Abstract
Background
High uptake of antiretroviral treatment (ART) is essential to reduce human immunodeficiency virus (HIV) transmission and related mortality; however, gaps in care exist. We aimed to construct the continuum of HIV care (CoC) in 2016 in 11 European Union (EU) countries, overall and by key population and sex. To estimate progress toward the Joint United Nations Programme on HIV/AIDS (UNAIDS) 90-90-90 target, we compared 2016 to 2013 estimates for the same countries, representing 73% of the population in the region.
Methods
A CoC with the following 4 stages was constructed: number of people living with HIV (PLHIV); proportion of PLHIV diagnosed; proportion of those diagnosed who ever initiated ART; and proportion of those ever treated who achieved viral suppression at their last visit.
Results
We estimated that 87% of PLHIV were diagnosed; 92% of those diagnosed had ever initiated ART; and 91% of those ever on ART, or 73% of all PLHIV, were virally suppressed. Corresponding figures for men having sex with men were: 86%, 93%, 93%, 74%; for people who inject drugs: 94%, 88%, 85%, 70%; and for heterosexuals: 86%, 92%, 91%, 72%. The proportion suppressed of all PLHIV ranged from 59% to 86% across countries.
Conclusions
The EU is close to the 90-90-90 target and achieved the UNAIDS target of 73% of all PLHIV virally suppressed, significant progress since 2013 when 60% of all PLHIV were virally suppressed. Strengthening of testing programs and treatment support, along with prevention interventions, are needed to achieve HIV epidemic control.
Keywords: HIV infection, continuum of care, sex, key population, Europe
Standardized definitions were used to estimate a 4-stage continuum of human immunodeficiency virus (HIV) care in 11 European Union (EU) countries in 2016. The EU is close to the 90-90-90 target, with the main challenge being the percentage of undiagnosed infections.
(See the Editorial Commentary by Granich and Gupta on pages 2917–9.)
Highly effective antiretroviral treatment (ART) reduces morbidity and increases the life expectancy of treated individuals living with human immunodeficiency virus (HIV), as it is highly effective in suppressing HIV viral load [1, 2]. Large studies on serodiscordant couples have provided evidence that virally suppressed individuals living with HIV have a negligible probability of transmitting the virus to their HIV-negative partner [3–5]. Nevertheless, in 2017 there were approximately 1.8 million new HIV infections worldwide, 160 000 of which were in the World Health Organization European region and 25 000 in the European Union (EU)/European Economic Area [6]. Given ART effectiveness, ongoing HIV transmission indicates gaps in HIV diagnosis and care, mainly attributed to late diagnosis [7], delayed ART initiation [8], and HIV care interruptions [9].
The HIV continuum of care (CoC) is a useful tool to provide a snapshot of the care continuum among people living with HIV (PLHIV) and to identify weaknesses and gaps within health systems. The 90-90-90 CoC target, 90% of PLHIV diagnosed, 90% of diagnosed being on ART, and 90% of those on ART being virally suppressed, sets important milestones toward achieving viral suppression for all PLHIV, thus saving lives and halting further transmission. Taken together, the target of 73% of those living with HIV being virally suppressed is expected to lead to a drastic decline in new infections by 2030 [10]. It is thus important for countries to regularly assess their progress toward achieving the 90-90-90 target.
Ideally, the HIV CoC should be informed uniformly by monitoring national cohorts. However, national cohorts are lacking. Thus, reduced cohorts that only include people accessing care are often used, risking to result in biased estimates [11, 12]. The EU CoC for 11 countries in 2013 was estimated using common definitions and recommendations to address these methodological and data challenges [13–15]. In 2013, Sweden and Denmark had reached the 90-90-90 target, whereas other countries had reached 90% in only 1 or 2 stages [15].
Since then, most European countries have adopted the test-and-treat guidelines [1, 16]; however, progress toward treatment and viral suppression targets may be uneven. Therefore, an assessment of the progress made in the EU is needed. Furthermore, national CoC figures are likely to mask differences between HIV key populations and sex within a given country; previous studies have reported such differences [17]. For example, men who have sex with men (MSM) have, in general, higher risk perception than other population groups and are consequently tested and diagnosed with HIV earlier after infection [18, 19]. Conversely, poor access to and retention in care for people who inject drugs (PWID) is a common problem worldwide, leading to potential lower levels of viral suppression in this group [20–23]. To date, there have not been studies on the HIV CoC stratified by sex in Europe.
Our aim was 2-fold: first, to provide updated estimates of national CoC for 2016 in 11 countries, representing 73% of the EU population, and to investigate country-specific progress toward the Joint United Nations Programme on HIV/AIDS (UNAIDS) 90-90-90 target since 2013, and second, to construct CoC by sex and key population in order to identify potential disparities in access to care and to assess the different needs of subgroups of PLHIV.
METHODS
Continuums of HIV care were constructed using data from the national HIV surveillance systems of Austria, Denmark, the Netherlands, and the United Kingdom. In these countries, national surveillance systems collect data on new HIV diagnoses and on longitudinal data and thus provided all necessary information. For Croatia, Germany, Greece, Italy, Spain, and Sweden, national surveillance and HIV cohort data were combined, while for France national surveillance, health insurance data, and cohort data were combined. Participating cohorts have been approved by ethics committees, national data protection agencies, or institutional review boards. Informed consent was obtained in all countries where it was required.
The 4-stage CoC was constructed for PLHIV in the participating countries at the end of 2016 overall, as well as disaggregated by sex and by key population (MSM, PWID, and Other). The Other group consisted of non-MSM, non-PWID; these were mainly people presumed to have acquired HIV via sex between men and women and were thus referred to as “Heterosexual” in this analysis. No specific CoC was constructed for individuals with an unknown mode of infection.
The 4-stage CoC was produced using standardized definitions [13, 24]. Stage 1 includes the estimated number of all PLHIV, diagnosed and undiagnosed, who were alive in each participating country at the end of 2016. Diagnosed individuals who had died or out-migrated before 31 December 2016 were excluded. In countries where data on out-migration were not available, the sample of individuals no longer living in the country was, when possible, estimated and excluded from the continuum (Supplement 1). For the majority of countries, the number of PLHIV was estimated by applying the incidence method of the European Centre for Disease Prevention and Control (ECDC) HIV modeling tool to surveillance data on newly diagnosed HIV cases, and 95% confidence intervals (CIs) were calculated using bootstrapping techniques [25]. Methods that have been used in each country are described in detail in Supplement 1.
Stage 2 included the proportion of PLHIV who were diagnosed. Uncertainty in this proportion was obtained by dividing the number of diagnosed individuals by the upper and lower CIs of the estimated PLHIV, when available. In the majority of the participating countries, the number of diagnosed individuals was derived directly from historical HIV surveillance data (Supplement 1).
Stage 3 was obtained based on cohort data and was defined as the proportion of those diagnosed who ever initiated ART, irrespective of treatment guidelines or ART regimens. A lower estimate was calculated using all diagnosed individuals as the denominator and all individuals who had ever been treated as the numerator. An upper estimate was calculated excluding individuals lost to follow-up. The proportion of diagnosed PLHIV who ever initiated ART was estimated as the midpoint between the lower and upper estimates.
Stage 4 was obtained based on cohort data and was defined as the proportion of those ever on ART who achieved viral suppression, that is, those with an HIV-RNA measurement ≤200 copies/mL or below the current assay detection limit at their last visit between 15 July 2015 and 31 December 2016. The threshold of ≤200 copies/mL was chosen to allow for improvements of the assay detection limit over time and to allow for comparisons with the 2013 EU CoC [15]. Individuals in care and on treatment, but without a viral load measurement available during that period, were considered as adherent and thus suppressed, while those without a viral load measurement during that period and not in care were considered as not virally suppressed. In a sensitivity analysis, assuming that all those without available measurements were not suppressed, the overall EU estimates remained unchanged (≤1% difference in any subgroup). As for stage 3, a lower estimate was calculated using all individuals who had ever been treated as the denominator and those virally suppressed as the numerator. An upper estimate was calculated excluding individuals lost to follow-up. The midpoint between these 2 estimates was considered the proportion of treated people who achieved viral suppression.
Estimates for stages 3 and 4 were provided directly from the cohorts’ representatives, except for Germany and Italy where raw data were used by the study team to derive these estimates.
In addition to the 4 stages of the CoC, the proportion of all PLHIV who achieved viral suppression, also called the substantive target, was estimated [26].
To produce estimates for the 11 participating countries, the number of individuals at each stage of the CoC from all countries was added. For stages 3 and 4, where the estimates corresponded to proportions out of previous stages rather than numbers, the numbers were estimated by multiplying the estimated proportion at a given stage with the number of individuals included in the previous one. Once the total number of individuals per stage was estimated, the proportion of individuals out of the previous stage was calculated, corresponding to a weighted average of all participating countries.
For Croatia, only overall and MSM estimates were available. For Sweden, only overall estimates were produced. In addition, only point estimates (ie, without a range) were available for Italy for stage 1, for Sweden for stages 3 and 4, and for the United Kingdom for stage 3.
Adult prevalence was estimated based on the population estimates of Eurostat for the participating EU countries, excluding individuals aged <15 years [27].
RESULTS
At the end of 2016, the total number of estimated PLHIV in the 11 participating countries was 702 848, corresponding to an adult prevalence of 0.23%. Prevalence ranged from 0.04% in Croatia to 0.38% in Spain. MSM accounted for 43% of all PLHIV, heterosexuals for 38%, and PWID for 11%; 8% of all PLHIV had unknown modes of infection. Women accounted for 25% of all PLHIV.
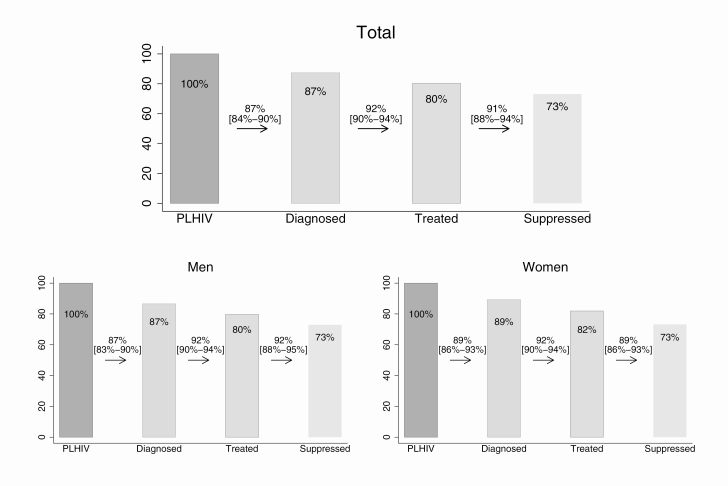
Overall, we estimated that 87% of PLHIV were diagnosed, 92% of those diagnosed had ever initiated ART, and 91% of those who had ever initiated ART were virally suppressed (Figure 1).
Figure 1.
Continuum of HIV care for 2016, overall and by sex in 11 European countries. The numbers between the bars correspond to proportions of the previous stage, while the numbers on the bars correspond to proportions of all PLHIV. Estimates by sex were not available for Croatia and Sweden. A slight underestimation of the variability is expected since only point estimates were available for Italy (stage 1) and Sweden (total population, stages 2, 3, and 4). The third bar corresponds to those ever treated. For the last bar, individuals with HIV-RNA measurement ≤200 copies/mL at last visit are considered suppressed. Abbreviation: PLHIV, people living with human immunodeficiency virus.
Of the total PLHIV, 73% were estimated to be virally suppressed. The 2013 corresponding CoC estimates for 10 of the 11 countries that participated (ie, all countries except Croatia) were 84%, 84%, and 85%, respectively. This shows a significant increase for the 2 last percentages between 2013 and 2016. Between 2013 and 2016, the absolute number of diagnosed individuals in these 10 countries increased by approximately 60 000 (from 552 631 in 2013 to 612 896 in 2016), the number undiagnosed decreased by almost 15 500 (103 869 in 2013 to 88 464 in 2016), and the number ever on ART increased by approximately 100 000 (from 464 256 in 2013 to 564 150 in 2016), leading to an increase in the number virally suppressed of approximately 120 000 (from 393 666 in 2013 to 513 691 in 2016).
There were no marked differences across the outcomes of the CoC between women (89% of female PLHIV were diagnosed, 92% of those diagnosed had ever initiated ART, and 89% of those who had ever initiated ART were virally suppressed) and men (87%, 92%, and 92%, respectively; Table 1, Figure 1).
Table 1.
Estimates of the 4 Stages of the Continuum of Human Immunodeficiency Virus care for 2016 in 11 European Countries by Key Population and Sex
| Country | Population | PLHIV [95% CI] | % Diagnosed [Estimated Range] | % Ever Treated [Estimated Range] | % Suppresseda [Estimated Range] | % Suppressed of PLHIV [Estimated Range] |
|---|---|---|---|---|---|---|
| Austria | ||||||
| MSM | 2920 [2781–3058] | 92.8 [88.6–97.4] | 93.9 [90.2–97.6] | 86.1 [77.3–95.0] | 75.0 [64.2–86.7] | |
| PWID | 1030 [997–1062] | 96.8 [93.9–100] | 92.8 [88.0–97.6] | 81.7 [74.9–88.4] | 73.4 [61.5–86.1] | |
| Heterosexualsb | 2786 [2648–2989] | 91.2 [85.0–95.9] | 95.0 [91.4–98.7] | 86.2 [78.5–93.9] | 74.7 [64.6–87.3] | |
| Men | 5338 [5134–5542] | 91.9 [88.5–95.6] | 93.2 [88.8–97.7] | 85.0 [76.4–93.6] | 72.8 [62.9–83.2] | |
| Women | 1739 [1631–1846] | 93.8 [88.4–100] | 94.0 [89.8–98.3] | 85.6 [78.5–92.7] | 75.5 [64.7–87.5] | |
| Total | 7079 [6946–7330] | 92.3 [89.2–94.1] | 93.4 [89.0–97.8] | 85.2 [76.9–93.4] | 73.4 [65.0–83.4] | |
| Croatia | ||||||
| MSM | 1112 [1012–1268] | 69.6 [61.0–76.5] | 95.9 [93.9–97.9] | 92.3 [90.6–94.1] | 61.6 [60.5–62.7] | |
| Total | 1488 [1356–1708] | 74.1 [64.5–81.3] | 96.2 [94.2–98.2] | 92.1 [90.2–94.1] | 65.7 [64.2–67.0] | |
| Denmark | ||||||
| MSM | 2934 [2598–3270] | 88.5 [79.4–100] | 98.4 [98.1–98.8] | 97.6 [97.1–98.2] | 85.1 [84.6–85.6] | |
| PWID | 452 [411–567] | 90.7 [72.3–99.8] | 95.0 [93.3–96.6] | 92.0 [89.8–94.1] | 79.3 [81.1] | |
| Heterosexuals | 2454 [1997–2919] | 94.2 [88.3–100] | 97.7 [97.2–98.2] | 94.7 [93.4–96.1] | 86.8 [85.1–88.0] | |
| Men | 4706 [4342–5269] | 90.1 [80.5–97.6] | 97.2 [96.7–97.6] | 96.8 [95.8–97.7] | 84.7 [83.8–85.5] | |
| Women | 1704 [1536–1872] | 90.1 [82.1–100] | 97.2 [96.7–97.6] | 93.6 [92.3–94.9] | 82.0 [80.8–83.1] | |
| Total | 6233 [5775–6690] | 92.7 [86.3–100] | 97.2 [96.7–97.6] | 95.9 [94.8–97.0] | 86.4 [85.4–87.3] | |
| France | ||||||
| MSM | 64 900 [63 600–66 200] | 85.9 [84.7–87.3] | 90.6 [85.9–91.9] | 96.8 [96.5–96.9] | 75.3 [74.6–76.0] | |
| PWID | 11 900 [11 500–12 300] | 97.4 [96.3–98.1] | 90.6 [87.2–93.9] | 95.3 [94.7–95.9] | 83.7 [82.0–86.2] | |
| Heterosexuals | 95 700 [93 500–98 100] | 84.9 [83.2–86.7] | 89.4 [88.6–90.3] | 93.9 [93.7–94.2] | 71.3 [70.8–71.8] | |
| Men | 115 600 [113 700–117 300] | 85.1 [84.0–86.4] | 89.8 [89.0–90.5] | 95.6 [95.5–95.8] | 73.1 [72.8–73.5] | |
| Women | 57 000 [56 000–57 900] | 88.2 [87.3–89.4] | 89.5 [88.1–90.9] | 93.8 [93.5–94.0] | 74.0 [73.2–74.9] | |
| Total | 172 700 [170 800–174 500] | 86.1 [85.2–87.1] | 89.7 [89.6–89.8] | 95.0 [94.9–95.2] | 73.4 [73.2–73.5] | |
| Germany | ||||||
| MSM | 545,00 [51 200–58 300] | 85.1 [85.1–90.6] | 94.2 [90.6–97.8] | 93.3 [90.2–96.5] | 74.8 [72.3–77.4] | |
| PWID | 8,800 [7,900–9,700] | 89.7 [81.4–100] | 91.8 [86.1–97.4] | 81.8 [75.0–88.5] | 67.4 [61.8–72.9] | |
| Heterosexuals | 18 500 [16 800–20 200] | 87.6 [80.2–96.4] | 94.7 [91.3–98.0] | 89.2 [86.1–92.3] | 74.0 [71.4–76.5] | |
| Men | 66 100 [61 400–71 200] | 85.8 [79.6–92.3] | 93.8 [90–97.5] | 91.7 [88.2–95.2] | 73.8 [71.0–76.6] | |
| Women | 15 600 [14 000–17 100] | 88.5 [80.7–98.6] | 94.4 [90.8–98.0] | 87.3 [83.5–91.2] | 72.9 [69.7–76.2] | |
| Total | 82 900 [77 100–89 400] | 86.6 [80.3–93.1] | 93.9 [90.2–97.6] | 90.8 [87.2–94.3] | 73.8 [70.9–76.7] | |
| Greece | ||||||
| MSM | 6767 [6519–7066] | 88.5 [84.8–91.9] | 89.0 [85.4–92.4] | 84.4 [80.1–88.7] | 66.5 [63.1–69.9] | |
| PWID | 2156 [2027–2336] | 59.9 [55.3–63.7] | 81.2 [74.1–88.3] | 73.7 [68.6–78.8] | 35.9 [33.4–38.3] | |
| Heterosexuals | 4406 [3922–4889] | 73.8 [66.5–82.9] | 91.3 [88.7–93.8] | 79.6 [69.7–89.5] | 53.6 [47.0–60.3] | |
| Men | 11 040 [10 627–11 624] | 79.7 [75.7–82.8] | 88.2 [84.2–92.1] | 82.2 [76.5–87.8] | 57.8 [53.8–61.8] | |
| Women | 2404 [2135–2680] | 72.0 [64.6–81.0] | 87.1 [82.0–92.2] | 79.5 [70.5–88.4] | 49.8 [44.2–55.4] | |
| Total | 12 953 [12 582–13 619] | 81.3 [77.3–83.7] | 88.3 [84.4–92.1] | 81.8 [75.6–87.9] | 58.7 [54.3–63.1] | |
| Italy | ||||||
| MSM | 48 458 | 83.9 | 88.7 [85.5–91.9] | 92.4 [91.2–93.5] | 68.8 [67.9–69.6] | |
| PWID | 26 481 | 95.9 | 85.3 [78.5–92.1] | 84.5 [78.3–90.7] | 69.1[64.0–74.2] | |
| Heterosexuals | 57 569 | 86.7 | 91.8 [89.0–94.6] | 89.6 [87.4–91.7] | 71.3 [69.6–73.0] | |
| Men | 104 255 | 86.7 | 89.2 [85.7–92.8] | 91.2 [89.1–93.2] | 70.6 [68.9–72.1] | |
| Women | 36 397 | 91.7 | 90.2 [86.4–94.0] | 86.4 [83.3–89.6] | 71.4 [68.9–74.1] | |
| Total | 140 652 | 88.0 | 89.4 [85.9–93.0] | 90.1 [87.8–92.4] | 70.9 [69.1–72.7] | |
| Netherlands | ||||||
| MSM | 13 876 [13 674–14 127] | 89.8 [88.2–91.1] | 97.8 [97.4–98.2] | 96.8 [95.7–97.9] | 84.4 [83.4–85.3] | |
| PWID | 360 [358–364] | 98.6 [97.5–99.2] | 96.7 [94.9–98.4] | 92.0 [88.1–95.8] | 87.5 [83.9–91.4] | |
| Heterosexuals | 7557 [7362–7757] | 87.6 [85.3–89.9] | 97.0 [96.0–97.9] | 92.4 [89.7–95.2] | 78.5 [76.2–80.1] | |
| Men | 18 588 [18 327–18 995] | 88.2 [86.3–89.5] | 97.5 [96.9–98.2] | 95.9 [94.2–96.9] | 81.6 [80.3–83.0] | |
| Women | 4257 [4157–4396] | 91.3 [88.4–93.5] | 97.1 [96.3–98.0] | 92.1 [89.5–94.8] | 80.6 [78.3–82.9] | |
| Total | 22 845 [22 530–23 269] | 88.8 [87.2–90.0] | 97.5 [96.8–98.1] | 95.1 [93.3–96.9] | 82.4 [80.8–83.9] | |
| Spain | ||||||
| MSM | 58 936 [56 222–63 156] | 83.5 [78.4–86.6] | 92.9 [91.3–94.6] | 88.6 [83.3–93.8] | 68.7 [64.6–72.7] | |
| PWID | 20 278 [20 091–20 614] | 97.6 [96.0–98.5] | 86.4 [82.0–90.8] | 79.2 [69.6–88.9] | 66.8 [58.7–75.0] | |
| Heterosexuals | 30 404 [28 620–32 270] | 83.0 [79.4–86.2] | 93.3 [91.0–95.6] | 83.9 [76.5–91.3] | 64.9 [59.2–70.7] | |
| Men | 119 937 [110 455–131 380] | 86.2 [82.3–88.8] | 92.5 [90.5–94.5] | 87.3 [81.3–93.2] | 69.6 [64.8–74.3] | |
| Women | 26 559 [23 962–29 527] | 86.3 [77.6–95.6] | 92.2 [89.7–94.6] | 82.6 [74.7–90.4] | 65.7 [59.4–71.9] | |
| Total | 146 500 [134 417–160 908] | 86.3 [82.1–88.8] | 92.5 [90.4–94.5] | 86.5 [80.2–92.8] | 68.8 [63.9–73.9] | |
| Sweden | ||||||
| MSM | … | … | 98.4 | 92.2 | … | |
| PWID | … | … | 93.4 | 87.8 | … | |
| Heterosexuals | … | … | 96.0 | 91.6 | … | |
| Men | … | … | 96.9 | 91.3 | … | |
| Women | … | … | 96.2 | 92.1 | … | |
| Total | 8,098 [8,143–8,061] | 89.2 [88.8–89.6] | 96.6 | 91.6 | … | |
| United Kingdom | ||||||
| MSM | 48 800 [46 400–53 600] | 88.0 [80.0–92.0] | 97.0 | 93.5 [90.0–97.0] | 79.3 [76.2–82.4] | |
| PWID | 2,500 [2,300–2,800] | 75.0 [67.0–81.0] | 93.0 | 88.0 [83.0–93.0] | 61.0 [57.6–64.5] | |
| Heterosexuals | 49 900 [49 100–51 700] | 91.0 [88.0–93.0] | 96.0 | 91.9 [87.0–96.0] | 79.9 [76.0–78.8] | |
| Men | 70 500 [67 800–75 400] | 90.0 [84.0–94.0] | 96.0 | 93.0 [89.0–97.0] | 80.4 [77.0–78.8] | |
| Women | 30 900 [30 300–31 700] | 92.0 [90.0–94.0] | 95.0 | 91.7 [87.0–96.0] | 80.5 [76.5–84.4] | |
| Total | 101 400 [98 600–106 400] | 91.0 [86.0–93.0] | 96.0 | 92.5 [88.0–97.0] | 80.4 [76.8–84.0] | |
| All countriesc | ||||||
| MSM | 303 203 | 86.0 [81.0–89.0] | 93.0 (91.0–95.0) | 93.0 [90.0–96.0] | 73.8 [71.5–76.1] | |
| PWID | 74 007 | 94.0 [91.0–96.0] | 88.0 [83.0–93.0] | 85.0 [78.0–91.0] | 69.6 [64.2–75.1] | |
| Heterosexuals | 269 571 | 86.0 [84.0–89.0] | 92.0 [91.0–94.0] | 91.0 [88.0–93.0] | 72.5 [70.1–73.9] | |
| Men | 516 064 | 87.0 [83.0–90.0] | 92.0 [90.0–94.0] | 92.0 [88.0–95.0] | 73.0 [70.3–75.5] | |
| Women | 176 560 | 89.0 [92.0–94.0] | 92.0 [90.0–94.0] | 89.0 [86.0–93.0] | 73.2 [70.2–76.2] | |
| Total | 702 848 | 87.0 [84.0–90.0] | 92.0 [90.0–94.0] | 91.0 [88.0–94.0] | 73.2 [70.7–75.7] |
Percentages correspond to proportions of individuals out of the previous stage, except for the last column where the percentage suppressed out of the total PLHIV is presented.
Abbreviations: CI, confidence interval; MSM, men who have sex with men; PLHIV, people living with human immunodeficiency virus; PWID, people who inject drugs.
aHIV-RNA measurement ≤200 copies/mL at last visit.
bNon-MSM, non-PWID, that is, mainly people presumed to have acquired HIV via sex between men and women.
cBased on available data: for Croatia only overall estimates and estimates for MSM were produced, and for Sweden only overall estimates were produced. The range of the number of PLHIV was not estimated since not all countries have provided lower and upper estimates. For stages 2–4, ranges were estimated based on available data.
The proportion diagnosed among estimated PLHIV ranged from 74% in Croatia to 93% in Denmark. Three of the 11 countries had achieved the first 90 target (Austria, Denmark, and the United Kingdom), and 8 countries were below it (Netherlands 89%, Sweden 89%, Italy 88%, Germany 87%, France 86%, Spain 86%, Greece 81%, and Croatia 74%). Of those diagnosed, the proportion ever on ART ranged from 88% in Greece to 97% in the Netherlands. Nine countries (Austria, Croatia, Denmark, France, Germany, Spain, Sweden, the Netherlands, and the United Kingdom) achieved ≥90% of those diagnosed ever on ART. The proportions virally suppressed among those who ever initiated ART ranged from 82% in Greece to 96% in Denmark. Eight countries (Croatia, Denmark, France, Germany, Italy, the Netherlands, Sweden, and the United Kingdom) achieved ≥90% of viral suppression. Only Denmark achieved ≥90% for each of the 3 continuum stages. For the substantive target, Austria, Denmark, France, Germany, the Netherlands, Sweden, and the United Kingdom reached ≥73% of all PLHIV virally suppressed, while Italy was very close at 71% (Supplements 2 and 3).
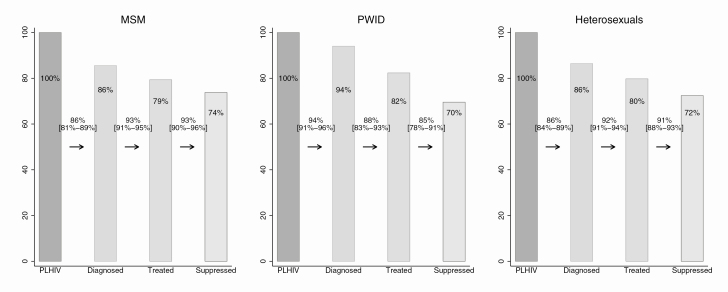
Regarding key population estimates, the highest percentage of PLHIV diagnosed was estimated among PWID (94%), followed by heterosexuals (86%) and MSM (86%) (Figure 2). PWID, however, had the lowest percentage of people diagnosed ever on ART and viral suppression after treatment initiation (88% treated and 85% virally suppressed). MSM and heterosexuals achieved higher percentages for these 2 stages; these figures were 93% and 93%, respectively, for MSM and 92% and 91% for heterosexuals. Overall, 74% of MSM, 72% of heterosexuals, and 70% of PWID living with HIV achieved viral suppression (Figure 2). There was considerable variation between key populations across countries (Table 1, Supplements 2 and 3). The largest differences were observed among PWID in all CoC stages. In Greece, 60% were diagnosed, 81% were treated, and 74% achieved viral suppression, while in the Netherlands, 99% were diagnosed, 97% were treated, and 92% achieved viral suppression. The proportion diagnosed among heterosexuals was also low in Greece (74%) and Spain (83%) compared with Denmark (94%), Austria, and the United Kingdom (91% in both). A low diagnosis proportion was estimated among MSM in Croatia (70%) as well (Supplements 2 and 3).
Figure 2.
Continuum of HIV care for 2016, by key population in 11 European countries. The numbers between the bars correspond to proportions of the previous stage, while the numbers on the bars correspond to proportions of PLHIV. Estimates by key population were not available for Sweden, while only estimates for MSM were available for Croatia. A slight underestimation of the variability of stage 1 is expected since only the point estimate was available for Italy. The third bar corresponds to those ever treated. For the last bar, individuals with HIV-RNA measurement ≤200 copies/mL at last visit are considered suppressed. Abbreviations: MSM, sex with men; PLHIV, people living with human immunodeficiency virus; PWID, persons who inject drugs.
DISCUSSION
At the end of 2016, the 11 participating countries, which represent close to three quarters of the EU population, were very close to reaching the UNAIDS 90-90-90 goal, with 87% of all PLHIV diagnosed, 92% of all diagnosed ever treated, and 91% of those ever treated being virally suppressed. Overall, an estimated 73% of PLHIV were virally suppressed, meaning that the substantive UNAIDS target was achieved; this proportion was 60% in 2013 [15]. A comparison of proportions and, most importantly, of absolute numbers of treated and virally suppressed individuals gives optimism that a decline in the number of new infections, as a result of an increase of PLHIV virally suppressed, is most likely in the participating EU countries.
In all stages of the CoC, substantial variation between countries was found: the percentage of diagnosed individuals varied between 74% in Croatia and 93% in Denmark, while the percentage of ever treated among diagnosed individuals varied between 88% in Greece and 98% in the Netherlands. Overall, the percentage of virally suppressed individuals ranged from 59% in Greece to 86% in Denmark. Some of these differences may be explained by the different percentages of missing data for viral load, especially when the lack of such data is informative. In Greece, for example, in 2016, due to bureaucratic/funding issues, mostly individuals with impaired health were prompted for an HIV-RNA measurement. In such cases, the percentage of PLHIV virally suppressed is expected to be underestimated. Thus, differences across countries partly reflect differences in healthcare systems and testing policies. Country-specific CoC estimates can point at specific weaknesses and lead the relevant authorities to adopt best practices. Ideally, these estimates are based on real-time or at least recent data (ie, previous year) so that corrective actions can be taken in time.
We found that a higher percentage of PWID had been diagnosed compared with MSM and heterosexuals. However, as MSM and heterosexuals had higher proportions of ever treated and virally suppressed, the proportion of PLHIV virally suppressed for these 2 groups was higher than for PWID (MSM, 74%; heterosexuals, 72%; PWID, 70%). Variation across countries within key populations partly reflects temporal differences in the epidemics. Unlike in the Netherlands, the outbreak in Greece among PWID is very recent, and most PWID still inject drugs, consequently they are less likely to be compliant with ART and achieve viral suppression.
In accordance with previous studies, the proportion of men who had been diagnosed was slightly lower than among women (87% in men vs 89% women) [28, 29]. HIV testing as a part of obstetrics exams may explain this small difference, although missed testing opportunities for women still exist [30]. The proportion diagnosed who had started treatment was the same (92% for both), and the proportion virally suppressed was slightly higher among men (92% for men vs 89% for women), resulting in the same overall proportions for men and women (73% for both).
The main challenge for all participating countries was the percentage of diagnosed individuals among PLHIV, overall and across key populations, except PWID. Among MSM, the ongoing higher HIV incidence may not be sufficiently covered by the recommendations for early testing [18, 19], which explains the higher undiagnosed percentage of MSM compared with PWID [20, 31, 32].
An important contribution of this analysis is that estimation of all stages of the CoC has been performed using unified definitions and common methods in the majority of participating countries. Nevertheless, there are some limitations. Estimation of the percentage ever treated among those diagnosed was based on cohort data. In some of the participating countries, cohorts represent a selected sample of diagnosed individuals [33]. In these countries, the percentage of individuals who ever initiated ART may have been overestimated. In Germany, for example, following a different methodology, the proportion of those ever treated has been estimated as 91% (84%–98%), slightly lower than the 94% (90%–98%) estimated by this analysis.
A significant challenge was how to accurately capture the number of PLHIV alive in each country, which required information on vital and migration status for all diagnosed individuals. Although, death numbers are expected to be accurate for most countries, data on out-migration are lacking. Therefore, the reported number of PLHIV may be slightly overestimated. Also, for small populations, PLHIV estimates have high levels of uncertainty, indicated by wider CIs. Another limitation of this study is its cross-sectional design. Recent studies have highlighted the importance of investigating, next to the standard CoC, time intervals that PLHIV spend in each stage [34]. A longitudinal CoC has been recently constructed [20, 34, 35].
Despite differences across countries, the 11 participating countries are on track to meet the 90-90-90 target by 2020. Ηowever, the participating countries mainly represent the Western EU countries, that seem to be closer to the 90-90-90 target compared with the Central and Eastern European countries [14]. In addition, as transmission due to undiagnosed HIV or unsuppressed viremia continues, both in the EU region and in neighboring countries, it may be difficult to substantially reduce HIV incidence without additional prevention measures, such as increased condom use, harm reduction programs for PWID, and national preexposure prophylaxis programs [36–39]. Indeed, the HIV epidemic has not been sufficiently restricted, with data indicating a slower than expected incidence decline [6]. It has been shown that for MSM, who represent more than 40% of all PLHIV in the participating countries, given immediate ART at diagnosis and no increase in condomless sex, an increase of up to 90% in the proportion of virally suppressed PLHIV within 1 year of infection is needed in order to reduce HIV incidence from 6/1000 to 1/1000 person-years in the United Kingdom [40]. In addition, late presentation remains high in most European countries, highlighting the need for timely HIV diagnosis [41]. Thus, strengthening of testing programs, including self-testing, and stronger treatment and adherence support, along with HIV transmission preventive measures, are needed to achieve epidemic control and, ultimately, zero new HIV transmission.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Austria: Austrian HIV Cohort Study Group
Steering committee members: Alexander Egle, Manfred Kanatschnig, Angela Öllinger, Armin Rieger, Brigitte Schmied, Elmar Wallner, Robert Zangerle.
Coordinating center: Medical University of Innsbruck (Robert Zangerle)
Funding: Austrian Agency for Health and Food Safety (AGES), hospitals running HIV treatment centers, pharmaceutical companies (equal contributions, irrespective of their market shares).
HIV treatment centers, *site coordinating physicians: LKH Innsbruck: Diyani Dewasurendra, Martin Gisinger, Maria Kitchen, Alexander Plattner, Elisabeth Rieser, Mario Sarcletti.* LKH Salzburg: Alexander Egle, Richard Greil,* Michaela Schachner. Kepler Universitätsklinikum Med Campus III. Linz: Angela Öllinger,* Matthias Skocic, Monika Müller. AKH Vienna: Regina Aichwalder, David Chromy, Katharina Grabmeier-Pfistershammer, Armin Rieger,* Michael Skoll, Veronique Touzeau. Otto-Wagner Hospital Vienna: Piotr Cichon, Brigitte Schmied,* Sonja Wolf-Nussmüller. Kaiser-Franz-Josef Hospital Vienna: Hermann Laferl, Alexander Zoufaly.* LKH Graz II, Standort West: Christina Genger-Hackl, Andreas Kapper, Thomas Schneeberger, Elisabeth Trattner, Elmar Wallner.* LKH Klagenfurt: Manfred Kanatschnig,* Georg Schober. Feldkirch: Michele Atzl,* Bernd Hartmann.
Virology: Elisabeth Puchhammer-Stöckl, Vienna; Jörg Berg, Kepler Universitäts-klinikum Med Campus III. Linz.
Data management: Heinz Appoyer (IT-related), Gisela Leierer (AHIVCOS), Michaela Rappold (AHIVCOS), Stefanie Strickner (AHIVCOS), Robert Zangerle (Medical University of Innsbruck).
Data safety and protection: Klaus Schindelwig, Innsbruck.
Scientific advisory board: Bruno Ledergerber, Zurich; Gerd Fätkenheuer, Cologne.
Croatia: Croatian HIV Cohort
This work was supported by the Croatian Science Foundation (project IP-2014–09-4461; principal investigator, Josip Begovac).
The Croatian HIV Cohort includes patients treated at the University Hospital of Infectious Diseases in Zagreb, Croatia (Josip Begovac, Davorka Lukas, Šime Zekan, Vanja Romih Pintar). This is the only treatment center in the country, and an electronic database is updated regularly and has been in use since 1997. Surveillance data were provided by the Croatian Institute of Public Health, Unit for HIV, Sexually Transmitted and Blood-Borne Infections, and HIV Registry (Tatjana Nemeth Blažić).
Denmark: Danish HIV Cohort Study
This work was supported by the Preben og Anne Simonsens Foundation.
The Danish HIV Cohort Study includes patients from the Departments of Infectious Diseases at Copenhagen University Hospitals, Rigshospitalet (J. Gerstoft, N. Obel) and Hvidovre (G. Kronborg), Odense University Hospital (C. Pedersen), Aarhus University Hospitals, Skejby (C.S. Larsen) and Aalborg (G. Pedersen), Herning Hospital (R. Mohey), Hillerød Hospital (L. Nielsen), Roskilde Hospital (L. Weise), Herlev University Hospital (B. Kvinesdal), Kolding Hospital (J. Jensen).
France: FHDH-ANRS CO4 Cohort
The FHDH ANRS CO4 cohort is funded by the ANRS, INSERM, and the French Ministry of Health.
Scientific committee: S. Abgrall, L. Bernard, E. Billaud, F. Boué, L. Boyer, A. Cabié, F. Caby, A. Canestri, D. Costagliola, L. Cotte, P. De Truchis, X. Duval, C. Duvivier, P. Enel, H. Fischer, J. Gasnault, C. Gaud, S. Grabar, M.A. Khuong, O. Launay, L. Marchand, M. Mary-Krause, S. Matheron, G. Melica-Grégoire, H. Melliez, J.L. Meynard, M. Nacher, J. Pavie, L. Piroth, I. Poizot-Martin, C. Pradier, J. Reynes, E. Rouveix, A. Simon, L. Slama, P. Tattevin, H. Tissot-Dupont.
COREVIH coordinating center: French Ministry of Health (J. Biga, T. Kurth), Technical Hospitalization Information Agency, ATIH (N. Jacquemet).
Statistical analysis center: UMRS 1136 INSERM et UPMC (D. Costagliola, principal investigator, S. Grabar, o-principal investigator, S. Abgrall, M. Guiguet, S. Leclercq, L. Lièvre, E. Marshall, M. Mary-Krause, H. Roul, H. Selinger-Leneman), INSERM-Transfert (V. Potard).
COREVIH: Paris area: Corevih Ile de France Centre (Paris-GH Pitié-Salpétrière: O. Benveniste, A. Simon, G. Breton, C. Lupin, E. Bourzam; Paris-Hôpital Saint-Antoine: P.M. Girard, L. Fonquernie, N. Valin, B. Lefebvre, M. Sebire; Paris-Hôpital Tenon: G. Pialoux, M.G. Lebrette, P. Thibaut, A. Adda, M. Hamidi, J. Cadranel, A. Lavolé, A. Parrot). Corevih Ile de France Est (Bobigny-Hôpital Avicenne: O. Bouchaud, N. Vignier, F. Méchaï, S. Makhloufi, P. Honoré; Bondy-Hôpital Jean Verdier; Paris-GH Lariboisière-Fernand Widal: J.F. Bergmann, V. Delcey, A. Lopes, P. Sellier, M. Parrinello; Paris-Hôpital Saint-Louis: E. Oksenhendler, L. Gerard, J.M. Molina, W. Rozenbaum, B. Denis, N. De Castro, C. Lascoux). Corevih Ile de France Nord (Paris-Hôpital Bichat-Claude Bernard: Y. Yazdanpanah, S. Matheron, S. Lariven, V. Joly, C. Rioux; St Denis-Hôpital Delafontaine: M.A. Khuong-Josses, M. Poupard, B. Taverne). Corevih Ile de France Ouest (Argenteuil-CH Victor Dupouy: L. Sutton, V. Masse, P. Genet, B. Wifaq, J. Gerbe; Boulogne Billancourt-Hôpital Ambroise Paré: E. Rouveix, S. Greffe, C. Dupont, A. Freire Maresca, E. Reimann; Colombes-Hôpital Louis Mourier: M. Bloch, F. Meier, E. Mortier, F. Zeng, B. Montoya; Garches-Hôpital Raymond Poincaré: C. Perronne P de Truchis, D. Mathez, D. Marigot-Outtandy, H. Berthé; Le Chesnay-Hôpital André Mignot: A. Greder Belan, A. Therby, C. Godin Collet, S.Marque Juillet, M. Ruquet, S. Roussin-Bretagne, P. Colardelle; Mantes La Jolie-CH François Quesnay: F. Granier, J.J. Laurichesse, V. Perronne; Meulan-CHI de Meulan les Mureaux: T. Akpan, M. Marcou; Nanterre-Hôpital Max Fourestier: V. Daneluzzi, J. Gerbe; Poissy-CHI de Poissy: C. Veyssier-Belot, H. Masson; St Germaine en Laye-CHI de St-Germain-en-Laye: Y. Welker, P. Brazille; Suresnes-Hôpital Foch: J.E. Kahn, D. Zucman, C. Majerholc, E. Fourn, D. Bornarel). Corevih Ile de France Sud (Clamart-Hôpital Antoine Béclère: F. Boué, S. Abgrall, V. Chambrin, I. Kansau, M. Raho-Moussa; Créteil-Hôpital Henri Mondor: J.D. Lelievre, G. Melica, M. Saidani, C. Chesnel, C. Dumont; Kremlin Bicêtre-Hôpital de Bicêtre: D. Vittecoq, O. Derradji, C. Bolliot, C. Goujard, E. Teicher, J. Gasnault, M. Mole, K. Bourdic; Paris-GH Tarnier-Cochin: D. Salmon, C. Le Jeunne, O. Launay, P. Guet, M.P. Pietri, E. Pannier Metzger, V. Marcou, P. Loulergue, N. Dupin, J.P. Morini, J. Deleuze, P. Gerhardt, J. Chanal; Paris-Hôpital Européen Georges Pompidou: L. Weiss, J. Pavie, M.L. Lucas, C. Jung, M. Ptak; Paris-Hôpital Hôtel Dieu: J.P. Viard, J. Ghosn, P. Gazalet, A. Cros, A. Maignan; Paris-Hôpital Necker adultes: C. Duvivier, O. Lortholary, C. Rouzaud, F. Touam, K. Benhadj; Paris-CMIP Pasteur: P.H. Consigny, P. Bossi, A. Gergely, G. Cessot, F. Durand).
Outside Paris area: Corevih Alsace (CH de Mulhouse: G. Beck-Wirth, C. Michel, M. Benomar; CHRU de Strasbourg: D. Rey, M. Partisani, C. Cheneau, M.L. Batard, P. Fischer). Corevih de l’Arc Alpin (CHU de Grenoble: P. Leclercq, M. Blanc, P. Morand, O. Epaulard, A. Signori-Schmuck). Corevih Auvergne-Loire (CHU de Clermont-Ferrand: H. Laurichesse, C. Jacomet, M. Vidal, D. Coban, S. Casanova; CHRU de Saint-Etienne: A. Fresard, C. Guglielminotti, E. Botelho-Nevers, A. Brunon-Gagneux, V. Ronat). Corevih Basse-Normandie (CHRU de Caen: R. Verdon, S. Dargère, E. Haustraete, P. Féret, P. Goubin). Corevih Bourgogne (CHRU de Dijon: P. Chavanet, A. Fillion, L. Piroth, D. Croisier, S. Gohier). Corevih Bretagne (CHU de Rennes: C. Arvieux, F. Souala, J.M. Chapplain, M. Ratajczak, J. Rohan). Corevih Centre Poitou-Charentes (CHRU de Tours). Corevih Franche-Comté (CH de Belfort: J.P. Faller, O. Ruyer, V. Gendrin, L. Toko; CHRU de Besançon: C. Chirouze, L. Hustache-Mathieu, J.F. Faucher, A. Proust, N. Magy-Bertrand, H. Gil, N. Méaux-Ruault). Corevih Haute-Normandie (CHRU de Rouen). Corevih Languedoc-Roussillon (CHU de Montpellier; CHU de Nîmes: A. Sotto, I. Rouanet, J.M. Mauboussin, R. Doncesco, G. Jacques). Corevih Lorraine Champagne Ardennes (Nancy-Hôpital de Brabois: T. May, C. Rabaud, M. Andre, M. Delestan, M.P. Bouillon; CHRU de Reims: F. Bani-Sadr, C. Rouger, J.L. Berger, Y. Nguyen). Corevih de Midi-Pyrénées Limousin (Toulouse CHU Purpan: B. Marchou, P. Delobel, G. Martin Blondel, L. Cuzin, N. Biezunski, L. Alric, D. Bonnet, M. Guivarch, A. Palacin, V. Payssan). Corevih Nord-Pas de Calais (CH de Tourcoing: H. Melliez, F. Ajana, A. Meybeck, N. Viget). Corevih PACA Est (Nice Hôpital Archet 1: C. Pradier, P. Pugliese, P.M. Roger, E. Rosenthal, J. Durant, E. Cua, A. Naqvi, I. Perbost, K. Risso; CH Antibes-Juan les Pins: D. Quinsat; CHI de Fréjus, St Raphaël: P. Del Giudice; CH de Grasse: P.Y. Dides). Corevih PACA Ouest (Marseille-Hôpital de la Conception: P. Enel, R. Sambuc, M.S. Antolini-Bouvenot, P. Druart, L. Meddeb, I. Ravaux, A. Menard, C. Tomei, C. Dhiver, H. Tissot-Dupont; Marseille-Hôpital Nord: J. Moreau, S. Mokhtari, M.J. Soavi, V. Thomas; Marseille-Hôpital Sainte-Marguerite: I. Poizot-Martin, S. Bregigeon, O. Faucher, V. Obry-Roguet, A.S. Ritleng, N. Petit; Marseille-Centre pénitentiaire des Baumettes: C. Bartoli, J.M. Ruiz, D. Blanc; CH d’Aix-En-Provence: T. Allegre, M. Sordage, J.M. Riou, C. Faudon; CH d’Avignon: B. Slama, H. Zerazhi, O. Boulat, S. Chebrek, M. Beyrne; CH de Digne Les Bains: P. Granet Brunello; CH de Gap: L. Pellissier, D. Bonnabel; CH de Martigues: R. Cohen Valensi, B. Mouchet, G. Mboungou; CHI de Toulon: A. Lafeuillade, E. Hope-Rapp, G. Hittinger, G. Philip, V. Lambry). Corevih Pays de la Loire (CHU de Nantes: F. Raffi, C. Allavena, E. Billaud, N. Hall, V. Reliquet). Corevih de la Vallée du Rhône (Lyon-Hôpital de la Croix-Rousse: C. Chidiac, L. Cotte, T. Ferry, T. Perpoint, P. Miailhes; Lyon-Hôpital Edouard Herriot: A. Boibieux, J.M. Livrozet, D. Makhloufi, F. Brunel, P. Chiarello).
Overseas: Corevih Guadeloupe (CHU de Pointe-à-Pitre: B. Hoen, I. Lamaury, I. Fabre, K. Samar, E. Duvallon; CH Saint-Martin: C. Clavel, S. Stegmann, V. Walter). Corevih Guyane (CH de Cayenne: M. Nacher, L. Adriouch, F. Huber, V. Vanticlke, P. Couppié). Corevih Martinique (CHU de Fort-de-France: A. Cabié, S. Abel, S. Pierre-François). Corevih de La Réunion (St Denis-CHU Félix Guyon: C. Gaud, C. Ricaud, R. Rodet, G. Wartel, C. Sautron; St Pierre-GH Sud Réunion: P. Poubeau, G. Borgherini, G. Camuset).
Germany: ClinSurv HIV
The clinical surveillance of HIV, ClinSurv HIV, is funded by the Robert Koch Institute, which is the German Public Health Institute.
Berlin: PD (stands for Privatdozent for men and Privatdozentin for women) Dr K. Arastéh, S. Kowohl Vivantes, Auguste-Viktoria-Clinic; Dr D. Schürmann, M. Warncke Charité, University Medicine Berlin. Bonn: Prof Dr J. Rockstroh, Dr J. Wasmuth, S. Hass University Medical Centre Bonn. Duesseldorf: PD Dr B.O. Jensen, C. Feind University Medical Centre Düsseldorf. Essen: Dr S. Esser, P. Schenk-Westkamp University Clinic Essen. Frankfurt: A. Haberl, C. Stephan HIV Center J.W. Goethe-University Frankfurt. Hamburg: Prof Dr A. Plettenberg, F. Kuhlendahl ifi (Institute for Interdisciplinary Medicine); Drs A. Adam, L. Weitner, K. Schewe, H. Goey; Drs S. Fenske, T. Buhk, Prof HJ. Stellbrink, PD C. Hoffmann, S Hansen at ICH (Infectious Diseases Centre) Study Centre Hamburg; PD Dr O Degen, M. Heuer at University Medical Centre Hamburg-Eppendorf. Hannover: Prof Dr M. Stoll, S. Gerschmann at Medical University Hannover. Kiel: Prof Dr H. Horst, S. Trautmann at University Clinic Schleswig-Holstein. Cologne: Prof Dr G. Fätkenheuer, D. Gillor at University Medical Centre Cologne. Munich: Prof Dr J. Bogner, B. Sonntag at University Hospital Munich. Regensburg: Prof Dr B. Salzberger at University Medical Centre Regensburg. Rostock: Dr C. Fritzsche at University Clinic Rostock.
Greece: AMACS Study
Steering committee: G. Adamis, A. Antoniadou, M. Chini, G. Chrysos, A. Gikas, H.A. Gogos, O. Katsarou, M. Lazanas, S. Metallidis, P. Panagopoulos, V. Paparizos, V. Papastamopoulos, D. Paraskevis, M. Psychogiou, H. Sambatakou (co-chair), N.V. Sipsas, G. Touloumi (chair).
Coordinating center: Department of Hygiene, Epidemiology and Medical Statistics, Medical School, National and Kapodistrian University of Athens, Greece (G. Touloumi, N. Pantazis, G. Vourli).
Participating centers: 4th Department of Internal Medicine, Medical School, National and Kapodistrian University of Athens, Attikon University Hospital (A. Antoniadou, A. Papadopoulos); Infectious Disease Unit, “Tzaneio” General Hospital of Piraeus (G. Chrysos, T. Nitsotolis); 1st Department of Propedeutic Medicine, Athens University, Medical School “Laikon” General Hospital (M. Psychogiou); 1st Department of Medicine, Infectious Diseases Unit, “G. Gennimatas” Athens General Hospital (G. Adamis, G. Xylomenos); 1st Department of Internal Medicine, Infectious Diseases Section, Patras University Hospital (H.A. Gogos, M.N. Marangos); Blood Transfusion Unit and National Reference Centre for Congenital Bleeding Disorders, Laikon General Hospital (O. Katsarou, A. Kouramba); Infectious Diseases Unit, Department of Pathophysiology, General Hospital of Athens “Laikon” and Medical School, National and Kapodistrian University of Athens, Athens, Greece (N.V. Sipsas, A. Kontos); Infectious Diseases Unit, Red Cross General Hospital of Athens (M. Chini, A. Lioni); First Internal Medicine Department, Infectious Diseases Division, Medical School, Aristotle University of Thessaloniki (S. Metallidis, O. Tsachouridou); AIDS Unit, Clinic of Venereologic & Dermatologic Diseases, Athens University, Medical School, Syngros Hospital (V. Paparizos, S. Kourkounti); HIV Unit, 2nd Department of Internal Medicine, Athens University, Medical School, Hippokration General Hospital (H. Sambatakou); Infectious Diseases & HIV Division, Department of Internal Medicine, Evaggelismos Athens General Hospital (V. Papastamopoulos); Infectious Diseases Unit, University General Hospital of Alexandroupolis, Democritus University of Thrace (P. Panagopoulos, A. Ganitis); Department of Internal Medicine, University Hospital of Heraklion, Heraklion, Crete, Greece (A. Gikas, E. Barbounakis).
Hellenic Society for the Study and Control of AIDS: M. Lazanas (chair), H. Gogos (co-chair).
Italy: ICoNA
Board of directors: A. d’Arminio Monforte (president), A. Antinori (vice-president), M. Andreoni, A. Castagna, F. Castelli, R. Cauda, G. Di Perri, M. Galli, R. Iardino, G. Ippolito, A. Lazzarin, G.C. Marchetti, G. Rezza, F. von Schloesser, P. Viale.
Scientific secretary: A. d’Arminio Monforte, A. Antinori, A. Castagna, F. Ceccherini-Silberstein, A. Cozzi-Lepri, E. Girardi, S. Lo Caputo, C. Mussini, M. Puoti, C.F. Perno.
Steering committee: A. Antinori, F. Bai, C. Balotta, A. Bandera, S. Bonora, M. Borderi, A. Calcagno, A. Capetti, M.R. Capobianchi, A. Castagna, F. Ceccherini-Silberstein, S. Cicalini, A. Cingolani, P. Cinque, A. Cozzi-Lepri, A. d’Arminio Monforte, A. Di Biagio, E. Girardi, N. Gianotti, A. Gori, G. Guaraldi, G. Lapadula, M. Lichtner, S. Lo Caputo, G. Madeddu, F. Maggiolo, G. Marchetti, L. Monno, C. Mussini, S. Nozza, C.F. Perno, C. Pinnetti, M. Puoti, E. Quiros Roldan, R. Rossotti, S. Rusconi, M.M. Santoro, A. Saracino, L. Sarmati.
Statistical and monitoring team: A. Cozzi-Lepri, I. Fanti, L. Galli, P. Lorenzini, A. Rodano’, M. Macchia, A. Tavelli.
Biological bank INMI: F. Carletti, S. Carrara, A. Di Caro, S. Graziano, F. Petroni, G. Prota, S. Truffa.
Participating physicians and centers: A. Giacometti, A. Costantini, V. Barocci (Ancona); G. Angarano, L. Monno, E. Milano (Bari); F. Maggiolo, C. Suardi (Bergamo); P. Viale, V. Donati, G. Verucchi (Bologna); F. Castelnuovo, C. Minardi, E. Quiros Roldan (Brescia); B. Menzaghi, C. Abeli (Busto Arsizio); B. Cacopardo, B. Celesia (Catania); J. Vecchiet, K. Falasca (Chieti); A. Pan, S. Lorenzotti (Cremona); L. Sighinolfi, D. Segala (Ferrara); P. Blanc, F. Vichi (Firenze); G. Cassola, C. Viscoli, A. Alessandrini, N. Bobbio, G. Mazzarello (Genova); M. Lichtner, L. Fondaco (Latina); P. Bonfanti, C. Molteni (Lecco); A. Chiodera, P. Milini (Macerata); G. Nunnari, G. Pellicanò (Messina); A. d’Arminio Monforte, M. Galli, A. Lazzarin, G. Rizzardini, M. Puoti, A. Castagna, E.S. Cannizzo, M.C. Moioli, R. Piolini, D. Bernacchia, S. Salpietro, C. Tincati (Milano); C. Mussini, C. Puzzolante (Modena); C. Migliorino, G. Lapadula (Monza); V. Sangiovanni, G. Borgia, V. Esposito, G. Di Flumeri, I. Gentile, V. Rizzo (Napoli); A.M. Cattelan, S. Marinello (Padova); A. Cascio, M. Trizzino (Palermo); D. Francisci, E. Schiaroli (Perugia); G. Parruti, F. Sozio (Pescara); G. Magnani, M.A. Ursitti (Reggio Emilia); M. Andreoni, A. Antinori, R. Cauda, A. Cristaudo, V. Vullo, R. Acinapura, D. Moschese, M. Capozzi, A. Mondi, A. Cingolani, M. Rivano Capparuccia, G. Iaiani, A. Latini, R. Gagliardini, M.M. Plazzi, G. De Girolamo, A. Vergori (Roma); M. Cecchetto, F. Viviani (Rovigo); G. Madeddu, A. De Vito (Sassari); B. Rossetti, F. Montagnani (Siena); A. Franco, R. Fontana Del Vecchio (Siracusa); C. Di Giuli (Terni); P. Caramello, G. Di Perri, S. Bonora, G.C. Orofino, M. Sciandra (Torino); M. Bassetti, A. Londero (Udine); V. Manfrin, G. Battagin (Vicenza); G. Starnini, A. Ialungo (Viterbo).
The Netherlands: ATHENA
The ATHENA database is maintained by Stichting HIV Monitoring and supported by a grant from the Dutch Ministry of Health, Welfare and Sport through the Centre for Infectious Disease Control of the National Institute for Public Health and the Environment.
Clinical centers
*Denotes site coordinating physician.
Amsterdam UMC, AMC site, Amsterdam: HIV treating physicians: M. van der Valk,* S.E. Geerlings, A. Goorhuis, J.W. Hovius, B. Lempkes, F.J.B. Nellen, T. van der Poll, J.M. Prins, P. Reiss, M. van Vugt, W.J. Wiersinga, F.W.M.N. Wit. HIV nurse consultants: M. van Duinen, J. van Eden, A. Hazenberg, A.M.H. van Hes, F.J.J. Pijnappel, S.Y. Smalhout, A.M. Weijsenfeld. HIV clinical virologists/chemists: S. Jurriaans, N.K.T. Back, H.L. Zaaijer, B. Berkhout, M.T.E. Cornelissen, C.J. Schinkel, K.C. Wolthers.
Amsterdam UMC, VUmc site, Amsterdam: HIV treating physicians: E.J.G. Peters,* M.A. van Agtmael, M. Bomers, K.C.E. Sigaloff. HIV nurse consultants: M. Heitmuller, L.M. Laan. HIV clinical virologists/chemists: C.W. Ang, R. van Houdt, M. Jonges, J. van Prehn.
Emma Kinderziekenhuis (Amsterdam UMC, AMC site): HIV treating physicians: T.W. Kuijpers, D. Pajkrt, H.J. Scherpbier. HIV nurse consultants: C. de Boer, A. van der Plas, A.M. Weijsenfeld.
Admiraal De Ruyter Ziekenhuis, Goes: HIV treating physicians: M. van den Berge,* A. Stegeman. HIV nurse consultants: S. Baas, L. Hage de Looff. HIV clinical virologists/chemists: B. Wintermans, J. Veenemans.
Catharina Ziekenhuis, Eindhoven: HIV treating physicians: M.J.H. Pronk,* H.S.M. Ammerlaan. HIV nurse consultants: E.S. de Munnik. HIV clinical virologists/chemists: A.R. Jansz, J. Tjhie, M.C.A. Wegdam, B. Deiman, V. Scharnhorst.
DC Klinieken Lairesse—Hiv Focus Centrum: HIV treating physicians: A. van Eeden,* M. van der Valk. HIV nurse consultants: W. Brokking, L.J.M. Elsenburg, H. Nobel. HIV clinical virologists/chemists: M. Damen.
ETZ (Elisabeth-TweeSteden Ziekenhuis), Tilburg: HIV treating physicians: M.E.E. van Kasteren,* M.A.H. Berrevoets, A.E. Brouwer. HIV nurse consultants: A. Adams, B.A.F.M. de Kruijf-van de Wiel, S. Keelan-Pfaf, B. van de Ven. Data collection: B.A.F.M. de Kruijf-van de Wiel, B. van der Ven. HIV clinical virologists/chemists: A.G.M. Buiting, J.L. Murck, D. Versteeg.
Erasmus MC, Rotterdam: HIV treating physicians: T.E.M.S. de Vries-Sluijs,* H.I. Bax, E.C.M. van Gorp, J.L. Nouwen, B.J.A. Rijnders, C.A.M. Schurink, A. Verbon, N.C. de Jong-Peltenburg. HIV nurse consultants: N. Bassant, J.E.A. van Beek, M. Vriesde, L.M. van Zonneveld. Data collection: H.J. van den Berg-Cameron, J. de Groot. HIV clinical virologists/chemists: C.A.B. Boucher, M.P.G. Koopmans, J.J.A. van Kampen.
Erasmus MC–Sophia, Rotterdam: HIV treating physicians: P.L.A. Fraaij, A.M.C. van Rossum, C.L. Vermont. HIV nurse consultants: L.C. van der Knaap, E. Visser.
Flevoziekenhuis, Almere: HIV treating physicians: J. Branger,* R.A. Douma. HIV nurse consultant: C.J.H.M. Duijf-van de Ven.
HagaZiekenhuis, Den Haag: HIV treating physicians: E.F. Schippers,* C. van Nieuwkoop. HIV nurse consultants: J.M. van IJperen, J. Geilings. Data collection: G. van der Hut. HIV clinical virologist/chemist: N.D. van Burgel.
HMC (Haaglanden Medisch Centrum), Den Haag: HIV treating physicians: E.M.S. Leyten,* L.B.S. Gelinck, F. Mollema. HIV nurse consultants: S. Davids-Veldhuis, G.S. Wildenbeest. HIV clinical virologists/chemists: E. Heikens.
Isala, Zwolle: HIV treating physicians: P.H.P. Groeneveld,* J.W. Bouwhuis, A.J.J. Lammers. HIV nurse consultants: S. Kraan, A.G.W. van Hulzen, M.S.M. Kruiper. Data collection: G.L. van der Bliek, P.C.J. Bor. HIV clinical virologists/chemists: P. Bloembergen, M.J.H.M. Wolfhagen, G.J.H.M. Ruijs.
Leids Universitair Medisch Centrum, Leiden: HIV treating physicians: F.P. Kroon,* M.G.J. de Boer, H. Scheper, H. Jolink. HIV nurse consultants: W. Dorama, N. van Holten. HIV clinical virologists/chemists: E.C.J. Claas, E. Wessels.
Maasstad Ziekenhuis, Rotterdam: HIV treating physicians: J.G. den Hollander,* R. El Moussaoui, K. Pogany. HIV nurse consultants: M. Kastelijns, J.V. Smit, E. Smit, D. Struik-Kalkman, C. Tearno. Data collection: T. van Niekerk. HIV clinical virologists/chemists: O. Pontesilli.
Maastricht UMC+, Maastricht: HIV treating physicians: S.H. Lowe,* A.M.L. Oude Lashof, D. Posthouwer. HIV nurse consultants: R.P. Ackens, K. Burgers, J. Schippers. Data collection: B. Weijenberg-Maes. HIV clinical virologists/chemists: I.H.M. van Loo, T.R.A. Havenith.
MC Zuiderzee, Lelystad: HIV treating physicians: S. Weijer.*
Medisch Centrum Leeuwarden, Leeuwarden: HIV treating physicians: M.G.A.van Vonderen,* L.M. Kampschreur. HIV nurse consultants: S. Faber, R. Steeman-Bouma. HIV clinical virologists/chemists: J Weel.
Medisch Spectrum Twente, Enschede: HIV treating physicians: G.J. Kootstra,* C.E. Delsing. HIV nurse consultants: M. van der Burg-van de Plas, H. Heins.
Noordwest Ziekenhuisgroep, Alkmaar: HIV treating physicians: W. Kortmann,* G. van Twillert,* R. Renckens. HIV nurse consultant and data collection: D. Ruiter-Pronk, F.A. van Truijen-Oud. HIV clinical virologists/chemists: J.W.T. Cohen Stuart, E.R. Jansen, M. Hoogewerf, W. Rozemeijer, W.A. van der Reijden, J.C. Sinnige.
OLVG, Amsterdam: HIV treating physicians: K. Brinkman,* G.E.L. van den Berk, W.L. Blok, P.H.J. Frissen, K.D. Lettinga, W.E.M. Schouten, J. Veenstra, S.M.E. Vrouenraets. HIV nurse consultants: C.J. Brouwer, G.F. Geerders, K. Hoeksema, M.J. Kleene, M. Knapen, I.B. van der Meché, E. Mulder-Seeleman, A.J.M. Toonen, S. Wijnands. HIV clinical virologists: D. Kwa.
Radboudumc, Nijmegen: HIV treating physicians: R. van Crevel,* K. van Aerde, A.S.M. Dofferhoff, S.S.V. Henriet, H.J.M. ter Hofstede, J. Hoogerwerf, M. Keuter, O. Richel. HIV nurse consultants: M. Albers, K.J.T. Grintjes-Huisman, M. de Haan, M. Marneef, R. Strik-Albers. HIV clinical virologists/chemists: J. Rahamat-Langendoen, F.F. Stelma. HIV clinical pharmacology consultant: D. Burger.
Rijnstate, Arnhem: HIV treating physicians: E.H. Gisolf,* R.J. Hassing, M. Claassen. HIV nurse consultants: G. ter Beest, P.H.M. van Bentum, N. Langebeek. HIV clinical virologists/chemists: R. Tiemessen, C.M.A. Swanink.
Spaarne Gasthuis, Haarlem: HIV treating physicians: S.F.L. van Lelyveld,* R. Soetekouw. HIV nurse consultants: L.M.M. van der Prijt, J. van der Swaluw. Data collection: N. Bermon. HIV clinical virologists/chemists: W.A. van der Reijden, R. Jansen, B.L. Herpers, D.Veenendaal.
Medisch Centrum Jan van Goyen, Amsterdam: HIV treating physicians D.W.M. Verhagen, F.N. Lauw. HIV nurse consultants: M.C. van Broekhuizen, M. van Wijk.
Universitair Medisch Centrum Groningen, Groningen: HIV treating physicians: W.F.W. Bierman,* M. Bakker, J. Kleinnijenhuis, E. Kloeze, A. Middel, E.H. Scholvinck, Y. Stienstra, A.R. Verhage, C.L. Vermont, K.M. Wouthuyzen-Bakker. HIV nurse consultants: A. Boonstra, H. de Groot-de Jonge, P.A. van der Meulen, D.A. de Weerd. HIV clinical virologists/chemists: H.G.M. Niesters, C.C. van Leer-Buter, M. Knoester.
Universitair Medisch Centrum Utrecht, Utrecht: HIV treating physicians: A.I.M. Hoepelman,* J.E. Arends, R.E. Barth, A.H.W. Bruns, P.M. Ellerbroek, T. Mudrikova, J.J. Oosterheert, M.J.A. de Regt, E.M. Schadd, M.A.D. van Zoelen. HIV nurse consultants: K. Aarsman, B.M.G. Griffioen-van Santen, I. de Kroon, C.S.A.M. van Rooijen. Data collection: M. van Berkel, C.S.A.M. van Rooijen. HIV clinical virologists/chemists: R. Schuurman, F. Verduyn-Lunel, A.M.J. Wensing.
Wilhelmina Kinderziekenhuis, UMC Utrecht, Utrecht: HIV treating physicians: L.J. Bont, S.P.M. Geelen, Y.G.T. Loeffen, T.F.W. Wolfs. HIV nurse consultants: N. Nauta.
Coordinating center: Director: P. Reiss. Deputy director: S. Zaheri. Data analysis: A.C. Boyd, D.O. Bezemer, A.I. van Sighem, C. Smit, F.W.M.N. Wit. Data management and quality control: M. Hillebregt, A. de Jong, T. Woudstra. Data monitoring: D. Bergsma, R. Meijering, L. van de Sande, T. Rutkens, S. van der Vliet. Data collection: L. de Groot, M. van den Akker, Y. Bakker, A. El Berkaoui, M. Bezemer, N. Brétin, E. Djoechro, J. Geerlinks, E. Kruijne, C. Lodewijk, E. Lucas, R. van der Meer, L. Munjishvili, F. Paling, B. Peeck, C. Ree, R. Regtop, Y. Ruijs, M. Schoorl, P. Schnörr, E. Tuijn, L. Veenenberg, E.C. Witte. Patient registration: Y. Ruijs, B. Tuk.
Spain: CoRIS
This work was supported by the Spanish Network of HIV/AIDS (RD12/0017/0018) and CIBER Epidemiología y Salud Pública, Spain. V. Hernando, O. Nuñez, A. Diaz.
Centers and investigators involved in CoRIS: Executive committee: Santiago Moreno, Julia del Amo, David Dalmau, Maria Luisa Navarro, Maria Isabel González, Jose Luis Blanco, Federico Garcia, Rafael Rubio, Jose Antonio Iribarren, Félix Gutiérrez, Francesc Vidal, Juan Berenguer, Juan González. Fieldwork, data management, and analysis: Paz Sobrino, Victoria Hernando, Belén Alejos, Débora Álvarez, Inma Jarrín, Cristina Moreno. BioBanK HIV: M. Ángeles Muñoz-Fernández, Isabel García-Merino, Coral Gómez Rico, Jorge Gallego de la Fuente, Almudena García Torre. Participating centers: Hospital General Universitario de Alicante (Alicante): Joaquín Portilla, Esperanza Merino, Sergio Reus, Vicente Boix, Livia Giner, Carmen Gadea, Irene Portilla, Maria Pampliega, Marcos Díez, Juan Carlos Rodríguez, Jose Sánchez-Payá. Hospital Universitari de Bellvitge (Hospitalet de Llobregat): Daniel Podzamczer, Elena Ferrerm Arkaitz Imaz, Evan Van Den Eyncle, Silvana Di Yacovo, Maria Sumoy. Hospital Universitario de Canarias (Santa Cruz de Tenerife): Juan Luis Gómez, Jehovana Hernández, María Remedios Alemán, María del Mar Alonso, María Inmaculada Hernández, Felicitas Díaz-Flores, Dácil García, Ricardo Pelazas. Hospital Universitario Central de Asturias (Oviedo): Victor Asensi, Eulalia Valle, José Antonio Cartón. Hospital Clínico San Carlos (Madrid): Vicente Estrada Pérez, Maria Jesus Téllez Molina, Jorge Vergas García, Elisa Pérez-Cecila Carrera. Hospital Doce de Octubre (Madrid): Rafael Rubio, Federico Pulido, Otilia Bisbal, Mariano Matarranz, Maria Lagarde, Rafael Rubio-Martín, Asunción Hernando, Laura Bermejo, Lourdes Dominguez. Hospital Universitario Donostia (San Sebastián): José Antonio Iribarren, Julio Arrizabalaga, María José Aramburu, Xabier Camino, Francisco Rodríguez-Arrondo, Miguel Ángel von Wichmann, Lidia Pascual Tomé, Miguel Ángel Goenaga, Mª Jesús Bustinduy, Harkaitz Azkune Galparsoro, Maialen Ibarguren, Mirian Aguado, Maitane Umerez. Hospital General Universitario de Elche (Elche): Félix Gutiérrez, Mar Masiá, Cristina López, Sergio Padilla, Andrés Navarro, Fernando Montolio, Catalina Robledano, Joan Gregori Colomé, Araceli Adsuar, Rafael Pascual, Federico Carlos, Maravillas Martinez, Jara Llenas García, Marta Fernández, Elena García. Hospital Germans Trías i Pujol (Badalona): Roberto Muga, Jordi Tor, Arantza Sanvisens. Hospital General Universitario Gregorio Marañón (Madrid): Juan Berenguer, Juan Carlos López Bernaldo de Quirós, Pilar Miralles, Isabel Gutiérrez, Margarita Ramírez, Belén Padilla, Paloma Gijón, Ana Carrero, Teresa Aldamiz-Echevarría, Francisco Tejerina, Francisco Jose Parras, Pascual Balsalobre, Cristina Diez. Hospital Universitari de Tarragona Joan XXIII, IISPV, Universitat Rovira i Virgili (Tarragona): Francesc Vidal, Joaquín Peraire, Consuelo Viladés, Sergio Veloso, Montserrat Vargas, Miguel López-Dupla, Montserrat Olona, Alba Aguilar, Joan Josep Sirvent, Verónica Alba, Olga Calavia. Hospital Universitario La Fe (Valencia): Marta Montero, José Lacruz, Marino Blanes, Eva Calabuig, Sandra Cuellar, José López, Miguel Salavert. Hospital Universitario La Paz/IdiPaz (Madrid): Juan González, Ignacio Bernardino de la Serna, José Ramón Arribas, María Luisa Montes, Jose Mª Peña, Blanca Arribas, Juan Miguel Castro, Javier Zamora, Ignacio Pérez, Miriam Estébanez, Silvia García, Marta Díaz, Natalia Stella Alcáriz, Jesús Mingorance, Dolores Montero, Alicia González, Maria Isabel de José. Hospital de la Princesa (Madrid): Ignacio de los Santos, Jesús Sanz, Ana Salas, Cristina Sarriá, Ana Gómez Berrocal, Lucio Garcia-Fraile. Hospital San Pedro-CIBIR (Logroño): José Antonio Oteo, José Ramón Blanco, Valvanera Ibarra, Luis Metola, Mercedes Sanz, Laura Pérez-Martínez. Hospital Universitario Miguel Servet (Zaragoza): Ascensión Pascual, Carlos Ramos, Piedad Arazo, Desiré Gil. Hospital Universitari Mutua de Terrassa (Terrassa): David Dalmau, Angels Jaén, Mireia Cairó, Daniel Irigoyen, Queralt Jordano, Mariona Xercavins, Javier Martinez-Lacasa, Pablo Velli, Roser Font, Montse Sanmartí, Laura Ibáñez. Complejo Hospitalario de Navarra (Pamplona): María Rivero, Marina Itziar Casado, Jorge Alberto Díaz, Javier Uriz, Jesús Repáraz, Carmen Irigoyen, María Jesús Arraiza. Hospital Parc Taulí (Sabadell): Ferrán Segura, María José Amengual, Gemma Navarro, Montserrat Sala, Manuel Cervantes, Valentín Pineda, Victor Segura, Marta Navarro, Esperanza Antón, Mª Merce Nogueras. Hospital Ramón y Cajal (Madrid): Santiago Moreno, José Luis Casado, Fernando Dronda, Ana Moreno, María Jesús Pérez Elías, Dolores López, Carolina Gutiérrez, Nadia Madrid, Angel Lamas, Paloma Martí, Alberto de Diaz, Sergio Serrrano, Lucas Donat. Hospital Reina Sofía (Murcia): Alfredo Cano, Enrique Bernal, Ángeles Muñoz. Hospital San Cecilio (Granada): Federico García, José Hernández, Alejandro Peña, Leopoldo Muñoz, Jorge Parra, Marta Alvarez, Natalia Chueca, Vicente Guillot, David Vinuesa, Jose Angel Fernández. Centro Sanitario Sandoval (Madrid): Jorge Del Romero, Carmen Rodríguez, Teresa Puerta, Juan Carlos Carrió, Mar Vera, Juan Ballesteros. Hospital de la Santa Creu i Sant Pau (Barcelona): Pere Domingo, Mª Antonia Sambeat, Karuna Lamarca, Gracia Mateo, Mar Gutiérrez, Irene Fernández. Hospital Universitario Santiago de Compostela (Santiago de Compostela): Antonio Antela, Elena Losada. Hospital Son Espases (Palma de Mallorca): Melchor Riera, Maria Peñaranda, Maria Leyes, Mª Angels Ribas, Antoni A Campins, Carmen Vidal, Leire Gil, Francisco Fanjul, Carmen Marinescu. Hospital Universitari Vall d´Hebron (Barcelona): Esteban Ribera. Hospital Virgen de la Victoria (Málaga): Jesús Santos, Manuel Márquez, Isabel Viciana, Rosario Palacios, Isabel Pérez, Carmen Maria González. Hospital Universitario Virgen del Rocío (Sevilla): Pompeyo Viciana, Manuel Leal, Luis Fernando López-Cortés, Nuria Espinosa. Hospital Universitario de Basurto (Bilbao): Josefa Muñoz, Miren Zuriñe Zubero, Josu Mirena Baraia-Etxaburu, Sofía Ibarra, Oscar Ferrero, Josefina López de Munain, Mª Mar Cámara, Iñigo López, Mireia de la Peña. Hospital Universitario Infanta Sofía (San Sebastián de los Reyes): Inés Suárez-García, Eduardo Malmierca. Hospital Universitario Costa del Sol (Marbella): Julián Olalla, Alfonso del Arco, Javier de la Torre, José Luis Prada, Zaira Caracuel. Hospital del Poniente (El Ejido): Ana Maria Lopez-Lirola, Ana Belén Lozano, Elisa Fernández, Inés Pérez, Juan Manuel Fernández. Hospital Universitario Santa Lucia (Cartagena): Onofre Juan Martínez, Francisco Jesús Vera, Lorena Martínez, Josefina García, Begoña Alcaraz, Amaya Jimeno. INIBIC-Complejo Hospitalario Universitario de A Coruña (A Coruña): Eva Poveda, Berta Pernas, Álvaro Mena, Marta Grandal, Ángeles Castro, José D. Pedreira. Hospital Clínico Universitario Virgen de la Arrixaca (Murcia): Carlos Galera, Helena Albendin, Asunción Iborra, Antonio Moreno, Maria Angeles Campillo, Asunción Vidal. Hospital Marina Baixa (Villajoyosa): Concha Amador, Francisco Pasquau, Javier Ena, Concha Benito, Vicenta Fenoll. Complejo Hospitalario de Jaén (Jaén): Mohamed Omar Mohamed-Balghata, Maria Amparo Gómez. Hospital San Agustín de Aviles (Avilés): Miguel Alberto de Zarraga, Maria Eugenia Rivas. Fundación Jiménez Diaz (Madrid): Miguel Górgolas.
Sweden: InfCare HIV
The InfCare HIV cohort is funded by the Swedish Association of Local Authorities and Regions and by the Swedish HIV clinics.
InfCare HIV steering committee: Anders Sönnerborg (director), Veronica Svedhem-Johansson, Leo Flamholc, Magnus Gisslén, Bo Hejdeman, Hans Norgren, Suzanne Wendahl.
InfCare HIV participitating centers: Karolinska University Hospital, South Hospital, Sahlgrenska University Hospital, Skane University Hospital, Borås Hospital, Eskilstuna Hospital, Falun Hospital, Gävle Hospital, Halmstad Hospital, Helsingborg Hospital, Kalmar Hospital, Karlskrona Hospital, Karlstad Hospital, Kristianstad Hospital, Linköping University Hospital, Ryhov County Hospital, Skövde Hospital, Sundsvall Hospital, Sunderbyn Hospital, Trollhättan Hospital, Uppsala University Hospital, University Hospital of Umeå, Visby Hospital, Västerås Central Hospital, Växjö Hospital, Örebro University Hospital, Östersund Hospital.
Acknowledgments. We would like to thank all the participants who took part in cohort studies in each country, as well as the personnel of these studies.
Disclaimer. This article presents independent results and research. The views expressed are those of the authors and not necessarily those of the Instituto de Salud Carlos III.
Financial support. This work was supported by the European Centre for Disease Prevention and Control through a framework contract (ECDC/2016/028).
Potential conflicts of interest. J. B. reports grants from the Croatian Science Foundation (project IP-2014–09–4461) during the conduct of the study, personal fees and nonfinancial support from MSD and Gilead, and grants and personal fees from ViiV outside the submitted work. D. C. reports human immunodeficiency virus-related research grants from Janssen and MSD France, personal fees from Janssen, MSD France, and Gilead for lectures, and personal fees from Merck Switzerland for consultancy outside the submitted work. E. G. reports grants from Gilead and Mylan and personal fees from ViiV, Mylan, and Gilead outside the submitted work. D. P. reports personal fees from Mylan outside the submitted work. K. P. has received a grant paid to her institution for this work from the European Centre for Disease Prevention and Control (ECDC) and has received honoraria from ViiV Healthcare, Gilead Sciences, and MSD, all of which are unrelated to this work. A. S. reports grants and personal fees from Gilead Sciences and GSK outside the submitted work. V. S. has served on advisory boards for ViiV Healthcare and Gilead and reports lecture fees from Gilead, Janssen, ViiV, and AbbVie (2018) outside the submitted work. A. v S. received funding paid to his institution from the ECDC to support this work. P. R. reports grants to his institution from Gilead Sciences, ViiV Healthcare, and Merck & Co and honoraria paid to his institution for advisory board participation from Gilead Sciences, ViiV Healthcare, Merck & Co, and Teva Pharmaceutical Industries outside the submitted work. G. T. has received grants paid to her institution and unrelated to this study from Gilead Sciences Europe, Bristol University, UCL, ECDC, and European Union and National funds and received a grant paid to her institution from University College London (UCL) to support this work. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Lundgren JD, Babiker AG, Gordin F, et al. Insight Start Study Group. Initiation of antiretroviral therapy in early asymptomatic HIV infection. N Engl J Med 2015; 373:795–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Eshleman SH, Hudelson SE, Redd AD, et al. Treatment as prevention: characterization of partner infections in the HIV prevention trials network 052 trial. J Acquir Immune Defic Syndr 2017; 74:112–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Campbell MS, Mullins JI, Hughes JP, et al. ; Partners in Prevention HSV/HIV Transmission Study Team Viral linkage in HIV-1 seroconverters and their partners in an HIV-1 prevention clinical trial. PLoS One 2011; 6:e16986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. The Lancet HIV. U=U taking off in 2017. Lancet HIV 2017; 4:e475. doi: 10.1016/S2352-3018(17)30183-2 [DOI] [PubMed] [Google Scholar]
- 5. Rodger AJ, Cambiano V, Bruun T, et al. ; PARTNER Study Group Risk of HIV transmission through condomless sex in serodifferent gay couples with the HIV-positive partner taking suppressive antiretroviral therapy (PARTNER): final results of a multicentre, prospective, observational study. Lancet 2019; 393:2428–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. European Centre for Disease Prevention and Control, World Health Organization Regional Office for Europe. HIV/AIDS surveillance in Europe 2018–2017 data. Copenhagen: WHO Regional Office for Europe, 2018. [Google Scholar]
- 7. Marks G, Crepaz N, Janssen RS. Estimating sexual transmission of HIV from persons aware and unaware that they are infected with the virus in the USA. AIDS 2006; 20:1447–50. [DOI] [PubMed] [Google Scholar]
- 8. Cohen MS, Chen YQ, McCauley M, et al. ; HPTN 052 Study Team Prevention of HIV-1 infection with early antiretroviral therapy. N Engl J Med 2011; 365:493–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Altice F, Evuarherhe O, Shina S, Carter G, Beaubrun AC. Adherence to HIV treatment regimens: systematic literature review and meta-analysis. Patient Prefer Adherence 2019; 13:475–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Joint United Nations Programme on HIV/AIDS (UNAIDS). 90-90-90: An ambitious target to help end the AIDS epidemic. Geneva: UNAIDS, 2014. Available from: http://www.unaids.org/sites/default/files/media_asset/90-90-90_en.pdf. Accessed 20 October 2019. [Google Scholar]
- 11. Drew RS, Rice B, Rüütel K, et al. HIV continuum of care in Europe and Central Asia. HIV Med 2017; 18:490–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Medland NA, McMahon JH, Chow EP, Elliott JH, Hoy JF, Fairley CK. The HIV care cascade: a systematic review of data sources, methodology and comparability. J Int AIDS Soc 2015; 18:20634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Gourlay AJ, Pharris AM, Noori T, et al. Towards standardized definitions for monitoring the continuum of HIV care in Europe. AIDS 2017; 31:2053–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. European Centre for Disease Prevention and Control. Continuum of HIV care. Monitoring implementation of the Dublin declaration on partnership to fight HIV/AIDS in Europe and Central Asia: 2018 progress report. Stockholm: ECDC, 2018. [Google Scholar]
- 15. Gourlay A, Noori T, Pharris A, et al. ; European HIV Continuum of Care Working Group The human immunodeficiency virus continuum of care in European Union countries in 2013: data and challenges. Clin Infect Dis 2017; 64:1644–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. World Health Organization. Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection 2016: recommendations for a public health approach. Geneva, Switzerland: WHO, 2016. [Google Scholar]
- 17. Brown AE, Attawell K, Hales D, et al. Monitoring the HIV continuum of care in key populations across Europe and Central Asia. HIV Medicine 2018; 19:431–39. [DOI] [PubMed] [Google Scholar]
- 18. Clifton S, Nardone A, Field N, et al. HIV testing, risk perception, and behaviour in the British population. AIDS 2016; 30:943–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Khawcharoenporn T, Chunloy K, Apisarnthanarak A. Uptake of HIV testing and counseling, risk perception and linkage to HIV care among Thai university students. BMC Public Health 2016; 16:556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Supervie V, Marty L, Lacombe JM, Dray-Spira R, Costagliola D; FHDH-ANRS CO4 study group Looking beyond the cascade of HIV care to end the AIDS epidemic: estimation of the time interval from HIV infection to viral suppression. J Acquir Immune Defic Syndr 2016; 73:348–55. [DOI] [PubMed] [Google Scholar]
- 21. Grau LE, Griffiths-Kundishora A, Heimer R, et al. Barriers and facilitators of the HIV care continuum in southern New England for people with drug or alcohol use and living with HIV/AIDS: perspectives of HIV surveillance experts and service providers. Addict Sci Clin Pract 2017; 12:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Risher K, Mayer KH, Beyrer C. HIV treatment cascade in MSM, people who inject drugs, and sex workers. Curr Opin HIV AIDS 2015; 10:420–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lesko CR, Tong W, Moore RD, Lau B. Retention, antiretroviral therapy use and viral suppression by history of injection drug use among HIV-infected patients in an urban HIV clinical cohort. AIDS Behav 2016; 21:1016–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Porter K, Gourlay A, Attawell K, et al. ; ECDC Dublin Declaration Monitoring Network Substantial heterogeneity in progress toward reaching the 90-90-90 HIV target in the WHO European Region. J Acquir Immune Defic Syndr 2018; 79:28–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. European Centre for Disease Prevention and Control. ECDC HIV modelling tool [software application] 2015 [Version 1.3.0 Stockholm ed.]. Stockholm: ECDC; Available from: http://ecdc.europa.eu/en/healthtopics/aids/Pages/hiv-modelling-tool.aspx. [Google Scholar]
- 26. European Centre for Disease Prevention and Control. Thematic report: HIV continuum of care. Monitoring implementation of the Dublin declaration on partnership to fight HIV/AIDS in Europe and Central Asia: 2014 progress report. Stockholm: ECDC, 2015. [Google Scholar]
- 27. European Commission, Eurostat: Your key to European statistics. Available at: https://ec.europa.eu/eurostat/web/population-demography-migration-projections/data/database. Accessed 24 October 2019.
- 28. Almirol EA, McNulty MC, Schmitt J, et al. Gender differences in HIV testing, diagnosis, and linkage to care in healthcare settings: identifying African American women with HIV in Chicago. AIDS Patient Care STDS 2018; 32: 399–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Porter K, Gourlay A. Men and the young are key to reaching the first 90. Lancet HIV 2017; 4:e479–80. [DOI] [PubMed] [Google Scholar]
- 30. Valle S, Pezzotti P, Floridia M, et al. Percentage and determinants of missed HIV testing in pregnancy: a survey of women delivering in the Lazio region, Italy. AIDS Care 2014; 26:899–906. [DOI] [PubMed] [Google Scholar]
- 31. Regine V, Dorrucci M, Pezzotti P, et al. People living with undiagnosed HIV infection and a low CD4 count: estimates from surveillance data, Italy, 2012 to 2014. Euro Surveill 2018; 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. European Centre for Disease Prevention and Control. HIV testing. Monitoring implementation of the Dublin declaration on partnership to fight HIV/AIDS in Europe and Central Asia: 2017 progress report. Stockholm: ECDC, 2017. [Google Scholar]
- 33. Vourli G, Pharris A, Cazein F, et al. Are European HIV cohort data within EuroCoord representative of the diagnosed HIV population? AIDS 2019; 33:133–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Jose S, Delpech V, Howarth A, et al. ; United Kingdom CHIC Study Steering Committee A continuum of HIV care describing mortality and loss to follow-up: a longitudinal cohort study. Lancet HIV 2018; 5:e301–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Lee H, Wu XK, Genberg BL, et al. ; Centers for AIDS Research Network of Integrated Clinical Systems Investigators Beyond binary retention in HIV care: predictors of the dynamic processes of patient engagement, disengagement, and re-entry into care in a US clinical cohort. AIDS 2018; 32:2217–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Bradley H, Rosenberg ES, Holtgrave DR. Data-driven goals for curbing the U.S. HIV epidemic by 2030. AIDS Behav 2019; 23:557–63. [DOI] [PubMed] [Google Scholar]
- 37. Scott N, Stoové M, Kelly SL, Wilson DP, Hellard ME. Achieving 90-90-90 human immunodeficiency virus (HIV) targets will not be enough to achieve the HIV incidence reduction target in Australia. Clin Infect Dis 2018; 66:1019–23. [DOI] [PubMed] [Google Scholar]
- 38. LeVasseur MT, Goldstein ND, Tabb LP, Olivieri-Mui BL, Welles SL. The effect of PrEP on HIV incidence among men who have sex with men in the context of condom use, treatment as prevention, and seroadaptive practices. J Acquir Immune Defic Syndr 2018; 77:31–40. [DOI] [PubMed] [Google Scholar]
- 39. Aspinall EJ, Nambiar D, Goldberg DJ, et al. Are needle and syringe programmes associated with a reduction in HIV transmission among people who inject drugs: a systematic review and meta-analysis. Int J Epidemiol 2014; 43:235–48. [DOI] [PubMed] [Google Scholar]
- 40. Phillips AN, Cambiano V, Miners A, et al. Potential impact on HIV incidence of higher HIV testing rates and earlier antiretroviral therapy initiation in MSM. AIDS 2015; 29:1855–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Mocroft A, Lundgren J, Antinori A, et al. Late presenters working group in COHERE in EuroCoord. Late presentation for HIV care across Europe: update from the Collaboration of Observational HIV Epidemiological Research Europe (COHERE) study, 2010 to 2013. Euro Surveill 2015; 20:2–12. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.