Abstract
Ovarian toxicity (ovotoxicity) is one of the major side effects of pharmaceutical compounds for women at or before reproductive age. The current gold standard for screening of compounds’ ovotoxicity largely relies on preclinical investigations using whole animals. However, in vivo models are time-consuming, costly, and harmful to animals. Here, we developed a 3-tiered ovotoxicity screening approach starting from encapsulated in vitro follicle growth (eIVFG) and screened for the potential ovotoxicity of 8 preclinical compounds from AstraZeneca (AZ). Results from Tiers 1 to 2 screenings using eIVFG showed that the first 7 tested AZ compounds, AZ-A, -B, -C, -D, -E, -F, and -G, had no effect on examined mouse follicle and oocyte reproductive outcomes, including follicle survival and development, 17β-estradiol secretion, ovulation, and oocyte meiotic maturation. However, AZ-H, a preclinical compound targeting the checkpoint kinase 1 inhibitor to potentiate the anticancer effects of DNA-damaging agents, significantly promoted granulosa cell apoptosis and the entire growing follicle atresia at clinically relevant concentrations of 1 and 10 μM. The more targeted explorations in Tier 2 revealed that the ovotoxic effect of AZ-H primarily resulted from checkpoint kinase 1 inhibition in granulosa cells. Using in vivo mouse model, the Tier 3 screening confirmed the in vitro ovotoxicities of AZ-H discovered in Tiers 1 and 2. Also, although AZ-H at 0.1 μM alone was not ovotoxic, it significantly exacerbated gemcitabine-induced ovotoxicities on growing follicles. Taken together, our study demonstrates that the tiered ovotoxicity screening approach starting from eIVFG identifies and prioritizes pharmaceutical compounds of high ovotoxicity concern.
Keywords: encapsulated in vitro follicle growth, ovotoxicity, tiered screening, pharmaceutical compound, checkpoint kinase inhibitor, fertility
The research and development (R&D) of new drugs is an extremely complex and expensive process. On average, it costs about $2.6 billion and more than 10 years for a new drug to be developed from its initial discovery to marketing approval (DiMasi et al., 2016). Of all the candidate compounds, approximately only 0.1% of them are able to pass preclinical evaluations for clinical trials, and 0.01%–0.02% of them will receive final approval from the Food and Drug Administration (Bakke et al., 1995; Eisenstein et al., 2005; Wong et al., 2019). Of all the failed compounds, more than 50% of them exhibit unintended toxicities to the tested cells, animals, and healthy volunteers or patients (Waring et al., 2015). Female reproductive toxicity is one of the major side effects of pharmaceutical compounds, particularly for the childhood, adolescent, and young adult populations, who are within or will be in reproductive age. However, the majority of recruited healthy volunteers or patients in clinical trials are adult males to protect the limited ovarian reserve and also avoid potential pregnancy in females (American Academy of Pediatrics, 2015). Therefore, the evaluation of compounds’ potential side effects on female reproductive system relies largely on preclinical investigations using whole animals.
The ovary is the primary female reproductive organ and functions to synthesize and secrete sex steroid hormones and to mature and ovulate germ cell oocyte for fertilization and pregnancy. Increasing evidence demonstrates that a broad spectrum of pharmaceutical compounds and environmental chemicals can result in female ovarian toxicity (ovotoxicity) and increase women’s risks of premature ovarian failure (POF), hormonal imbalance, and sub- or infertility (Bhattacharya and Keating, 2012; Vabre et al., 2017). Thus far, the lack of optimal in vitro models makes the gold standard for preclinical testing of the ovotoxicity of chemicals rely primarily on whole laboratory animals. However, in vivo models are time- and effort-consuming and costly, and it is also unethical to sacrifice a large number of animals for human benefits. Moreover, the specific upstream reproductive endpoints in the ovary, such as follicle development, hormone secretion, and oocyte maturation and ovulation, are difficult to monitor in real time without dissecting whole animals. Ovarian cell lines including both follicular somatic cells (eg, granulosa cell lines) and denuded oocytes have been cultured in vitro for ovotoxicity testing (Havelock et al., 2004; Pocar et al., 2001, 2003). However, the three-dimensional (3D) follicular cell-/tissue-specific architecture is missing in these cultures. More importantly, normal follicle development and oocyte maturation require orchestrated bidirectional communications between somatic cells and their enclosed oocyte (Biggers et al., 1967; Buccione et al., 1990; Eppig et al., 2005; Gui and Joyce, 2005; Su et al., 2007), suggesting that the traditional cultures of individual type of ovarian cells cannot reconstitute the physiology necessary for testing the ovotoxicity of chemical exposure as with intact ovaries or follicles in vivo.
The method of in vitro ovarian follicle culture has been used for examining the effect of xenobiotic exposures on female ovarian function, suggesting a robust model for in vitro ovotoxicity testing (Rasmussen et al., 2017; Stefansdottir et al., 2014; Wang et al., 2018b; Xiao et al., 2017b; Zhou and Flaws, 2017; Zhou et al., 2015; Zhou and Shikanov, 2018). In our previous studies, we used the alginate hydrogel encapsulation method to culture both mouse and human preantral follicles in vitro (Xiao et al., 2015a,b, 2017b), which is termed encapsulated in vitro follicle growth (eIVFG). The eIVFG maintains the 3D architecture of follicles and recapitulates most of key events of folliculogenesis and oogenesis in vivo, including follicle growth and development from the primary or secondary stage to antral stage for maturation, hormone secretion, and oocyte maturation and ovulation (Xiao et al., 2015a,b, 2017b). In this study, we further developed a novel-tiered ovotoxicity screening approach starting from eIVFG and screened for the potential ovotoxicity of 8 preclinical compounds from AstraZeneca (AZ). The in vivo or clinically relevant exposure concentrations were used, which were determined based on the in vivo pharmacokinetic data in various species (eg, mouse, rat, and dog) from AZ or previously published results. In summary, our results indicate that the tiered ovotoxicity screening approach allows us to identify and prioritize chemicals of high ovotoxicity concern for more targeted, sophisticated, and mechanistic evaluations.
MATERIALS AND METHODS
Animals
The CD-1 mouse breeding colony (Charles River Laboratory, Wilmington, Massachusetts) was maintained in the animal facility at the University of South Carolina. All mice were housed in polypropylene cages and provided with food and water ad libitum. Animals were kept on a 12-h light/dark cycle (7:00 am to 7:00 pm) at 23 ± 1°C with 30%–50% relative humidity. All methods used in this study were approved by the University of South Carolina Institutional Animal Care and Use Committee (IACUC) and corresponded to the National Institutes of Health (NIH) guidelines and public law.
Follicle isolation, encapsulation, and culture
Immature ovarian follicles at multilayered secondary stage (150–180 μm, type 5b) were mechanically isolated from 15- or 16-day-old CD-1 female mice as we previously described (Xiao et al., 2015a, 2017b). Only follicles that displayed intact morphology were selected for encapsulation and culture. Selected follicles were encapsulated individually in 0.5% alginate hydrogel (Sigma-Aldrich, St Louis, Missouri). Follicles were placed in maintenance media containing minimal essential medium (αMEM with GlutaMAX, Gibco, Thermo Fisher Scientific, Inc, Waltham, Massachusetts) and 1% fetal bovine serum (FBS, Sigma-Aldrich) for 0.5 h after encapsulation. Encapsulated follicles were then individually placed in 96-well plates, with each well containing 100 μl growth media (50% αMEM with GlutaMAX and 50% Nutrient Mixture [F-12 with GlutaMAX, Gibco, Thermo Fisher Scientific, Inc] supplemented with 3-mg/ml bovine serum albumin [Thermo Fisher Scientific, Inc], 20-mIU/ml recombinant follicle-stimulating hormone [A. F. Parlow, National Hormone and Peptide Program, National Institute of Diabetes and Digestive and Kidney Diseases, Bethesda, Maryland], 1-mg/ml bovine fetuin [Sigma-Aldrich], 5 μg/ml insulin, 5 μg/ml transferrin, and 5 ng/ml selenium [Sigma-Aldrich]). Follicles were cultured at 37°C in 5% CO2 for 8 days and half of the growth media (50 µl) was replaced every other day. For all experiments, follicles were randomly distributed to experimental groups with each group having 8–12 follicles and 3–5 replicates were performed.
Follicle growth and survival assessment
Follicles cultured using eIVFG were imaged at each media change using an Olympus CKX53 Inverted Microscope with 10× and 20× objectives (Olympus Corporation, Hachioji-shi, Tokyo, Japan). Follicles were considered dead if they had unhealthy appearing oocytes and/or granulosa cells, or if the integrity of the oocyte and somatic cell interface was visibly compromised. Follicle growth curves were obtained by plotting the average follicle diameter, which was calculated by averaging 2 perpendicular measurements from basement membrane to basement membrane of each follicle using ImageJ software ( NIH, Bethesda, Maryland). Follicle survival rate was calculated by dividing the number of survived follicles on days 0, 2, 4, 6, and 8 to the number of cultured follicles on day 0. For each experimental group, the follicle growth curve and survival rates on days 0, 2, 4, 6, 8 were plotted, and the lethal concentration 50 (LC50) on in vitro cultured follicles was calculated using GraphPad Prism (GraphPad Software, San Diego, California).
Hormone measurements
The 17β-estradiol (E2) concentrations in the conditioned follicle culture media on day 8 of eIVFG were collected and measured using ELISA kits (Calbiotech, Spring Valley, California) according to the manufacturer’s instructions. Briefly, the antiestradiol capture antibodies precoated wells were incubated with E2 standards, conditioned follicle culture media, and Estradiol Biotin Reagent for 45 min. Next, the Estradiol Enzyme Reagent was added and incubated for another 45 min. After washing the wells with the washing buffer for 3 times, the solution of TMB reagent was added and incubated at room temperature for 20 min, resulting in the development of blue color. Last, the reaction was stopped by the addition of Stop Solution and the absorbance was measured using a BioTek Synergy HT microplate reader (BioTek Instruments, Inc, Winooski, Vermont) at 450 nm within 15 min. All assays were run in duplicate.
In vitro ovulation, oocyte maturation, and oocyte size measurement
On day 8 of eIVFG, follicles were mechanically removed from alginate beads and then incubated in in vitro maturation media (αMEM with 10% FBS, 1.5-IU/ml human chorionic gonadotropin [hCG, Sigma-Aldrich], and 10-ng/ml epidermal growth factor [from R&D Systems, Minneapolis, Minnesota]) for 16 h at 37°C in 5% CO2 in air. Oocytes were denuded from surrounding cumulus cells using 0.3% hyaluronidase (Sigma-Aldrich). Oocytes were considered to be arrested at prophase I in the germinal vesicle stage if the nucleus was intact, and were considered to have undergone germinal vesicle breakdown if the nucleus was not visible. If a polar body (PB) was present in the perivitelline space, the oocytes were classified as metaphase II (MII). Fragmented or shrunken oocytes were classified as degenerated (D). For all MII oocytes, the oocyte diameter was obtained from 2 perpendicular measurements, including the zona pellucida. The first measurement detected the widest diameter of oocyte and the second measurement originated at a right angle from the midpoint of the first measurement. The final oocyte diameter was calculated by averaging the 2 obtained measurements.
AZ compound exposure and ovotoxicity testing
All 8 tested pharmaceutical compounds were kindly gifted from AZ (Cambridge, United Kingdom) from projects that closed because of nonfemale reproductive toxicity safety reasons identified during preclinical or clinical development. Because the Material Transfer Agreement with AZ, we named them from AZ-A to AZ-H instead of using their original AZ library IDs. However, for the compounds of AZ-A and AZ-H that are commercially available now for scientific research, we started to use their original AZ library IDs (AZD8542 for AZ-A and AZD7762 for AZ-H) and also introduced their molecular targets from the Tier 2 screening. All tested AZ compounds were dissolved in 100% dimethyl sulfoxide (DMSO, Sigma-Aldrich) to make a stock concentration at 200 mM and the stock solutions were stored at −20°C and away from light. For ovotoxicity testing, the stock solutions were diluted to the targeted exposure concentrations in follicle culture media. In Tier 1 ovotoxicity screening, follicles were treated with each tested candidate compound at 10 µM from days 2 to 8 of eIVFG. We started compound exposure from day 2 instead day 0 to exclude any dead follicles within the first 2 days of eIVFG that might have resulted from mechanical manipulation during follicle isolation. In Tier 2 ovotoxicity screening, follicles were treated with AZ-A as the negative control and AZ-H as the positive control at 0, 0.1, 1, and 10 µM for 24 h from day 2 of eIVFG, followed by a continuous culture without AZ compound until day 8 of eIVFG. The rationale for determining in vitro compound exposure concentrations was described in the Results section. For both Tiers 1 and 2 screenings, the assessments of follicle and oocyte reproductive outcomes were performed as described above, including follicle survival, follicle development, E2 secretion, and in vitro ovulation and oocyte maturation.
Chk1 or Chk2 inhibitor treatment and ovotoxicity testing
Another set of multilayered secondary follicles were isolated and cultured using eIVFG. Follicles were treated with 3-specific checkpoint kinase 1 (Chk1) inhibitors, including Rabusertib at 2 μM, CHIR-124 at 0.2 μM, and MK-8776 at 20 μM, and 1 checkpoint kinase 2 (Chk2) inhibitor, BML-277 at 5 μM from days 2 to 8 of eIVFG. The follicle survival and development were examined as described above. The concentrations we used were either based on human plasma/serum levels in clinical trials or based on previous results that showed effective checkpoint kinase (Chk) inhibition in in vitro cultured cell lines. The detailed justification was described in the Results section.
In vivo animal exposure
To investigate whether the gained ovotoxicities of AZ-H in Tiers 1 and 2 screenings could be validated using in vivo animal models, 21-day-old CD-1 female mice were intraperitoneally injected with 25 mg/kg of AZ-H dissolved in 50-μl DMSO. We used 21-day-old mice because their ovaries contain all developmental stages of preantral and antral follicles, indicating a good in vivo animal model to study the impact of xenobiotic exposure on female ovarian functions (Wang et al., 2018b, 2019). The same volume of DMSO was used as the vehicle control. AZ-A that showed negative ovotoxicity in Tiers 1 and 2 screenings was used as negative control and the exposure dose of AZ-A was at 20 mg/kg through intraperitoneal injection. The rationale of the in vivo dose selection for both AZ-A and AZ-H was described in the Results section. Ovaries were collected 24 h postinjection for histology and TUNEL staining.
Gemcitabine and doxorubicin treatment
Follicles were treated with different concentrations of gemcitabine (GEM, Sigma-Aldrich) at 0, 0.02, 0.1, and 0.5 μM, and doxorubicin (DOX, Sigma-Aldrich) at 0, 10, 20, and 50 nM alone or with AZ-H at 0.1 μM for 24 h, followed by a continuous culture using compound-free growth media until day 8 of eIVFG. The follicle and oocyte reproductive outcomes were assessed as described above, including follicle survival and follicle development. The specific rationale of using GEM and DOX for cotreatment experiments and determination of concentration range were described in the Results section.
Histology and TUNEL assay
For in vitro exposure experiments, follicles were collected at 24 h after vehicle or AZ compound treatment and fixed overnight at 4°C in 4% paraformaldehyde (PFA)-Cacodylate-Ca2+ Fixation Buffer (4% PFA [Sigma-Aldrich], 0.1-M sodium cacodylate [Electron Microscopy Sciences, Hatfield, Pennsylvania], 0.1 M sucrose [Sigma-Aldrich], 10-mM calcium chloride [Thermo Fisher Scientific, Inc], pH = 7.4). After washing with 1× phosphate-buffered saline (PBS, Thermo Fisher Scientific, Inc) 3 times, fixed follicles were dehydrated in ascending concentrations of ethanol (50%–100%) and embedded in paraffin (Sigma-Aldrich). For in vivo exposure experiments, ovaries were collected 24 h after vehicle or AZ compound injection, fixed in Shandon Formal-Fixx 10% Neutral Buffered Formalin solution (Thermo Fisher Scientific, Inc) for 24 h, and embedded in paraffin. Both embedded follicles and ovaries were sectioned at the thickness of 5 μm with a RM 2165 microtome (Leica Microsystems, Nussloch, Germany). For histology staining, paraffin sections of follicles or ovaries were stained with hematoxylin and eosin (Thermo Fisher Scientific, Inc) as we previously described (Xiao et al., 2015a). The DeadEnd Fluorometric TUNEL System Kit (Promega, Madison, Wisconsin) was used to detect apoptotic cells according to the manufacturer’s instructions. Briefly, paraffin sections of follicles or ovaries were deparaffinized, rehydrated, and fixed in 4% formaldehyde in 1× PBS. After permeabilizing by 20-µg/ml Proteinase K solution for 10 min, the TdT reaction mix was added to the ovarian/follicular sections and incubated for 1 h at 37°C in a humidified chamber. The sections were counter-stained using Vectashield Mounting Medium with DAPI (Vector Laboratories, Burlingame, California) and TUNEL positive signals were analyzed under Olympus BX51 microscope (Olympus Corporation, Hachioji-shi, Tokyo, Japan) and quantified by ImageJ software. The relative TUNEL fluorescence intensity was calculated according to the following formula: intensity = total TUNEL positive signal pixel intensity/area.
Statistical analyses
Follicle growth, survival, hormone secretion, and oocyte reproductive outcomes were analyzed from 3 independent cultures in which 8–12 follicles were included in each experimental group and replicate. Follicle growth pattern and survival rates on the same day, hormone secretion on day 8, ovulated MII oocyte percentage and diameter, and TUNEL positive signal in different drug-treated groups were analyzed using 1-way ANOVA. The post hoc test was performed to compare the difference between 2 groups if a significant difference was observed. The significance level was set at p < .05.
RESULTS
The Development of a Tiered Ovotoxicity Screening
To efficiently and effectively identify and prioritize pharmaceutical compounds of high ovotoxicity concerns for more advanced assessments, we developed a tiered ovotoxicity screening starting from eIVFG (Figure 1). In Tier 1, follicles are treated with each candidate compound at a single high dose from days 2 to 8 of eIVFG. During the entire compound exposure window, the follicle and oocyte reproductive outcomes are evaluated, including follicle survival, follicle growth and development, sex steroid hormone secretion, and in vitro ovulation and oocyte maturation. If no ovotoxic outcome is discovered, the tested compound is identified as low ovotoxicity concern. In contrast, if any adverse readouts are detected, the candidate compound is considered as a suspect ovotoxic chemical and advanced to Tier 2 screening with more sophisticated testing, such as the dose-response exposure, specific window exposure, or more in-depth cellular or molecular targeted toxicity investigations. The specific experimental design in Tier 2 depends on the ovotoxicity results obtained in Tier 1. For compounds that exhibit negative ovotoxic outcomes in Tier 2, they are reconsidered as low ovotoxicity concern. However, the tested compounds can be further advanced to Tier 3 screening with in vivo animal exposure and toxicity validation when they show consistent ovotoxicities between Tiers 1 and 2. If a tested compound exhibits consistent ovotoxicities in all 3 tiers, it is identified as an ovotoxic chemical. If a compound shows inconsistent ovotoxicities between in vitro and in vivo models, more factors will be considered and investigated, such as liver metabolism, pharmacokinetics/toxicokinetics, local, or systemic effects.
Figure 1.
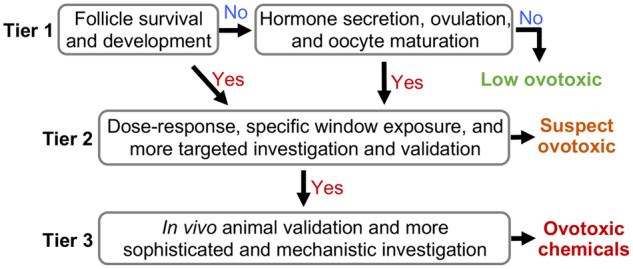
The tiered ovotoxicity screening strategy for testing the effect of pharmaceutical compounds on female reproductive health and fertility.
Effects of AZ Compounds on Follicle Survival, Development, and Hormone Secretion in Tier 1 Screening
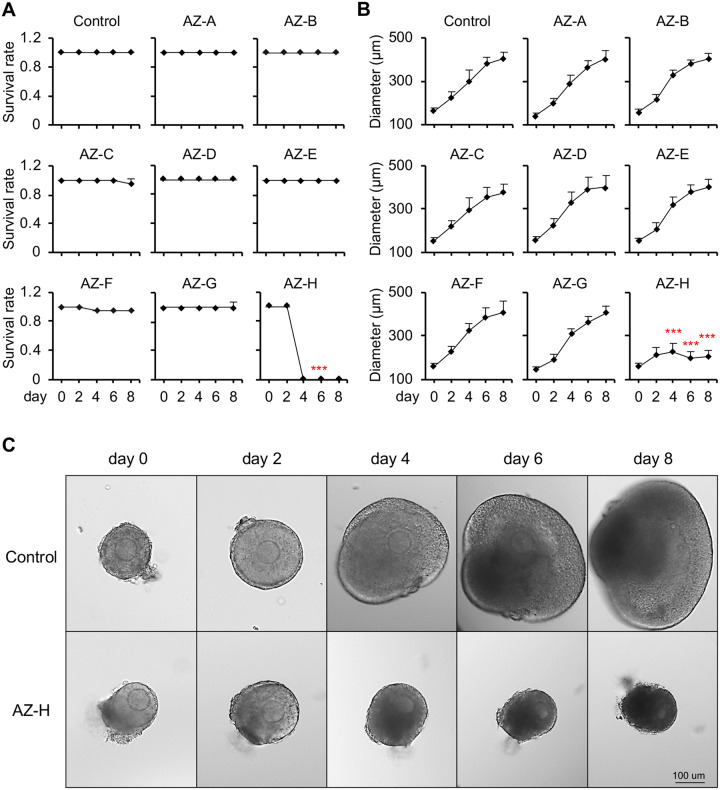
We used 8 AZ compounds (AZ-A to AZ-H) to test the performance of the developed tiered ovotoxicity screening as described in Figure 1. Based on our internal pharmacokinetic results in various species and published data, the majority of tested compounds have the maximum plasma or serum concentrations up to 10 µM. In the Tier 1 screening, we therefore treated cultured follicles with each candidate compound at a high concentration at 10 µM from days 2 to 8 of eIVFG. In the control group, the alginate hydrogel encapsulation maintained the 3D architecture of cultured follicles and supported follicle growth from multilayered secondary stage on day 0 to antral stage on day 8 during eIVFG (Figure 2A). The follicle diameter increased from 161.9 ± 15.3 μm on day 0 to 405.4 ± 28.4 μm on day 8, and the follicle survival rate was 100% on day 8 (Figure 2A). Follicles treated with all 8 AZ compounds at 10 µM except AZ-H showed comparable follicle survival rates and growth patterns to the control group (Figs. 2A and 2B). However, follicles treated with AZ-H at 10 µM resulted in 100% follicle death starting from day 4 of eIVFG (Figs. 2A–C).
Figure 2.
Effect of 8 AstraZeneca (AZ) compounds on follicle survival and development during encapsulated in vitro follicle growth (eIVFG). A and B, Follicle survival rates (A) and follicle diameters (B) during eIVFG after AZ compound exposure (AZ-A to AZ-H) at 10 µM from days 2 to 8. C, Representative images of follicles during eIVFG treated with vehicle or AZ-H at 10 µM from days 2 to 8. Error bar: SD; ***p < .001 compared with control group; scale bar: 100 μm. N = 8–12 follicles in each experimental group and 3 replicates were performed.
To investigate the effects of AZ compounds on ovarian steroidogenesis, we collected conditioned follicle culture media on day 8 and measured the concentration of E2, an important sex steroid hormone synthesized and secreted from growing follicles that supports the functions of ovary/follicles, downstream reproductive organs, and systemic health (Buyuk et al., 2010). ELISA results indicated that there was no significant difference in the E2 secretion levels between control group and follicles treated with all tested AZ compounds except AZ-H (Figure 3). The extremely low concentrations of E2 in AZ-H treatment group reflected the above findings that all treated follicles were dead on day 8. These results indicate that there is a low ovotoxicity concern for the AZ compounds of AZ-A, -B, -C, -D, -E, -F, and -G. However, AZ-H has suspected ovotoxicity and requires further investigations.
Figure 3.
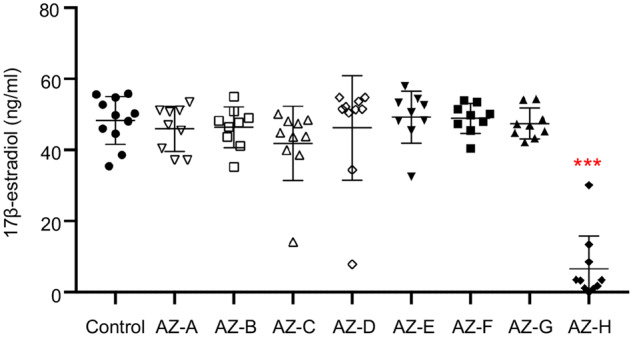
Effect of 8 AstraZeneca (AZ) compounds (AZ-A to AZ-H) on 17β-estradiol secretion on day 8 of encapsulated in vitro follicle growth. Error bar: SD; ***p < .001 compared with control group. N = 8–12 follicles in each experimental group and 3 replicates were performed.
Effects of AZ Compounds on Follicle Ovulation and Oocyte Meiotic Maturation in Tier 1 Screening
We next treated grown antral follicles with hCG on day 8 to determine the effect of AZ compound exposure during folliculogenesis and oogenesis on follicle ovulation and oocyte meiotic maturation. Results showed that 88.9 ± 0.2% of antral follicles in the control group ruptured and ovulated MII oocytes with the first PB extrusion (Figs. 4A and 4B). The ovulated MII oocytes had normal morphological appearance and the average MII oocyte diameter was 89.4 ± 3.5 μm (Figure 4C). Because there was 100% of follicle death on day 8 after AZ-H treatment (Figure 2), no follicle was collected for in vitro ovulation and oocyte maturation (red crosses in Figs. 4B and 4C). For the follicles treated with all the other 7 AZ-compounds, they had comparable MII oocyte percentage, morphological appearance, and oocyte size compared with the control group (Figure 4), suggesting that these 7 AZ-compounds do not interfere with follicle ovulation and oocyte meiotic maturation.
Figure 4.
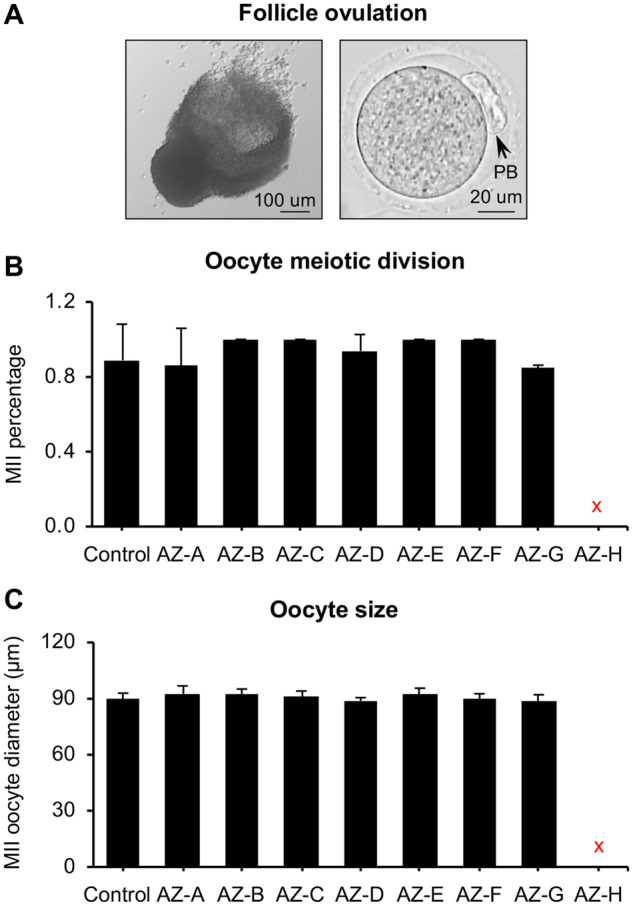
Effect of 8 AstraZeneca (AZ) compounds (AZ-A to AZ-H) on in vitro ovulation and oocyte meiotic maturation. A, Representative images of a ruptured follicle (left) and ovulated metaphase II (MII) oocyte (right) after treatment with human chorionic gonadotropin for 14 h. B and C, Oocyte MII percentages (B) and MII oocyte diameter (C) after in vitro ovulation and oocyte maturation. Scale bar: 100 μm for ruptured follicle and 20 μm for MII oocyte; error bar: SD. The red cross marks indicated no follicles were selected for in vitro ovulation because of the 100% follicle death upon AZ-H treatment. N = 8–12 follicles in each experimental group and 3 replicates were performed. Abbreviation: PB, polar body.
Effects of AZ-H on Follicle Survival and Development in Tier 2 Screening
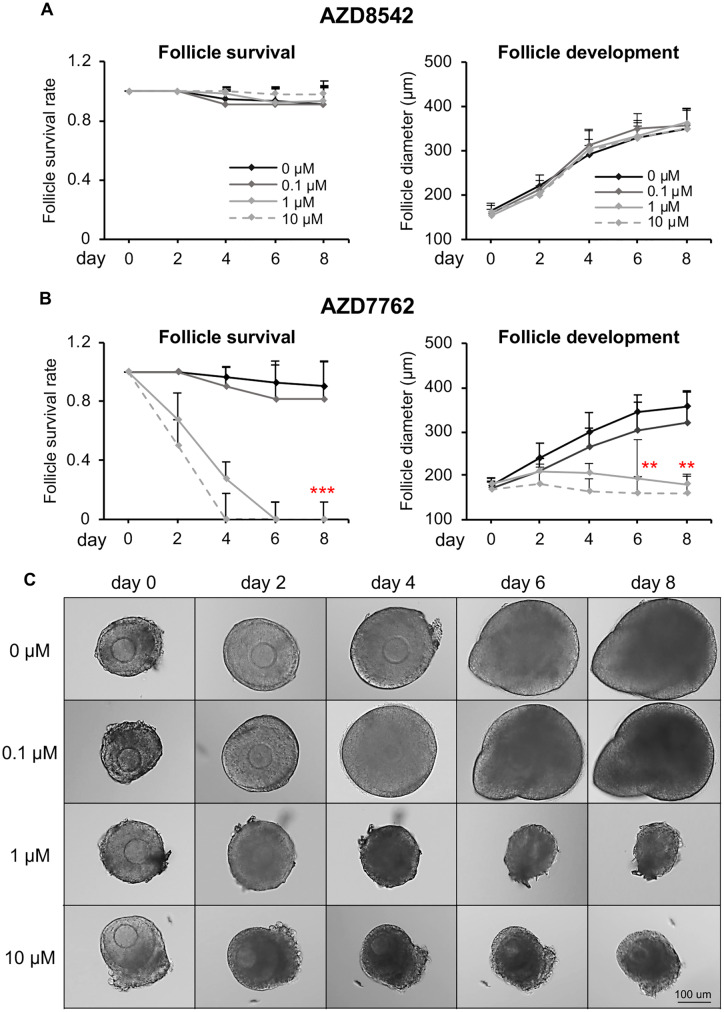
Based on the ovotoxicity screening results in Tier 1, we next selected the ovotoxic compound AZ-H for a more advanced Tier 2 screening. We also chose AZ-A that did not show ovotoxicity in Tier 1 as the negative control and used DMSO as the vehicle control. AZ-A and AZ-H have the AZ compound library ID as AZD8542 and AZD7762 (so referred to below), respectively. AZD8542 was designed as a hedgehog pathway antagonist to inhibit tumor progression by targeting fibroblasts (Hwang et al., 2012); and AZD7762 was designed as a Chk1/2 inhibitor to potentiate the therapeutic effect of DNA-damaging agents on tumor cells (Zabludoff et al., 2008). Based on the results from previous clinical trials, the maximal plasma concentration of AZD7762 was between 0.83 and 1.10 μM in humans (Sausville et al., 2014; Seto et al., 2013) and 9 μM in dogs (unpublished data from AZ). Therefore, we performed a dose-response study by treating cultured follicles at 0, 0.1, 1, and 10 μM in Tier 2. We also chose a more specific exposure window by treating follicles for 24 h because results from Tier 1 indicated that AZD7762 caused remarkable morphological changes to follicles within 24 h (Figure 2C). For the compound of AZD8542, previous studies reported that the in vitro treatment of AZD8542 at 0.1–1 μM effectively inhibited the hedgehog pathway in human pancreatic stellate cells 24 h after compound treatment (Hwang et al., 2012). Therefore, we used the same exposure concentration range and window as the AZD7762.
Consistent to the ovotoxicity screening results in Tier 1, follicles treated with all tested concentrations of AZD8542 had comparable follicle survival rates (Figure 5A, left panel), follicle development patterns (Figure 5A, right panel), secreted E2 levels (Figure 6A), and in vitro ovulation and oocyte meiotic division outcomes (Figure 6B) to the control group. However, for the follicles treated with AZD7762 at 1 or 10 µM, all follicles were dead on day 4 or 6 (Figs. 5B and 5C) and the E2 secretion levels also significantly decreased on day 8 (Figure 6A). When follicles were treated with AZD7762 at 0.1 µM, the follicle survival rate and terminal diameter on day 8 decreased by 9.9% and 10.4%, respectively, compared with the control group, but the changes were not statistically significant (Figure 5B). At the exposure concentration of 0.1 µM, both the E2 secretion levels and MII oocyte percentages after in vitro ovulation were comparable with the control group (Figure 6). These results indicate that Tiers 1 and 2 screenings have consistent results and AZD7762 exhibits ovotoxicities when the exposure level is at or higher than 1 µM.
Figure 5.
Effect of AZD8542 and AZD7762 on follicle survival and development during encapsulated in vitro follicle growth (eIVFG). A and B, Follicle survival rates and follicle diameters during eIVFG upon different concentrations of AZD8542 (A) and AZD7762 (B) treatment. C, Representative images of follicles during eIVFG treated with AZD7762 at 0, 0.1, 1, and 10 µM for 24 h. Scale bar: 100 μm; error bar: SD; **p < .01; ***p < .001 compared with control group. N = 8–12 follicles in each experimental group and 3 replicates were performed.
Figure 6.
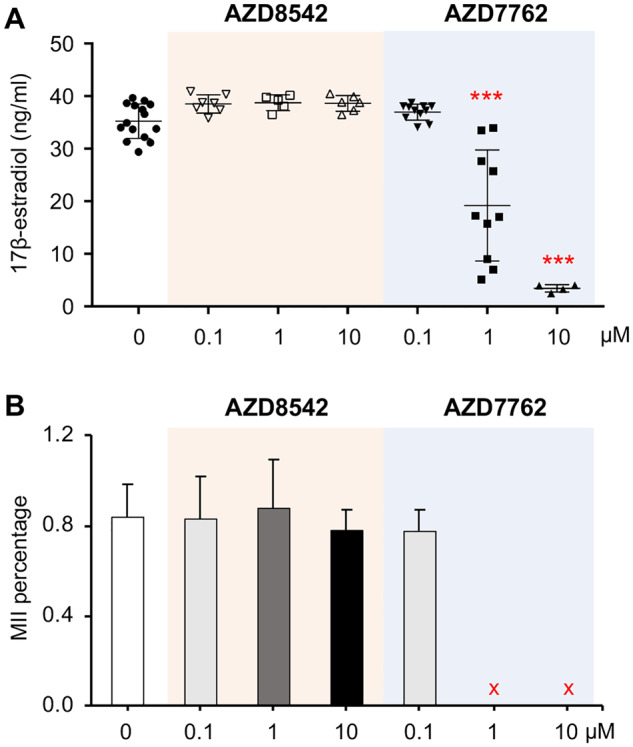
Effect of AZD7762 and AZD8542 on 17β-estradiol (E2) secretion and oocyte maturation in vitro. A, E2 secretion levels on day 8 of encapsulated in vitro follicle growth (eIVFG) after follicles were treated with different concentrations of AZD8542 and AZD7762 for 24 h. B, Oocyte metaphase II (MII) percentages from follicles treated with different concentrations of AZD8542 and AZD7762 for 24 h. Error bar: SD; ***p < .001 compared with control group. The red cross marks indicated no follicles were selected for in vitro ovulation because of the 100% follicle death upon AZ-H treatment. N = 8–12 follicles in each experimental group and 3 replicates were performed.
AZD7762 Targeted Granulosa Cells to Promote Follicle Atresia
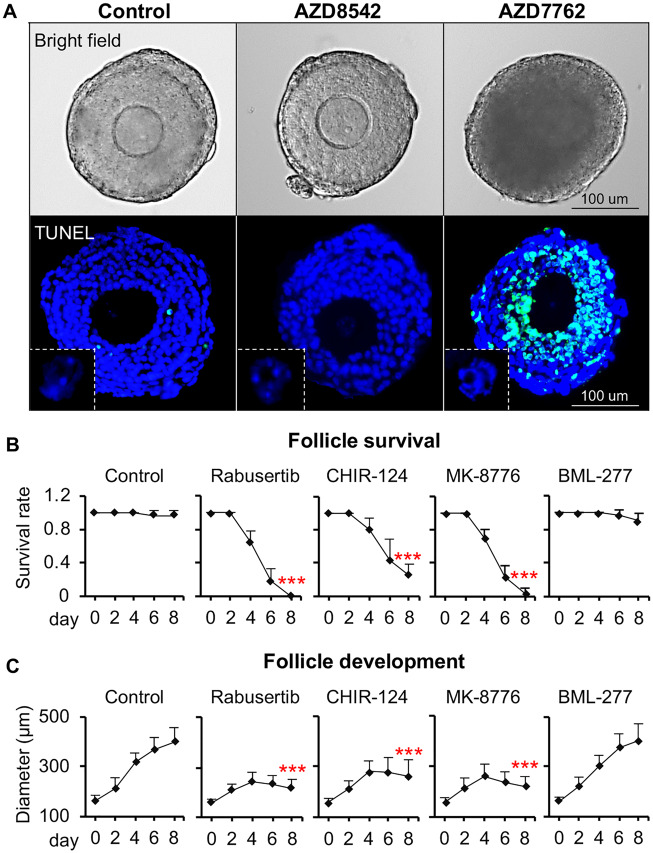
We next selected the ovotoxic AZD7762 for more targeted histological staining and TUNEL assay. DMSO was used as the vehicle control and AZD8542 was used as the negative control. At 24-h postvehicle (DMSO) or 10-µM AZD8542 treatment, follicles had comparable morphology and TUNEL stainings to the control group (Figure 7A). However, follicles treated with AZD7762 at 10 µM showed markedly increased granulosa cell apoptosis indicated by the DNA fragmentation in TUNEL staining (Figure 7A). Differing from some ovotoxic chemicals such as DOX that damage the outer layers of the theca cells and granulosa cells and then promote the entire follicle atresia (Xiao et al., 2017b), AZD7762 resulted in a greater degree of apoptosis in the inner layers of the granulosa cells (Figure 7A). Moreover, up to 24 h exposure of AZD7762, TUNEL staining results indicated that the central oocytes did not show obvious DNA damage compared with their surrounding somatic cells (Figure 7, white inserts). These data indicate that AZD7762 primarily targets inner granulosa cells to promote the entire follicle atresia.
Figure 7.
Target inhibition of AZD7762 on ovarian follicle. A, Representative images of follicles in bright field and follicular cell apoptosis after follicles were treated with AZD7762 and AZD8542 exposure at 10 µM for 24 h in vitro. Blue: DAPI; green: DNA fragmentation revealed by TUNEL staining. Scale bar: 100 μm. White squares indicated the TUNEL staining of oocytes. N = 5–10 follicles for each experimental group and 3 replicates were performed. B and C, Effect of 3 Chk1 inhibitors (Rabusertib at 2 μM, CHIR-124 at 0.2 Μm, and MK-8776 at 20 μM) and 1 Chk2 inhibitor (BML-277 at 5 µM) on follicle survival (B) and development (C) during encapsulated in vitro follicle growth. Error bar: SD; ***p < .001 compared with control group. N = 8–12 follicles in each experimental group and 3 replicates were performed.
The Inhibition of Chk1 but Not Chk2 Resulted in AZD7762-induced Ovotoxicities
Because AZD7762 was found to have an equal inhibitory potency for Chk1 and Chk2 (Ma et al., 2011; Morgan et al., 2010; Wang et al., 2018a; Zabludoff et al., 2008), we next treated follicles with more specific Chk inhibitors to differentiate the role of Chk1 and Chk2 in AZD7762-induced ovotoxicities. The tested inhibitors included Chk1 inhibitors Rabusertib, CHIR-124, and MK-8776, and Chk2 inhibitor, BML-277. The exposure concentrations were determined either based on human plasma/serum levels for the cases of Rabusertib and MK-8776 (Karp et al., 2012; King et al., 2014; Wang et al., 2014; Wehler et al., 2017) or based on previous in vitro studies that showed effective Chk inhibition in cultured cells for the cases of CHIR-124 and BML-277 (Arienti et al., 2005; Boudny et al., 2019; Dai et al., 2011; Tse et al., 2007; Tuppi et al., 2018). The ovotoxicity screening results revealed that all 3 tested Chk1 inhibitors exhibited similar ovotoxicities as AZD7762. Specifically, upon the treatment of Rabusertib at 2 µM, CHIR-124 at 0.2 µM, and MK-8776 at 20 µM, the follicle survival rates were decreased to 0, 25%, and 4%, respectively, on day 8 of eIVFG (Figs. 7B and 7C). Interestingly, follicles treated with the specific Chk2 inhibitor BML-277 at 5 µM had comparable follicle survival rates and growth patterns to the control group (Figs. 7B and 7C). These results suggest that the inhibition of Chk1 but not Chk2 is responsible for the AZD7762-induced ovotoxicities on growing follicles.
AZD7762 Consistently Promoted Growing Follicle Atresia In Vivo
To further validate the ovotoxicities of AZD7762 observed in Tiers 1 and 2 studies, we next performed in vivo animal exposure in Tier 3 screening. In previous rodent models, animals were intravenously or intraperitoneally injected with AZD7762 at 10–25 mg/kg, which effectively potentiated chemotherapeutical drug-induced tumor cell death (Itamochi et al., 2014; Ma et al., 2012; Quin et al., 2016; Zabludoff et al., 2008). We therefore treated 21-day-old CD-1 female mice with AZD7762 at 25-mg/kg body weight once. Meanwhile, DMSO was used as the vehicle control and AZD8542 as the negative control. The treatment dose of AZD8542 was at 20 mg/kg which has been demonstrated to significantly inhibit tumor growth in an in vivo colon cancer mouse model (Hwang et al., 2012). Both histology and TUNEL staining of ovaries collected 24 h after vehicle or AZ compounds injection indicated that AZD8542-treated ovaries had similar ovary or follicle morphology and comparable levels of ovarian cell apoptosis compared with the vehicle-treated ovaries (Figure 8). However, AZD7762 significantly increased granulosa cell apoptosis in the secondary and antral stages of follicles, which was characterized by the pyknotic and fragmented nuclei in histological staining (Figure 8a, black squares) and the DNA-fragmented nuclei in TUNEL staining (Figure 8A, white squares). Similar to the in vitro ovotoxic patterns (Figure 7), in vivo results also showed that AZD7762 caused a greater degree of apoptosis in the inner layers of the granulosa cells compared with outer cell layers (Figure 8A, white squares). With respect to the earlier stage of primordial follicles that we cannot examine using eIVFG, both histological and TUNEL staining results indicated that there was no significant difference between all treatment groups (Figure 8A, red squares). These results indicate that the obtained ovotoxicities from eIVFG could be validated in in vivo animal models, and AZD7762 primarily targets growing follicles but not primordial follicles to result in ovotoxicities.
Figure 8.
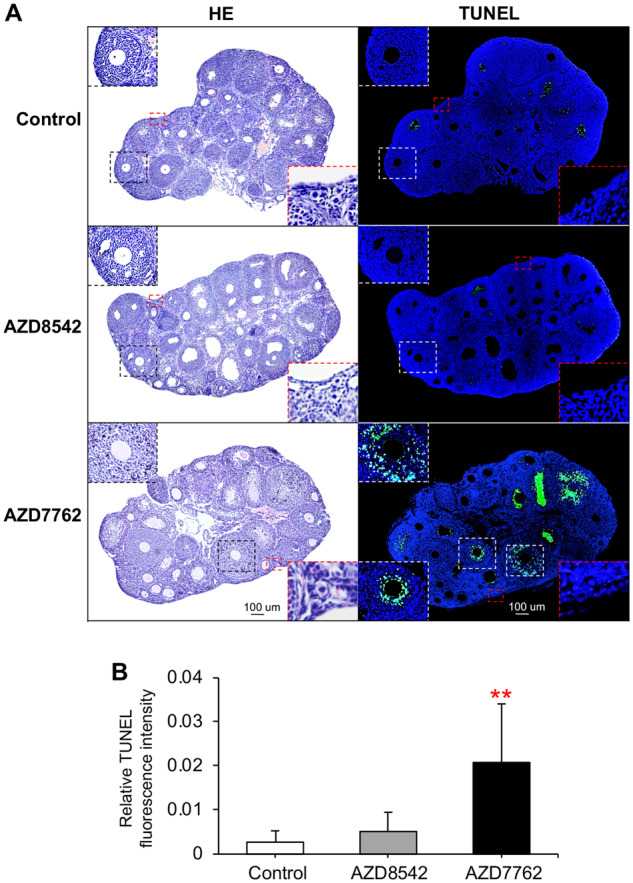
Effect of AZD7762 and AZD8542 on ovarian follicle atresia in vivo. A, Representative ovary histological and TUNEL staining images 24 h after treatment of vehicle, AZD7762 at 25 mg/kg, and AZD8542 at 20 mg/kg through intraperitoneal injection. Black squares indicated the histological staining of growing follicles, white squares indicated the TUNEL staining of growing follicles, and red squares indicated the histological staining and the TUNEL staining of primordial follicles. B, Relative TUNEL fluorescent intensity in the ovaries treated with vehicle, AZD7762, and AZD8542. Scale bar: 100 μm. Error bar: SD; **p < .01. Blue: DAPI; green: DNA fragmentation revealed by TUNEL staining. N = 3–7 mice/ovaries in each treatment group.
AZD7762 Exacerbated Ovotoxicity of GEM but Not DOX During eIVFG
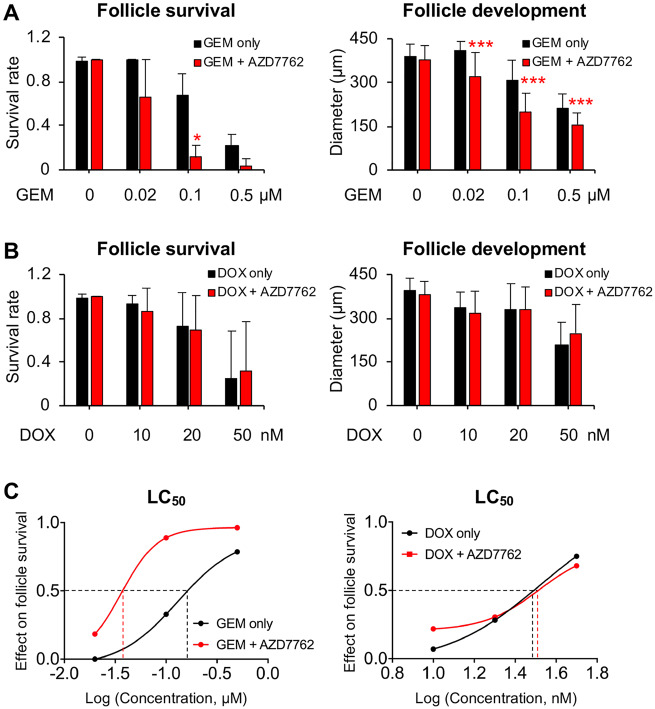
Because AZD7762 was designed as a Chk1 inhibitor to enhance DNA-damaging agent-induced cancer cell apoptosis (Zabludoff et al., 2008), we next determined whether cotreatment with AZD7762 exacerbated chemodrug-induced ovotoxicities. We chose GEM as the cotreated chemotherapeutic drug because it was used by most of previous studies to test the synergistic anticancer effect of AZD7762 (Liu et al., 2017; Morgan et al., 2010; Sausville et al., 2014; Seto et al., 2013; Zabludoff et al., 2008) and GEM has also been reported to cause ovotoxicity by promoting growing follicle atresia (Yuksel et al., 2015). In addition, we tested the potential enhancing effect of AZD7762 on DOX, another commonly used chemotherapeutic drug showing ovotoxicities on growing follicles (Ben-Aharon et al., 2010; Roti Roti et al., 2012; Wang et al., 2019; Xiao et al., 2017b). Both GEM and DOX showed dose-dependent ovotoxicities on cultured follicles during eIVFG as we and others previously demonstrated (Figs. 9A and 9B) (Xiao et al., 2017b; Yuksel et al., 2015). The treatment of AZD7762 at 0.1 µM did not affect follicle development and survival (Figure 2); however, it significantly promoted GEM-induced follicle atresia and inhibition of follicle growth and development, particularly when GEM was at or above 0.1 µM (Figure 9A). The LC50 of GEM on follicle survival was 0.13 µM (Figure 9C), however, it significantly decreased to 0.04 µM after follicles were cotreated with 0.1 µM AZD7762 (Figure 9C). Interestingly, both the follicle survival rates and follicle terminal diameters were similar between the DOX only and DOX and AZD7762 cotreatment groups (Figure 9B), suggesting that there is no exacerbating effect of AZD7762 on DOX-induced ovotoxicity. Taken together, these results indicate that the cotreatment of AZD7762 can exacerbate chemotherapy-induced ovotoxicity, but the effect depends on the specific anticancer agents, which have distinct cell-killing mechanisms.
Figure 9.
Effect of AZD7762 on exacerbating chemotherapeutic chemical-induced ovotoxicity. A and B, Follicle survival rates and terminal diameters on day 8 of eIVFG after follicles were treated with gemcitabine (GEM) at 0, 0.02, 0.1, and 0.5 µM (A) or doxorubicin (DOX) at 0, 10, 20, and 50 nM (B) alone or cotreated with AZD7762 at 0.1 µM. C, Effect of different doses of chemicals on follicle survival and LC50 value for log of concentration. Error bar: SD; *p < .05 and ***p < .001 compared with control group. N = 8–10 follicles in each experimental group and 3 replicates were performed.
DISCUSSION
Ovotoxicity is one of the major off-target effects of pharmaceutical compounds. Previous studies have revealed that multiple drugs, such as chemotherapeutics, can damage ovarian follicles and increase the risk of POF, early menopause, and infertility in both reproductive aged women and prepubertal girls (Spears et al., 2019). The current gold standard for ovotoxicity testing relies on whole laboratory animals. However, it is challenging to use in vivo models to examine the specific and dynamic follicle and oocyte reproductive outcomes without dissecting whole animals, and it is also not feasible to use in vivo models to screen for the ovotoxicity of all hundreds of even thousands of candidate compounds. Tiered screening strategies have been increasingly employed in toxicity testing (Becker et al., 2007; Bus and Becker, 2009; Doe et al., 2006; Krewski et al., 2010). Compared with traditional methods using whole animals, the tiered screening is more efficient and cost-effective to identify and prioritize chemicals of low or high toxicity concern for the following more targeted and sophisticated assessments.
Previous studies have demonstrated that in vitro ovarian follicle culture is a robust model for ovotoxicity testing (Rasmussen et al., 2017; Stefansdottir et al., 2014; Wang et al., 2018b; Xiao et al., 2015a, 2017b; Zhou and Flaws, 2017; Zhou et al., 2015; Zhou and Shikanov, 2018). Here, we developed a tiered ovotoxicity screening approach to identify pharmaceutical compounds of high ovotoxicity concern. The tiered screening begins with eIVFG in Tier 1, a 3D in vitro follicle growth method we have previously developed (Xiao et al., 2015a,b, 2017b), as a highly sensitive but less sophisticated screening method. If the tested compounds present negative ovotoxicities, they will be considered as low ovotoxicity concern. In contrast, for compounds that do not pass Tier 1 screening, more complex approaches will be used to further determine the likelihood and extent of the potential ovotoxicity in the Tiers 2 and 3 screenings. In addition, the results obtained in Tier 1 will also be used to determine which specific tests should be conducted in Tiers 2 and 3.
Our Tier 1 screening results showed that all the tested AZ compounds except AZD7762 (AZ-H) had no impact on the follicle and oocyte health (Figs. 2–4). However, AZD7762 is a suspect ovotoxic chemical. In Tier 2, we chose AZD7762 with suspicious ovotoxicity and AZD8542 with negative ovotoxicity for a more sophisticated dose-response study and obtained consistent ovotoxic results to Tier 1 (Figs. 5 and 6). Moreover, we found that AZD7762 primarily damages granulosa cells but not oocytes to induce the entire follicle atresia (Figure 7A). Furthermore, we validated the positive and negative ovotoxicities of AZD7762 and AZD8542, respectively, using in vivo animal models in Tier 3 (Figure 8). These results suggest that eIVFG has a great potential to serve as an effective and efficient in vitro model for identifying and prioritizing chemicals of high ovotoxicity concern.
AZD7762 was originally designed as a selective ATP-competitive Chk1 inhibitor to abrogate S or G2 phase checkpoints to enhance DNA-damaging agent-induced cancer cell death (Zabludoff et al., 2008). It was later found that AZD7762 also had an equal inhibiting potency on Chk2 (Ma et al., 2011; Morgan et al., 2010; Wang et al., 2018a; Zabludoff et al., 2008). In the Tier 2 screening, we therefore further differentiated whether the AZD7762-induced ovotoxicities were caused by the inhibitory effect of Chk1 and/or Chk2 by using more specific Chk inhibitors. Our results showed that the Chk2-specific inhibitor, BML-277, was not ovotoxic (Figs. 7B and 7C) (Arienti et al., 2005), suggesting that the AZD7762-induced granulosa cell apoptosis was not directly caused by its inhibitory effect on Chk2. These results are also consistent with previous findings that the Chk2 deficiency does not impair female mouse fertility (Bolcun-Filas et al., 2014; Hirao et al., 2002; Takai et al., 2002).
There is limited data regarding the role of Chk1 in ovarian follicle development and survival because Chk1-deficiency is embryonically lethal during the peri-implantation period (Liu et al., 2000; Takai et al., 2000). To determine whether the AZD7762-induced follicle death is caused by Chk1 inhibition, we tested 3 Chk1-specific inhibitors in the Tier 2 screening and found that all 3 Chk1 inhibitors showed consistent ovotoxicities to AZD7762 (Figs. 7B and 7C), indicating that inhibition of Chk1 may play essential roles in AZD7762-induced granulosa cell death. Because granulosa cells are mitotically active during folliculogenesis, we speculate that the inhibition of Chk1 and subsequent lack of Chk1-mediated cell cycle arrest and DNA repair, upon AZD7762 or other Chk1 inhibitor treatment, result in excessive accumulation of DNA damage, which triggers granulosa cell apoptosis. Chk1 has also been reported to regulate cell cycle transition during normal mitotic division, such as the G1/S transition, S phase progression, mitotic entry, and mitosis (Patil et al., 2013; Zhang and Hunter, 2014). As granulosa cells are actively proliferating during folliculogenesis (Lu et al., 2005), it is also possible that the inhibition of Chk1 disrupts cell cycle transition during granulosa cell proliferation, leading to apoptosis, and the subsequent entire follicle atresia. However, the underlying molecular mechanism of Chk1 inhibition and granulosa cell apoptosis requires further investigations.
In addition to inhibiting Chk1, it is also possible that the granulosa cell apoptosis induced by AZD7762 or other Chk1 inhibitors is caused by other off-target effects. For example, rabusertib, a specific Chk1 inhibitor, has been found to not only inhibit the phosphorylation of Chk1 at Ser296, the major mechanism to potentiate DNA-damaging agent-induced cancer cell death, but also directly result in cancer cell DNA damage and apoptosis (van Harten et al., 2019; Wang et al., 2014). Moreover, although 2 Phase I clinical trials demonstrated that AZD7762 could enhance the anticancer effect of 2 widely used therapeutic chemicals, GEM and irinotecan (Ho et al., 2011; Sausville et al., 2014), it was not advanced to Phase II stage due to the unintended cardiotoxicity (Ho et al., 2011; Reichert et al., 2016; Sausville et al., 2014). It was hypothesized that AZD7762 may inhibit other noncheckpoint kinases or ATP-dependent nonkinase proteins to cause cytotoxicities in cardiomyocytes (Ma et al., 2011; Manic et al., 2015), because another Chk1-specific inhibitor, MK-8776, did not show cardiotoxicity (Daud et al., 2015). Taken together, these results indicate that further studies are necessary to elucidate the specific underlying molecular mechanism of Chk1 inhibitor-induced ovotoxicities. Transgenic mice with conditional deletion of Chk1 in granulosa cells will help to confirm the role of Chk1 in granulosa cell survival and proliferation.
GEM has been demonstrated to induce cancer cell DNA damage and activate Chk1 but not Chk2 through phosphorylation (Isono et al., 2017; Morgan et al., 2006). It has been well demonstrated that cotreatment with AZD7762 abrogated Chk1-mediated cell cycle arrest and potentiated GEM-induced cancer cell apoptosis (Isono et al., 2017; Landau et al., 2012; Liu et al., 2017). For noncancerous cells, it was expected that there is a functional G1 checkpoint pathway allowing for DNA damage repair and cell survival (Abraham, 2001; Zhang and Hunter, 2014), thus the cotreatment with AZD7762 will only or primarily potentiate the cytotoxicities of DNA-damaging agents on cancer cells (Zabludoff et al., 2008). However, our results revealed that although AZD7762 at 0.1 µM alone did not induce follicle atresia as the higher concentrations at 1 and 10 µM did, the cotreatment with AZD7762 at 0.1 µM significantly enhanced GEM-induced growing follicle death (Figure 9). These results suggest that the exacerbating effect of AZD7762 on GEM’s ovotoxicities might be primarily induced by inhibiting Chk1-mediated cell cycle arrest and DNA repair. Interestingly, with respect to DOX which has been found to damage growing follicles through inducing granulosa cell apoptosis (Xiao et al., 2017b), the cotreatment with AZD7762 did not show any exacerbating effect (Figure 9). These results suggest that unlike the GEM-induced DNA damage and Chk1-mediated cell cycle arrest or apoptosis, DOX may use different molecular mechanisms to promote granulosa cell apoptosis in growing follicles.
The in vivo animal models in Tier 3 demonstrated that the clinically relevant exposure level of AZD7762 promoted the death of growing follicles but not primordial follicles (Figure 8), indicating that the dormant state of primordial follicles, particular the mitotically inactive pregranulosa cells, are less sensitive to AZD7762. Similarly, previous studies also found that several chemotherapeutic chemicals, such as cyclophosphamide, primarily damaged growing follicles but not primordial follicles, and the depletion or reduction of growing follicles could in turn overactivate primordial follicles, exhausting the ovarian reserve (Gonfloni et al., 2009; Kim et al., 2013, 2019; Nguyen et al., 2018). Therefore, although AZD7762 did not directly affect primordial follicle survival, its damaging effect on growing follicles will also increase the risk of POF, early menopause, and infertility through overactivating primordial follicles and diminishing the ovarian reserve.
One of the limitations of our study is that there is no liver metabolism in the eIVFG system. However, some chemicals may require liver metabolic activation to exhibit ovotoxicity or liver detoxification may inactivate the parental compound which is ovotoxic. To resolve this issue, 1 potential approach is to include the metabolite(s) in addition to the parental compound for in vitro compound exposure. A more encouraging approach is to use the emerging microfluidic technology to interconnect in vitro cultured ovarian tissues with liver organoids or cell lines to incorporate a more in vivo-like liver metabolism and pharmacokinetics into ovotoxicity testing. For example, we recently created a microfluidic platform that can integrate 2 or 5 different tissues together to study the tissue-tissue communications (Xiao et al., 2017a). However, its high cost and low-throughput properties limit its application in high-throughput ovotoxicity screening, which requires further improvement. Another limitation here is that eIVFG supports follicle growth starting from the primary or secondary stage. However, due to the technical difficulties, it is challenging to individually culture primordial follicles in vitro. Previous studies have cultured mouse neonatal whole ovaries or ovarian explants (Kim et al., 2013), which is a good model to study the impact of pharmaceutical compounds on primordial follicle survival and activation.
In summary, our study demonstrates that eIVFG is a robust in vitro model for testing the effects of pharmaceutical compounds on female ovarian functions and fertility. The developed tiered ovotoxicity screening can efficiently and effectively help us identify and prioritize candidate compounds of high ovotoxicity concern for subsequent more targeted, sophisticated, and mechanistic in vitro and in vivo ovotoxicity assessments. In additional to pharmaceutical compounds, the developed tiered ovotoxicity screening method also provides good models for investigating the impact of environmental contaminants on female ovarian functions and fertility.
ACKNOWLEDGMENTS
We thank all insightful suggestions and comments from the internal approval committee members at AstraZeneca.
AUTHOR CONTRIBUTIONS
J.X., Y.W., A.E.K., Y.Z., Y.L., and J.Z. contributed to the experimental execution, data collection and analysis, and manuscript writing. K.M., K.F., Q.Z., and T.K.W. contributed to the experimental design, data analysis, and manuscript writing. S.X. conceived of the project, designed experiments, collected, analyzed and interpreted data, wrote the manuscript, and provided final approval of the manuscript.
FUNDING
Arnold School of Public Health Start Up Fund at the University of South Carolina; National Institutes of Health (P01ES028942 and K01ES030014 to S.X., and UG3ES029073 to T.K.W.); National Science Foundation (1832901 to S.X.).
DECLARATION OF CONFLICTING INTERESTS
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
REFERENCES
- Abraham R. T. (2001). Cell cycle checkpoint signaling through the ATM and ATR kinases. Gene Dev. 15, 2177–2196. [DOI] [PubMed] [Google Scholar]
- American Academy of Pediatrics. (2016). Ethical Considerations for Including Women as Research Participants. Pediatrics 137, e20153990. [Google Scholar]
- Arienti K. L., Brunmark A., Axe F. U., McClure K., Lee A., Blevitt J., Neff D. K., Huang L., Crawford S., Pandit C. R., et al. (2005). Checkpoint kinase inhibitors: SAR and radioprotective properties of a series of 2-arylbenzimidazoles. J. Med. Chem. 48, 1873–1885. [DOI] [PubMed] [Google Scholar]
- Bakke O. M., Manocchia M., de Abajo F., Kaitin K. I., Lasagna L. (1995). Drug safety discontinuations in the United-Kingdom, the United-States, and Spain from 1974 through 1993—A regulatory perspective. Clin. Pharmacol. Ther. 58, 108–117. [DOI] [PubMed] [Google Scholar]
- Becker R. A., Plunkett L. M., Borzelleca J. F., Kaplan A. M. (2007). Tiered toxicity testing: Evaluation of toxicity-based decision triggers for human health hazard characterization. Food Chem. Toxicol. 45, 2454–2469. [DOI] [PubMed] [Google Scholar]
- Ben-Aharon I., Bar-Joseph H., Tzarfaty G., Kuchinsky L., Rizel S., Stemmer S. M., Shalgi R. (2010). Doxorubicin-induced ovarian toxicity. Reprod. Biol. Endocrinol. 8, 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharya P., Keating A. F. (2012). Impact of environmental exposures on ovarian function and role of xenobiotic metabolism during ovotoxicity. Toxicol. Appl. Pharmacol. 261, 227–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biggers J. D., Whittingham D. G., Donahue R. P. (1967). The pattern of energy metabolism in the mouse oocyte and zygote. Proc. Natl. Acad. Sci. U.S.A. 58, 560–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolcun-Filas E., Rinaldi V. D., White M. E., Schimenti J. C. (2014). Reversal of female infertility by Chk2 ablation reveals the oocyte DNA damage checkpoint pathway. Science 343, 533–536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boudny M., Zemanova J., Khirsariya P., Borsky M., Verner J., Cerna J., Oltova A., Seda V., Mraz M., Jaros J., et al. (2019). Novel CHK1 inhibitor MU380 exhibits significant single-agent activity in TP53-mutated chronic lymphocytic leukemia cells. Haematologica 104, 2443–2455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buccione R., Schroeder A. C., Eppig J. J. (1990). Interactions between somatic-cells and germ-cells throughout mammalian oogenesis. Biol. Reprod. 43, 543–547. [DOI] [PubMed] [Google Scholar]
- Bus J. S., Becker R. A. (2009). Toxicity testing in the 21st century: A view from the chemical industry. Toxicol. Sci. 112, 297–302. [DOI] [PubMed] [Google Scholar]
- Buyuk E., Nejat E., Neal-Perry G. (2010). Determinants of female reproductive senescence: Differential roles for the ovary and the neuroendocrine axis. Semin. Reprod. Med. 28, 370–379. [DOI] [PubMed] [Google Scholar]
- Dai B. J., Zhao X. F., Mazan-Mamczarz K., Hagner P., Corl S., Bahassi E. M., Lu S., Stambrook P. J., Shapiro P., Gartenhaus R. B. (2011). Functional and molecular interactions between ERK and CHK2 in diffuse large B-cell lymphoma. Nat. Commun. 2, 402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daud A. I., Ashworth M. T., Strosberg J., Goldman J. W., Mendelson D., Springett G., Venook A. P., Loechner S., Rosen L. S., Shanahan F., et al. (2015). Phase I dose-escalation trial of checkpoint kinase 1 inhibitor MK-8776 as monotherapy and in combination with gemcitabine in patients with advanced solid tumors. J. Clin. Oncol. 33, 1060–1066. [DOI] [PubMed] [Google Scholar]
- DiMasi J. A., Grabowski H. G., Hansen R. W. (2016). Innovation in the pharmaceutical industry: New estimates of R&D costs. J. Health Econ. 47, 20–33. [DOI] [PubMed] [Google Scholar]
- Doe J. E., Boobis A. R., Blacker A., Dellarco V., Doerrer N. G., Franklin C., Goodman J. I., Kronenberg J. M., Lewis R., McConnell E. E., et al. (2006). A tiered approach to systemic toxicity testing for agricultural chemical safety assessment. Crit. Rev. Toxicol. 36, 37–68. [DOI] [PubMed] [Google Scholar]
- Eisenstein E. L., Lemons P. W. II, Tardiff B. E., Schulman K. A., Jolly M. K., Califf R. M. (2005). Reducing the costs of phase III cardiovascular clinical trials. Am. Heart J. 149, 482–488. [DOI] [PubMed] [Google Scholar]
- Eppig J. J., Pendola F. L., Wigglesworth K., Pendola J. K. (2005). Mouse oocytes regulate metabolic cooperativity between granulosa cells and oocytes: Amino acid transport. Biol. Reprod. 73, 351–357. [DOI] [PubMed] [Google Scholar]
- Gonfloni S., Di Tella L., Caldarola S., Cannata S. M., Klinger F. G., Di Bartolomeo C., Mattei M., Candi E., De Felici M., Melino G., et al. (2009). Inhibition of the c-Abl-TAp63 pathway protects mouse oocytes from chemotherapy-induced death. Nat. Med. 15, 1179–1185. [DOI] [PubMed] [Google Scholar]
- Gui U. M., Joyce L. A. (2005). RNA interference evidence that growth differentiation factor-9 mediates clocyte regulation of cumulus expansion in mice. Biol. Reprod. 72, 195–199. [DOI] [PubMed] [Google Scholar]
- Havelock J. C., Rainey W. E., Carr B. R. (2004). Ovarian granulosa cell lines. Mol. Cell. Endocrinol. 228, 67–78. [DOI] [PubMed] [Google Scholar]
- Hirao A., Cheung A., Duncan G., Girard P. M., Elia A. J., Wakeham A., Okada H., Sarkissian T., Wong J. A., Sakai T., et al. (2002). Chk2 is a tumor suppressor that regulates apoptosis in both an ataxia telangiectasia mutated (ATM)-dependent and an ATM-independent manner. Mol. Cell. Biol. 22, 6521–6532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho A. L., Bendell J. C., Cleary J. M., Schwartz G. K., Burris H. A., Oakes P., Agbo F., Barker P. N., Senderowicz A. M., Shapiro G. (2011). Phase I, open-label, dose-escalation study of AZD7762 in combination with irinotecan (irino) in patients (pts) with advanced solid tumors. J. Clin. Oncol. 29, 3033. [Google Scholar]
- Hwang R. F., Moore T. T., Hattersley M. M., Scarpitti M., Yang B., Devereaux E., Ramachandran V., Arumugam T., Ji B., Logsdon C. D., et al. (2012). Inhibition of the hedgehog pathway targets the tumor-associated stroma in pancreatic cancer. Mol. Cancer Res. 10, 1147–1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isono M., Hoffmann M. J., Pinkerneil M., Sato A., Michaelis M., Cinatl J., Niegisch G., Schulz W. A. (2017). Checkpoint kinase inhibitor AZD7762 strongly sensitises urothelial carcinoma cells to gemcitabine. J. Exp. Clin. Cancer Res. 36, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itamochi H., Nishimura M., Oumi N., Kato M., Oishi T., Shimada M., Sato S., Naniwa J., Sato S., Kudoh A., et al. (2014). Checkpoint kinase inhibitor AZD7762 overcomes cisplatin resistance in clear cell carcinoma of the ovary. Int. J. Gynecol. Cancer 24, 61–69. [DOI] [PubMed] [Google Scholar]
- Karp J. E., Thomas B. M., Greer J. M., Sorge C., Gore S. D., Pratz K. W., Smith B. D., Flatten K. S., Peterson K., Schneider P., et al. (2012). Phase I and pharmacologic trial of cytosine arabinoside with the selective checkpoint 1 inhibitor Sch 900776 in refractory acute leukemias. Clin. Cancer Res. 18, 6723–6731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S. Y., Cordeiro M. H., Serna V. A., Ebbert K., Butler L. M., Sinha S., Mills A. A., Woodruff T. K., Kurita T. (2013). Rescue of platinum-damaged oocytes from programmed cell death through inactivation of the p53 family signaling network. Cell Death Differ. 20, 987–997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S. Y., Nair D. M., Romero M., Serna V. A., Koleske A. J., Woodruff T. K., Kurita T. (2019). Transient inhibition of p53 homologs protects ovarian function from two distinct apoptotic pathways triggered by anticancer therapies. Cell Death Differ. 26, 502–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King C., Diaz H., Barnard D., Barda D., Clawson D., Blosser W., Cox K., Guo S., Marshall M. (2014). Characterization and preclinical development of LY2603618: A selective and potent Chk1 inhibitor. Invest. New Drugs 32, 213–226. [DOI] [PubMed] [Google Scholar]
- Krewski D., Acosta D. Jr, Andersen M., Anderson H., Bailar J. C. III, Boekelheide K., Brent R., Charnley G., Cheung V. G., Green S. Jr, et al. (2010). Toxicity testing in the 21st century: A vision and a strategy. J. Toxicol. Environ. Health B Crit. Rev. 13, 51–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landau H. J., McNeely S. C., Nair J. S., Comenzo R. L., Asai T., Friedman H., Jhanwar S. C., Nimer S. D., Schwartz G. K. (2012). The checkpoint kinase inhibitor AZD7762 potentiates chemotherapy-induced apoptosis of p53-mutated multiple myeloma cells. Mol. Cancer Ther. 11, 1781–1788. [DOI] [PubMed] [Google Scholar]
- Liu Q. H., Guntuku S., Cui X. S., Matsuoka S., Cortez D., Tamai K., Luo G. B., Carattini-Rivera S., DeMayo F., Bradley A., et al. (2000). Chk1 is an essential kinase that is regulated by ATR and required for the G(2)/M DNA damage checkpoint. Gene Dev. 14, 1448–1459. [PMC free article] [PubMed] [Google Scholar]
- Liu Y., Li Y., Wang X., Liu F., Gao P., Quinn M. M., Li F., Merlino A. A., Benes C., Liu Q., et al. (2017). Gemcitabine and Chk1 inhibitor AZD7762 synergistically suppress the growth of Lkb1-deficient lung adenocarcinoma. Cancer Res. 77, 5068–5076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu C. L., Yang W., Hu Z. Y., Liu Y. X. (2005). Granulosa cell proliferation differentiation and its role in follicular development. Chin. Sci. Bull. 50, 2665–2671. [Google Scholar]
- Ma C. X., Janetka J. W., Piwnica-Worms H. (2011). Death by releasing the breaks: Chk1 inhibitors as cancer therapeutics. Trends Mol. Med. 17, 88–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Z., Yao G., Zhou B., Fan Y., Gao S., Feng X. (2012). The Chk1 inhibitor AZD7762 sensitises p53 mutant breast cancer cells to radiation in vitro and in vivo. Mol. Med. Rep. 6, 897–903. [DOI] [PubMed] [Google Scholar]
- Manic G., Obrist F., Sistigu A., Vitale I. (2015). Trial watch: Targeting ATM-Chk2 and ATR-Chk1 pathways for anticancer therapy. Mol. Cell. Oncol. 2, e1012976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan M. A., Parsels L. A., Parsels J. D., Lawrence T. S., Maybaum J. (2006). The relationship of premature mitosis to cytotoxicity in response to checkpoint abrogation and antimetabolite treatment. Cell Cycle 5, 1983–1988. [DOI] [PubMed] [Google Scholar]
- Morgan M. A., Parsels L. A., Zhao L., Parsels J. D., Davis M. A., Hassan M. C., Arumugarajah S., Hylander-Gans L., Morosini D., Simeone D. M., et al. (2010). Mechanism of radiosensitization by the Chk1/2 inhibitor AZD7762 involves abrogation of the G2 checkpoint and inhibition of homologous recombinational DNA repair. Cancer Res. 70, 4972–4981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen Q. N., Zerafa N., Liew S. H., Morgan F. H., Strasser A., Scott C. L., Findlay J. K., Hickey M., Hutt K. J. (2018). Loss of puma protects the ovarian reserve during DNA-damaging chemotherapy and preserves fertility. Cell Death Dis. 9, 618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patil M., Pabla N., Dong Z. (2013). Checkpoint kinase 1 in DNA damage response and cell cycle regulation. Cell. Mol. Life Sci. 70, 4009–4021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pocar P., Augustin R., Gandolfi F., Fischer B. (2003). Toxic effects of in vitro exposure to p-tert-octylphenol on bovine oocyte maturation and developmental competence. Biol. Reprod. 69, 462–468. [DOI] [PubMed] [Google Scholar]
- Pocar P., Perazzoli F., Luciano A. M., Gandolfi F. (2001). In vitro reproductive toxicity of polychlorinated biphenyls: Effects on oocyte maturation and developmental competence in cattle. Mol. Reprod. Dev. 58, 411–416. [DOI] [PubMed] [Google Scholar]
- Quin J., Chan K. T., Devlin J. R., Cameron D. P., Diesch J., Cullinane C., Ahern J., Khot A., Hein N., George A. J., et al. (2016). Inhibition of RNA polymerase I transcription initiation by CX-5461 activates non-canonical ATM/ATR signaling. Oncotarget 7, 49800–49818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmussen L. M., Sen N., Vera J. C., Liu X., Craig Z. R. (2017). Effects of in vitro exposure to dibutyl phthalate, mono-butyl phthalate, and acetyl tributyl citrate on ovarian antral follicle growth and viability. Biol. Reprod. 96, 1105–1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichert Z. R., Wahl D. R., Morgan M. A. (2016). Translation of targeted radiation sensitizers into clinical trials. Semin. Radiat. Oncol. 26, 261–270. [DOI] [PubMed] [Google Scholar]
- Roti Roti E. C., Leisman S. K., Abbott D. H., Salih S. M. (2012). Acute doxorubicin insult in the mouse ovary is cell- and follicle-type dependent. PLoS One 7, e42293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sausville E., Lorusso P., Carducci M., Carter J., Quinn M. F., Malburg L., Azad N., Cosgrove D., Knight R., Barker P., et al. (2014). Phase I dose-escalation study of AZD7762, a checkpoint kinase inhibitor, in combination with gemcitabine in us patients with advanced solid tumors. Cancer Chemother. Pharmacol. 73, 539–549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seto T., Esaki T., Hirai F., Arita S., Nosaki K., Makiyama A., Kometani T., Fujimoto C., Hamatake M., Takeoka H., et al. (2013). Phase I, dose-escalation study of AZD7762 alone and in combination with gemcitabine in Japanese patients with advanced solid tumours. Cancer Chemother. Pharmacol. 72, 619–627. [DOI] [PubMed] [Google Scholar]
- Spears N., Lopes F., Stefansdottir A., Rossi V., De Felici M., Anderson R. A., Klinger F. G. (2019). Ovarian damage from chemotherapy and current approaches to its protection. Hum. Reprod. Update 25, 673–693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefansdottir A., Fowler P. A., Powles-Glover N., Anderson R. A., Spears N. (2014). Use of ovary culture techniques in reproductive toxicology. Reprod. Toxicol. 49, 117–135. [DOI] [PubMed] [Google Scholar]
- Su Y. Q., Sugiura K., Wigglesworth K., O'Brien M. J., Affourtit J. P., Pangas S. A., Matzuk M. M., Eppig J. J. (2007). Oocyte regulation of metabolic cooperativity between mouse cumulus cells and oocytes: BMP15 and GDF9 control cholesterol biosynthesis in cumulus cells. Development 135, 111–121. [DOI] [PubMed] [Google Scholar]
- Takai H., Naka K., Okada Y., Watanabe M., Harada N., Saito S., Anderson C. W., Appella E., Nakanishi M., Suzuki H., et al. (2002). Chk2-deficient mice exhibit radioresistance and defective p53-mediated transcription. EMBO J. 21, 5195–5205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takai H., Tominaga K., Motoyama N., Minamishima Y. A., Nagahama H., Tsukiyama T., Ikeda K., Nakayama K., Nakanishi N., Nakayama K. (2000). Aberrant cell cycle checkpoint function and early embryonic death in Chk1(−/−) mice. Gene Dev. 14, 1439–1447. [PMC free article] [PubMed] [Google Scholar]
- Tse A. N., Rendahl K. G., Sheikh T., Cheema H., Aardalen K., Embry M., Ma S., Moler E. J., Ni Z. J., Lopes de Menezes D. E., et al. (2007). CHIR-124, a novel potent inhibitor of Chk1, potentiates the cytotoxicity of topoisomerase I poisons in vitro and in vivo. Clin. Cancer Res. 13, 591–602. [DOI] [PubMed] [Google Scholar]
- Tuppi M., Kehrloesser S., Coutandin D. W., Rossi V., Luh L. M., Strubel A., Hotte K., Hoffmeister M., Schafer B., De Oliveira T., et al. (2018). Oocyte DNA damage quality control requires consecutive interplay of Chk2 and Ck1 to activate p63. Nat. Struct. Mol. Biol. 25, 261–269. [DOI] [PubMed] [Google Scholar]
- Vabre P., Gatimel N., Moreau J., Gayrard V., Picard-Hagen N., Parinaud J., Leandri R. D. (2017). Environmental pollutants, a possible etiology for premature ovarian insufficiency: A narrative review of animal and human data. Environ. Health 16, 37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Harten A. M., Buijze M., van der Mast R., Rooimans M. A., Martens-de Kemp S. R., Bachas C., Brink A., Stigter-van Walsum M., Wolthuis R. M. F., Brakenhoff R. H. (2019). Targeting the cell cycle in head and neck cancer by Chk1 inhibition: A novel concept of bimodal cell death. Oncogenesis 8, 1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang F. Z., Fei H. R., Cui Y. J., Sun Y. K., Li Z. M., Wang X. Y., Yang X. Y., Zhang J. G., Sun B. L. (2014). The checkpoint 1 kinase inhibitor LY2603618 induces cell cycle arrest, DNA damage response and autophagy in cancer cells. Apoptosis 19, 1389–1398. [DOI] [PubMed] [Google Scholar]
- Wang L., Wang Y., Chen A., Jalali A., Liu S., Guo Y., Na S., Nakshatri H., Li B. Y., Yokota H. (2018. a). Effects of a checkpoint kinase inhibitor, AZD7762, on tumor suppression and bone remodeling. Int. J. Oncol. 53, 1001–1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Liu M., Johnson S. B., Yuan G., Arriba A. K., Zubizarreta M. E., Chatterjee S., Nagarkatti M., Nagarkatti P., Xiao S. (2019). Doxorubicin obliterates mouse ovarian reserve through both primordial follicle atresia and overactivation. Toxicol. Appl. Pharmacol. 381, 114714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Liu M., Zhang J., Liu Y., Kopp M., Zheng W., Xiao S. (2018. b). Multidrug resistance protein 1 deficiency promotes doxorubicin-induced ovarian toxicity in female mice. Toxicol. Sci. 163, 279–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waring M. J., Arrowsmith J., Leach A. R., Leeson P. D., Mandrell S., Owen R. M., Pairaudeau G., Pennie W. D., Pickett S. D., Wang J., et al. (2015). An analysis of the attrition of drug candidates from four major pharmaceutical companies. Nat. Rev. Drug Discov. 14, 475–486. [DOI] [PubMed] [Google Scholar]
- Wehler T., Thomas M., Schumann C., Bosch-Barrera J., Vinolas Segarra N., Dickgreber N. J., Dalhoff K., Sebastian M., Corral Jaime J., Alonso M., et al. (2017). A randomized, phase 2 evaluation of the Chk1 inhibitor, LY2603618, administered in combination with pemetrexed and cisplatin in patients with advanced nonsquamous non-small cell lung cancer. Lung Cancer 108, 212–216. [DOI] [PubMed] [Google Scholar]
- Wong C. H., Siah K. W., Lo A. W. (2019). Estimation of clinical trial success rates and related parameters. Biostatistics 20, 273–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao S., Coppeta J. R., Rogers H. B., Isenberg B. C., Zhu J., Olalekan S. A., McKinnon K. E., Dokic D., Rashedi A. S., Haisenleder D. J., et al. (2017. a). A microfluidic culture model of the human reproductive tract and 28-day menstrual cycle. Nat. Commun. 8, 14584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao S., Duncan F. E., Bai L., Nguyen C. T., Shea L. D., Woodruff T. K. (2015. a). Size-specific follicle selection improves mouse oocyte reproductive outcomes. Reproduction 150, 183–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao S., Zhang J., Liu M., Iwahata H., Rogers H. B., Woodruff T. K. (2017. b). Doxorubicin has dose-dependent toxicity on mouse ovarian follicle development, hormone secretion, and oocyte maturation. Toxicol. Sci. 157, 320–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao S., Zhang J., Romero M. M., Smith K. N., Shea L. D., Woodruff T. K. (2015. b). In vitro follicle growth supports human oocyte meiotic maturation. Sci. Rep. 5, 17323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuksel A., Bildik G., Senbabaoglu F., Akin N., Arvas M., Unal F., Kilic Y., Karanfil I., Eryilmaz B., Yilmaz P., et al. (2015). The magnitude of gonadotoxicity of chemotherapy drugs on ovarian follicles and granulosa cells varies depending upon the category of the drugs and the type of granulosa cells. Hum. Reprod. 30, 2926–2935. [DOI] [PubMed] [Google Scholar]
- Zabludoff S. D., Deng C., Grondine M. R., Sheehy A. M., Ashwell S., Caleb B. L., Green S., Haye H. R., Horn C. L., Janetka J. W., et al. (2008). AZD7762, a novel checkpoint kinase inhibitor, drives checkpoint abrogation and potentiates DNA-targeted therapies. Mol. Cancer Ther. 7, 2955–2966. [DOI] [PubMed] [Google Scholar]
- Zhang Y., Hunter T. (2014). Roles of Chk1 in cell biology and cancer therapy. Int. J. Cancer 134, 1013–1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou C., Flaws J. A. (2017). Effects of an environmentally relevant phthalate mixture on cultured mouse antral follicles. Toxicol. Sci. 156, 217–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou H., Malik M. A., Arab A., Hill M. T., Shikanov A. (2015). Hydrogel based 3-dimensional (3D) system for toxicity and high-throughput (HTP) analysis for cultured murine ovarian follicles. PLoS One 10, e0140205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou H., Shikanov A. (2018). Three-dimensional hydrogel-based culture to study the effects of toxicants on ovarian follicles. Methods Mol. Biol. 1758, 55–72. [DOI] [PubMed] [Google Scholar]