Abstract
Objectives
Rapid antigen testing (RAT) for coronavirus disease 2019 (COVID-19) has lower sensitivity but high accuracy during the early stage when compared to reverse transcription quantitative polymerase chain reaction (RT-qPCR). The aim of this study was to investigate the concordance between RAT and RT-qPCR results, and their prediction of disease transmission.
Methods
This single-center retrospective observational study of inpatients with COVID-19 was conducted from March 6 to June 14, 2020. Nasopharyngeal swabs were used to perform RAT and RT-qPCR. The primary endpoint was concordance between RAT and RT-qPCR results. The secondary endpoints were the factors causing disagreement in the results and the estimated transmissibility in RT-qPCR-positive patients with mild symptoms.
Results
Overall, 229 samples in viral transport medium (VTM) were obtained from 105 patients. The positive and negative concordance rates for VTM were 41% vs 99% (κ = 0.37) and 72% vs 100% (κ = 0.50) for samples collected on disease days 2–9. An increased body temperature (odds ratio 0.54) and absence of drugs with potential antiviral effect (odds ratio 0.48) yielded conflicting results. RAT was associated with the ability to end isolation (OR 0.11, 95% confidence interval 0.20–0.61).
Conclusions
RAT and RT-qPCR results were highly consistent for samples collected at the appropriate time and could be useful for inferring the possibility of transmissibility.
Keywords: COVID-19, Antigen test, Appropriate timing for antigen test, Estimating transmissibility
Introduction
Nucleic acid detection by reverse transcription quantitative polymerase chain reaction (RT-qPCR) is the prime diagnostic modality for coronavirus disease 2019 (COVID-19) (Hanson et al., 2020). However, besides laboratories and major hospitals, few facilities have the necessary equipment for RT-PCR. Even if RT-qPCR can be performed, the test requires capital investment and significant manpower. Moreover, point-of-care RT-PCR testing equipment that eliminates the need for extraction and the preparation of reaction reagents have been developed; however, their installation cost remains a problem. In Japan, there is a shortage of devices and reagents for such testing equipment because the high demand exceeds the supply. Thus, on May 13, 2020, Japan approved rapid antigen testing (RAT) targeted towards the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) nucleoprotein, using immunochromatography (ESPLINE SARS-CoV-2; Fujirebio Inc. Japan) (Japanese Ministry of Health, Labor, and Welfare (JMHLW), 2020a). However, in the pre-approval trial with PCR as control, the positive and negative concordance rates on 72 nasopharyngeal swabs (NPS) were 37% and 98%, respectively. When using NPS in viral transport medium (VTM), the rates were 67% and 100%, respectively. Due to these low positive concordance rates, the PCR test was initially recommended in addition to a negative rapid test result. However, since acceptable positive and negative concordance rates could be obtained in the early stages of COVID-19 (days 2–9) when the viral load is sufficient, the Japanese Ministry of Health, Labor, and Welfare guidelines of June 16, 2020 allowed for a confirmatory decision to be made based on RAT of samples obtained during the early stages of COVID-19 (JMHLW, 2020b).
The aim of this study was to investigate the concordance between RAT and RT-qPCR results. Factors associated with disagreement between RAT and PCR results were also analyzed, and the predictive ability of RAT for disease transmissibility was investigated according to the governmental policy that allows patients to discontinue isolation if their temperature is <37.5 °C and other symptoms are improving, on or after day 11 of the disease (JMHLW, 2020c).
Methods
Patients and definitions
This was a single-center, retrospective observational study of confirmed COVID-19 patients whose NPS specimens were collected and stored between March 6 and June 14, 2020. Information disclosure forms were published on the hospital’s clinical department webpage, and patients who opted out were excluded. This study was approved by the ethics review board of the hospital (NCGM-G-003587-00).
The following data were retrieved from the medical records: age, sex, race, height, weight, body mass index, smoking and medical history, complications, use of drugs with potential antiviral effects, artificial ventilation with intubation, and extracorporeal membrane oxygenation, vital signs on the day of sampling (peak body temperature, final blood pressure measurement, final pulse measurement, final respiratory rate, final transdermal oxygen saturation), use of oxygen on the day of sampling, blood test results on the day of sampling (white blood cell count, differential blood count (lymphocyte fraction, neutrophil–lymphocyte ratio), lactate dehydrogenase, C-reactive protein, and d-dimer), computed tomography images, imaging findings of pneumonia, sample collection date, and date of onset. The drugs with potential antiviral effects administered were favipiravir, lopinavir–ritonavir, hydroxychloroquine (HCQ), and ciclesonide inhalation; other drugs including remdesivir were regarded as unknown because clinical trial participants were also included in the study. Disease severity was classified as moderate (needed oxygen), severe (underwent tracheal intubation and ventilator management), or mild (remaining manifestations) following Japanese guidelines (JMHLW, 2020c). Patients with mild disease could discontinue isolation if their temperature was <37.5 °C on or after day 11 of the disease.
Laboratory tests and definitions
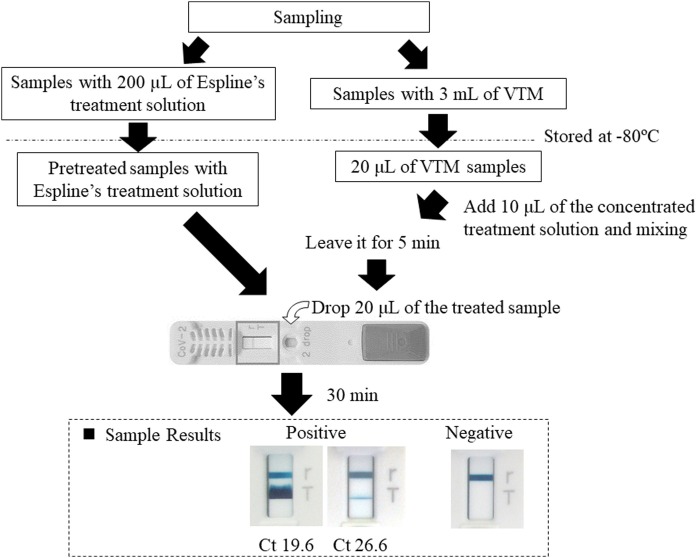
Samples obtained from confirmed COVID-19 inpatients in another study (NCGM-G-003472-02) were collected and stored in deep freezers at −80 °C after receiving their written informed consent. Universal Transport Medium (1 ml or 3 ml; COPAN Diagnostics Inc., USA) was used as the VTM. If the amount of VTM was 1 ml, it was diluted with 2 ml of sterile saline and 500 μl was dispensed into screw-top tubes. For RAT, the nasopharynx was swabbed using the kit’s swab (developed in approximately 200 μl of reagent). After the SARS-CoV-2 RAT, the remaining sample was dispensed into screw-top tubes. The stored NPS samples in VTM and RAT reagent were tested as shown in Figure 1 . SARS-CoV-2 RT-qPCR tests were performed using N and N2 primers (National Institute of Infectious Disease, 2020). For residual reagents, 140 μl was used for nucleic acid extraction with the QIAamp Viral RNA Mini Kit (QIAGEN, Hilden, Germany) to obtain 60 μl of nucleic acid extract. For VTM samples, 200 μl was used to obtain 60 μl of the nucleic acid extract with the QIAsymphony DSP Virus/Pathogen Mini Kit (Qiagen). For small samples, VTM samples were diluted in sterile distilled water, while the reagent samples were diluted with reagent to obtain the required amount; 5 μl of the nucleic acid extract was used for qPCR. RT-PCR was performed using the Applied Biosystems 7500 Real-Time PCR System (Applied Biosystems, Foster City, CA, USA) or QuantStudio 5 (Applied Biosystems). Using the calculated cycle threshold (Ct) value and PCR amplification efficiency of the N2 primer set, a correlation equation for the Ct value and number of RNA copies was determined. The Ct value obtained from the reference material (SeraCare, AccuPlexTM SARS-CoV-2 Reference Material Kit) was used to perform a correction to calculate the number of RNA copies (<1% per test was treated as negative).
Figure 1.
Rapid antigen testing procedures.
VTM, viral transport medium; Ct, cycle threshold.
Samples that were antigen-positive but PCR-negative were retested using the same RT-qPCR equipment, but using a kit with a different primer (SARS-CoV-2 Direct Detection RT-qPCR Kit, Takara Bio, Japan). The SARS-CoV-2 RT-qPCR and RAT were performed by SRL Inc. (Tokyo, Japan).
The primary outcome measures were the positive (sensitivity) and negative (specificity) concordance rates and coefficients of SARS-CoV-2 detection results using RT-qPCR and the ESPLINE kit, calculated for all of the samples collected at the appropriate time (disease day 2–9) and samples collected at later stages from onset (disease days other than 2–9). VTM was also examined in a similar manner using only the first sample.
The secondary outcomes were the factors influencing disagreement in the results. The patients were divided into two groups: those with agreement between RT-qPCR and RAT (concordant) and those with disagreement (discordant). Factors ending the isolation of PCR-positive patients were also analyzed.
Statistical methods
Discrete data were expressed as the number and percentage (n, %) and compared using Fisher’s exact test, while continuous data were expressed as the median and interquartile range (IQR) and compared using the Mann–Whitney U-test. The Benjamini–Hochberg correction was performed for multiple comparisons of three or more groups. We calculated the positive (sensitivity) and negative (specificity) concordance rates with qPCR results, and a 95% confidence interval (CI) for each, consistent with Cohen’s kappa and Gwet’s AC1 statistic (AC1) (Gwet, 2008) using SAS version 9.4 (SAS Institute, USA). The presence or absence of disagreement between RAT and RT-qPCR results was set as the outcome. Factors associated with the outcome were also identified using univariate logistic regression analysis, and factors at p < 0.1 were included in the multivariate logistic regression analysis (stepwise method). Multivariate logistic regression was also used to analyze factors associated with ending the isolation in PCR-positive patients. All p-values were two-tailed, and p < 0.05 was considered statistically significant. All statistical analyses were performed using IBM SPSS Statistics for Windows, version 26.0 (IBM Corp., Armonk, NY, USA).
Results
Overall, 229 VTM and 40 reagent samples were obtained from 105 and 13 patients, respectively. Further, 35 of both VTM and reagent samples were obtained from nine patients. The patient characteristics are shown in Table 1 . Drugs with potential antiviral effects were used in 57 (54%) patients, 56% of whom used HCQ, while five used two or more drugs. The median peak body temperature on the day of sampling was 36.5 °C (range 36.3–36.6 °C), and three patients (7.5%) had temperatures ≥37.5 °C. The VTM samples were collected on median disease day 13 (range day 9–17), and 59 (26%) were taken at the appropriate time. Overall, 51 samples (22%) were taken from patients who needed oxygen during the sample collection.
Table 1.
Patient characteristics and their status on the day of sampling.
| Total | Mild | Moderate | Severe | |
|---|---|---|---|---|
| Number | 105 | 74 | 19 | 12 |
| Age (years) | 53 (36–68) | 48(31–62) | 65 (48–74) | 68 (55–79) |
| Sex, male | 72 (68.6%) | 49 (66%) | 14 (74%) | 9 (75%) |
| Nationality, Japanese | 98 (93.3%) | 68 (92%) | 18 (95%) | 12 (100%) |
| Height (m) | 1.65 (1.60–1.72) | 1.65 (1.60–1.71) | 1.68 (1.58–1.74) | 1.66 (1.59–1.74) |
| Weight (kg) | 64.4 (53.5–75.0) | 64.4 (52.9–74.6) | 63.8 (50.7–75.2) | 65.6 (61.1–75.7) |
| Body mass index (kg/m2) | 22.7 (20.6–26.4) | 22.6 (20.4–26.2) | 22.2 (20.3–25.7) | 23.7 (21.7–27.1) |
| Past medical history | ||||
| Bronchial asthma | 6 (5.7%) | 5 (6.8%) | 1 (5.3%) | 1 (8.3%) |
| Cancer | 7 (6.7%) | 6 (8.1%) | 0 (0%) | 1 (8.3%) |
| Immunocompromised | 6 (5.7%) | 4 (5.4%) | 1 (5.3%) | 1 (8.3%) |
| Diabetes mellitus | 15 (14.3%) | 6 (8.1%) | 4 (21%) | 5 (41%) |
| Smoking | ||||
| Never | 47 (44.8%) | 36 (49%) | 6 (32%) | 5 (41%) |
| Current smoker | 24 (22.9%) | 16 (22%) | 6 (32%) | 2 (17%) |
| Ex-smoker | 22 (21.0%) | 14 (19%) | 5 (26%) | 3 (25%) |
| Unknown | 12 (11.4%) | 8 (11%) | 2 (11%) | 2 (17%) |
| Drugs with potential antiviral effects | 57 (54.3%) | 29 (39%) | 16 (84%) | 12 (100%) |
| Pneumonia | 86 (82.0%) | 55 (74%) | 19 (100%) | 12 (100%) |
| VTM samples, n | 229 | 166 | 40 | 23 |
| Day of illness on sampling day | 13 (9–17) | 13 (9–17) | 13 (11–18) | 17 (13–23) |
| Peak body temperature on sampling day | 36.6 (36.4–37.0) | 36.6 (36.4–36.9) | 36.6 (36.3–37.1) | 36.9 (36.5–37.6) |
| Respiratory rate at sampling | 18 16–18 | 17 (16–18) | 18 (16–20) | 20 (18–24) |
| Oxygen demand at sampling | 51 (22.2%) | 0 | 29 (72.5%) | 22 (95.7%) |
| Reagent samples, n | 40 | 36 | 4 | 0 |
| Day of illness on sampling day | 14 (9–18) | 13 (8–17) | 28 (25–35) | – |
| Peak body temperature on sampling day | 36.5 (36.3–36.6) | 36.5 (36.2–36.7) | 36.5 (36.3–36.6) | – |
| Respiratory rate at sampling | 17 (16–18) | 17 (16–18) | 17 (15–18) | – |
| Oxygen demand at sampling | 2 (5.0%) | 0 | 2 (50.0%) | – |
VTM, viral transport medium. Data are expressed as n (%) or median (interquartile range), or as specified.
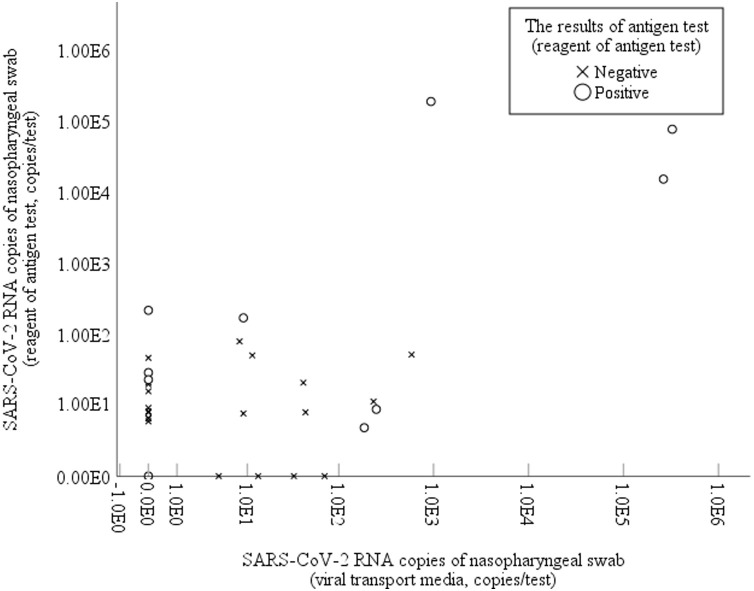
The VTM PCR results differed from the reagent sample results in 19 (60%) samples, including 15 reagent-positive and VTM-negative samples and four VTM-positive and reagent-negative samples. There was no significant correlation in the number of viral copies between VTM and reagent samples (p = 0.19). Result disagreement was particularly notable in VTM samples with a significantly lower number of copies (0.0 vs 42.9, p < 0.001) (Supplementary Material Figure 1).
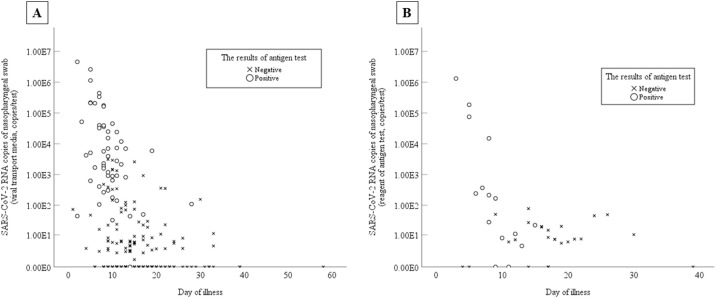
When VTM PCR results were regarded as the gold standard, the positive and negative coincidence rates of RAT were 35% and 78% (Table 2 A), while when reagent PCR results were regarded as the gold standard, they were 41% and 75% (Table 2B). When samples were collected at the appropriate timing for RAT (disease day 2–9), the positive concordance rate with VTM PCR results as the gold standard was low at 57%, but was high (90%, κ = 0.57, AC1 = 0.76) when reagent PCR results were the gold standard, showing good agreement. The positive and negative concordance rates of all RT-qPCR VTM samples with RAT and of samples collected at the appropriate timing for RAT were 41% vs 99% and 72% vs 100%, respectively (Table 2C). Regarding viral load and disease day with VTM, a significant difference was found in the median number of viral copies between the appropriate and non-appropriate timing groups, with the copies/test being 7.1 × 102 (range 3.1–3.6 × 104) and 0 (0–24), respectively (p < 0.001). The median Ct with VTM was 27.5 (23.3–32.0) and 34.0 (30.3–36.6), respectively, with a significantly higher viral load in RT-qPCR-positive samples (p < 0.001). A similar trend was observed in reagent samples (Ct 32.2 vs 37.4, p < 0.001). Both VTM and reagent samples tended to be positive in the early stages of the disease (Figure 2 ).
Table 2.
Antigen and PCR test result concordance for each type of sample.
| (A) Antigen test (reagent) vs RT-qPCR (VTM) | |||
|---|---|---|---|
| All | RT-qPCR |
||
| Positive | Negative | Total | |
| Antigen + | 6 | 4 | 10 |
| Antigen – | 11 | 14 | 25 |
| Positive concordance rate, % (95% CI) | 35 (13–58) | ||
| Negative concordance rate, % (95% CI) | 78 (59–97) | ||
| Cohen’s kappa (standard error) | 0.13 (0.15) | ||
| Gwet’s AC1 statics (standard error) | 0.19 (0.18) | ||
| Appropriate timing | RT-qPCR | ||
| (Between 2 and 9 days from symptom onset) | |||
| Positive | Negative | Total | |
| Antigen + | 4 | 2 | 6 |
| Antigen – | 3 | 0 | 3 |
| Positive concordance rate, % (95% CI) | 57 (20–94) | ||
| Negative concordance rate, % (95% CI) | 0 (0–59) | ||
| Cohen’s kappa (standard error) | −0.36 (0.17) | ||
| Gwet’s AC1 statics (standard error) | 0.072 (0.39) | ||
| Not appropriate timing | RT-qPCR | ||
| (>9 days from symptom onset) | |||
| Positive | Negative | Total | |
| Antigen + | 2 | 2 | 4 |
| Antigen – | 8 | 14 | 22 |
| Positive concordance rate, % (95% CI) | 20 (0.0–45) | ||
| Negative concordance rate, % (95% CI) | 88 (71–100) | ||
| Cohen’s kappa (standard error) | 0.085 (0.17) | ||
| Gwet’s AC1 statics (standard error) | 0.37 (0.20) | ||
| (B) Antigen test (reagent) vs RT-qPCR (reagent) | |||
|---|---|---|---|
| All | RT-qPCR |
||
| Positive | Negative | Total | |
| Antigen + | 13 | 2 | 15 |
| Antigen – | 19 | 6 | 25 |
| Positive concordance rate, % (95% CI) | 41 (24–58) | ||
| Negative concordance rate, % (95% CI) | 75 (45–100) | ||
| Cohen’s kappa (standard error) | 0.087 (0.10) | ||
| Gwet’s AC1 statics (standard error) | −0.019 (0.17) | ||
| Appropriate timing | RT-qPCR | ||
| (Between 2 and 9 days from symptom onset) | |||
| Positive | Negative | Total | |
| Antigen + | 9 | 1 | 10 |
| Antigen – | 1 | 2 | 3 |
| Positive concordance rate, % (95% CI) | 90 (71–100) | ||
| Negative concordance rate, % (95% CI) | 67 (13–100) | ||
| Cohen’s kappa (standard error) | 0.57 (0.27) | ||
| Gwet’s AC1 statics (standard error) | 0.76 (0.17) | ||
| Not appropriate timing | RT-qPCR | ||
| (>9 days from symptom onset) | |||
| Positive | Negative | Total | |
| Antigen + | 4 | 1 | 5 |
| Antigen – | 18 | 4 | 22 |
| Positive concordance rate, % (95% CI) | 18 (2.1–34) | ||
| Negative concordance rate, % (95% CI) | 80 (45–100) | ||
| Cohen’s kappa (standard error) | −0.0079 (0.085) | ||
| Gwet’s AC1 statics (standard error) | −0.40 (0.18) | ||
| (C) Antigen test (VTM) vs RT-qPCR (VTM) | |||
|---|---|---|---|
| All | RT-qPCR |
||
| Positive | Negative | Total | |
| Antigen + | 52 | 1a | 53 |
| Antigen – | 76 | 100 | 176 |
| Positive concordance rate, % (95% CI) | 41 (32–49) | ||
| Negative concordance rate, % (95% CI) | 99 (97–100) | ||
| Cohen’s kappa (standard error) | 0.37 (0.046) | ||
| Gwet’s AC1 statics (standard error) | 0.36 (0.064) | ||
| Appropriate timing | RT-qPCR | ||
| (Between 2 and 9 days from symptom onset) | |||
| Positive | Negative | Total | |
| Antigen + | 34 | 0 | 34 |
| Antigen – | 13 | 11 | 24 |
| Positive concordance rate, % (95% CI) | 72 (60–85) | ||
| Negative concordance rate, % (95% CI) | 100 (100–100) | ||
| Cohen’s kappa (standard error) | 0.50 (0.11) | ||
| Gwet’s AC1 statics (standard error) | 0.61 (0.11) | ||
| Not appropriate timing | RT-qPCR | ||
| (>9 days from symptom onset) | |||
| Positive | Negative | Total | |
| Antigen + | 18 | 1 | 19 |
| Antigen – | 63 | 89 | 152 |
| Positive concordance rate, % (95% CI) | 22 (13–31) | ||
| Negative concordance rate, % (95% CI) | 99 (97–100) | ||
| Cohen’s kappa (standard error) | 0.22 (0.051) | ||
| Gwet’s AC1 statics (standard error) | 0.36 (0.077) | ||
RT-qPCR, reverse transcription quantitative polymerase chain reaction; VTM, viral transport medium; CI, confidence interval.
RT-qPCR was positive by another RT-qPCR kit (SARS-CoV-2 Direct Detection RT-qPCR Kit, Takara Bio).
Figure 2.
Correlations between severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) viral load and day after the onset of coronavirus disease 2019 (COVID-19) symptoms with the results of rapid antigen testing. (A) Samples in viral transport medium; the rapid antigen testing results for viral transport medium samples. (B) Samples in the reagent of the rapid antigen test (ESPLINE kit); the rapid antigen testing results for the reagent samples.
Among samples with at least 102 and 103 copies/test, the positive concordance rates with RAT were 67% (95% CI 56–78%) and 85% (95% CI 74–95%), with a κ of 0.68 and 0.73 and AC1 of 0.79, and 0.86, respectively. When the samples were collected at the appropriate time, the respective positive concordance rates increased to 92% (95% CI 83–100%) and 97% (95% CI 90–100%), with κ coefficients of 0.86 and 0.76 and AC1 of 0.87 and 0.76, respectively.
The positive and negative concordance rates when only the first samples were examined for reagent PCR as the gold standard and with VTM, as well as when the samples were collected at the appropriate timing for RAT, are shown in Supplementary Material Table S1A and B. VTM RAT and RT-qPCR results were discordant in 77 (34%) PCR-positive and RAT-negative samples. The median Ct value for discordant samples was significantly higher than that for concordant samples: 35.8 (range 32.3–37.0) vs 26.6 (range 23.3–29.3) (p < 0.001). The univariate analyses showed that sample collection timing, not using drugs with potential antiviral effects, increasing body temperature, and respiratory rate of ≥20/min influenced RAT and PCR results (Supplementary Material Table S2). In the multivariate analysis, RAT and PCR result agreement was significantly associated with body temperature (rise per °C) (odds ratio (OR) 0.54, 95% CI 0.33–0.89, p = 0.017) and not using drugs with potential antiviral effects (OR 0.48, 95% CI 0.27–0.87, p = 0.015). Even after excluding the unknown cases, the OR was 0.49 (95% CI 0.26–0.92) in the multivariate analysis, indicating that not using drugs with potential antiviral effects affected the disagreement between PCR and RAT results (Supplementary Material Table S3).
Of the 96 samples from patients whose isolation could be discontinued when the sample was collected, 38 were PCR-positive, with a significantly higher Ct value than that in the mild patient group whose isolation could not be discontinued (36.0 vs 28.3, p < 0.001). Of the 88 samples from mild PCR-positive cases, 86 were assessed as being able to end isolation. Only RAT results were significantly associated with being able to end isolation in both the univariate and multivariate logistic regression analysis models (OR 0.11, 95% CI 0.20–0.61) (Supplementary Material Table S4).
Discussion
In this study, although the positive concordance rates of RAT of NPS and RT-qPCR tests using samples stored in VTM and ESPLINE reagent samples were low, the positive concordance rates and κ coefficients increased when the analysis was limited to samples collected at the appropriate time. Moreover, the concordance with VTM RAT results was relatively high when it was examined using at least 102 copies/test in VTM RT-qPCR as the criterion for positivity, with κ coefficients and AC1 of 0.68 and 0.79, respectively. Previous studies comparing PCR and RAT have also reported increased sensitivity with RAT for specimens with high copy numbers (low Ct value) (Mak et al., 2020, Porte et al., 2020, Scohy et al., 2020). Although Scohy et al. reported low overall sensitivity of 30.2% with COVID-19 Ag Respi-Strip (Scohy et al., 2020), the positive rate of RAT on samples with low Ct values was high, and samples with Ct values <25 (equivalent to >104 copies/ml) had 100% sensitivity. Furthermore, in samples collected at the appropriate time with high copy numbers, the positive concordance rate increased to 92% and 97% with at least 102 and 103 copies/test, respectively, and the κ coefficients were 0.86 and 0.76, showing an extremely high level of agreement. A study that evaluated rapid fluorescence immunochromatography of nasopharyngeal samples collected in the early stages of COVID-19 (median day 2) reported a sensitivity of 93.9% and specificity of 100% (Porte et al., 2020). As the fluorescent color in this study was determined by a dedicated reader, these results cannot be considered equivalent to those of the previous study, but it does indicate that the disease stage influences the RAT results. With other antigens, the binding of antibodies produced by the patient at the antigen–antibody reaction site can produce false-negatives (Sadamoto et al., 1993). In SARS-CoV-2, mucosal antibody production has been reported after about a week (Cervia et al., 2020). The mucosal antibodies described in this report are against the spikes, which are different from the nucleoproteins targeted by the ESPLINE test. However, in antibody tests using ELISA, nucleoproteins tended to increase earlier than spike proteins (Van Elslande et al., 2020). As the rate of antibody acquisition is also high, there remains the possibility that the production of mucosal antibodies may affect RAT results.
Although the detection limits of each SARS-CoV-2 test method remain unclear, the necessary viral loads set by the Japanese Ministry of Health, Labor, and Welfare are 101, 102, and 103 copies/test for PCR, loop-mediated isothermal amplification, and RAT, respectively (JMHLW, 2020d). However, in the former two tests, NPS are generally stored after being placed in VTM. The amount of VTM is usually 1–3 ml, implying a dilution of 5–15 times compared to 200 μl of RAT reagent. In the present study, the large number of samples that were reagent-positive and VTM-negative among the VTM and reagent samples collected on the same day suggests that disparities in sample viral loads can have a major impact on the results (Supplementary Material Figure S1). Conversely, with RT-qPCR, some VTM samples were positive while the reagent sample was negative. This is thought to be because the reagent swab was not sampled in exactly the same manner as VTM, despite being collected on the same day. Other reports have speculated that the cause of false-negatives with PCR is an insufficient sampling (Piras et al., 2020, Rhee et al., 2020). Similar to the present study, comparisons of VTM and RAT results with reagents showed extremely low concordance rates for RT-qPCR using both samples, which is considered undesirable.
The disagreement between RAT and PCR test results of samples stored in VTM was associated with low body temperature and the use of drugs with potential antiviral effects. The most commonly used drugs with potential antiviral effects were HCQ, followed by ciclesonide inhalation and favipiravir. HCQ has been shown to suppress the virus in vitro (Colson et al., 2020), and a small open-label non-randomized clinical trial reported the contribution of HCQ to the rapid decrease in viral load to negative (Gautret et al., 2020). However, in randomized controlled trials, neither this effect nor dose-dependent changes have been observed, leading to the conclusion that HCQ cannot be expected to suppress the viral load clinically (Borba et al., 2020, Hernandez et al., 2020, Tang et al., 2020). Nevertheless, in molecular simulations, HCQ binds efficiently to the NTD-N protein (Amin and Abbas, 2020), which suggests that it may affect the ESPLINE kit, which targets the nucleoproteins. One possible reason for the discrepancy in results is that the number of viral copies is a confounding factor, although it was significantly higher when drugs with potential antiviral effects were included (data not shown). Further studies are needed to assess the effects of drugs with potential antiviral effects on RAT.
In mild cases, the virus could not be cultured after 8–12 days from onset (Centers for Disease Control and Prevention (CDC), 2020; Singanayagam et al., 2020; Wolfel et al., 2020). Epidemiological data and mathematical models of contacts have also indicated that secondary infections from contacts are extremely rare after 5–10 days from onset (Cheng et al., 2020, Ferretti et al., 2020). Based on these findings, Britain, the World Health Organization, the USA, and other countries changed their conditions for ending isolation after a certain number of days from onset (CDC, 2020; Department of Health and Social Care, 2020; World Health Organization, 2020). On June 12, 2020, Japan added “72 h after becoming asymptomatic and 10 days after onset” to its criteria for ending isolation (JMHLW, 2020c). In the present study, the SARS-CoV-2 gene was detected in 44% of samples from those meeting the conditions for ending isolation – mild cases with no fever and 10 days from onset. Similar situations have been observed in many countries, with positive PCR results occurring long after 10 days from onset, when patients are believed to no longer be infectious (Agarwal et al., 2020, Shi et al., 2020).
In high-prevalence situations, the guidelines of the Infectious Diseases Society of America (Hanson et al., 2020) allow for the screening of all inpatients. However, screening using PCR may lead to the diagnosis of patients who have no infectivity. Isolating patients creates problems such as the wastage of personal protective equipment, reduced quality of patient care, consumption of limited resources such as private rooms, and increasing psychological burden on the patient (Rhee et al., 2020). Although it may be possible to estimate the transmissibility from the difference in viral load, it is unrealistic to carry out precise quantification of all the tests. It may also be possible to estimate the viral load using the Ct value (Singanayagam et al., 2020, Tom and Mina, 2020). However, it is difficult to generalize a cut-off because the Ct value differs with reagents and test equipment (Chang et al., 2020).
Based on the multivariate analysis in the present study, RAT may be useful for determining whether patients have transmissible disease or not. However, this study did not include samples from the pre-syndromic phase. Mathematical models and viral culture have shown that COVID-19 is transmissible for about 10 days before onset (He et al., 2020, Singanayagam et al., 2020). However, because the pre-syndromic phase is short, the probability that asymptomatic PCR-positive patients are in this phase may be low. Of the 43 patients who were diagnosed in an asymptomatic state in the cruise ship outbreak, 10 (23%) were reported to be pre-syndromic (Tabata et al., 2020). However, few asymptomatic patients detected by chance in group screenings were in the pre-syndromic phase (Lavezzo et al., 2020, Lytras et al., 2020, Nishiura et al., 2020, Sutton et al., 2020). Furthermore, according to Singanayagam et al., the RT-qPCR Ct values of pre-syndromic samples that were positive in viral cultures were relatively low (<30) (Singanayagam et al., 2020). This suggests that RAT may sufficiently capture the pre-syndromic state with viable viral shedding. Although we await the clinical assessments of RAT in the pre-syndromic state, very few patients are encountered in this state during screenings.
While immunochromatography produces a certain number of false-positives, there are no reports of PCR-negative and RAT-positive cases in studies on RAT using immunochromatography, suggesting very few false-positives (Kashiwagi et al., 2020, Mak et al., 2020, Porte et al., 2020, Scohy et al., 2020). In the present study, however, there was disagreement with one VTM sample result, which was positive when tested with an RT-qPCR kit using a different primer–probe set. Moreover, two samples of rapid test reagents that were PCR-negative and RAT-positive became negative.
Limitations
This study, which used stored samples, included multiple samples from the same person, and thus did not completely reflect the conditions at diagnosis. However, the examination of the initial samples only showed similar trends in the positive and negative concordance rates and κ coefficients. RT-qPCR results with ≥102 copies/test taken at the appropriate time as the criterion for positivity also had a high concordance level. Nevertheless, because there was a delay in collecting many of the samples following the onset of symptoms, we had insufficient samples to demonstrate the performance of RAT in the very early stages of the disease.
In the assessment of transmissibility, animal studies (Sia et al., 2020) and human epidemiological data (Rhee et al., 2020) have shown growth in viral cultures as an indicator of transmissibility. Viral cultures were not conducted in this study. The study ultimately only showed that RAT can estimate the period when a patient is thought to be infectious and did not show how viral cultures and RAT results are related.
Conclusions
The concordance rates between RAT and PCR tests were not very high, but concordance increased in samples taken at the appropriate time. Agreement with RT-qPCR was high when there were at least 102 copies/test. As diluting samples with VTM can be a problem, higher concordance rates can be expected as more studies adopt the ESPLINE method. Because RAT is less sensitive than RT-qPCR and the appropriate timing of sampling is limited, these results suggest that in mild cases that are RT-qPCR-positive, a negative RAT may indicate a low level of transmissibility. This could serve as a reference when assessing the infection status of patients who are positive during PCR screening.
Funding
This work was supported by Fujirebio Inc., Japan and was used to conduct the current research and prepare the article.
Conflict of interest
K.Y. has received research grants from Fujirebio Inc. for the submitted work. S.Y. is an employee of Fujirebio Inc. K.Y. has received research grants from Mizuho Medy CO. Ltd., Japan and N.O. declares grants from Sanofi K.K., Japan and Eiken Chemical Co. Ltd., Japan outside the submitted work.
Acknowledgements
We thank the staff at the Disease Control and Prevention Center, Department of Respirology, National Center for Global Health and Medicine, and those at the AIDS Clinical Center, National Center for Global Health and Medicine for collecting the clinical samples.
Footnotes
Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.ijid.2020.12.079.
Appendix A. Supplementary data
The following are Supplementary data to this article:
References
- Agarwal V., Venkatakrishnan A.J., Puranik A., Lopez-Marquez A., Challener D.W., Horo J.C.O. Long-term SARS-CoV-2 RNA shedding and its temporal association to IgG seropositivity. Cell Death Discov. 2020;6:138. doi: 10.1038/s41420-020-00375-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amin M., Abbas G. Docking study of chloroquine and hydroxychloroquine interaction with RNA binding domain of nucleocapsid phospho–protein—an in silico insight into the comparative efficacy of repurposing antiviral drugs. J Biomol Struct Dyn. 2020:1–13. doi: 10.1080/07391102.2020.1775703. preprint. [DOI] [PubMed] [Google Scholar]
- Borba M.G.S., Val F.F.A., Sampaio V.S., Alexandre M.A.A., Melo G.C., Brito M. Effect of high vs low doses of chloroquine diphosphate as adjunctive therapy for patients hospitalized with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection: a randomized clinical trial. JAMA Netw Open. 2020;3 doi: 10.1001/jamanetworkopen.2020.8857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention . 2020. Symptom-Based Strategy to Discontinue Isolation for Persons with COVID-19. Available at: https://www.cdc.gov/coronavirus/2019–ncov/community/strategy–discontinue–isolation.html. [Accessed 14 August 2020] (Updated July 22, 2020). [Google Scholar]
- Cervia C., Nilsson J., Zurbuchen Y., Valaperti A., Schreiner J., Wolfensberger A. Systemic and mucosal antibody responses specific to SARS-CoV-2 during mild versus severe COVID-19. J Allergy Clin Immunol. 2020 doi: 10.1016/j.jaci.2020.10.040. S0091-6749(20)31623-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang M.C., Hur J., Park D. Interpreting the COVID-19 test results: a guide for physiatrists. Am J Phys Med Rehabil. 2020;99:583–585. doi: 10.1097/PHM.0000000000001471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng H.Y., Jian S.W., Liu D.P., Ng T.C., Huang W.T., Lin H.H. Contact tracing assessment of COVID-19 transmission dynamics in Taiwan and risk at different exposure periods before and after symptom onset. JAMA Intern Med. 2020;180:1156–1163. doi: 10.1001/jamainternmed.2020.2020. [published online ahead of print, 2020 May 1] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colson P., Rolain J.M., Lagier J.C., Brouqui P., Raoult D. Chloroquine and hydroxychloroquine as available weapons to fight COVID-19. Int J Antimicrob Agents. 2020;55 doi: 10.1016/j.ijantimicag.2020.105932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Department of Health and Social Care . Department of Health and Social Care; London: 2020. Department of Health and Social Care Statement from the UK Chief Medical Officers on Extension of Self–Isolation Period: 30 July, 2020. 30 July, 2020. Available at: https://www.gov.uk/government/news/statement-from-the-uk-chief-medical-officers-on-extension-of-self-isolation-period-30-july-2020, [Accessed 2 October 2020] [Google Scholar]
- Ferretti L., Wymant C., Kendall M., Zhao L., Nurtay A., Abeler-Dörner L. Quantifying SARS-CoV-2 transmission suggests epidemic control with digital contact tracing. Science. 2020;368 doi: 10.1126/science.abb6936. eabb6936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gautret P., Lagier J.C., Parola P., Hoang V.T., Meddeb L., Mailhe M. Hydroxychloroquine and azithromycin as a treatment of COVID-19: results of an open–label non–randomized clinical trial. Int J Antimicrob Agents. 2020;56 doi: 10.1016/j.ijantimicag.2020.105949. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Gwet K.L. Wiley–Interscience; New York: 2008. Intrarater Reliability. Wiley Encyclopedia of Clinical Trials; pp. 473–485. [DOI] [Google Scholar]
- Hanson K.E., Caliendo A.M., Arias C.A., Englund J.A., Lee M.J., Loeb M. Infectious diseases society of America guidelines on the diagnosis of COVID-19. Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa760. ciaa760 preprint. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He X., Lau E.H.Y., Wu P., Deng X., Wang J., Hao X. Temporal dynamics in viral shedding and transmissibility of COVID-19. Nat Med. 2020;26:672–675. doi: 10.1038/s41591–020–0869–5. [published correction appears in Nat Med. 2020 August 17] [DOI] [PubMed] [Google Scholar]
- Hernandez A.V., Roman Y.M., Pasupuleti V., Barboza J.J., White C.M. Hydroxychloroquine or chloroquine for treatment or prophylaxis of COVID-19: a living systematic review. Ann Intern Med. 2020;173:287–296. doi: 10.7326/M20–2496. [DOI] [PubMed] [Google Scholar]
- Japanese Ministry of Health, Labor, and Welfare . 2020. Approval of In vitro Diagnostics for the Novel Coronavirus Infection. Available at: https://www.mhlw.go.jp/content/11124500/000632304.pdf. [Accessed 30 May 2020] [Google Scholar]
- Japanese Ministry of Health, Labor, and Welfare . 2020. Guidelines Regarding Use of SARS-CoV-2 Antigen Detection Kits (revised on June 16, 2020) [in Japanese] Available at: https://www.mhlw.go.jp/content/000640554.pdf, [Accessed 2 October 2020] [Google Scholar]
- Japanese Ministry of Health, Labor, and Welfare . 2020. Clinical Management of Patients with COVID-19: a Guide for Front–Line Healthcare Workers Version 2.1. Available at: https://www.mhlw.go.jp/content/000646531.pdf, [Accessed 2 October 2020] [Google Scholar]
- Japanese Ministry of Health, Labor, and Welfare . 2020. Addition of Quantitative Testing of Pathogen Antigens in Testing for Novel Coronavirus Infection (Draft) (25/June/2020) [in Japanese] Available at: https://www.mhlw.go.jp/content/10906000/000643018.pdf, [Accessed 2 October 2020] [Google Scholar]
- Kashiwagi K., Ishii Y., Aoki K., Yagi S., Maeda T., Miyazaki T. Immunochromatographic test for the detection of SARS-CoV-2 in saliva. J Infect Chemother. 2020 doi: 10.1016/j.jiac.2020.11.016. S1341-321X(20)30423-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavezzo E., Franchin E., Ciavarella C., Cuomo-Dannenburg G., Barzon L., Vecchio C.D. Suppression of COVID-19 outbreak in the Italian municipality of Vo’. Nature. 2020;584:425–429. doi: 10.1038/s41586-020-2488-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lytras T., Dellis G., Flountzi A., Hatzianastasiou S., Nikolopoulou G., Tsekou K. High prevalence of SARS-CoV-2 infection in repatriation flights to Greece from three European countries. J Travel Med. 2020;27 doi: 10.1093/jtm/taaa054. taaa054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mak G.C., Cheng P.K., Lau S.S., Wong K.K., Lau C.S., Lam E.T. Evaluation of rapid antigen test for detection of SARS-CoV-2 virus. J Clin Virol. 2020;129 doi: 10.1016/j.jcv.2020.104500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Institute of Infectious Disease . 2020. Manual for the Detection of Pathogen 2019–nCoV Ver.2.6. Available at: https://www.niid.go.jp/niid/images/epi/corona/2019-nCoVmanual20200217-en.pdf, [Accessed 2 October 2020] [Google Scholar]
- Nishiura H., Kobayashi T., Miyama T., Suzuki A., Jung S.M., Hayashi K. Estimation of the asymptomatic ratio of novel coronavirus infections (COVID-19) Int J Infect Dis. 2020;94:154–155. doi: 10.1016/j.ijid.2020.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piras A., Rizzo D., Uzzau S., De Riu G., Rubino S., Bussu F. Inappropriate nasopharyngeal sampling for SARS-CoV-2 detection is a relevant cause of false negative reports. Otolaryngol Head Neck Surg. 2020;163:459–461. doi: 10.1177/0194599820931793. [DOI] [PubMed] [Google Scholar]
- Porte L., Legarraga P., Vollrath V., Aguilera X., Munita J.M., Araos R. Evaluation of novel antigen–based rapid detection test for the diagnosis of SARS-CoV-2 in respiratory samples. Int J Infect Dis. 2020;99:328–333. doi: 10.1016/j.ijid.2020.05.098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhee C., Kanjilal S., Baker M., Klompas M. Duration of SARS-CoV-2 infectivity: when is it safe to discontinue isolation? Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa1249. ciaa1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadamoto S., Ikeda R., Nishikawa A., Shinoda T. Evidence for interference by immune complexes in the serodiagnosis of cryptococcosis. Microbiol Immunol. 1993;37:129–133. doi: 10.1111/j.1348–0421.1993.tb03189.x. [DOI] [PubMed] [Google Scholar]
- Scohy A., Anantharajah A., Bodéus M., Kabamba–Mukadi B., Verroken A., Rodriguez–Villalobos H. Low performance of rapid antigen detection test as frontline testing for COVID-19 diagnosis. J Clin Virol. 2020;129 doi: 10.1016/j.jcv.2020.104455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi D., Wu W., Wang Q., Xu K., Xie J., Wu J. Clinical characteristics and factors associated with long–term viral excretion in patients with severe acute respiratory syndrome coronavirus 2 infection: a single–center 28–day study. J Infect Dis. 2020;222:910–918. doi: 10.1093/infdis/jiaa388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sia S.F., Yan L.M., Chin A.W.H., Fung K., Choy K.T., Wong A.Y.L. Pathogenesis and transmission of SARS-CoV-2 in golden hamsters. Nature. 2020;583:834–838. doi: 10.1038/s41586–020–2342–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singanayagam A., Patel M., Charlett A., Bernal J.L., Saliba V., Ellis J. Duration of infectiousness and correlation with RT–PCR cycle threshold values in cases of COVID-19, England, January to May 2020. Euro Surveill. 2020;25:2001483. doi: 10.2807/1560–7917.ES.2020.25.32.2001483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutton D., Fuchs K., D’Alton M., Goffman D. Universal screening for SARS-CoV-2 in women admitted for delivery. N Engl J Med. 2020;382:2163–2164. doi: 10.1056/NEJMc2009316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabata S., Imai K., Kawano S., Ikeda M., Kodama T., Miyoshi K. Clinical characteristics of COVID-19 in 104 people with SARS-CoV-2 infection on the Diamond Princess cruise ship: a retrospective analysis. Lancet Infect Dis. 2020;20:1043–1050. doi: 10.1016/S1473–3099(20)30482–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang W., Cao Z., Han M., Wang Z., Chen J., Sun W. Hydroxychloroquine in patients with mainly mild to moderate coronavirus disease 2019: open label, randomised controlled trial. BMJ. 2020;369:m1849. doi: 10.1136/bmj.m1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tom M.R., Mina M.J. To interpret the SARS-CoV-2 test, consider the cycle threshold value. Clin Infect Dis. 2020;71:2252–2254. doi: 10.1093/cid/ciaa619. ciaa619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Elslande J., Decru B., Jonckheere S., Van Wijngaerden E., Houben E., Vandecandelaere P. Antibody response against SARS-CoV-2 spike protein and nucleoprotein evaluated by four automated immunoassays and three ELISAs. Clin Microbiol Infect. 2020;26:1557.e1–1557.e7. doi: 10.1016/j.cmi.2020.07.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfel R., Corman V.M., Guggemos W., Seilmaier M., Zange S., Müller M.A. Virological assessment of hospitalized patients with COVID-2019. Nature. 2020;581:465–469. doi: 10.1038/s41586-020-2196-x. [DOI] [PubMed] [Google Scholar]
- World Health Organization . WHO; Geneva: 2020. Clinical Management of COVID-19 (Interim Guidance) 27 May, Available at: https://www.who.int/publications/i/item/clinical-management-of-covid-19 [Accessed 2 October 2020] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.