Abstract
Background
Mycobacterium abscessus causes chronic pulmonary infections. Owing to its resistance to most classes of antibiotics, treatment is complex and cure rates are only 45%. Tigecycline is active against M. abscessus, but severe toxicity and the need for IV administration limit its use.
Objectives
To assess the potential of inhaled tigecycline as a treatment for M. abscessus pulmonary disease, by measuring its efficacy in a mouse model of chronic M. abscessus pulmonary disease, establishing the intracellular activity of tigecycline against M. abscessus in human macrophages and measuring the activity of tigecycline in the sputum of cystic fibrosis patients.
Methods
We infected GM-CSF knockout mice with M. abscessus by intrapulmonary aerosol. Infected mice were treated with tigecycline in 0.25, 1.25 and 2.5 mg doses, by inhalation, or untreated, for 28 days. Tigecycline was added to human peripheral blood-derived macrophages infected with M. abscessus to assess its intracellular activity. We performed a time–kill kinetics experiment of tigecycline against M. abscessus with and without sputum of cystic fibrosis patients.
Results
Inhaled tigecycline proved highly effective against M. abscessus in GM-CSF knockout mice. The effect was dose dependent. Tigecycline showed potent activity against M. abscessus in macrophages and retained most of its activity in the presence of sputum of cystic fibrosis patients.
Conclusions
Inhaled tigecycline may represent a viable treatment option for M. abscessus pulmonary disease, where treatment outcomes are currently very poor. A stable and safe formulation is required to proceed to further pharmacodynamic studies and ultimately clinical trials.
Introduction
Mycobacterium abscessus causes severe, chronic pulmonary infections in susceptible hosts. For its intrinsic resistance to most classes of antibiotics, M. abscessus has been rightfully dubbed an ‘antibiotic nightmare’.1 Treatment regimens for M. abscessus pulmonary disease typically involve an intensive phase first, with multiple IV antibiotics, which include imipenem, amikacin and tigecycline, with oral azithromycin. The second, continuation phase consists of multiple oral and inhaled agents whose selection is guided, in part, by susceptibility testing. These typically include azithromycin, clofazimine, moxifloxacin, minocycline, linezolid and inhaled amikacin.2,3 For most of the oral drugs and inhaled amikacin, efficacy is unproven and in vitro data suggest limited to no activity.4–6 Perhaps as a result, treatment outcomes in M. abscessus pulmonary disease are very poor, with treatment success rates of 45%.7
IV tigecycline became a drug of choice after a retrospective case series showed favourable responses to tigecycline-containing regimens in 16 of 26 patients (62%) with M. abscessus pulmonary disease who received >1 month of tigecycline treatment.8 Yet adverse events were reported in 94% of patients, most commonly nausea and vomiting. Ten of the 36 patients (28%) had to stop tigecycline therapy early (within the first month), because of these adverse events and more than half of the patients treated for >1 month received reduced doses of tigecycline (i.e. <100 mg/day) and anti-emetics to ameliorate nausea and vomiting.8
In vitro pharmacodynamic studies using the hollow-fibre model confirmed the efficacy of tigecycline against M. abscessus. Yet this study showed that increasing the daily dose to at least 200 mg would be required to optimize tigecycline’s efficacy.9
Based on the toxicity profile and requirement of higher doses, an inhaled formulation of tigecycline could be attractive to deliver high doses at the site of infection, maximizing its activity while minimizing systemic toxicity. In the current study, we have tested our hypotheses that inhaled tigecycline is effective and well-tolerated in the treatment of M. abscessus pulmonary disease in a validated mouse model.
Materials and methods
Antibiotics and bacterial strains
Tigecycline (Tygacil®, powder for IV infusion; Pfizer) was obtained from the Department of Pharmacy at Radboud University Medical Center (Nijmegen, the Netherlands). We obtained the M. abscessus subspecies abscessus CIP104536 type strain (synonym ATCC 19977) from the Pasteur Institute (Paris, France) and M. abscessus subspecies abscessus #21, a clinical isolate with smooth colony morphology,10 from the University of Colorado Hospital Clinical Microbiology Laboratory. Identification of both isolates was confirmed by WGS; the raw sequence files (FASTQ) were archived in the NCBI Sequence Read Archive (project number PRJNA566387).
Tigecycline MIC measurements
Tigecycline MICs were determined by broth microdilution in CAMHB,11 using SensiTitre RGMYCO plates with freeze-dried tigecycline at final concentrations ranging from 0.015 to 4 mg/L (Trek Diagnostics/ThermoFisher, Landsmeer, the Netherlands). For intracellular and time–kill assays, the MIC for M. abscessus CIP104536 was determined using an in-house broth microdilution assay in CAMHB.
Intracellular antimycobacterial activity
Peripheral blood mononuclear cells were obtained from three healthy volunteers (Sanquin Bloodbank, Nijmegen, the Netherlands). Informed consent was obtained for use of their blood for scientific purposes, as approved by the Ethics Committee of Radboud University Medical Center. The isolation and differentiation protocol was executed as previously described.12 On the day of infection, M. abscessus CIP104536 was added at a ratio of 1:1 (cells:bacteria) for 1 h at 37°C to allow phagocytosis before the medium was changed to RPMI 1640 supplemented with 10% human pooled serum. After the 1 h incubation, tigecycline was added at 2 × MIC (8 mg/L) concentration; an untreated control was also included. After 1 day, 2 days and 3 days, the numbers of extracellular and intracellular bacteria were quantified as previously described.12
Time–kill kinetics in cystic fibrosis sputum
We performed in duplicate a time–kill kinetics experiment with tigecycline at 2 × MIC (8 mg/L) concentration against M. abscessus CIP104536 in CAMHB with and without 10% (v/v) sputum of cystic fibrosis patients, using previously published methodology.6,13 Sputum of 18 cystic fibrosis patients was pooled and sterilized by overnight UV irradiation; sterility was checked by 24 h incubation on 5% Columbia sheep blood agar. At the start of the experiment and after 1, 2, 3, 4, 5 and 7 days of incubation at 30°C, samples were taken from the culture bottles and plated in triplicate on Middlebrook 7H10 agar for cfu counting.
Mouse model experiment
Mice
GM-CSF knockout mice14 were bred at Colorado State University animal housing facilities. Prior to infection, mice were verified as genetic knockout through PCR genotyping. University facilities cared for the animals in line with both Association for Assessment and Accreditation of Laboratory Animal Care (AAALAC) and NIH guidelines.
Bacteria
M. abscessus #21 was grown in Middlebrook 7H9 broth supplemented with OADC and 0.05% Tween 80 in a shaker incubator at 37°C. After ∼24 h of incubation, when the culture reached OD600 between 0.6 and 0.7 (∼1 × 107 cfu/mL), bacteria were aliquotted in preparation for infection. Aliquots were serially diluted into 1:5 dilutions and plated on Middlebrook 7H11 (M7H11) selective agar (BD Difco) supplemented with 10% OADC enrichment (BD Difco) and cfu were enumerated after 3–4 days of colony growth. All cfu values were converted to log10 cfu/mL.
Infection
GM-CSF knockout mice were infected with 1 × 106 cfu of M. abscessus #21 in a 50 μL saline solution, through the intrapulmonary aerosol route using an FMJ-250 high-pressure syringe device (PennCentury, Philadelphia, PA, USA) with an attached MicroSprayer (MicroSprayer, model IA-C; PennCentury) as previously described.10 The mice were temporarily anaesthetized using a mixture of isoflurane and oxygen. The mouse was quickly placed abdomen-down with its teeth suspended up at a 45° angle, and its tongue gently extended out. The MicroSprayer tip was then inserted into the upper trachea and bacteria inoculum sprayed out in the 50 μL dose.10 After the 50 μL administration, the mouse was placed back into the appropriate cage and monitored as it regained consciousness.
Drug treatment
Tigecycline was aliquotted into separate labelled vials, which were wrapped in aluminium foil and stored in a temperature- and humidity-controlled room to maintain drug stability. The appropriate vial was reconstituted in 300 μL of 0.9% endotoxin-free saline solution (TekNova) immediately before administration to minimize drug degradation.15 In two separate experiments, the mice received tigecycline via intrapulmonary aerosol delivery using the MicroSprayer device and methodology as explained above.10 All treatment groups received one 50 μL dose 5 days a week for a total duration of 4 weeks. As control groups, we treated 8 infected mice with aerosolized endotoxin-free saline solution (0.9%; TekNova; six mice) and another group of 10 mice was infected but not treated.
Bacterial load determination
One day and 10 days after infection, as well as 4 weeks after initiation of therapy, mice were euthanized by CO2 inhalation followed by cervical dislocation. During the necropsies, the lungs were examined for gross pathology and photographed. Thereafter, lungs were prepared for enumeration of bacterial burden and histopathology.
Mouse lung tissues were homogenized using a Next Advance Bullet Blender (Averill Park, NY, USA). Briefly, the left lobe of the lung was placed in a 1.5 mL sterile, safe-lock Eppendorf tube containing 0.5 mL of sterile 1 × PBS and 3 × 3.2 mm sterile stainless-steel beads and homogenized for 4 min at 8000 rpm in the Bullet Blender. After homogenization, each sample was used for bacterial enumeration. Briefly, serial dilutions of homogenized organs were prepared and cultured on the in-house-prepared M7H11 selective agar supplemented with 10% OADC and 10 μg/mL cycloheximide (Goldbio) and 50 μg/mL carbenicillin (Goldbio) to prevent fungal and bacterial contamination. Plates were incubated for 3–4 days at 37°C, after which the cfu in each plate were enumerated, with the data expressed as mean ± SEM log10 cfu for each group.
To prevent a drug carry-over effect onto agar plates, the samples were concurrently plated on in-house-prepared M7H11 agar plates supplemented with 0.4% activated charcoal (Sigma–Aldrich). To further determine whether residual drug in tissue homogenate was inhibiting colony formation on agar plates, separate 100 μL lung tissue homogenates were spiked with 100 μL of 104 M. abscessus #21. After sample homogenizing, the 200 μL sample was used for dilution and plating. Plates were incubated at 37°C for 72 h and then cfu were counted.
Histology
The right lung lobe was immediately placed into 4% paraformaldehyde diluted in PBS during the necropsy. Tissues were then paraffin embedded and sectioned onto charged slides (6–10 μm) to be stained by haematoxylin and eosin (H&E).
Ethics
The Colorado State University Animal Users Committee (IACUC) approved all experimental protocols involving the animals.
Statistical analysis
For the macrophage infection, a factorial ANOVA with post hoc Tukey’s honest significance test was used to assess differences in total mycobacterial load between treated and untreated macrophages. Calculations were performed in R, version 3.5.2. For the mouse model experiments, cfu data were analysed using Graph Pad Prism version 8.1.1; the statistical analysis was performed using Dunnett’s multiple comparisons test as part of a one-way ANOVA test.
Results
In vitro and ex vivo assays
The tigecycline MIC for the M. abscessus #21 clinical isolate used for the mouse model experiments and that for the M. abscessus CIP104536 type strain was 0.5 mg/L using the SensiTitre RGMYCO assay (both isolates also showed inducible macrolide resistance); the tigecycline MIC for M. abscessus CIP104536 using the in-house method was 4 mg/L.
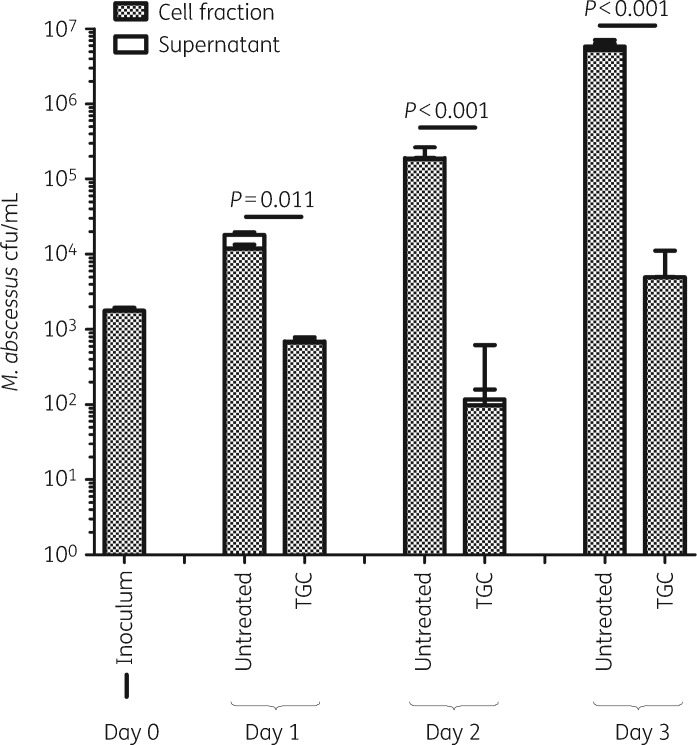
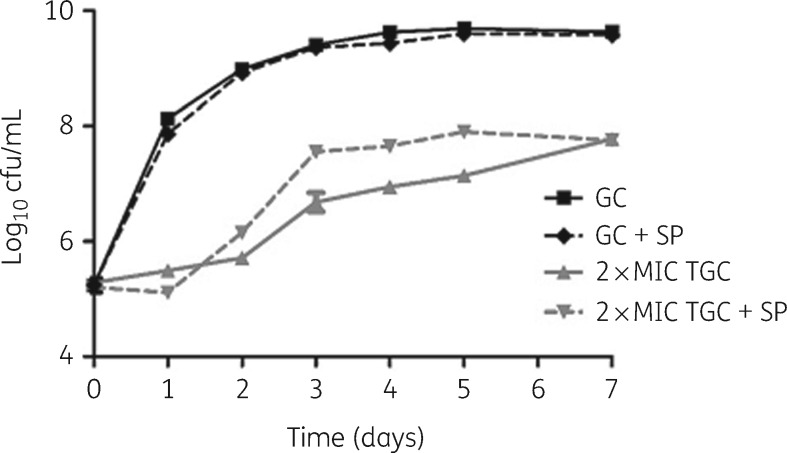
Tigecycline exhibited significant activity against M. abscessus CIP104536 in human macrophages, evidenced by a 3 log10 kill after 3 days (Figure 1). In the time–kill assay, after 1 day of unhindered activity, the sputum of cystic fibrosis patients temporarily inhibited tigecycline activity, causing a 1 log10 cfu difference in kill rate on Days 3–5 (Figure 2).
Figure 1.
Intracellular activity of tigecycline against M. abscessus CIP104536. TGC, tigecycline.
Figure 2.
Time–kill kinetics of tigecycline against M. abscessus CIP104536 with and without cystic fibrosis sputum. GC, growth control; TGC, tigecycline; +SP, with 10% (v/v) cystic fibrosis sputum.
Mouse model experiments
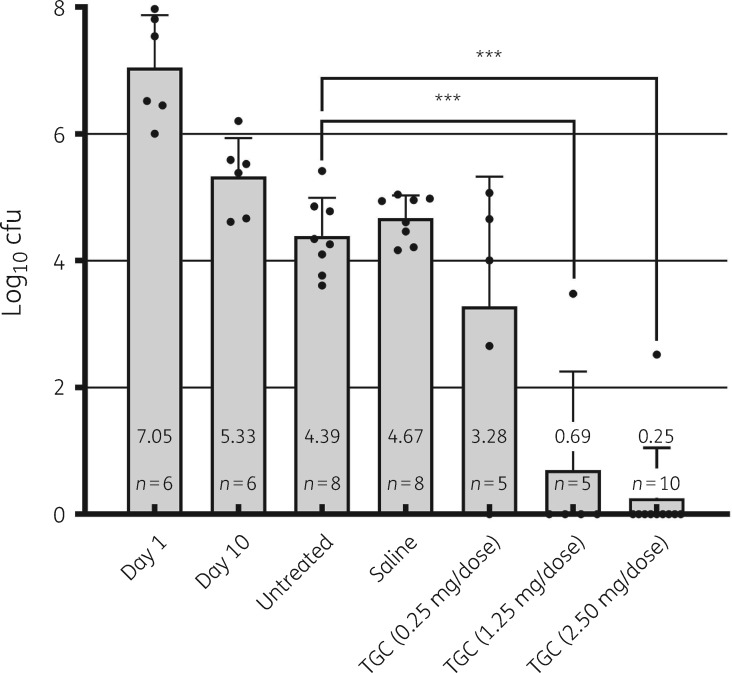
Inhaled high-dose tigecycline proved effective in vivo, in a dose-dependent manner (Figure 3). In the 2.5 mg dose group, one animal had countable colonies of M. abscessus, owing to pulmonary abscess formation. The effect of both 1.25 mg and 2.50 mg tigecycline doses on lung cfu counts resulted in a significant reduction of 3.98 and 4.42 log10 cfu, respectively, when compared with the untreated control group (P < 0.0001). Charcoal-supplemented M7H11 plates and the spiked lung tissue samples did not yield evidence for significant carry-over or residual drug activity in the homogenized tissue (data not shown). There were no signs of distress or other drug toxicity in the tigecycline-treated mice.
Figure 3.
Bacterial load in the lungs of mice from the two experiments. Single data points in each group are represented by filled black circles. The y-axis represents the mean value and SEM of log10 cfu in the lungs. The x-axis represents each group and timepoint or group treatment in the study. Day 1 and Day 10 are timepoints included as infection control groups. All treatments were initiated on Day 10 and each group, excluding the untreated group, received an intrapulmonary aerosol dose of either saline or tigecycline at low/medium/high concentrations (0.25/1.25/2.50 mg/50 μL dose, respectively). Dunnett’s multiple comparisons test: ***P < 0.0001. TGC, tigecycline.
Histology
Histopathological analysis of the lung sections obtained from M. abscessus-infected GM-CSF knockout mice showed granuloma or pre-granuloma formations, influx of inflammatory cells, mainly plasma cells and neutrophils, in parenchyma and influx of lymphocytes in perivascular zones and an increase in foamy cells in granulomatous-like formations (Figure 4). Lung tissue of tigecycline-treated mice showed increased necrotic debris in the alveolar lumen and proteinaceous oedema residue, and occasionally alveoli were lined by hypertrophied cells with vesicular nuclei, suggesting type II cell hyperplasia.
Figure 4.
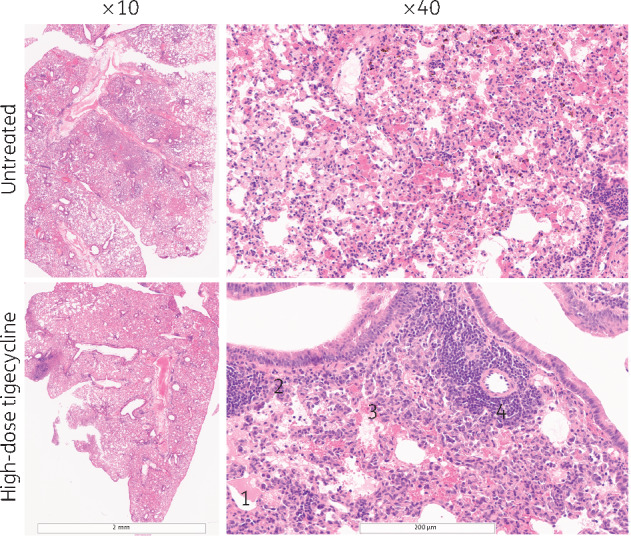
Representative images of H&E-stained lung-tissue sections from M. abscessus-infected GM-CSF knockout mice not receiving treatment (top row) and receiving high-dose tigecycline (bottom row) at ×10 and ×40 magnification. Histology shows granuloma-like formations and perivascular lymphocyte infiltration. Representative histological features are shown in the bottom right-hand photograph: 1, oedema and granulomatous formation with numerous foamy cells; 2, type II cell hyperplasia; 3, necrotic debris; and 4, perivascular lymphocyte cuffs.
Discussion
Inhaled tigecycline proved highly efficacious in a mouse model of chronic pulmonary M. abscessus disease. This offers a new and interesting lead for an infectious disease where active antibiotics are few and treatment outcomes are dismal despite long-term complex multidrug regimens.7 The possible clinical potential of inhaled tigecycline in pulmonary M. abscessus disease in humans is further supported by four other experimental findings. First, tigecycline shows antimycobacterial activity in human macrophages (Figure 1), which is a key asset in the treatment of intracellular pathogens like mycobacteria. Second, owing to the frequent occurrence of M. abscessus pulmonary disease in cystic fibrosis patients,3,16 it is important to know that the activity of tigecycline is decreased but not annulled in the presence of cystic fibrosis sputum (Figure 2), although the clinical implications of this in vitro finding have not been studied.13 Third, the tigecycline susceptibility of the strain used in the mouse model experiment was within the normal distribution reported by Wallace et al.17 (≤0.06–1 mg/L); the higher MIC using the in-house assay likely results from tigecycline degradation in aqueous environments.15 Last, we noted no signs of distress or inhalation-associated toxicity. Histology did not show airway damage in response to tigecycline inhalation. This is in line with previous observations in mice treated with inhaled doxycycline,18 as well as recent clinical experience with topical tigecycline application.19
The effect of inhaled tigecycline was dose dependent, which echoes the previously recorded association between tigecycline AUC/MIC ratio and bactericidal effect in the hollow-fibre model.9 This association is difficult to extract from the current experiments, as we have no pharmacokinetic data and only three dose groups, of which the two lowest dose groups are small in size. The effect of inhaled tigecycline seems not to result from carry-over, as cfu counts were similar on Middlebrook 7H11 with and without activated charcoal. In fact, the antibacterial effect of the inhaled tigecycline may have been underestimated in the mouse model experiment. Tigecycline rapidly degrades in aqueous environments,15 so the dose applied in the mouse model experiments was likely lower than the calculated dose. An inhaled formulation of tigecycline should be designed to prevent drug degradation prior to and during administration; owing to its instability in aqueous environments,15 a dry powder inhalation might be a preferred formulation.
The observation that anatomical changes like abscess formation impede the activity of inhaled tigecycline in the mouse model is relevant; M. abscessus pulmonary disease affects patients with underlying structural abnormalities of the lungs or may itself lead to such abnormalities, particularly fibro-cavitary lesions.20 If the activity or deposition of inhaled tigecycline is limited within such lesions, that might affect its use. Similarly, the efficacy may be different in the altered chemical environment of the lungs of cystic fibrosis patients. Mouse models mimicking cystic fibrosis have recently been applied to model M. abscessus lung infections and treated with inhaled antibiotics;21 this may be an interesting model for further preclinical development of inhaled tigecycline.
One of the major limitations of the current study is the lack of pharmacokinetic analyses in the mouse model experiments. Measuring plasma concentrations could have helped to establish which fraction of the inhaled dose of tigecycline makes it into the bloodstream, which could have helped in estimating the likelihood of systemic adverse events as well as the likelihood of causing tigecycline resistance in commensal flora. Second, we used different M. abscessus isolates for in vivo and in vitro and ex vivo studies. The M. abscessus #21 isolate was previously shown to yield a stable chronic infection in GM-CSF knockout mice.10,22 For in vitro and ex vivo work we used the M. abscessus CIP104536 type strain, to align with previous studies6,9 and for reproducibility. Also, the inherent limitations of in vitro studies and mouse models apply here; for example, the applied doses cannot easily be translated into a dose suitable for use in clinical trials.
The clinical relevance of the observations on safety and efficacy of inhaled tigecycline against M. abscessus can only be proven in clinical trials. These will have to start with creating a stable formulation coupled with an effective inhalation device, followed by Phase 1 trials of safety and tolerability of this novel formulation, before moving into actual efficacy studies.
In summary, we show that inhaled tigecycline yields a very potent effect in GM-CSF knockout mice with chronic M. abscessus pulmonary disease. In an infection where treatment outcomes are so very poor, inhaled tigecycline may represent a viable treatment option. A stable and safe formulation is required to proceed to further pharmacodynamic studies and ultimately clinical trials.
Acknowledgements
We wish to thank Dr Mary Jackson of Colorado State University and Dr Nancy Madinger of the University of Colorado Hospital Clinical Microbiology Laboratory for providing the M. abscessus #21 isolate.
Funding
This work was supported by a personal grant for Jakko van Ingen, from the Netherlands Organization for Scientific Research (NWO/ZonMW grant Veni 016.176.024); Mercedes Gonzalez-Juarrero and in vivo studies herein are supported by the National Institute of Allergy and Infectious Diseases, National Institutes of Health (grant number R01AI120670).
Transparency declarations
None to declare.
References
- 1. Nessar R, Cambau E, Reyrat JM et al. Mycobacterium abscessus: a new antibiotic nightmare. J Antimicrob Chemother 2012; 67: 810–18. [DOI] [PubMed] [Google Scholar]
- 2. Haworth CS, Banks J, Capstick T et al. British Thoracic Society guidelines for the management of non-tuberculous mycobacterial pulmonary disease (NTM-PD). Thorax 2017; 72 Suppl 2: ii1–64. [DOI] [PubMed] [Google Scholar]
- 3. Floto RA, Olivier KN, Saiman L et al. US Cystic Fibrosis Foundation and European Cystic Fibrosis Society consensus recommendations for the management of non-tuberculous mycobacteria in individuals with cystic fibrosis. Thorax 2016; 71 Suppl 1: i1–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ferro BE, Srivastava S, Deshpande D et al. Amikacin pharmacokinetics/pharmacodynamics in a novel hollow-fiber Mycobacterium abscessus disease model. Antimicrob Agents Chemother 2016; 60: 1242–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ferro BE, Srivastava S, Deshpande D et al. Moxifloxacin’s limited efficacy in the hollow-fiber model of Mycobacterium abscessus disease. Antimicrob Agents Chemother 2016; 60: 3779–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ruth MM, Sangen JJN, Pennings LJ et al. Minocycline has no clear role in the treatment of Mycobacterium abscessus disease. Antimicrob Agents Chemother 2018; 62: e01208–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kwak N, Dalcolmo MP, Daley CL et al. Mycobacterium abscessus pulmonary disease: individual patient data meta-analysis. Eur Respir J 2019; 54: 1801991. [DOI] [PubMed] [Google Scholar]
- 8. Wallace RJ Jr, Dukart G, Brown-Elliott BA et al. Clinical experience in 52 patients with tigecycline-containing regimens for salvage treatment of Mycobacterium abscessus and Mycobacterium chelonae infections. J Antimicrob Chemother 2014; 69: 1945–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ferro BE, Srivastava S, Deshpande D et al. Tigecycline is highly efficacious in Mycobacterium abscessus pulmonary disease. Antimicrob Agents Chemother 2016; 60: 2895–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. De Groote MA, Jackson M, Gonzalez-Juarrero M et al. Optimization and lead selection of benzothiazole amide analogs toward a novel antimycobacterial agent. Front Microbiol 2018; 9: 2231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.CLSI. Susceptibility Testing of Mycobacteria, Nocardiae, and Other Aerobic Actinomycetes—Third Edition: M24. 2018. [PubMed]
- 12. Ruth MM, Magombedze G, Gumbo T et al. Minocycline treatment for pulmonary Mycobacterium avium complex disease based on pharmacokinetics/pharmacodynamics and Bayesian framework mathematical models. J Antimicrob Chemother 2019; 74: 1952–61. [DOI] [PubMed] [Google Scholar]
- 13. King P, Lomovskaya O, Griffith DC et al. In vitro pharmacodynamics of levofloxacin and other aerosolized antibiotics under multiple conditions relevant to chronic pulmonary infection in cystic fibrosis. Antimicrob Agents Chemother 2010; 54: 143–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Dranoff G, Mulligan R. Activities of granulocyte-macrophage colony-stimulating factor revealed by gene transfer and gene knockout studies. Stem Cells 1994; 12: 173–83. [PubMed] [Google Scholar]
- 15. Jitkova Y, Gronda M, Hurren R et al. A novel formulation of tigecycline has enhanced stability and sustained antibacterial and antileukemic activity. PLoS One 2014; 9: e95281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Adjemian J, Olivier KN, Prevots DR. Epidemiology of pulmonary nontuberculous mycobacterial sputum positivity in patients with cystic fibrosis in the United States, 2010-2014. Ann Am Thorac Soc 2018; 15: 817–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wallace RJ, Brown-Elliott BA, Crist CJ et al. Comparison of the in vitro activity of the glycylcycline tigecycline (formerly GAR-936) with those of tetracycline, minocycline, and doxycycline against isolates of nontuberculous mycobacteria. Antimicrob Agents Chemother 2002; 46: 3164–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Tata PR, Pardo-Saganta A, Prabhu M et al. Airway-specific inducible transgene expression using aerosolized doxycycline. Am J Respir Cell Mol Biol 2013; 49: 1048–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. van Wijk F, Waterval J, van Aerde K et al. Successful systemic and topical treatment of Mycobacterium abscessus otomastoiditis. Antimicrob Agents Chemother 2019; 64: e01203–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Stout JE, Koh WJ, Yew WW. Update on pulmonary disease due to non-tuberculous mycobacteria. Int J Infect Dis 2016; 45: 123–34. [DOI] [PubMed] [Google Scholar]
- 21. Banaschewski B, Verma D, Pennings LJ et al. Clofazimine inhalation suspension for the aerosol treatment of pulmonary nontuberculous mycobacterial infections. J Cyst Fibros 2019; 18: 714–20. [DOI] [PubMed] [Google Scholar]
- 22. De Groote MA, Johnson L, Podell B et al. GM-CSF knockout mice for preclinical testing of agents with antimicrobial activity against Mycobacterium abscessus. J Antimicrob Chemother 2014; 69: 1057–64. [DOI] [PubMed] [Google Scholar]