Abstract
Background
High diagnostic accuracy for pneumonia, absence of radiation exposure and repeatability are intrinsic features of lung ultrasonography making it an attractive tool in the assessment of patients with COVID-19 pneumonia. The aim of our prospective, observational study was to detect COVID-19-associated sonographic features and assess the potential value of LUS in predicting adverse events.
Methods
From March 12th to April 20th 2020 patients admitted to two medium-intensive wards with a discharge diagnosis of COVID-19 pneumonia were enrolled and underwent lung ultrasonography. The prognostic value of several ultrasonographic scores at admission and after 72 hours from the first examination (the total score, the anterolateral score, the number of positive region and the presence of consolidation) were analysed with logistic regression along with other potential prognostic factors. The primary outcome was a composite of death and transfer to Intensive Care Unit (ICU), while the secondary was continuous positive airways pressure (CPAP) support.
Results
190 patients were enrolled in the study. The primary outcome was seen in 25 patients (13%), the secondary outcome in 36 (22%). At multivariate regression no sonographic score at admission was independently correlated with the primary outcome while the total score, the anterolateral score, the number of positive regions were associated with CPAP support. When considering the subgroup of patients undergoing lung ultrasonography after 72 hours (128 patients) the total score was independently associated with both the primary and secondary outcome.
Conclusion
Lung ultrasonography can be a promising prognostic tool in patients admitted to non-ICU units for COVID-19 pneumonia.
1. Introduction
From February to March 2020, Italy became the most affected country worldwide by coronavirus disease (COVID-19). Our healthcare system was put under enormous pressure, especially in Lombardia which accounts for almost half of the deaths of the entire country [1]. In this setting, early identification of suspected cases, assessment of disease severity and monitoring patients affected by COVID-19 pneumonia became crucial. Lung computed tomography (CT) is considered the gold standard to detect pulmonary lesions in patients with COVID-19 pneumonia: patients typically show bilateral, multilobar ground-glass opacities, with a prevalent peripheral distribution, which can progress to crazy paving and consolidations [2,3]. Nevertheless, the pandemic spread of the disease in many cases did not allow a standardized CT-based diagnostic approach due to either overcrowding of Emergency Department (ED) and high risk of transporting contagious and unstable patients to radiology department.
In the last decades, lung ultrasound (LUS) represented a reliable imaging tool to differentiate causes of acute dyspnoea and acute respiratory failure [4] and to monitor lung involvement in hospitalized patients [5,6]. Moreover it was shown to be an accurate tool for diagnosis and follow-up of pneumonia [7,8]. It is performed at bedside, usually with portable US devices, thus minimizing the risk associated with transfer of infectious and potentially critical/unstable patients. It is known that one of the major LUS limits is its possibility to only detect abnormalities reaching the pleural line; nevertheless, COVID-19 pulmonary involvement seems to start from the peripheral lung regions [9], allowing a reliable US assessment. For these reasons, LUS encountered growing enthusiasm for its application in COVID-19 affected patients. Peng et al. first described their experience in the use of LUS in China, suggesting its use as alternative to other conventional imaging methods [10]. Two recent studies reported a good correlation between LUS and CT findings [11,12].
The most frequently reported LUS features in COVID-19 patients are the following: interstitial involvement represented by B-lines pattern (i.e. the presence of at least three B-lines in a lung scan) which can progress up to confluent B-lines also called “white lung”; pleural line abnormalities, like thickened, irregular or fragmented pleural line; consolidations, ranging from small, subpleural consolidation to large consolidation with air bronchograms [13], [14], [15], [16].
In last months a lot of studies aimed to detect prognostic factors in COVID-19 pneumonia; most of them were demographic, clinical and laboratory parameters [17], [18], [19]. LUS may potentially be used to identify patients who are at risk of adverse outcomes; in fact a severe pulmonary involvement by LUS may be associated with a more severe disease course.
As shown, literature on LUS in COVID-19 patients is rapidly growing; however few studies evaluated the prognostic value of LUS in COVID-19 patients [20], [21], [22], [23]. All these studies enrolled a significant percentage of patients who were hospitalized in Intensive Care Units (ICU). An Italian study outlined the prognostic role of LUS in a cohort of patients in the Emergency Department [24].
In our study we aimed to detect COVID-19 associated sonographic features and assess the potential value of LUS in predicting adverse events in a cohort of patients hospitalized in a medium-intensity department.
2. Materials and methods
The study is part of a single-centre, prospective, observational, cohort study of all the adult COVID-19 patients admitted to Luigi Sacco Hospital in Milan, Italy, since February 21st 2020; the observation of the cohort was censored on April 20th 2020. COVID-19 infection was defined by a positive RT-PCR assay according to WHO criteria [25]. The data extracted from the patients’ clinical charts on a daily basis and stored in an ad hoc database included demographic, clinical, radiological and pharmacological data. The study was approved by the local Ethics Committee and informed consent was obtained (Protocol Number 16088/2020).
LUS was routinely performed in the subgroup of patients admitted to our two Internal Medicine Departments which were converted into COVID-19 medium-intensity care units. Thus we considered adult patients consecutively hospitalized with a diagnosis (imaging/clinical derived) of COVID-19 related pneumonia.
Pre-existing conditions that may mislead the evaluation of lung ultrasound (i.e. congestive heart failure, lung neoplasms, pre-existing lung interstitial diseases) were considered exclusion criteria.
Age, Charlson Comorbidity Index (CCI), presence of hypertension, obesity (expressed as body mass index, BMI) and arterial oxygen partial pressure/fractional inspired oxygen ratio (P/F) were analysed because of the increasing evidences of their connection to poor outcome in COVID-19 patients.
2.1. Lung ultrasound
As defined by local clinical practise protocol, patients underwent lung ultrasound (LUS) examination within 48 hours from admission.
A subgroup of patients underwent other LUS examinations, the first one performed after 72 hours from admission LUS. Other LUS exams were performed according to physician decision. Every performed LUS was recorded in the patient electronic clinical record; images (pictures or videos) were properly stored.
LUS was performed by physicians trained in point of care ultrasound and particularly in lung examinations. The physicians were not blind to the clinical and radiological status of patients. Nevertheless, a second, blind independent observer with more than 10 years certified experience in lung ultrasound reviewed 5% of the recorded scans to assess reproducibility of LUS. The scans were randomly chosen for the review.
LUS was performed with different models of portable ultrasound devices: Philips CX50, GE Logiq F6 and Vinno 8, using convex probes for a thorough evaluation of both lungs. US machines setting were optimized following the subsequent modalities: low mechanical index (0.7 or less); a single focus, positioned on the pleural line; no harmonic modality; no persistence.
The exam was conducted by dividing the chest wall in 12 regions, six for each lung: two anterior (upper and lower), two lateral and two posterior regions as described before [26]. In details, the anterior axillary line was used to divide anterior and lateral regions, the posterior axillary line divided lateral and posterior regions, while the internipple line split upper and lower areas.
Anterolateral and posterior regions were evaluated in supine and sitting position, respectively. When patients were unable to maintain a sitting position, posterior regions were evaluated with the patients in left and right lateral decubitus.
We decided to exclude the left inferior anterior region because the presence of the heart might compromise the correct evaluation and the allocation of a reliable score, therefore leaving a total of 11 areas.
Each region was scored as follows:
-
-
Score 0: regular pleural line, presence of horizontal artefacts (A-lines)
-
-
Score 1: at least 3 B-lines in at least one scan of the region; the B-lines do not merge one in the other. Small subpleural consolidations ≤1 cm diameter may be present.
-
-
Score 2: multiple, converging B-lines, usually determining a so-called “white lung”. Small subpleural consolidations ≤1 cm diameter may be present.
-
-
Score 3: presence of at least one consolidation with major axis >1 cm.
The presence of pleural effusion was reported on the report form.
For each LUS we considered for analysis the total score, the total number of positive regions (NPR, number of regions with score ≥1), the anterolateral score and the presence or absence of consolidative lesions (score 3 in at least one region). The total score was calculated by summing the scores of all 11 lung regions (range: 0- 33); the anterolateral score was derived by summing the anterior and lateral regional scores (range: 0-21).
2.2. Endpoints
In this study we analysed the association between the severity of pulmonary involvement by LUS at admission (as assessed by the total score, the NPR, the anterolateral scores, the presence of consolidations) and adverse outcomes in COVID-19 pneumonia. The primary outcome was death or ICU transfer. The secondary outcome was non-invasive mechanical ventilation support with continuous positive airway pressure (CPAP). In a subgroup of patients, we analysed the association between the burden of pulmonary involvement at LUS performed after 72 hours from the first examination (as assessed by total score) and the same outcomes.
2.3. Statistical analysis
Continuous variables were reported as medians and interquartile ranges. Categorical data were expressed as counts (percentages).
To assess the predictive value of the four ultrasound scores for the two outcomes, univariate and multivariate logistic regression analyses were conducted. We performed univariate analyses considering the ultrasound scores and the following parameters as potential confounders: age (≤65, 66-75, >75 years), CCI (0; 1-2; 3-11), presence of hypertension, obesity (BMI ≤25; 25-30; >30) and P/F (≤200, 200-300, >300). Only the variables statistically significant in univariate analysis were entered in multivariate models. Four separate multivariate analyses were performed using one of four LUS scores and the potential confounders statistically significant at the univariate stage.
Following the same statistical approach, we performed an additional analysis including only the patients who underwent LUS after 72 hours.
Results were expressed as odds ratio (OR) and 95% confidence interval (95% CI). P value <0.05, two tailed, was considered statistical significant.
In addition, for the total LUS scores at admission and after 72 hours, receiver operating characteristic (ROC) curve analyses were also performed to describe the ability of the score to predict the primary outcome. Sensitivity and specificity, with their 95% CIs, were calculated.
SAS software (release 9.4; SAS Institute, Inc., Cary, North Carolina) was used to perform statistical analysis
3. Results
During the study period 243 patients were admitted to Internal Medicine COVID-19 wards with a diagnosis of COVID-19-related pneumonia; among them 6 met the exclusion criteria. Two hundred thirty-seven patients were eligible for the study; however 47 of them were excluded (30 patients did not undergo LUS at admission and 17 underwent LUS but incomplete reports made them not suitable for attribution of LUS scores).
The overall characteristics of the 190 patients included in the study are shown in Table 1 .
Table 1.
Population characteristics.
| Overall population (no. 190) | |
|---|---|
| AGE - median (IQR) – years | 62 (49-73) |
| ≤65 years old – no. (%) | 115 (61) |
| 66-75 years old – no. (%) | 35 (18) |
| >75 years old – no. (%) | 40 (21) |
| SEX: MALE – no. (%) | 112 (59) |
| COEXISTING CONDITIONS - n (%) | |
| Charlson Comorbidity Index (CCI) – median (IQR) | 1 (0-4) |
| CCI 0 – no. (%) | 69 (36) |
| CCI 1-2 – no. (%) | 51 (27) |
| CCI 3-11 – no. (%) | 70 (37) |
| Hypertension | 63 (33) |
| CAD | 14 (7) |
| Diabetes | 25 (14) |
| Active cancer | 6 (3) |
| Obesity BMI > 30 Kg/m2 | 20 (14) |
| BMI > 25; ≤ 30 Kg/m2 | 53 (36) |
| Pre-existing pulmonary disease | 20 (11) |
| PRESENTING SYMPTOMS – no. (%) | |
| Fever | 170 (89) |
| Dyspnoea | 77 (41) |
| Cough | 108 (57) |
| Arthralgia/myalgia | 7 (4) |
| Headache | 9 (5) |
| Fatigue | 21 (11) |
| DAYS FROM SYMPTOMS ONSET TO HOSPITAL ADMISSION - median (IQR) | 8 (5-12) |
| LABORATORY FINDINGS AT ADMISSION - median (IQR) | |
| White-cell count (× 10 9 /liter) | 6000 (4775-8018) |
| Neutrophil count (× 10 9/liter) | 4250 (2772-6550) |
| Lymphocyte count (× 10 9/liter) | 1185 (830-1534) |
| CRP (mg/L) | 62 (19-141) |
| Lactate dehydrogenase (U/liter) | 284 (218-383) |
| D-dimer (ng/L) | 676 (335-1215) |
| IL-6 (ng/L) | 27 (13-68) |
| P/F categories (mmHg) – no. (%) | |
| ≤200 | 36 (19) |
| 200-300 | 30 (16) |
| >300 | 120 (65) |
| P/F at admission (mmHg) – median (IQR) | 343 (230-380) |
| CLINICAL FEATURES | |
| NEWS at admission - median (IQR) | 3 (2-5) |
| Systolic blood pressure < 90 mmHg at admission - no. (%) | 3 (1.6) |
| Respiratory rate > 24 at admission - no. (%) | 36 (18.9) |
| Fever > 38°C at admission - no. (%) | 22 (11.6) |
| Oxygen therapy at admission - no. (%) | 123 (64.7) |
| RADIOLOGICAL FINDINGS - no. (%) | |
| Interstitial involvement/consolidation | 157 (84.0) |
| Bilateral involvement | 112 (59.9) |
| OUTCOME - no. (%) | |
| Death or transfer to ICU | 25 (13.2) |
| Death | 19 (10) |
| Transfer to ICU | 10 (5.3) |
| CPAP | 53 (27.9) |
| CPAP, no death nor ICU | 36 (18.9) |
| No death or transfer to ICU, nor CPAP | 129 (67.9) |
| LENGTH OF HOSPITAL STAY (days) – median (IQR) | 12 (7-20) |
IQR=Interquartile Range; CAD=Coronary Artery Disease; BMI=Body Mass Index; CRP=C-Reactive Protein; IL-6=Interleukin 6; P/F=arterial oxygen partial pressure/fractional inspired oxygen ratio; NEWS=national early warning score; ICU=intensive care unit; CPAP=continuous positive air pressure.
The median age was 62 years (IQR 49-73) with a prevalence of male sex (59%).
Ten patients were transferred to ICU and 19 patients died; the primary composite outcome was observed in 25 patients (13.2%). Of the 165 remaining patients, 36 (21.8 %) were treated with CPAP.
The median time from the onset of symptoms to hospital admission was 8 days (IQR 5-12) and the median length of hospital stay was 12 days (IQR 7-20).
All enrolled patients underwent a chest X-ray in the Emergency Department: in 157 patients (84%) it showed the presence of interstitial and/or alveolar involvement, in 112 cases (59.9%) the involvement detected on X-ray was bilateral.
3.1. Lung ultrasound
LUS findings are presented in Table 2 .
Table 2.
Lung ultrasound characteristics.
| Overall population (no. 190) | |
|---|---|
| BILATERAL INVOLVEMENT - no. (%) | 173 (91.1) |
| CONSOLIDATIONS – no. (%) | 46 (24.2) |
| No. Consolidations ≥2 – no. (%) | 31 (16.3) |
| Bilateral – no. (%) | 31 (16.3) |
| PLEURAL EFFUSION - no. (%) | 12 (6.3) |
| TOTAL SCORE – median (IQR) | 10 (6-16) |
| NPR SCORE – median (IQR) | 7 (5-10) |
| ANTEROLATERAL SCORE - median (IQR) | 5 (3-9) |
| REGIONAL INVOLVEMENT – no. (%) Consolidations – no. (%) |
|
| RIGHT UPPER ANTERIOR | 89 (46.8) 1 (0.5) |
| RIGHT LOWER ANTERIOR | 102 (53.7) 0 |
| RIGHT UPPER LATERAL | 106 (55.8) 1 (0.5) |
| RIGHT LOWER LATERAL | 130 (68.4) 3 (1.6) |
| RIGHT UPPER POSTERIOR | 121 (63.7) 3 (1.6) |
| RIGHT LOWER POSTERIOR | 160 (84.2) 37 (19.5) |
| LEFT UPPER ANTERIOR | 81 (42.6) 0 |
| LEFT UPPER LATERAL | 120 (63.2) 0 |
| LEFT LOWER LATERAL | 140 (73.7) 7 (3.7) |
| LEFT UPPER POSTERIOR | 112 (58.9) 3 (1.6) |
| LEFT LOWER POSTERIOR | 163 (85.8) 33 (17.4) |
NPR=Number of Positive Region; IQR=inter-quartile range.
Pathological findings (B-lines pattern/consolidations, i.e. LUS score ≥ 1) were observed in 182 patients (95.8%) (Fig. 1 ). One hundred seventy-three patients (91.1%) presented a bilateral lung involvement. No patient without bilateral involvement met the primary or secondary outcome.
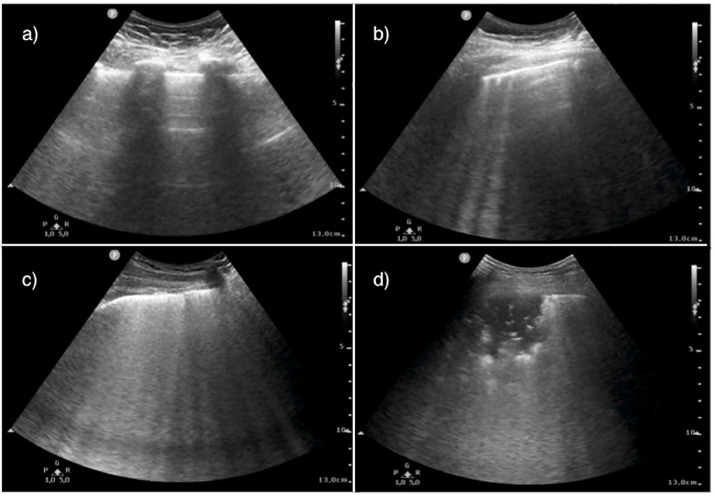
Fig. 1.
Lung ultrasound (LUS) patterns. a) Score 0, physiological pattern, regular pleural line with horizontal artefacts (A pattern); b) Score 1, presence of at least three vertical scattered artefacts (B lines); c) Score 2, multiple converging B lines (“white lung”); d) Score 3, subpleural consolidation >1 cm.
Pleural effusion was observed in a minority of patients (6.3%).
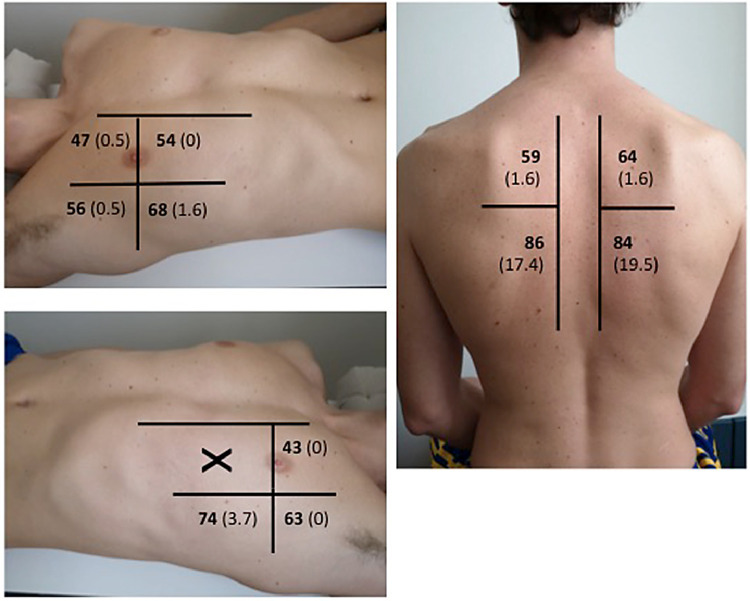
Consolidations were observed in 46 patients (24.2%). The analysis of regional score shows that the inferior-posterior regions were more frequently involved; these were as well the regions more frequently interested by consolidations (Fig. 2 ).
Fig. 2.
Analysis of regional score: percentage of patients with positive region (score >l) in bold. Percentage of patients with consolidation in brackets.
The median time of symptomatic disease before the first LUS was 9 days. In 73 out of 190 patients the examination at admission was performed within 7 days from symptoms’ onset. In this subset of population LUS showed a significantly lower total score (9 vs. 12; p 0.0128), NPR (6 vs. 8; p 0.0069) and anterolateral score (4 vs. 7; p 0.0067). The prevalence of consolidations at LUS was not significantly reduced in patients who had symptoms for less than 7 days (19.2% vs. 27.6%; p not significant).
One hundred twenty-eight patients were followed with LUS performed 3 days after the first examination. In this subset of patients, 10 reached the primary outcome; of the remaining 118 patients, 26 underwent CPAP. In 66 patients (51.5%) a worse total score was observed; 39 patients (30.5%) showed an improvement in total score, while in 23 patients (18%) LUS findings remained stable.
LUS scores stratified by the occurrence of the primary and secondary outcomes are shown in Table 3 .
Table 3.
LUS scores and outcomes.
| LUS AT ADMISSION | |||
|---|---|---|---|
| PRIMARY OUTCOME | Total (no. 190) | Non D/ICU (no. 165) | D/ICU (no. 25) |
| Total score – median (IQR) | 10 (6-16) | 10 (6-15) | 15 (12-20) |
| NPR score – median (IQR) | 7 (5-10) | 7 (4-10) | 10 (8-11) |
| Anterolateral score - median (IQR) | 5 (3-9) | 5 (2-8) | 9 (7-12) |
| Consolidation – no. (%) | 46 (24.2) | 38 (23.0) | 8 (32.0) |
| SECONDARY OUTCOME | Total (no. 165) | Non CPAP (no. 129) | CPAP (no. 36) |
| Total score – median (IQR) | 10 (6-15) | 8 (5-12) | 16 (12-18) |
| NPR score – median (IQR) | 7 (4-10) | 6 (4-9) | 10 (7-11) |
| Anterolateral score - median (IQR) | 5 (2-8) | 4 (2-7) | 8 (6-10) |
| Consolidation – no. (%) | 38 (23.0) | 22 (17.1) | 16 (44.4) |
| LUS AFTER 72 HOURS | |||
| PRIMARY OUTCOME | Total (no. 128) | Non D/ICU (no. 118) | D/ICU (no. 10) |
| Total score – median (IQR) | 11 (6-18) | 11 (6-16) | 22 (22-24) |
| SECONDARY OUTCOME | Total (no. 118) | Non CPAP (no. 92) | CPAP (no. 26) |
| Total score – median (IQR) | 11 (6-16) | 8 (5-14) | 18 (14-20) |
LUS=lung ultrasound; NPR=number of positive region; D/ICU=death or transfer to ICU; CPAP=continuous positive airways pressure; IQR=inter-quartile range.
The results of univariate and multivariate analyses for both primary and secondary outcome are summarized in Table 4 and Supplementary Table 1.
Table 4.
LUS at admission: univariate and multivariate analysis for primary and secondary outcome.
| PRIMARY OUTCOME (NUMBER OF PATIENTS=190) | ||||
|---|---|---|---|---|
| UNIVARIATE ANALYSIS |
MULTIVARIATE ANALYSES° |
|||
| OR (95% CI) | p value | OR (95% CI) | p value | |
| TOTAL SCORE | 1.18# (1.09-1.28) | <0.0001 | - | ns |
| NPR SCORE | 1.36# (1.14-1.64) | 0.0008 | - | ns |
| ANTEROLATERAL SCORE | 1.25# (1.11-1.40) | 0.0002 | - | ns |
| CONSOLIDATION presence vs absence |
1.57 (0.63-3.93) | 0.3321 | - | - |
| AGE (years) | 0.0007 | ns | ||
| 66-75 vs ≤65 | 1.72 (0.49-6.11) | - | ||
| >75 vs ≤65 | 6.44 (2.42-17.10) | |||
| P/F (mmHg) | <0.0001 | |||
| 200-300 vs >300 | 4.60 (1.24-17.10) | + | + | |
| ≤200 vs >300 | 16.43 (5.39-50.04) | |||
| CCI | 0.0046 | ns | ||
| 1-2 vs 0 | 4.47 (0.86-23.12) | - | ||
| 3-11 vs 0 | 10.74 (2.38-48.57) | |||
| BMI (Kg/m2) | 0.7878 | |||
| 25-30 vs ≤25 | 0.76 (0.24-2.42) | - | - | |
| >30 vs ≤25 | 1.29 (0.32-5.31) | |||
| HYPERTENSION presence vs absence |
1.16 (0.48-2.78) | 0.7461 | - | - |
| SECONDARY OUTCOME (NUMBER OF PATIENTS=165) | ||||
|---|---|---|---|---|
| UNIVARIATE ANALYSIS |
MULTIVARIATE ANALYSES§ |
|||
| OR (95% CI) | p value | OR (95% CI) | p value | |
| TOTAL SCORE | 1.19# (1.10-1.28) | <0.0001 | 1.14# (1.04-1.24) | 0.0033 |
| NPR SCORE | 1.36# (1.17-1.58) | <0.0001 | 1.26# (1.06-1.49) | 0.0091 |
| ANTEROLATERAL SCORE | 1.26# (1.13-1.40) | <0.0001 | 1.20# (1.06-1.36) | 0.0037 |
| CONSOLIDATION presence vs absence |
3.89 (1.74-8.67) | 0.0009 | - | ns |
| AGE (years) | 0.1112 | |||
| 66-75 vs ≤65 | 2.35 (0.95-5.83) | - | - | |
| >75 vs ≤65 | 2.08 (0.79-5.49) | |||
| P/F (mmHg) | <0.0001 | |||
| 200-300 vs >300 | 3.05 (1.07-8.69) | * | * | |
| ≤200 vs >300 | 25.11 (7.88-79.95) | |||
| CCI | 0.2816 | |||
| 1-2 vs 0 | 0.45 (0.16-1.26) | - | - | |
| 3-11 vs 0 | 0.96 (0.41-2.20) | |||
| BMI (Kg/m2) | 0.1695 | |||
| 25-30 vs ≤25 | 0.59 (0.25-1.38) | - | - | |
| >30 vs ≤25 | 0.27 (0.06-1.27) | |||
| HYPERTENSION presence vs absence |
1.04 (0.47-2.27) | 0.9299 | - | - |
LUS=lung ultrasound; NPR=number of positive region; P/F=arterial oxygen partial pressure/fractional inspired oxygen ratio; CCI=Charlson Comorbidity Index; BMI=Body Mass Index; D/ICU=death or transfer to ICU; CPAP=continuous positive airways pressure; OR=Odds Ratio; 95% CI=95% confidence interval; ns=not statistically significant.
OR for 1-unit increase in LUS scores.
LUS scores adjusted for age, P/F ratio and CCI.
LUS scores adjusted for P/F ratio.
P/F was the only factor associated to primary outcome in each multivariate analysis for different LUS scores. Therefore OR (95% CI) and p values for P/F in multivariate models are the same of univariate analysis.
P/F was the only confounder associated to secondary outcome in each multivariate analysis for different LUS scores. OR (95% CI) and p values for P/F in multivariate models are reported in Supplementary Table 1.
The total LUS score, the anterolateral score and the NPR at admission were significantly associated with the risk of death or transfer to ICU (D-ICU). Among the confounders, age, P/F ratio and CCI were all associated to D-ICU at the univariate analysis, whereas BMI and hypertension were not.
The three LUS variables associated to the primary outcome at the univariate analysis were evaluated in different multivariate analyses with age, P/F ratio and CCI. In all three cases, the only factor associated with the primary outcome was P/F ratio.
Total score, anterolateral score, NPR and presence of consolidation were all associated to the secondary outcome at univariate analysis. Among the five confounders included in the analysis, only P/F ratio was associated to this outcome in the univariate analysis, whereas age, CCI, BMI and hypertension were not. At the multivariate analyses total score, anterolateral score and NPR maintained their significant association with the use of CPAP together with P/F ratio.
The results in the subgroup evaluated with a second LUS after 72 hours are reported in Table 5 . In these patients the total score was associated to the primary outcome at univariate analysis together with P/F ratio. In the multivariate analysis, LUS score at 72 hours remained the only independent factor associated to D-ICU.
Table 5.
LUS after 72 hours: univariate and multivariate analysis for primary and secondary outcome.
| PRIMARY OUTCOME (NUMBER OF PATIENTS=128) | ||||
|---|---|---|---|---|
| UNIVARIATE ANALYSIS |
MULTIVARIATE ANALYSIS° |
|||
| OR (95% CI) | p value | OR (95% CI) | p value | |
| TOTAL SCORE | 1.36# (1.12-1.66) | 0.0022 | 1.36# (1.12-1.66) | 0.0022 |
| AGE (years) | 0.0643 | |||
| 66-75 vs ≤65 | 1.04 (0.11-9.84) | - | - | |
| >75 vs ≤65 | 4.94 (1.21-20.09) | |||
| P/F (mmHg) | 0.0217 | ns | ||
| 200-300 vs >300 | 4.47 (0.84-23.90) | - | ||
| ≤200 vs >300 | 9.44 (1.88-47.45) | |||
| CCI | 0.0701 | |||
| 1-2 vs 0 | 2.97 (0.26-34.09) | - | - | |
| 3-11 vs 0 | 9.53 (1.12-80.89) | |||
| BMI (Kg/m2) | 0.8718 | |||
| 25-30 vs ≤25 | 0.78 (0.17-3.47) | - | - | |
| >30 vs ≤25 | 1.28 (0.22-7.29) | |||
| HYPERTENSION presence vs absence |
1.35 (0.36-5.07) | 0.6559 | - | - |
| SECONDARY OUTCOME (NUMBER OF PATIENTS=118) | ||||
|---|---|---|---|---|
| UNIVARIATE ANALYSIS |
MULTIVARIATE ANALYSIS§ |
|||
| OR (95% CI) | p value | OR (95% CI) | p value | |
| TOTAL SCORE | 1.26# (1.14-1.40) | <0.0001 | 1.24# (1.11-1.39) | 0.0001 |
| AGE (years) | 0.2091 | |||
| 66-75 vs ≤65 | 2.49 (0.84-7.39) | - | - | |
| >75 vs ≤65 | 1.83 (0.60-5.54) | |||
| P/F (mmHg) | 0.0003 | 0.0271 | ||
| 200-300 vs >300 | 1.98 (0.61-6.43) | 0.70 (0.16-2.95) | ||
| ≤200 vs >300 | 27.69 (5.43-141.20) | 8.77 (1.54-49.85) | ||
| CCI | 0.3296 | |||
| 1-2 vs 0 | 0.55 (0.17-1.74) | - | - | |
| 3-11 vs 0 | 1.36 (0.52-3.55) | |||
| BMI (Kg/m2) | 0.1176 | |||
| 25-30 vs ≤25 | 0.46 (0.17-1.22) | - | - | |
| >30 vs ≤25 | 0.26 (0.05-1.27) | |||
| HYPERTENSION presence vs absence |
1.43 (0.59-3.45) | 0.4231 | - | - |
LUS=lung ultrasound; P/F=arterial oxygen partial pressure/fractional inspired oxygen ratio; CCI=Charlson Comorbidity Index; BMI=Body Mass Index; D/ICU=death or transfer to ICU; CPAP=continuous positive airways pressure; OR=Odds Ratio; 95% CI=95% confidence interval; ns=not statistically significant.
OR for 1-unit increase in total score.
Total score adjusted for P/F ratio. At multivariate analysis only total score resulted statistically significant.
Total score adjusted for P/F ratio.
When considering the secondary outcome total score at 72 hours correlated to CPAP treatment; once again, the only confounder associated at the univariate analysis was P/F ratio. Total score at 72 hours and P/F ratio maintained the association to the use of CPAP at the multivariate analysis.
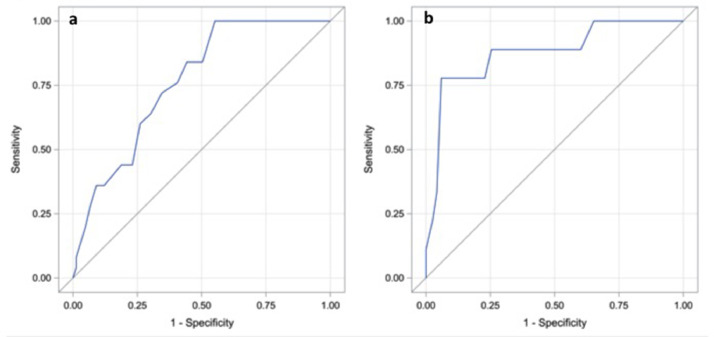
The ROC curve analysis results for the total LUS score at admission and after 72 hours are shown in Fig. 3 . A total LUS score of 9 at admission is a reliable cut-off value to rule out death and ICU transfer (sensitivity 100%; specificity 45%). When considering LUS performed after 72 hours a cut-off value of 17 can accurately predict the primary outcome (sensitivity 89%; specificity 85%).
Fig. 3.
a) LUS at admission: Receiver operating characteristic (ROC) curve analysis for the primary outcome (Area Under the Curve = 0.7625). b) LUS after 72 hours: ROC curve analysis for the primary outcome (Area Under the Curve = 0.8757).
Among the 2090 scanned areas of admission LUS in the 190 patients, 110 were blindly reviewed. In 102 of the reviewed scans (92.7%) there was a perfect inter-observer reproducibility (same score allocated by the two independent observers).
4. Discussion
The main finding of our study in a population of consecutive patients with COVID-19 related pneumonia is that LUS performed after 72 hours is a reliable prognostic tool allowing to identify patients undergoing death or transfer to ICU. Furthermore even LUS at admission proved to be a valuable tool in predicting the development of respiratory failure needing treatment with continuous positive airway pressure.
In recent months several studies characterized the sonographic features of patients with COVID-19 pneumonia. Peng et al. showed that common findings were the presence of a focal or diffuse B-line pattern, a thickened and irregular pleural line and consolidations ranging from small subpleural to large translobar ones [10]. Our study showed the presence of focal or diffuse B-line pattern in almost all hospitalized patients with COVID-19 related pneumonia; in more than 90% of them pulmonary involvement was bilateral. These findings confirm what previously reported in small populations of recent studies where focal or diffuse B-lines and subpleural consolidations were the most frequently detected abnormalities and a pulmonary bilateral involvement on LUS was present in almost all the cases [11, 16]. The reported greater percentage of patients with consolidation as compared to our study (50% versus 24%) probably reflects a population with an increased number of critical patients and different time intervals between the onset of symptoms and ultrasound examinations [11, 16]. In our population the most frequently involved pulmonary regions on LUS were the infero-posterior areas, while pleural effusion was detected only in a minority of patients, thus confirming previous results [16].
To our knowledge this is the first prospective study assessing the potential role of LUS as a prognostic tool in a population of patients admitted to non-ICU units for COVID-19- related pneumonia. Zieleskiewicz et al. and Deng et al. showed that LUS scores highly correlated with CT scores [20,21]; moreover, the last study showed that the use of a score cut-off was able to distinguish between more and less severe critical patients. Lichter et al. showed that LUS score at admission could accurately predict death and need for mechanical ventilation [22]. Bonadia et al. showed that patients with poor outcome (death or transfer to ICU) had worse LUS scores in the ED [24]. We assessed the potentials of four different LUS scores at admission. From our results, although P/F at admission was the only factor independently correlated with ICU/death at multivariate analyses, three out of four sonographic scores (total score, NPR and anterolateral score) were independently associated to the secondary outcome in different multivariate models. This finding suggests a potential role of LUS at admission in identifying patients who are at risk of developing an acute respiratory failure with the need of treatment with CPAP. Of notice, the predictive role of the anterolateral score (limited to the seven anterolateral regions) could be relevant in particular settings, as in unstable or bedridden patients, where the evaluation of posterior regions is often unfeasible. Moreover, the correlation of total LUS score after 72 hours with ICU/death suggests that ultrasonographic monitoring accurately reflects disease progression. This result indicates the potential value of LUS as a tool for dynamic lung monitoring, thus extending what reported by Deng et al. and Dargent et al. [21,23] to a less critical population.
Finally, none of the patients without bilateral involvement at LUS showed an adverse event (death, transfer to ICU and treatment with continuous positive airway pressure). Further studies are warranted to find out if LUS can be used as a rule out tool in settings such as Emergency Departments where it is crucial to identify patients who can be safely discharged.
These data together with the known practical advantages of being repeatable and quickly performed at bedside (thus limiting infectious patient transportation) confirm the potentiality of LUS in aiding the clinician in the risk stratification of the patient with COVID-19 pneumonia.
4.1. Limits
This study was monocentric so that the results may not be generalized for several reasons, starting from the prompt availability of portable machines (one every 15 beds) and the routinely use of LUS in both the wards before COVID-19 emergency.
Furthermore, physicians performing the examination were not blinded to patient clinical and radiological data.
Finally, 30 patients didn't undergo LUS at admission probably as a consequence of the high workload of healthcare staff due to the huge number of daily admissions during the first days of COVID-19 emergency. Moreover in 17 patients incomplete LUS reports made examinations not suitable for attribution of LUS scores.
5. Conclusions
Our data suggest that LUS can be a promising prognostic tool in patients admitted to non-ICU units for COVID-19 pneumonia.
Further studies are warranted to confirm the same results and to assess the prognostic role of LUS in other settings such as Emergency Department or ICU units.
Funding
None.
Author contribution
F.C., E.C., and C.C. conceived the idea and designed the project. F.L., M.B., G.R., M.D.M., M.A.M. and G.V. carried out the data collection. M.B., F.L., D.T. and A.M.B. analysed the data and prepared the figures. F.L., M.B., F.C., G.C. and C.C. drafted the manuscript. G.C. conducted the statistical analyses. All the authors have revised the manuscript critically, approved the version submitted for publication and have agreed to be accountable for all aspects of the work.
Declaration of Competing Interest
The authors declare that they have no conflict of interests.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.ejim.2020.12.012.
Appendix. Supplementary materials
References
- 1.Istituto Superiore di Sanità. Characteristics of SARS-CoV-2 patients dying in Italy Report based on available data on June 2020.
- 2.Chung M, Bernheim A, Mei X, Zhang N, Huang M, Zeng X, Cui J, Xu W, Yang Y, Fayad ZA, Jacobi A, Li K, Li S, Shan H. CT imaging features of 2019 novel coronavirus (2019 nCoV) Radiology. 2020;295:202–207. doi: 10.1148/radiol.2020200230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shi H, Han X, Jiang N, Cao Y, Alwalid O, Gu J, et al. Radiological findings from 81 patients with COVID-19 pneumonia in Wuhan, China: a descriptive study. Lancet Infect Dis. 2020;20(4):425–434. doi: 10.1016/S1473-3099(20)30086-4. Apr. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lichtenstein DA, Mezière GA. Relevance of lung ultrasound in the diagnosis of acute respiratory failure the BLUE protocol. Chest. 2008;134(1):117–125. doi: 10.1378/chest.07-2800. Jul. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mojoli F, Bouhemad B, Mongodi S, et al. Lung ultrasound for critically Ill patients. Am J Respir Crit Care Med. 2019;199(6):701–714. doi: 10.1164/rccm.201802-0236CI. 2019. [DOI] [PubMed] [Google Scholar]
- 7.Mayo PH, Copetti R, Feller-Kopman D, Mathis G, Maury E, Mongodi S, et al. Thoracic ultrasonography: a narrative review. Intensive Care Med. 2019;45(9):1200–1211. doi: 10.1007/s00134-019-05725-8. Sep. [DOI] [PubMed] [Google Scholar]
- 6.Ticinesi A, Lauretani F, Nouvenne A, Mori G, Chiussi G, Maggio M, Meschi T. Lung ultrasound and chest x-ray for detecting pneumonia in an acute geriatric ward. Medicine. 2016;95(27):e4153. doi: 10.1097/MD0000000000004153. Jul. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chavez MA, Shams N, Ellington LE, Naithani N, Gilman RH, Steinhoff MC, Santosham M, Black RE, Price C, Gross M, Checkley W. Lung ultrasound for the diagnosis of pneumonia in adults: a systematic review and meta-analysis. Respir Res. 2014;15(1):50. doi: 10.1186/1465-9921-15-50. Apr. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Di Serafino M, Notaro M, Rea G, Iacobellis F, Delli Paoli V, Acampora C, Ianniello S, Brunese L, Romano L, Vallone G. The lung ultrasound: facts or artifacts? In the era of COVID-19 outbreak. Radiol Med. 2020:1–16. doi: 10.1007/s11547-020-01236-5. Jun. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Peng QY, Wang XT, Zhang LN. Chinese critical care ultrasound study group (CCUSG). Findings of lung ultrasonography of novel corona virus pneumonia during the 2019 – 2020 epidemic. Intensive Care Med. 2020;46(5):849–850. doi: 10.1007/s00134-020-05996-6. May. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nouvenne A, Davìd Zani M, Milanese G, Parise A, Baciarello M, Bignami EG, Odone A, Sverzellati N, Meschi T, Ticinesi A. Lung ultrasound in Covid-19 Pneumonia: correlations with chest CT on hospital admission. Respiration. 2020:1–8. doi: 10.1159/000509223. Jun. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lomoro P, Verde F, Zerboni F, Simonetti I, Borghi C, Fachinetti C, Natalizi A, Martegani A. Eur J Radiol Open. 2020;7 doi: 10.1016/j.ejro.2020.100231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kulkarni S, Down B, Jha S. Point-of-care (POC) lung ultrasound in intensive care during the COVID-19 pandemic. Clinic Radiol. 2020 doi: 10.1016/j.crad.2020.05.001. May, Online ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mohamed MFH, Al-Shokri S, Yousaf Z, Danjuma M, Parambil J, Mohamed S, Mubasher M, Dauleh MM, Hasanain B, Al Kahlout MA, Abubeker IY. Frequency of abnormalities detected by point-of-care lung ultrasound in symptomatic COVID-19 patients: systematic review and meta-analysis. Am J Trop Med Hyg. 2020 doi: 10.4269/ajtmh.20-0371. doi: 19.4269/ajtmh.20-0371. Online ahead of print Jun. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gargani L, Soliman-Aboumarie H, Volpicelli G, Corradi F, Pastore MC, Cameli M. Why, when, and how to use lung ultrasound during the COVID-19 pandemic: enthusiasm and caution. Eur Heart J Cardiovasc Imaging. 2020 doi: 10.1093/ehjci/jeaa163. Jun, Online ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xing C, Li Q, Du H, Kang W, Lian J, Yuan L. Lung ultrasound findings in patients with COVID-19 pneumonia. Crit Care. 2020;24(1):174. doi: 10.1186/s13054-020-02876-9. 10.1186s13054-020-02876-9, Apr. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhou Y, He Y, Yang H, Yu H, Wang T, Chen Z, Yao R, Liang Z. Development and validation a nomogram for predicting the risk of severe COVID-19: a multi-center study in Sichuan, China. PLoS One. 2020;15(5) doi: 10.1371/journal.pone.0233328. May. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, Xiang J, Wang Y, Song B, Gu X, Guan L, Wei Y, Li H, Wu X, Xu J, Tu S, Zhang Y, Chen H, Cao B. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054–1062. doi: 10.1016/S0140-6736(20)30566-3. Mar. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Giacomelli A, Ridolfo AL, Milazzo L, Oreni L, Bernacchia D, Siano M, Bonazzetti C, Covizzi A, Schiuma M, Passerini M, Piscaglia M, Coen M, Gubertini G, Rizzardini G, Cogliati C, Brambilla AM, Colombo R, Castelli A, Rech R, Riva A, Torre A, Meroni L, Rusconi S, Antinori S, Galli M. 30-day mortality in patients hospitalized with COVID-19 during the first wave of the Italian epidemic: a prospective cohort study. Pharmacol Res. 2020;158 doi: 10.1016/j.phrs.2020.104931. Aug. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zieleskiewicz L, Markarian T, Lopez A, Taguet C, Mohammedi N, Boucekine M, Baumstarck K, Besch G, Mathon G, Duclos G, Bouvet L, Michelet P, Allaouchiche B, Chaumoître K, Di Bisceglie M, Leone M, AZUREA Network Comparative study of lung ultrasound and chest computed tomography scan in the assessment of severity of confirmed COVID-19 pneumonia. Intensive Care Med. 2020:1–7. doi: 10.1007/s00134-020-06186-0. Jul. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Deng Q, Zhang Y, Wang H, Chen L, Yang Z, Peng Z, Liu Y, Feng C, Huang X, Jiang N, Wang Y, Guo J, Sun B, Zhou Q. Semiquantitative lung ultrasound scores in the evaluation and follow-up of critically ill patients with COVID-19: a single center study. Acad Radiol. 2020;S1076-6332(20):30404–30409. doi: 10.1016/j.acra.2020.07.002. Jul. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lichter Y, Topilsky Y, Taieb P, Banai A, Hochstadt A, Merdler I, Gal Oz A, Vine J, Goren O, Cohen B, Sapir O, Granot Y, Mann T, Friedman S, Angel Y, Adi N, Laufer-Perl M, Ingbir M, Arbel Y, Matot I, Szekely Y. Lung ultrasound predicts clinical course and outcomes in COVID-19 patients. Intensive Care Med. 2020 doi: 10.1007/s00134-020-06212-1. Aug, Online ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dargent A, Chatelain E, Kreitmann L, Quenot JP, Cour M, Argaud L; COVID-LUS study group Lung ultrasound score to monitor COVID-19 pneumonia progression in patients with ARDS. PLoS One. 2020;15(7) doi: 10.1371/journal.pone.0236312. Jul. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bonadia N, Carnicelli A, Piano A, Buonsenso D, Gilardi E, Kadhim C, Torelli E, Petrucci M, Di Maurizio L, Biasucci DG, Fuorlo M, Forte E, Zaccaria R, Franceschi F. Lung ultrasound findings are associated with mortality and need for intensive care admission in COVID-19 patients evaluated in the emergency department. Ultrasound Med Biol. 2020;46(11):2927–2937. doi: 10.1016/j.ultrasmedbio.2020.07.005. Nov. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.World Health Organization. Global surveillance for COVID-19 caused by human infection with COVID-19 virus. Interim guidance. 2020 Mar. WHO/2019-nCoV/SurveillanceGuidance/2020.6.
- 26.Bouhemad B, Mongodi S, Via G, Rouquette I. Ultrasound for “lung monitoring” of ventilated patients. Anesthesiology. 2015;122(2):437–447. doi: 10.1097/ALN.0000000000000558. Feb. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.