Abstract
Aims
To examine whether test utilization and prevalence of ischemia with positron emission tomography (PET) myocardial perfusion imaging (MPI) follow the previously described trends with single photon computed tomography (SPECT).
Methods and results
MPI studies performed between January 2003 and December 2017 were identified. Number of PET and SPECT MPI studies performed per year was determined. Trends in the proportion of studies showing any ischaemia (>0%) with both modalities were compared before and after adjusting for baseline differences in patient characteristics using propensity scores. Interaction between imaging modality and year of testing was examined using modified Poisson regression. A total of 156 244 MPI studies were performed (30% PET and 70% SPECT). Between 2003 and 2017, the number of PET studies increased from 18 to 61 studies/1000 patient encounters, while SPECT volumes declined from 169 to 34/1000 patient encounters (P < 0.001 for within-group comparisons). The prevalence of any ischaemia in SPECT-tested patients declined from 53.9% to 28.3% between 2003 and 2017, whereas ischaemia prevalence in PET-tested patients declined from 57.2% to 38.2% (P < 0.001 for within-modality comparisons), with more PET studies showing ischaemia compared to SPECT [relative risk (RR) 1.44, 95% confidence interval (CI) 1.42–1.47; P < 0.001]. After propensity score matching of 26 066 patients tested with SPECT with 26 066 patients tested with PET, the between-modality difference in ischaemia prevalence was significantly attenuated, with a slightly higher overall likelihood of detecting ischaemia with PET compared to SPECT (RR 1.08, 95% CI 1.05–1.11; P < 0.001).
Conclusions
Utilization of PET MPI at a large-volume referral centre increased significantly between 2003 and 2017. Despite a significant decrease in the prevalence of ischaemia with SPECT and PET during the same period, the decline was less with PET, perhaps related to baseline risk of tested patients.
Keywords: myocardial perfusion imaging, positron emission tomography, ischaemia, coronary artery disease
Introduction
Several studies have reported a decline in the prevalence of inducible ischaemia with myocardial perfusion imaging (MPI) over the past three decades, irrespective of coronary artery disease (CAD) status in tested patients.1–4 While the factors contributing to this change are incompletely identified, various mechanisms have been posited as potential explanations.5,6 Widespread use of protective cardiac medications, including statins and aspirin,7,8 and improved cardiovascular risk factor control,9,10 have likely contributed to a decline in the severity and extent of obstructive CAD angiographically3 and an improvement in overall outcomes of patients with acute myocardial infarction and CAD in general.11,12 In addition, the recent introduction of Appropriate Use Criteria for imaging13 and radiologic benefits managers may have also had an impact on the types of patients referred for MPI testing.14
Whereas the decline in ischaemic burden with single photon emission computed tomography (SPECT) MPI has been well described, little is known as to whether similar trends exist with positron emission tomography (PET). Differences in diagnostic accuracy15 and patient referral patterns16 between PET and SPECT may lead to differences in rates of detectable ischaemia between the two modalities. With the growing use of PET MPI in contemporary nuclear cardiology practices, a ‘test substitution’ phenomenon may take place, where certain patient profiles may potentially lead to the preferential use of PET instead of SPECT,17 which also may cause the prevalence of ischaemia to vary between the two modalities.
Defining utilization patterns and temporal trends of ischaemia with PET MPI will not only complement existing data on SPECT, but can also provide additional insight into mechanisms underlying the observed decline in ischaemic burden with SPECT. Accordingly, we sought to examine changes in test use over time with PET and SPECT, and to compare trends in the detection of ischaemia between the two modalities using data from a large-volume centre that routinely offers both modalities to patients with known or suspected CAD.
Methods
Data source and study population
We identified consecutive, clinically indicated PET or SPECT MPI studies performed between 1 January 2003 and 31 December 2017 using the electronic database of Saint Luke’s Mid America Heart Institute nuclear laboratory (Kansas City, MO, USA). The database includes information on MPI studies performed at multiple sites throughout the health system, including four hospital-based sites, where both PET and SPECT capabilities are offered routinely, and five community-based facilities offering only SPECT MPI. All studies are electronically transmitted to the central nuclear cardiology laboratory at Saint Luke’s Mid America Heart Institute, where image processing takes place and study interpretation is performed by experienced nuclear cardiologists. Data on patient demographics, cardiovascular risk factors, and study characteristics are prospectively entered into the database by trained staff.
For patients who underwent serial MPI testing during the study, the initial and all subsequent studies were included in the analysis, given the study objective of tracking changes in the prevalence of inducible ischaemia over time. Studies performed for indications other than assessment of ischaemia (including detection of myocardial viability, evaluation of inflammation/cardiac sarcoidosis, integrity of cardiac sympathetic innervation, or evaluation for cardiac amyloidosis) and those with missing data were excluded. The remaining studies constituted the ‘main analytic cohort’, where trends in test utilization and prevalence of ischaemia were examined. Afterwards, a propensity score analysis was used to ensure comparisons between comparable patients evaluated with PET vs. SPECT testing. Prior to propensity score matching, exercise SPECT studies and those performed at sites that did not offer PET were excluded, to maximize comparability of patients in the PET and SPECT groups. Studies included after propensity score matching constituted the ‘matched cohort’.
The study was reviewed and approved by the Saint Luke’s Hospital Institutional Review Board; waiver for written informed consent was granted based on the retrospective design of the study.
MPI methods
PET MPI was performed using a dedicated cardiac PET scanner (ACCEL, CTI, Knoxville, TN, USA) with 68Ge line source attenuation correction, or using a variety of hybrid PET/CT systems (Biograph 16 or 64, Siemens Healthineers, Knoxville, TN, USA). For SPECT MPI, imaging was completed using either small field-of-view Anger cameras (CardioMD, Philips, Milpitas, CA, USA) with 153Gd line source attenuation correction, hybrid large field-of-view SPECT/CT systems (Symbia Intevo, Siemens, Munich, Germany), large field-of-view Anger cameras without attenuation correction (ECAM, Siemens, Munich, Germany), or solid-state detector cameras (D-SPECT, Spectrum Dynamics, Sarasota, FL, USA). Supplementary data online, Table S1 summarizes the nuclear equipment used at each of the participating sites.
All PET studies were performed with pharmacologic stress, using either dipyridamole, adenosine, or regadenoson, whereas SPECT studies were performed either with exercise, pharmacologic stress—using any of the aforementioned coronary vasodilators or dobutamine—or a combination of low-level exercise with vasodilator stress.
82Rb was used for PET imaging; 99mTc, 201Tl, or a combination of both were used with SPECT imaging. Rest/stress protocol was used with all PET MPI studies, while a combination of rest/stress, stress/rest, and stress-only protocols was used with SPECT MPI. Stress testing and image acquisition were completed in accordance with published guidelines.18–21
PET and SPECT image interpretation
The extent of ischaemia for both PET and SPECT was recorded using a semi-quantitative method, where each of the 17-myocardial segments (or 20 segments in studies performed prior to September 2009) was scored on a (0–4) scale by visual inspection, with higher scores indicating more severe reduction in tracer uptake. Segments were scored both with stress and at rest, where applicable. Subsequently, summed rest, summed stress, and summed difference scores (SDS) were calculated by combining segmental scores. Percentage of ischaemic myocardium was calculated by dividing SDS by 68 (or 80 in studies performed prior to September 2009) and multiplying by 100%. Based on prior work, this variable was used to create categories of ischaemia severity as follows: none = 0, mild >0 to 5%, moderate >5 to 10%, and severe >10%.1–3,22 Image processing and interpretation was performed using various versions of the QPS software (Cedars-Sinai Medical Center, LA, CA, USA).
Test utilization and prevalence of ischaemia
We examined changes in PET and SPECT utilization over time. To account for growth in practice size over time, the number of MPI studies performed in the in- and out-patient settings every year was standardized to the total number of patient encounters in the respective setting in that year, obtained from the hospital’s administrative database. For hospital encounters, we used the total number of discharges from acute care facilities, whereas the number of patient encounters in cardiology clinics was used for outpatient encounters.
Afterwards, we determined the proportion of MPI studies with any detectable ischaemia in each year, by dividing the number of ischaemic PET (or SPECT) studies by the total number of PET (or SPECT) studies performed in a given year. The proportion of studies demonstrating moderate-to-severe ischaemia with each modality was calculated in an analogous fashion. All comparisons were first performed in the main cohort and then repeated within the propensity score-matched cohort.
Statistical analyses
Baseline demographics, clinical characteristics, and imaging findings for patients who underwent PET and SPECT were presented as means ± standard deviation for continuous variables, and as counts and percentages for categorical variables. Baseline characteristics were compared using standardized difference.23 A standardized difference <10% has been shown to indicate negligible differences in covariate balance between groups.24
For the purpose of creating the propensity score-matched cohort, we included 28 covariates likely to influence the selection of PET testing, based on clinical experience. These variables were included in a non-parsimonious, multivariable logistic regression model with PET MPI testing as the outcome. Included covariates were age, sex, body mass index, family history of CAD, smoking, diabetes, hypertension, hyperlipidaemia, peripheral arterial disease, history of congestive heart failure, cardiomyopathy, history of stroke, obstructive sleep apnoea, chronic obstructive pulmonary disease, asthma, atrial fibrillation, history of ventricular tachycardia, intracardiac defibrillator (ICD) implantation, inpatient status, typical angina at presentation, dyspnoea, history of prior CAD, prior percutaneous coronary intervention (PCI), prior coronary artery bypass grafting (CABG), history of coronary calcification, abnormal baseline electrocardiogram (ECG), baseline ST/T ECG abnormality, and left bundle branch block. A propensity score—defined as the probability of being assigned to PET after adjusting for covariates—was determined for each patient and was used to create a 1:1 match between the PET and SPECT cohorts. Greedy matching of SPECT and PET patients was performed, using a calibre of 0.2 times the standard deviation of the logit of the linear predictor,25 and patients were additionally matched by year of testing. Covariate balance in the matched cohort was verified by comparing standardized differences before and after matching.
Modified Poisson regression was used to determine the effect of testing modality, year of testing, and the interaction between these variables on the prevalence of ischaemia within the matched cohort. Within each modality, we calculated the relative risk for detecting ischaemia in each year after 2003, using 2003 as a reference. Pre-specified subgroup analyses were then conducted within the matched cohort to compare temporal trends of ischaemia in males vs. females, patients seen in the outpatient vs. hospital setting, patients with vs. without diabetes, and those with vs. without prior CAD, using three-way interactions between year of testing, testing modality, and each of the grouping factors mentioned. All analyses were two-tailed and evaluated at a significance level of 0.05. Analyses were performed using SAS 9.4 (SAS Institute, Cary, NC, USA).
Results
Study population
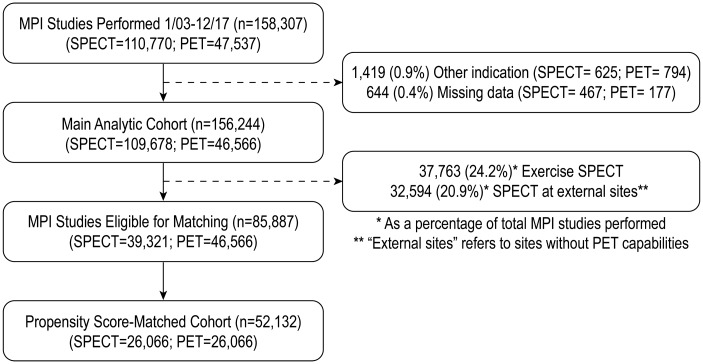
A total of 158 307 MPI studies were performed between 1 January 2003 and 31 December 2017, including 47 537 (30%) PET and 110 770 (70%) SPECT studies. After excluding 1419 studies (0.9%; 794 PET and 625 SPECT) that were performed for indications other than ischaemia detection, and 644 studies (0.4%; 177 PET and 467 SPECT) with missing data on ischaemia, a total of 156 244 studies were included in the main cohort for analysis of test volumes and ischaemia trends. For the purpose of creating cohorts of comparable patients tested with PET or vasodilator SPECT, we excluded 32 594 (20.9% of total MPI volume) SPECT studies performed at sites that did not offer PET and 37 763 exercise SPECT studies (24.2% of the total MPI volume). After 1:1 propensity score matching to facilitate this comparison, there remained 52 132 studies, with 26 066 studies in each group (Figure 1).
Figure 1.
Consort diagram for inclusion and exclusion criteria in the study.
Baseline characteristics
In the overall cohort before propensity score matching, there were significant differences in baseline characteristics between patients tested with PET and SPECT (Table 1). Patients who were tested with PET were older, more likely to have been tested in the inpatient setting, and had a greater burden of comorbidities—including diabetes, hypertension, hyperlipidaemia, prior CAD and revascularization, congestive heart failure, cardiomyopathy, atrial fibrillation, prior stroke, peripheral arterial disease, chronic obstructive pulmonary disease, obstructive sleep apnoea, left bundle branch block, abnormal baseline ECG, and implanted ICDs, but were also less likely to be smokers. The described differences were no longer found after propensity score matching, with standardized differences <10% for all covariates (Supplementary data online, Figure S1). After matching, the mean age of tested patients was 67 ± 11.8 years, the majority were men (52.6%), and most patients underwent testing in the outpatient setting (75.2%). Prior CAD was present in more than half of the patients (54.3%), with prior PCI or CABG performed in 34% and 16.5% of patients, respectively.
Table 1.
Baseline demographic and clinical patient characteristics by imaging modality (PET vs. SPECT) before and after propensity score matching
| Main cohort |
Matched cohort |
|||||
|---|---|---|---|---|---|---|
| PET (n = 46 566) | SPECT (n = 109 678) | Stand. Diff. (%) | PET (n = 26 066) | SPECT (n = 26 066) | Stand. Diff. (%) | |
| Age | 68.9 ± 11.7 | 62.6 ± 12.5 | 51.9 | 67.1±11.8 | 67±11.8 | 0.5 |
| Men | 25 135 (54) | 61 751 (56.3) | 4.7 | 13 700 (52.6) | 13 710 (52.6) | 0.1 |
| BMI (kg/m2) | 29.6 ± 6.1 | 30.3 ± 16.9 | 5.2 | 30.7±6.1 | 30.5±7.5 | 1.9 |
| Inpatient status | 15 132 (32.5) | 17 995 (16.4) | 38.1 | 6649 (25.5) | 6269 (24.1) | 3.4 |
| Known CAD | 28 675 (61.6) | 48 183 (43.9) | 35.9 | 14 250 (54.7) | 14 035 (53.8) | 1.7 |
| Prior PCI | 17 992 (38.6) | 29 606 (27) | 25.0 | 8989 (34.5) | 8713 (33.4) | 2.2 |
| Prior CABG | 9041 (19.4) | 13 135 (12) | 20.6 | 4420 (17) | 4197 (16.1) | 2.3 |
| Diabetes | 14 576 (31.3) | 21 944 (23.7) | 18.4 | 8294 (31.8) | 8365 (32.1) | 0.7 |
| Hyperlipidemia | 38 407 (82.5) | 81 956 (74.7) | 19.0 | 21 112 (81) | 21 133 (81.1) | 0.2 |
| Hypertension | 38 389 (82.4) | 77 252 (70.4) | 28.6 | 21 222 (81.4) | 21 136 (81.1) | 0.8 |
| Family history | 22 056 (47.4) | 55 537 (50.6) | 6.5 | 12 708 (48.8) | 12 725 (48.8) | 0.1 |
| Smoking | 6357 (13.7) | 19 308 (17.6) | 10.9 | 4064 (15.6) | 4169 (16) | 1.1 |
| Abnormal baseline ECG | 36 582 (78.6) | 75 501 (68.8) | 22.2 | 20 395 (78.2) | 20 429 (78.4) | 0.3 |
| Typical angina | 828 (1.8) | 2504 (2.3) | 10.0 | 537 (2.1) | 532 (2) | 1.9 |
| Dyspnoea | 23 348 (50.1) | 50 407 (46) | 8.4 | 13 364 (51.3) | 13 398 (51.4) | 0.3 |
| History of CAC | 4934 (10.6) | 10 196 (9.3) | 4.3 | 2577 (9.9) | 2651 (10.2) | 0.9 |
| PAD | 9238 (19.8) | 11 222 (10.2) | 27.1 | 4618 (17.7) | 4474 (17.2) | 1.5 |
| CHF | 6166 (13.2) | 6980 (6.4) | 23.3 | 3202 (12.3) | 3157 (12.1) | 0.5 |
| Cardiomegaly | 1446 (3.1) | 3127 (2.9) | 1.5 | 1039 (4.0) | 1104 (4.2) | 1.3 |
| LBBB | 2850 (6.1) | 3383 (3.1) | 14.5 | 1541 (5.9) | 1528 (5.9) | 0.2 |
| IVCD | 1758 (3.8) | 3375 (3.1) | 3.8 | 1068 (4.1) | 1042 (4) | 0.5 |
| Baseline ST/T abnormality | 12 152 (26.1) | 30 040 (27.4) | 2.9 | 7527 (28.9) | 7387 (28.3) | 1.2 |
| Atrial fibrillation | 10 439 (22.4) | 13 014 (11.9) | 28.3 | 5378 (20.6) | 5163 (19.8) | 2.1 |
| Ventricular tachycardia | 1558 (3.3) | 1804 (1.6) | 10.9 | 846 (3.2) | 756 (2.9) | 2.0 |
| Asthma | 5138 (11) | 10 608 (9.7) | 4.5 | 3069 (11.8) | 3061 (11.7) | 0.1 |
| COPD | 6677 (14.3) | 9695 (8.8) | 17.2 | 3607 (13.8) | 3608 (13.8) | 0 |
| OSA | 9080 (19.5) | 15 390 (14) | 14.7 | 5256 (20.2) | 5177 (19.9) | 0.8 |
| Prior stroke | 6600 (14.2) | 8740 (8) | 19.9 | 3476 (13.3) | 3430 (13.2) | 0.5 |
| Cardiomyopathy | 8799 (18.9) | 11 728 (10.7) | 23.3 | 4794 (18.4) | 4563 (17.5) | 2.3 |
| Syncope | 2756 (5.9) | 4749 (4.3) | 7.2 | 1469 (5.6) | 1470 (5.6) | 0 |
| Palpitations | 5719 (12.3) | 8631 (7.9) | 14.7 | 2888 (11.1) | 2861 (11) | 0.3 |
| ICD | 1906 (4.1) | 1490 (1.4) | 16.9 | 940 (3.6) | 868 (3.3) | 1.5 |
| Pharmacologic stress | 46 566 (100) | 59 088 (53.9) | 131 | 26 066 (100) | 26 066 (100) | 0 |
| Ischaemia | 17.5 | 7.7 | ||||
| None | 25 779 (55.4) | 70 123 (63.9) | 15 010 (57.6) | 15 850 (60.8) | ||
| Mild | 8325 (17.9) | 17 574 (16) | 4585 (17.6) | 4543 (17.4) | ||
| Moderate–severe | 12 462 (26.8) | 21 981 (20) | 6471 (24.8) | 5673 (21.6) | ||
| % ischaemia | 4.3 ± 8.5 | 3.3 ± 7.8 | 12.3 | 4.1 ± 8.5 | 3.5 ± 8.3 | 6.4 |
| SRS | 2 ± 5.8 | 1.7 ± 4.7 | 5.7 | 1.8 ± 5.8 | 2.4 ±5.6 | 10.5 |
| LVEF (rest) | 58.6 ± 36.5 | 66 ± 15.9 | 26.5 | 58.7 ± 47.3 | 63.6 ± 18 | 13.6 |
Continuous variables presented as mean ± standard deviation; categorical variables as percentages. Standardized difference (Stand. Diff) expressed as a percentage.
BMI, body mass index; CABG, coronary artery bypass grafting; CAC, coronary artery calcification; CAD, coronary artery disease; CHF, congestive heart failure; COPD, chronic obstructive pulmonary disease; ICD, intracardiac defibrillator; IVCD, interventricular conduction delay; LBBB, left bundle branch block; LVEF, left ventricular ejection fraction; OSA, obstructive sleep apnoea; PAD, peripheral arterial disease; PCI, percutaneous coronary intervention; SRS, summed rest score.
PET and SPECT test utilization
Total utilization of MPI decreased significantly starting in 2005 from 191 studies per 1000 patient encounters to 75 studies per 1000 patient encounters in 2017 (Figure 2). This was mainly driven by a significant decline in SPECT MPI utilization, which changed from 169 to 34 studies per 1000 patient encounters during the study period. In contrast, PET utilization increased from 18 studies per 1000 patient encounters to 61 studies per 1000 patient encounters in 2009 before plateauing at 41 studies per 1000 patient encounters from 2013 to 2017. Years 2011–2012 were a notable exception to the described trends, with a precipitous drop in PET utilization and a compensatory increase in SPECT volumes, corresponding to an FDA safety recall of rubidium generators.26
Figure 2.
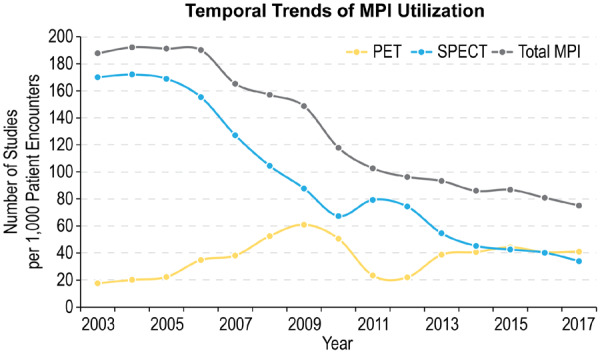
Temporal trends of MPI utilization with PET and SPECT.
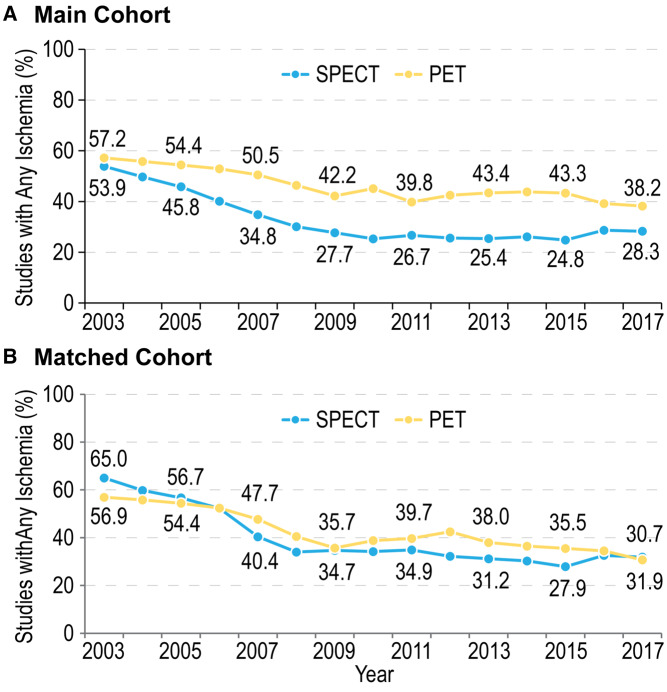
Prevalence of ischaemia with PET and SPECT
The prevalence of detectable ischaemia declined significantly over time with both PET and SPECT (Figure 3A). In the main cohort, the proportion of PET studies showing any ischaemia decreased from 57.2% in 2003 to 38.2% in 2017 (P < 0.0001), with a more pronounced decrease seen in SPECT during the same period (from 53.9% to 28.3%; P < 0.0001). Similarly, the proportion of PET studies revealing moderate–severe ischaemia of 5% or greater declined between 2003 and 2017 from 37.6% to 27.2% (P < 0.0001), with a similar but more marked reduction in the SPECT group from 34.5% to 16.7%, respectively (P < 0.0001) (Supplementary data online, Figure S2A).
Figure 3.
Change in the prevalence of ischaemia over time with PET and SPECT, (A) in the main cohort and (B) in the matched cohort. The proportion of studies showing any ischaemia declined over time with both PET and SPECT (P < 0.0001 for both), with a more pronounced decline observed with SPECT in the unmatched cohort (A). After propensity score matching, temporal decline persisted with both modalities; however, the between-modality difference in prevalence of ischaemia was significantly attenuated (B).
After propensity score matching, a temporal decline in the prevalence of any ischaemia persisted in non-exercise stress tests with both modalities, with a decrease in the proportion of studies showing any ischaemia from 56.9% in 2003 to 30.7% in 2017 with PET and from 65% to 31.9% with SPECT, P < 0.0001 for both comparisons (Figure 3B). A similar pattern was observed for studies showing moderate–severe ischaemia: decline from 37.4% to 21% with PET and from 42.8% to 19.5% with SPECT, P<0.0001 for both comparisons (Supplementary data online, Figure S2B). However, the between-modality difference in ischaemia prevalence observed in the unmatched cohort was significantly attenuated after matching.
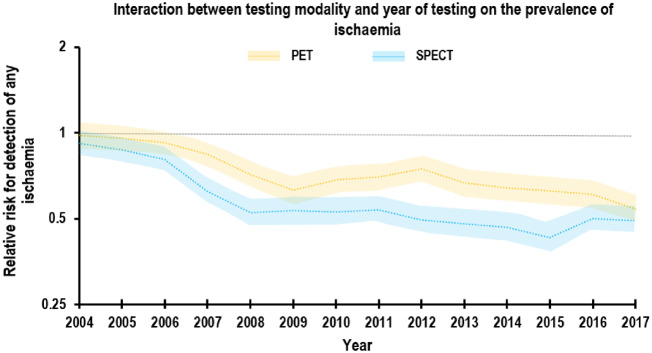
In a model adjusted for year of testing, imaging modality and interaction term between year × modality, PET was associated with a higher likelihood of detecting ischaemia in the main cohort [relative risk (RR) 1.44, 95% confidence interval (CI) 1.42–1.47; P < 0.0001]. After propensity score matching, however, the point estimate for the testing modality main effect was significantly smaller (RR 1.08, 95% CI 1.05–1.11; P < 0.0001), but still suggested higher likelihood of detecting ischaemia with PET, although the difference between PET and SPECT in the likelihood of detecting ischaemia was clinically marginal (Figure 4).
Figure 4.
Interaction between testing modality and year of testing on the prevalence of ischaemia. The likelihood of detecting any ischaemia declined initially then plateaued with both modalities, with a statistically significant, but clinically marginal differnce between PET and SPECT (P for modality × year interaction <0.001).
Similar temporal trends were observed when subgroups of interest were examined within the matched cohort—including gender, hospitalization status, history of CAD, and diabetes—showing similar rates of decline in burden of ischaemia over time with both modalities (P-values of 0.87, 0.08, 0.19, and 0.07, respectively) across these subgroups (Supplementary data online, Figure S3).
Discussion
In the first evaluation of temporal trends with PET MPI at a large-volume nuclear laboratory, we report an increase in the use of PET between 2003 and 2017, with a concomitant drop in SPECT volumes. Moreover, the frequency of PET or SPECT MPI studies showing any ischaemia has progressively declined during the last 15 years, with a less pronounced decline seen in PET studies. After accounting for patient-level characteristics associated with modality selection, however, the rate of decline in ischaemia prevalence was nearly similar between PET and vasodilator SPECT.
By presenting the unmatched PET–SPECT comparison, we aimed to provide an unadjusted crude description of utilization patterns and trends in ischaemia prevalence for both modalities that reflects the experience of all patients undergoing radionuclide perfusion imaging. Comparing trends after propensity score matching assessed whether non-exercise stress testing revealed differential rates of ischaemia detection between PET and SPECT. Our results suggest that the difference observed in the unmatched cohort is largely driven by the higher risk profile of patients being referred to PET (who often are unable to exercise), and is less likely due to unique attributes of any of the imaging modalities.
The current findings corroborate previously published data and show a consistent decline in ischaemic burden with MPI over time.1–4 However, our data show ischaemia continues to be prevalent among patients referred for MPI testing, with roughly a third of studies in 2017 in the overall cohort showing some degree of ischaemia and one in five studies showing at least moderate ischaemia. Such rates are in line with rates published in prior studies,1,3 but are significantly higher than those reported when patients with known CAD were excluded.2,4
The decline in ischaemic burden over time is likely multifactorial and is in part related to improved outcomes of patients with atherosclerotic heart disease and reduction in cardiovascular risk,12,27 increased use of secondary prevention medications such as statins, improved control of cardiovascular risk factors, and advances in stent technology for patients undergoing PCIs.7–10 These factors have likely reduced the burden of atherosclerosis and prevalence of typical angina among patients referred for non-invasive stress testing, thereby leading to less inducible ischaemia with a variety of functional imaging modalities.28
Additionally, patterns of test utilization in this study suggest that test substitution has taken place between PET and SPECT at participating centres, with the exception of 2011–2012 period during which ‘reverse test substitution’ took place due to Rb-82 shortage. Older patients and those with CAD, prior revascularization and greater burden of comorbidities were preferentially referred to PET as providers gained confidence with PET technology, and as a result, less sick patients were tested with SPECT. As such, ‘test substitution’ may be another potential explanation for the disproportionate reduction in ischaemia with SPECT in the unmatched analysis. Of note, the number of cardiac CT angiography studies performed at our institution did not significantly increase during the study period, in contrast to the trend seen at many centres, and therefore did not contribute to the test substitution phenomenon observed.
We also observed an overall decline in total testing volume. Besides improved secondary prevention treatments and stent technology, it is likely the release of Appropriate Use Criteria for Single Photon Emission Computed Tomography29 in 2005 has contributed to the observed decline in SPECT MPI utilization at our institution, corresponding to a national trend that has also been shown previously by others.1,30,31 The impact of the release of appropriate use criteria and changes in the prevalence of ischaemic studies, however, is likely more complex and warrants further discussion. When practices that are considered ‘inappropriate’ according to appropriate use criteria—including routine testing in asymptomatic patients post coronary revascularization or prior to low-risk non-cardiac surgeries—are ordered less frequently, it would be expected that the proportion of studies with inducible ischaemia will increase, as studies performed for ‘inappropriate’ indications are less likely to be abnormal.13 As such, our results may appear to be incongruent with this expectation. However, it should be acknowledged that the impact of appropriate use criteria adoption has likely happened in the context of several other concurrent changes, including radiology benefits managers (independent private entities within the U.S. health system utilized by private payers and government insurance in more recent years, that work with ordering physician to pre-authorize advanced imaging services to limit over-utilization of covered services) and others, many of which could also potentially affect rates of ischaemia with MPI testing.
Study limitations
This study reflects a large single-centre experience, and is thereby subject to referral bias and may have limited generalizability. Nonetheless, the current data describe experience from one of the few large-volume nuclear laboratories in the USA, with greater than 40 000 PET MPI studies included, and is the largest formal evaluation of temporal trends with PET. Second, information on insurance status of patients who underwent PET or SPECT were unavailable, and this might be a source of unmeasured residual confounding between the PET and the SPECT cohorts. If insurance status was a confounder, patients who are uninsured or with public insurance may have been more frequently tested with the less expensive SPECT modality. To the extent these patients have higher baseline ischaemic risk, the SPECT group may have had a higher pre-test probability for ischaemia detection despite propensity score matching, which did not account for insurance status. Third, residual confounding may explain the marginal difference in ischaemia prevalence between PET and SPECT, even after adjusting for potential biases in patient referral based on their perceived cardiovascular risk. Lastly, the change in SPECT imaging technology during the study period, with introduction of solid-state detector cameras around 2009 is potentially another confounder that may affect the interpretation of the present study. However, the downward trend of ischaemia prevalence was consistent throughout the study period, and it is unlikely that changes in SPECT instrumentation during the study have affected the prevalence of ischaemia.
Conclusion
Utilization of PET MPI has increased over time at a large-volume nuclear laboratory, whereas SPECT use declined significantly during the same period. Such changes were accompanied by a significant decline in the frequency of ischaemic studies with both PET and SPECT, even though this decline was less pronounced with PET. The observed difference in ischaemia prevalence between PET and SPECT is likely related to the higher risk profile of patients referred to PET.
Funding
Research reported in this publication was supported by the National Heart, Lung And Blood Institute of NIH under the award number T32HL110837 (F.J.A., K.K.P.) and R01HL123980 (P.S.C). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Health.
Conflict of interest: J.A.S receives research grant support from Bayer and is the PI of an analytic center for the American College of Cardiology. He serves as a consultant to United Healthcare, Bayer, Janssen, AstraZeneca and Novartis. He has an equity interest in Health Outcomes Sciences and serves on the Board of Blue Cross Blue Shield of Kansas City. T.M.B receives research grant support from Bracco, Jubilant DraxImage, and GE Healthcare. He serves as a consultant to Curium, GE Healthcare, and Mallinckrodt. He has ownership interest in Cardiovascular Imaging Technologies. He has intellectual property rights for Imagen Pro/MD/Q/3D/SPECT software. Other authors have no conflicts.
Supplementary Material
References
- 1. Jouni H, Askew JW, Crusan DJ, Miller TD, Gibbons RJ. Temporal trends of single-photon emission computed tomography myocardial perfusion imaging in patients with coronary artery disease: a 22-year experience from a tertiary academic medical center. Circ Cardiovasc Imaging 2017;10:1–10. [DOI] [PubMed] [Google Scholar]
- 2. Jouni H, Askew JW, Crusan DJ, Miller TD, Gibbons RJ. Temporal trends of single-photon emission computed tomography myocardial perfusion imaging in patients without prior coronary artery disease: a 22-year experience at a tertiary academic medical center. Am Heart J 2016;176:127–33. [DOI] [PubMed] [Google Scholar]
- 3. Duvall WL, Rai M, Ahlberg AW, O’Sullivan DM, Henzlova MJ. A multi-center assessment of the temporal trends in myocardial perfusion imaging. J Nucl Cardiol 2015;22:539–51. [DOI] [PubMed] [Google Scholar]
- 4. Rozanski A, Gransar H, Hayes SW, Min J, Friedman JD, Thomson LEJ et al. Temporal trends in the frequency of inducible myocardial ischemia during cardiac stress testing: 1991 to 2009. J Am Coll Cardiol 2013;61:1054–65. [DOI] [PubMed] [Google Scholar]
- 5. Thompson RC, Allam AH. More risk factors, less ischemia, and the relevance of MPI testing. J Nucl Cardiol 2015;22:552–4. [DOI] [PubMed] [Google Scholar]
- 6. Beller GA. How should we interpret the decrease in annual volume of stress imaging tests for evaluation of suspected or known coronary artery disease with fewer high-risk test results? Circ Cardiovasc Imaging 2017;10:e006702. [DOI] [PubMed] [Google Scholar]
- 7. Luepker RV, Steffen LM, Duval S, Zantek ND, Zhou X, Hirsch AT. Population trends in aspirin use for cardiovascular disease prevention 1980–2009: the Minnesota Heart Survey. J Am Heart Assoc 2015;4:e002320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Salami JA, Warraich H, Valero-Elizondo J, Spatz ES, Desai NR, Rana JS et al. National trends in statin use and expenditures in the US adult population from 2002 to 2013: insights from the medical expenditure panel survey. JAMA Cardiol 2017;2:56–65. [DOI] [PubMed] [Google Scholar]
- 9. Luepker RV, Steffen LM, Jacobs DR Jr, Zhou X, Blackburn H. Trends in blood pressure and hypertension detection, treatment, and control 1980 to 2009: the Minnesota Heart Survey. Circulation 2012;126:1852–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Benjamin EJ, Virani SS, Callaway CW, Chang AR, Cheng S, Chiuve SE et al. Heart disease and stroke statistics—2018 update: a report from the American Heart Association. Circulation 2018;137:e67–s492. [DOI] [PubMed] [Google Scholar]
- 11. Yeh RW, Sidney S, Chandra M, Sorel M, Selby JV, Go AS. Population trends in the incidence and outcomes of acute myocardial infarction. N Engl J Med 2010;362:2155–65. [DOI] [PubMed] [Google Scholar]
- 12. Ford ES, Ajani UA, Croft JB, Critchley JA, Labarthe DR, Kottke TE et al. Explaining the decrease in U.S. deaths from coronary disease, 1980-2000. N Engl J Med 2007;356:2388–98. [DOI] [PubMed] [Google Scholar]
- 13. Elgendy IY, Mahmoud A, Shuster JJ, Doukky R, Winchester DE. Outcomes after inappropriate nuclear myocardial perfusion imaging: a meta-analysis. J Nucl Cardiol 2016;23:680–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hendel RC. Utilization management of cardiovascular imaging pre-certification and appropriateness. JACC Cardiovasc Imaging 2008;1:241–8. [DOI] [PubMed] [Google Scholar]
- 15. Mc Ardle BA, Dowsley TF, deKemp RA, Wells GA, Beanlands RS. Does rubidium-82 PET have superior accuracy to SPECT perfusion imaging for the diagnosis of obstructive coronary disease? A systematic review and meta-analysis. J Am Coll Cardiol 2012;60:1828–37. [DOI] [PubMed] [Google Scholar]
- 16. Hachamovitch R, Nutter B, Hlatky MA, Shaw LJ, Ridner ML, Dorbala S et al. Patient management after noninvasive cardiac imaging results from SPARC (Study of myocardial perfusion and coronary anatomy imaging roles in coronary artery disease). J Am Coll Cardiol 2012;59:462–74. [DOI] [PubMed] [Google Scholar]
- 17. Bateman TM, Dilsizian V, Beanlands RS, DePuey EG, Heller GV, Wolinsky DA. American Society of Nuclear Cardiology and Society of Nuclear Medicine and Molecular Imaging Joint Position Statement on the Clinical Indications for Myocardial Perfusion PET. J Nucl Med 2016;57:1654–6. [DOI] [PubMed] [Google Scholar]
- 18. Henzlova MJ, Duvall WL, Einstein AJ, Travin MI, Verberne HJ. ASNC imaging guidelines for SPECT nuclear cardiology procedures: stress, protocols, and tracers. J Nucl Cardiol 2016;23:606–39. [DOI] [PubMed] [Google Scholar]
- 19. Dilsizian V, Bacharach SL, Beanlands RS, Bergmann SR, Delbeke D, Dorbala S et al. ASNC imaging guidelines/SNMMI procedure standard for positron emission tomography (PET) nuclear cardiology procedures. J Nucl Cardiol 2016;23:1187–226. [DOI] [PubMed] [Google Scholar]
- 20. Verberne HJ, Acampa W, Anagnostopoulos C, Ballinger J, Bengel F, De Bondt P et al. EANM procedural guidelines for radionuclide myocardial perfusion imaging with SPECT and SPECT/CT: 2015 revision. Eur J Nucl Med Mol Imaging 2015;42:1929–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Flotats A, Knuuti J, Gutberlet M, Marcassa C, Bengel FM, Kaufmann PA et al. Hybrid cardiac imaging: SPECT/CT and PET/CT. A joint position statement by the European Association of Nuclear Medicine (EANM), the European Society of Cardiac Radiology (ESCR) and the European Council of Nuclear Cardiology (ECNC). Eur J Nucl Med Mol Imaging 2011;38:201–12. [DOI] [PubMed] [Google Scholar]
- 22. Patel MR, Bailey SR, Bonow RO, Chambers CE, Chan PS, Dehmer GJ et al. ACCF/SCAI/AATS/AHA/ASE/ASNC/HFSA/HRS/SCCM/SCCT/SCMR/STS 2012 appropriate use criteria for diagnostic catheterization: a report of the American College of Cardiology Foundation Appropriate Use Criteria Task Force, Society for Cardiovascular Angiography and Interventions, American Association for Thoracic Surgery, American Heart Association, American Society of Echocardiography, American Society of Nuclear Cardiology, Heart Failure Society of America, Heart Rhythm Society, Society of Critical Care Medicine, Society of Cardiovascular Computed Tomography, Society for Cardiovascular Magnetic Resonance, and Society of Thoracic Surgeons. J Am Coll Cardiol 2012;59:1995–2027. [DOI] [PubMed] [Google Scholar]
- 23. Austin P. Using standardized difference to compare the prevalence of a binary variable between two groups in observational research. Commun Stat Simul Comput 2009;38:1228–34. [Google Scholar]
- 24. Austin PC. An introduction to propensity score methods for reducing the effects of confounding in observational studies. Multivariate Behav Res 2011;46:399–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Austin PC. Optimal caliper widths for propensity-score matching when estimating differences in means and differences in proportions in observational studies. Pharm Stat 2011;10:150–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. https://wayback.archive-it.org/7993/20171105113250/https://www.fda.gov/Drugs/DrugSafety/DrugSafetyPodcasts/ucm266403.htm (10 June 2019, date last accessed).
- 27. Tu JV, Khan AM, Ng K, Chu A. Recent temporal changes in atherosclerotic cardiovascular diseases in Ontario: clinical and health systems impact. Can J Cardiol 2017;33:378–84. [DOI] [PubMed] [Google Scholar]
- 28. Carpeggiani C, Landi P, Michelassi C, Sicari R, Picano E. The declining frequency of inducible myocardial ischemia during stress echocardiography over 27 consecutive years (1983–2009). Int J Cardiol 2016;224:57–61. [DOI] [PubMed] [Google Scholar]
- 29. Brindis RG, Douglas PS, Hendel RC, Peterson ED, Wolk MJ, Allen JM et al. ACCF/ASNC appropriateness criteria for single-photon emission computed tomography myocardial perfusion imaging (SPECT MPI): a report of the American College of Cardiology Foundation Quality Strategic Directions Committee Appropriateness Criteria Working Group and the American Society of Nuclear Cardiology endorsed by the American Heart Association. J Am Coll Cardiol 2005;46:1587–605. [DOI] [PubMed] [Google Scholar]
- 30. McNulty EJ, Hung YY, Almers LM, Go AS, Yeh RW. Population trends from 2000–2011 in nuclear myocardial perfusion imaging use. JAMA 2014;311:1248–9. [DOI] [PubMed] [Google Scholar]
- 31. Levin DC, Parker L, Halpern EJ, Rao VM. Recent trends in imaging for suspected coronary artery disease: what is the best approach? J Am Coll Radiol 2016;13:381–6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.