Abstract
Coronavirus disease 2019 (COVID-19) has played havoc on this world’s health and economics since its outbreak in December 2019. Reverse transcription-polymerase chain reaction (RT-PCR) has been the gold standard to diagnose severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Still, few false-positive reports are emerging up that add to the physicians’ dilemma and maintenance of health statistics.
Keywords: Coronavirus disease 2019, Reverse transcription-polymerase chain reaction, False-positive RT-PCR
Introduction
Coronaviruses (CoVs) are enveloped viruses, which have a positive single-stranded RNA genome and are pathogenic towards human beings [1]. Although the gold standard for detecting SARS-COV-2 infection is a quantitative RT-PCR assay [2], it has started to face rare cases of false-positive reports. Here, we discuss such a confusing case of probable false-positive RT-PCR for SARS-COV-2.
Case Presentation
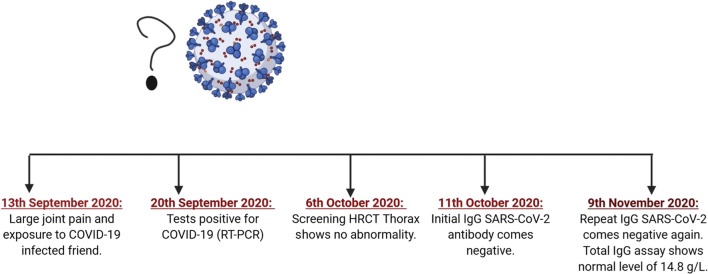
A 27-year-old male working at Andhra Pradesh presented to his doctor-on-call within his working area with a week history of large joint pains, 2-day history of fever with dry cough, and body pain. His pains seemed to have worsened since his fever started, and it was quite intense, although clinical examination did not reveal any inflamed joints or any chest findings. From his history, it became evident that he had been in contact with one of his colleagues 7 days back who recently tested COVID-19 RT-PCR positive. His RT-PCR for SARS-COV-2 came positive on September 20, 2020, and he maintained the home isolation protocols as laid down by the government. No repeat COVID-19 test was done by his employer, since he was labeled as a mild COVID-19 patient and, as per Indian government directive, a repeat test for COVID-19 is not indicated in mild infections. His joint pains persisted for two more months, and no other viral tests were done at that time of initial presentation. His computerized tomography (CT) scan of thorax, initial IgG SARS-COV-2 (October 11, 2020), repeat IgG SARS-COV-2 (November 9, 2020), and total IgG titer (November 9, 2020) showed normal CT findings, initial and repeat IgG SARS-COV-2 (both done 1 month apart) index value of 0.03 (non-reactive), and total IgG 14.8 g/L respectively (Fig. 1).
Fig. 1.
HRCT thorax, initial and repeat IgG SARS-COV-2, and total IgG level
The timeline of presentation and tests done is depicted in Fig. 2 below.
Fig. 2.
Timeline of events. Created with BioRender.com
Discussion and Conclusion
The first thing that we have to remember is that no test is 100% perfect [3]. The real-time RT-PCR results using primers in various genes can easily get affected by the viral RNA sequence variations [4]. As per guidelines laid down by CDC (Centers for Disease Control and Prevention), the negative template control (NTC) sample should be negative, showing no fluorescence growth curves that cross the threshold line [5]. Sample contamination can lead to false-positive results with one or more of the primer and probe NTC reactions [5]. Confirmatory reporting guidelines should be maintained to avoid risks to patients emerging out of a false positive report. The FDA recommendations of using 100 contiguous bases for nucleic acid-based molecular diagnostics for viral disease infections are often not met by the probes used in CDC rRT-PCR test kits for the SARS-CoV-2 assay, which are mostly about 25 bases long [6]. Traditionally, a PCR is supposed to be followed by a second separation technique that should be used for confirmation, which is not done in rRT-PCR used in screening or rapid diagnostic purposes [7]. Amplification errors are also possible if short probes are used in systems going beyond the cycle threshold (Ct) of 40 used in general for amplification [7]. Detection of another non-SARS-CoV-2 virus/microorganism has also been postulated as one of the probable hypotheses of false-positive reports along with the interference of pure technical artifacts and chances of cross-contamination [8]. Sample mix-ups, data error, and software problems can also lead to false-positive results [9]. These studies have cited cross-contamination and carryover contamination to be the most probable reason for these false-positive results [9].
The patient who gets falsely stamped as COVID-19 positive faces tremendous mental agony, social stigma, and a very high risk of actually getting the infection if he or she is segregated or isolated in COVID-19 wards for observation. This might be due to the long contagious period of SARS-CoV-2-infected patients, who might be present in those wards, which was found to be 20 days in one large scale study from China of 301 patients [10]. This study also showed a false positive RT-PCR test in 30% (21 patients) of the study population who tested positive on third time testing, although their two previous reports came negative.
There has been a case report suggesting the potential role of CT chest in diagnosing a COVID-19 suspected patient whose RT-PCR came negative for the first four times, and CT chest showed the typical findings of patchy ground-glass opacity on admission, progressing rapidly to segmental mixed consolidation and ground-glass opacity 3 days post-admission [11]. This case and similar others [12] highlight the use of CT thorax as an aid to isolate suspected cases of COVID-19 infections.
The above instances shed light on few vital points for our health care setup: (a) organizing wards where suspected cases can be isolated till reports are available to curtail down the spread, (b) a repeat test of RT-PCR at 24 h and probably 48 h to minimize both false negative and false positive reports, and (c) making a CT thorax mandatory to aid in diagnosis and also prognosis by looking into the CT severity score [13].
Taking into consideration all the above discussions, we are left with few new unsolved questions for our false-positive case other than the normal probability of sampling error:
Was there any simultaneous RNA viral infection like Chikungunya at that time that could have cross-reacted, considering the typical prolonged joint pains he faced and the endemic zone of Andhra Pradesh state (where he worked) for Chikungunya fever [14]?
Is there any subset of patients who, even after having normal titers of total IgG, cannot mount up proper antibody response?
A case report from Singapore showed false-positive dengue rapid antigen tests due to underlying COVID-19 infection [15]. Hence, false-positive reports due to interaction between two RNA viruses are known. False-positive results have also been reported from pre-surgical cases, from 0.3 to 3% [8, 16].
Our case report is another example of rare false-positive RT-PCR test for SARS-COV-2 reported from India. The significance of these false-positive reports is far-reaching, with effects on the mental health of the patient and loss of work power for the growing society in the face of this pandemic. Proper confirmatory tests must be performed before delivering positive reports in the SARS-COV-2 infection.
Author’s Contribution
The whole manuscript was written and revised by the single corresponding author.
Data Availability
Available on request with the author.
Compliance with Ethical Standards
Conflict of Interest
The author declares that he has no conflicts of interest.
Consent
Prior written informed consent was taken from the patient.
Ethical Clearance
Not required as per law of the land, since it is a case report.
Consent for Publication
Prior written informed consent to publish in medical journal for knowledge sharing purpose was taken.
Footnotes
This article is part of the Topical Collection on Covid-19
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Naqvi AAT, Fatima K, Mohammad T, et al. Insights into SARS-CoV-2 genome, structure, evolution, pathogenesis and therapies: structural genomics approach. Biochim Biophys Acta Mol basis Dis. 2020;1866(10):165878. doi: 10.1016/j.bbadis.2020.165878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Oliveira BA, Oliveira LC, Sabino EC, Okay TS. SARS-CoV-2 and the COVID-19 disease: a mini review on diagnostic methods. Rev Inst Med Trop Sao Paulo. 2020;62:e44. doi: 10.1590/S1678-9946202062044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Watson J, Whiting PF, Brush JE. Interpreting a covid-19 test result. BMJ. 2020;369. Available at: https://www.bmj.com/content/369/bmj.m1808. [DOI] [PubMed]
- 4.Phan T. Genetic diversity and evolution of SARS-CoV-2. Infect Genet Evol. 2020;81:104260. doi: 10.1016/j.meegid.2020.104260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.https://www.cdc.gov/coronavirus/2019-ncov/lab/index.html?CDC_AA_refVal=https%3A%2F%2Fwww.cdc.gov%2Fcoronavirus%2F2019-ncov%2Flab%2Frt-pcr-detection-instructions.html. Accessed 9 Nov 2020.
- 6.Lee SH. Testing for SARS-CoV-2 in cellular components by routine nested RT-PCR followed by DNA sequencing. Int J Geriatr Rehab. 2020;2:69–96. [Google Scholar]
- 7.www.aacc.org. (n.d.). False positive results in real-time reverse transcription-polymerase chain reaction (rRT-PCR) for SARS-CoV-2? | AACC.org. [online] Available at: https://www.aacc.org/science-and-research/scientific-shorts/2020/false-positive-results-in-real-time-reverse-transcription-polymeras. Accessed 9 Nov. 2020.
- 8.Katz AP, Civantos FJ, Sargi Z, Leibowitz JM, Nicolli EA, Weed D, Moskovitz AE, Civantos AM, Andrews DM, Martinez O, Thomas GR. False-positive reverse transcriptase polymerase chain reaction screening for SARS-CoV-2 in the setting of urgent head and neck surgery and otolaryngologic emergencies during the pandemic: clinical implications. Head Neck. 2020;42:1621–1628. doi: 10.1002/hed.26317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cohen AN, Kessel B. False positives in reverse transcription PCR testing for SARS-CoV-2. 2020. 10.1101/2020.04.26.20080911.
- 10.Xiao AT, Tong YX, Gao C, Zhu L, et al. Dynamic profile of RT-PCR findings from 301 COVID-19 patients in Wuhan, China: a descriptive study. J Clin Virol. 2020;127:104346. doi: 10.1016/j.jcv.2020.104346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Feng H, Liu Y, Lv M, Zhong J. A case report of COVID-19 with false negative RT-PCR test: necessity of chest CT. Jpn J Radiol. 2020;38(5):409–410. doi: 10.1007/s11604-020-00967-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xie X, Zhong Z, Zhao W, et al. Chest CT for typical coronavirus disease 2019 (COVID-19) pneumonia: relationship to negative RT-PCR testing. Radiology. 2020;296(2):E41–E45. doi: 10.1148/radiol.2020200343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yang R, Li X, Liu H, et al. Chest CT Severity Score: an imaging tool for assessing severe COVID-19. Radiol Cardiothorac Imaging. 2(2):e200047. [DOI] [PMC free article] [PubMed]
- 14.Mohan A, Kiran DH, Manohar IC, Kumar DP. Epidemiology, clinical manifestations, and diagnosis of Chikungunya fever: lessons learned from the re-emerging epidemic. Indian J Dermatol. 2010;55(1):54–63. doi: 10.4103/0019-5154.60355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Correction to Lancet Infect Dis 2020; published online Aug 3. Lancet Infect Dis. 2020;20(10), e250. 10.1016/S1473-3099(20)30589-2. [DOI] [PMC free article] [PubMed]
- 16.Albendin-Iglesias H, Mira-Bleda E, Roura-Piloto AE, et al. Usefulness of the epidemiological survey and RTePCR test in pre-surgical patients for assessing the risk of COVID-19. J Hosp Infect. 2020;105(4):773–775. doi: 10.1016/j.jhin.2020.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Available on request with the author.