in the United States (2020–2030). Large reductions in incidence are possible, but without elimination of disparities in healthcare access, we found that wide disparities persisted for black compared with white populations in particular (lowest IRR, 1.69 [95% credible interval, 1.19–2.30]).
Keywords: HIV/AIDS, simulation modeling, United States, racial/ethnic inequities
Stalled progress in reducing new human immunodeficiency virus (HIV) infections is a primary motivator for the United States’ (US) ambitious plan to “End the HIV Epidemic” (EHE) [1, 2]. Though the tools to reach these ambitious targets are available, there are wide disparities in access to HIV prevention and treatment services in white vs black and Hispanic populations, particularly in young men who have sex with men (MSM) [1, 3]. Black–white and Hispanic–white disparities in the US in HIV incidence and prevalence have been documented since 1984 [4]. Compounded by lack of healthcare access, assortative sexual mixing patterns [5], racism, poverty, and stigma [6], these disparities have widened. New HIV diagnoses among white MSM decreased 16% between 2010 and 2016, while HIV diagnoses have remained stable among black MSM and increased by 18% among Hispanic MSM [7]. Despite representing < 1% of the total population [2], black and Hispanic MSM accounted for > 45% of new HIV diagnoses in 2017, while white MSM (0.9% of the total population) contributed 17% of new diagnoses [8]. Likewise, black and Hispanic Americans accounted for 31% of the US population but 69% of new HIV diagnoses in 2018 [9].
In a series of recent articles [10–15], we modeled the impact of combinations of evidence-based strategies to prevent, diagnose, and treat HIV/AIDS in 6 US cities comprising 12 of 48 EHE-targeted counties and 24% of national HIV prevalence [1]. We found that if these strategies could be implemented at “ideal” (90% target population coverage) levels, the 90% absolute incidence reduction target from 2020 to 2030 could be approached in Baltimore, Maryland (84% [credible intervals {CrI} 71%–87%]); Atlanta, Georgia (74% [CrI 67%–81%]); and Miami, Florida (78% [CrI 52%–87%]), with 42%–58% reductions projected in other cities (Seattle, Washington; Los Angeles, California; and New York City, New York) where incidence is already at a lower level [15]. Importantly, modeled strategies did not account for explicit efforts to reduce racial/ethnic healthcare disparities; rather, we assumed these strategies would reach racial/ethnic groups in proportion with their baseline levels of access. Herein, our objective was to define the potential impact of implementing such strategies on racial disparities in HIV incidence, absent additional targeted efforts to address racial/ethnic disparities in access to HIV/AIDS prevention and treatment efforts.
METHODS
We used a dynamic, deterministic compartmental HIV transmission model to replicate the city-level HIV microepidemics in Atlanta, Baltimore, Los Angeles, Miami (Dade County), New York City, and Seattle (King County). Details are available in prior publications [10, 12].
The model tracked HIV-susceptible individuals through infection, diagnosis, treatment with antiretroviral therapy (ART), and ART dropout. In each city, the adult population (aged 15–64 years) was partitioned by biological sex (male, female), HIV risk group (MSM, people who inject drugs [PWID], MSM-PWID, and heterosexual), race/ethnicity (black/African American [black], Hispanic/Latinx [Hispanic], and non-Hispanic white/others [white]), and sexual risk behavior level (high risk vs low risk). The model captured heterogeneity in the risk of HIV transmission, aging (via differential maturation and mortality rates for people living with HIV and the general population across cities), and observed racial/ethnic disparities in access to health and prevention services, including HIV testing, ART, syringe service programs (SSPs), medication for opioid use disorder (MOUD), and targeted preexposure prophylaxis (PrEP) for high-risk MSM.
Racial/ethnic- and risk behavior–specific linkage to HIV care and use of PrEP, SSP, and MOUD were drawn from local, state, and national surveillance sources [10]. We estimated stratified, regional ART initiation and persistence rates through separate analysis of HIV Research Network data [11]. In the absence of comprehensive city-level HIV testing data [10], we back-calculated HIV testing rates from high-quality race/ethnicity- and risk behavior–specific surveillance data for new diagnoses published by local public health departments [12]. We accounted for people living with HIV in-migration and population growth, also stratified by race/ethnicity [10].
We focused on the unique combinations of evidence-based interventions identified for each city, delivered at ideal levels (ie, 90% target population coverage) [15], in comparison to the status quo scenario holding access to care constant at 2015 levels (with 2017 levels for PrEP) [13]. We projected stratified incidence rates per 100 000 individuals aged 15–64 years and calculated incidence rate ratios (IRRs) [16] for blacks and Hispanics vs whites under status quo and ideal implementation conditions, from 2020 to 2030. We present mean estimates and 95% CrIs for incidence rates and IRRs using the results of 2000 Monte Carlo simulations, drawing key parameter values from their theoretical distributions.
RESULTS
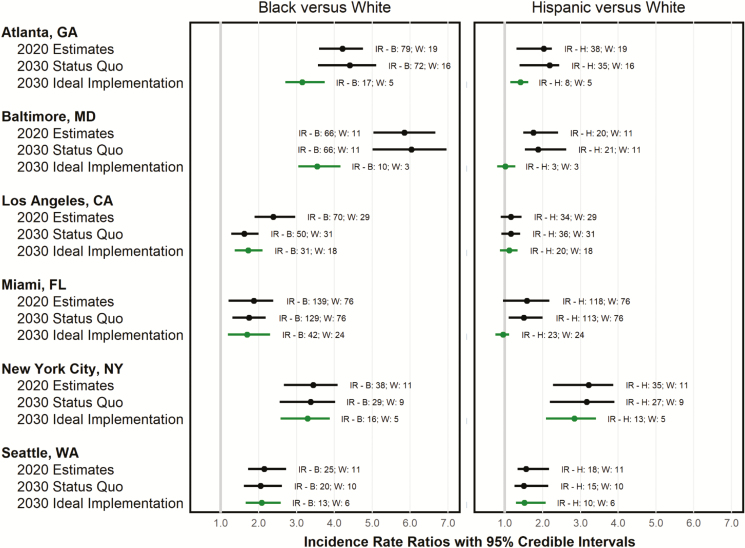
In 2020, estimated IRRs between black and white populations (holding access to care at 2015 levels [2017 for PrEP]) ranged from 1.87 (CrI, 1.21–2.39) in Miami to 5.85 (CrI, 5.03–6.66) in Baltimore. Between Hispanics and whites, we found a range of 1.16 (CrI, 0.89–1.45) in Los Angeles, to 3.22 (CrI, 2.28–3.87) in New York City (Figure 1).
Figure 1.
Projected change in the racial/ethnic human immunodeficiency virus (HIV) incidence rate and incidence rate ratio under status quo (black) and city-specific optimal combination implementation strategies delivered at ideal levels (gray) for 6 United States cities, 2020–2030. All figures are model-based projections; “status quo” entails holding HIV service access constant at 2015 (preexposure prophylaxis: 2017) levels; “ideal implementation” entails implementing city-specific combinations of between 9 and 13 evidence-based interventions at ideal levels (ie, reaching 90% of each intervention’s target population). Abbreviations: B, black/African American; CA, California; FL, Florida; GA, Georgia; H, Hispanic/Latinx; IR, incidence rate (per 100 000 individuals); MD, Maryland; NY, New York; W, white/other; WA, Washington.
Combination implementation strategies to address HIV/AIDS implemented at ideal levels resulted in substantial declines in projected IRR between blacks and whites in Baltimore (39.0% reduction [CrI, 32.4%–46.2%]), Los Angeles (27.3% reduction [CrI, 21.7%–35.8%]), and Atlanta (25.0% reduction [CrI, 14.6%–31.3%]), but this ratio was relatively stable in the other cities. Nowhere did the IRR between blacks and whites approach parity; Los Angeles and Miami were closest at (1.73 [CrI, 1.37–2.10]) and (1.69 [CrI, 1.19–2.30]), respectively. Nonetheless, absolute incidence rate declines among blacks were large across cities, ranging from a 46.2% reduction (CrI, 36.4%–56.8%) in Seattle to 84.5% (CrI, 75.2%–89.3%) in Baltimore, with rates per 100 000 diminishing from 24.6 to 13.2 and 66.0 to 10.2, respectively (Figure 1).
Declines in the IRR were greater for Hispanics, with Miami (0.95 [CrI, 0.75–1.12]), Baltimore (1.01 [CrI, 0.80, 1.28]), and Los Angeles (1.12 [CrI, 0.87, 1.34]) all at or approaching parity with whites. Similarly, reductions in the absolute incidence rate across all cities but Los Angeles were greater among Hispanics and ranged from 40.6% (CrI, 26.9%–55.2%) in Los Angeles to 85.6% (CrI, 77.0%–90.0%) in Baltimore, with rates per 100 000 diminishing from 33.7 to 19.8 and 20.0 to 2.9, respectively.
Finally, the proportion of black and Hispanic MSM among total new HIV infections was projected to decrease in Los Angeles and Miami (by 1.4% and 2.4%, respectively) and increase in the other cities from 2.1% in New York City to 7.8% in Baltimore.
DISCUSSION
This study demonstrates that simply implementing biomedical interventions without addressing social determinants of health such as poverty, stigma, racism, and access to care among others may achieve large reductions in absolute incidence rates but will not eliminate the current racial/ethnicity disparities in HIV incidence in the US. While it was not surprising that maintaining the status quo in service delivery did not eliminate disparities in any city, even with city-specific combinations of strategies implemented at ideal levels, wide disparities in HIV incidence would largely remain, with Hispanic populations approaching parity with whites in only Miami, Baltimore, and Los Angeles. These disparities in incidence will persist so long as disparities in access to services remain, a result reinforced by the very limited extent of sexual mixing across ethnic groups, particularly in MSM populations.
Increasing access to HIV prevention and care services will undoubtedly reduce incidence rates in all racial/ethnic groups; however, eliminating disparities will require additional efforts outside of typical medical care settings. The Ryan White HIV/AIDS Program has made progress in reducing HIV-related health disparities by reaching beyond the boundaries of clinical care and developing an evidence base for patient-centered interventions that can improve outcomes among underserved race/ethnic groups [17]. The effectiveness, potential for scale-up, resource requirements, and costs of such interventions represent key considerations for EHE-targeted counties moving forward.
Despite reductions in absolute incidence across all 3 race/ethnic groups, our results suggested that black and Hispanic MSM would remain a large proportion of all new HIV infections. Achieving substantial reductions in incidence disparity is made more difficult given the greater HIV prevalence in these populations, the greater likelihood of having relationships with partners from one’s own racial/ethnic group, and poorer engagement in care in these populations, resulting in larger pools of virally unsuppressed people living with HIV/AIDS [18]. As a result, black and Hispanic MSM face a much greater probability of HIV acquisition compared to whites [19], making PrEP a prevention strategy with the potential to deliver substantial benefits in this population. Increasing access to PrEP among black and Hispanic MSM (to reduce large disparities that have been estimated at the national level) [20] should be prioritized as one means of reducing disparities.
This study had several limitations, as noted in previous studies [10, 12, 14, 15]. In particular, the evidence base on HIV-related service access by race/ethnicity is incomplete; poor data on population-level HIV testing rates in the US and on stratified data for PrEP uptake among race/ethnic groups reduced our precision and will challenge the national response. Given the wide disparities in PrEP access by race/ethnicity [20], this lack of geographic precision may have understated our estimates of disparities in HIV incidence, which were conservative compared to disparities in new diagnoses reported in publicly available data and local surveillance reports [10, 12].
Addressing the root causes of disparities in addition to inequities in access to health systems will likely not only reduce disparities in HIV incidence, but also impact other syndemics that share these underlying risk factors. Zero-discrimination targets have been proposed as part of the national HIV/AIDS strategy in the past, and should be made an explicit feature of the EHE strategy if it is to succeed.
Notes
Localized HIV Modeling Study Group. Czarina N. Behrends, PhD, Department of Healthcare Policy and Research, Weill Cornell Medical College; Carlos Del Rio, MD, Hubert Department of Global Health, Emory Center for AIDS Research, Rollins School of Public Health, Emory University; Julia C. Dombrowski, MD, Department of Medicine, Division of Allergy and Infectious Diseases, adjunct in Epidemiology, University of Washington and Deputy Director, HIV/STD Program, Public Health–Seattle & King County; Daniel J. Feaster, PhD, Department of Public Health Sciences, Leonard M. Miller School of Medicine, University of Miami; Kelly A. Gebo, MD, Bloomberg School of Public Health, Johns Hopkins University; Matthew Golden, MD, primary with Department of Medicine, Division of Allergy and Infectious Diseases, University of Washington and Director, HIV/STD Program, Public Health–Seattle & King County; Gregory Kirk, MD, Bloomberg School of Public Health, Johns Hopkins University; Brandon D. L. Marshall, PhD, Department of Epidemiology, Brown School of Public Health, Rhode Island; Shruti H. Mehta, PhD, Bloomberg School of Public Health, Johns Hopkins University; Lisa R Metsch, PhD, Department of Sociomedical Sciences, Mailman School of Public Health, Columbia University; Julio Montaner, MD, BC Centre for Excellence in HIV/AIDS, Faculty of Medicine, University of British Columbia; Bohdan Nosyk, PhD, BC Centre for Excellence in HIV/AIDS, Faculty of Health Sciences, Simon Fraser University; Ankur Pandya, PhD, T. H. Chan School of Public Health, Harvard University; Bruce R. Schackman, PhD, Department of Healthcare Policy and Research, Weill Cornell Medical College; Steven Shoptaw, PhD, Centre for HIV Identification, Prevention and Treatment Services, School of Medicine, University of California, Los Angeles; Steffanie A. Strathdee, PhD, School of Medicine, University of California, San Diego.
Author contributions. B. N. conceptualized the study and wrote the first draft of the article. E. K., X. Z., M. P., and B. E. assisted with analyses and contributed to manuscript development. J. E. M., C. N. B., C. D. R., D. J. F., M. G., B. D. L. M., S. H. M., Z. F. M., L. R. M., A. P., B. R. S., S. S., and S. A. S. aided in the interpretation of results and provided critical revisions to the manuscript. B. N. secured funding for the study. All authors approved the final draft.
Financial support. This study was funded by the National Institute on Drug Abuse (NIDA) (grant number R01DA041747). B. R. S. received additional support from the Center for Health Economics of Treatment Interventions for Substance Use Disorder, HCV, and HIV (grant number P30DA040500). S. S. is supported by the University of California, Los Angeles Center for HIV Identification, Prevention and Treatment Services (National Institutes of Health [NIH] grant number P30 MH058107). S. A. S. is supported by a NIDA Method to Extend Research in Time (MERIT) award (award number R37DA019829).
Potential conflicts of interest. C. N. B. reports grants from NIDA, during the conduct of the study. C. D. R. reports grants from NIH/NIDA, and grants from NIH/National Institute of Allergy and Infectious Diseases, during the conduct of the study. M. G. reports grants to the University of Washington from Hologic, outside the submitted work. B. D. L. M. reports grants from Simon Fraser University, during the conduct of the study; and grants from NIH, outside the submitted work. S. H. M. reports personal fees from Gilead Sciences, outside the submitted work. B. R. S. reports grants from NIDA, during the conduct of the study. S. S. reports grants from the National Institute of Mental Health Center for HIV Identification, Prevention, and Treatment Services, outside the submitted work. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Fauci AS, Redfield RR, Sigounas G, Weahkee MD, Giroir BP. Ending the HIV epidemic: a plan for the United States. JAMA 2019; 321:844–5. [DOI] [PubMed] [Google Scholar]
- 2. El-Sadr WM, Mayer KH, Rabkin M, Hodder SL. AIDS in America—back in the headlines at long last. N Engl J Med 2019; 380:1985–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Gant Z, Dailey A, Hu X, Johnson AS. HIV care outcomes among Hispanics or Latinos with diagnosed HIV infection—United States, 2015. Morb Mortal Wkly Rep 2017; 66:1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Chapin-Bardales J, Rosenberg ES, Sullivan PS. Trends in racial/ethnic disparities of new AIDS diagnoses in the United States, 1984–2013. Ann Epidemiol 2017; 27:329–34.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Mustanski B, Birkett M, Kuhns LM, Latkin CA, Muth SQ. The role of geographic and network factors in racial disparities in HIV among young men who have sex with men: an egocentric network study. AIDS Behav 2015; 19:1037–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Sullivan PS, Rosenberg ES, Sanchez TH, et al. Explaining racial disparities in HIV incidence in black and white men who have sex with men in Atlanta, GA: a prospective observational cohort study. Ann Epidemiol 2015; 25:445–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Centers for Disease Control and Prevention. HIV and gay and bisexual men Available at: https://www.cdc.gov/hiv/group/msm/index.html. Accessed 8 January 2020.
- 8. Centers for Disease Control and Prevention. HIV surveillance report Vol. 29 2017. Available at: http://www.cdc.gov/hiv/library/reports/hiv-surveillance.html. Accessed 8 January 2020. [Google Scholar]
- 9. Centers for Disease Control and Prevention. HIV surveillance report (preliminary). Vol. 30 2018. Available at: http://www.cdc.gov/hiv/library/reports/hiv-surveillance.html. Accessed 26 February 2020. [Google Scholar]
- 10. Krebs E, Enns B, Wang L, et al. Localized HIV Modeling Study Group Developing a dynamic HIV transmission model for 6 U.S. cities: an evidence synthesis. PLoS One 2019; 14:e0217559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wang L, Krebs E, Min JE, et al. HIV Research Network Combined estimation of disease progression and retention on antiretroviral therapy among treated individuals with HIV in the USA: a modelling study. Lancet HIV 2019; 6:e531–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Zang X, Krebs E, Min JE, et al. Localized HIV Modeling Study Group Development and calibration of a dynamic HIV transmission model for 6 US cities. Med Decis Making 2020; 40:3–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Nosyk B, Zang X, Krebs E, et al. Ending the epidemic in America will not happen if the status quo continues: modeled projections for human immunodeficiency virus incidence in 6 US cities. Clin Infect Dis 2019; 69:2195–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Krebs E, Zang X, Enns B, et al. Localized Economic Modeling Study Group The impact of localized implementation: determining the cost-effectiveness of HIV prevention and care interventions across six United States cities. AIDS 2020; 34:447–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Nosyk B, Zang X, Krebs E, et al. What will it take to “End the HIV epidemic” in the US? An economic modeling study in 6 cities [manuscript published online ahead of print 5 March 2020]. Lancet HIV 2020. doi:10.1016/S2352-3018(20)30033-3. [Google Scholar]
- 16. Harper S, King NB, Meersman SC, Reichman ME, Breen N, Lynch J. Implicit value judgments in the measurement of health inequalities. Milbank Q 2010; 88:4–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Mandsager P, Marier A, Cohen S, Fanning M, Hauck H, Cheever LW. Reducing HIV-related health disparities in the Health Resources and Services Administration’s Ryan White HIV/AIDS Program. Am J Public Health 2018; 108:S246–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Rosenberg ES, Millett GA, Sullivan PS, Del Rio C, Curran JW. Understanding the HIV disparities between black and white men who have sex with men in the USA using the HIV care continuum: a modeling study. Lancet HIV 2014; 1:e112–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. McCree DH, Williams AM, Chesson HW, et al. Changes in disparities in estimated HIV incidence rates among black, Hispanic/Latino, and white men who have sex with men (MSM) in the United States, 2010–2015. JAIDS 2019; 81:57–62. [DOI] [PubMed] [Google Scholar]
- 20. Harris NS, Johnson AS, Huang Y-LA, et al. Vital signs: status of human immunodeficiency virus testing, viral suppression, and HIV preexposure prophylaxis—United States, 2013–2018. Morb Mortal Wkly Rep 2019; 68:1117. [DOI] [PMC free article] [PubMed] [Google Scholar]