Abstract
Over a century ago, Élie Metchnikoff described the macrophages’ ability to phagocytose. Propelled by advances in technology enabling phenotypic and functional analyses at unpreceded resolution, a recent renaissance in macrophage research has shed new light on these ‘big eaters’. We here give an overview of cardiac macrophages’ provenance in the contexts of cardiac homeostasis and stress. We highlight the recently identified mechanism by which these cells regulate electrical conduction in the atrioventricular node and discuss why we need a deeper understanding of monocytes and macrophages in systolic and diastolic dysfunctions.
Keywords: Macrophages, Monocytes, Myocardial infarction, Conduction, Diastolic dysfunction
Macrophages and their precursors
Macrophages are versatile immune cells strategically positioned throughout the body. They are crucially involved during organ development as well as tissue homeostasis and repair, and they exert multiple functions in inflammation.1 In the 1960s, van Furth and Cohn2 proposed that macrophages originate from circulating blood monocytes. An integral part of the vertebrate innate immune system, monocytes comprise 5–20% of peripheral blood mononuclear cells in humans and about 2–4% of blood leucocytes in mice. During embryonic development, monocytes are produced in the Foetal liver, and in adult haematopoiesis they arise from haematopoietic stem cells (HSCs) in the bone marrow. During this process, HSCs differentiate into common myeloid progenitors (CMPs), at which point they no longer express the surface markers CD117 (c-kit), Sca-1, and CD34. By increasing CD16/32, CMPs differentiate to granulocyte-macrophage progenitors.3 Further differentiation steps can include generating a monocyte-macrophage dendritic cell precursor4 and a common monocyte progenitor.5 This linear development may vary depending on heterogeneity in precursor populations and earlier lineage commitment.6 In addition, certain inflammatory triggers, such as atherosclerosis or myocardial infarction (MI), trigger extramedullary monocyte production in the spleen.7 , 8
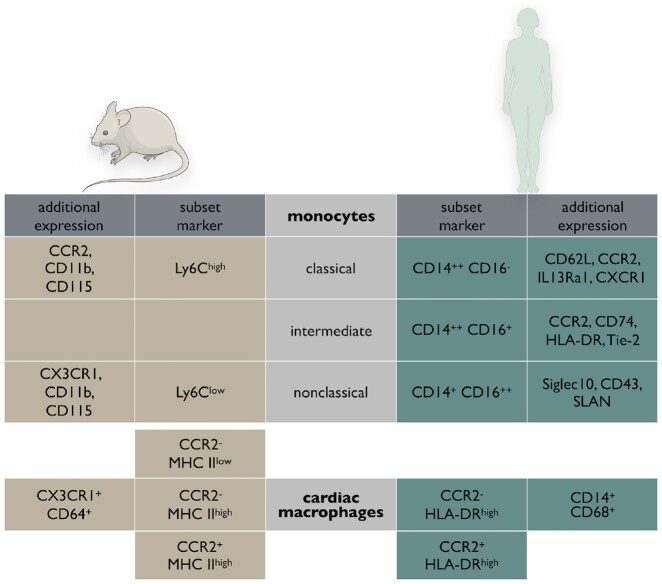
In humans, three monocyte subsets can be classified based on expression of CD14 and CD16: classical (CD14++CD16−), non-classical (CD14+CD16++), and intermediate (CD14++CD16+) monocytes.9 Recent cytometer time-of-flight mass cytometry and transcriptional profiling by single-cell RNA sequencing (RNA-seq) suggest the intermediate population in particular appears to be substantially heterogeneous.10 , 11 In mice, mature monocytes can be identified by their expression of CD11b and CD115 and distinguished by the surface marker Ly-6C (Figure 1). Ly-6Chigh chemokine (C–C motif) receptor-2 (CCR2)high chemokine (C–X3–C motif) receptor-1 (CX3CR1)low monocytes, which are considered the murine equivalent to human classical monocytes, preferentially accumulate at sites of inflammation. Ly-6Clow CCR2low CX3CR1high monocytes, on the other hand, patrol the vasculature via LFA-1 integrin, remove damaged endothelial cells and maintain vascular integrity and homeostasis. “Non-classical” Ly6Clow monocytes differentiate from Ly6Chigh monocytes, in a process that depends on Nr4a1,12 and have previously been described as ‘vascular macrophages’. Transcriptionally, however, they cluster with monocytes lacking core macrophage transcripts such as Mer tyrosine kinase (MertK).13
Figure 1.
Overview and comparison of monocyte subsets (top) and cardiac macrophages (bottom) defined in humans and mice with their respective surface markers.
Genetic lineage tracing in mice revealed that macrophages can also arise independently from monocytes: FMS-like tyrosine kinase 3 (FLT-3), also known as CD135, is a cytokine receptor expressed on definitive but not primitive macrophages. While the latter are progeny of primitive haematopoietic progenitor cells that are present in the embryonic yolk sac, definitive haematopoietic progenitor cells can be found in both embryos (yolk sac, foetal liver) and adults (bone marrow and spleen).14 Experiments involving Flt3-Cre mice demonstrated that the heart (and a variety of other organs) contains macrophages originating from both primitive and definitive haematopoietic lineages.15 In addition, Mass et al. recently demonstrated that during pre-natal development, erythro-myeloid progenitors generate ‘premacrophages’ (pMacs), macrophage precursors that simultaneously colonize different organs of the whole mouse embryo and then derive their tissue-specific identity locally.16
Macrophages are best defined by their function (e.g. phagocytosis, immunity); surface (e.g. CD68, F4/80) or transcriptional markers (e.g. MertK); morphology (e.g. phagosome inclusion) or location in the investigated organ.17 As they are highly plastic, the widely used M1/M2 subset classification, which is certainly applicable for the in vitro conditions it was defined by, holds a number of shortcomings when it comes to in vivo macrophage subsets and phenotypes. In order to develop a classification that better captures in vivo phenotypes, a common macrophage-activation nomenclature was recently proposed that suggests the description of macrophages based on origin, activation, and a collection of molecular markers.18 Such markers may include transcription factors, SOCS proteins, chemokines, cytokines, scavenger receptors, or amino acid metabolism.18 While this novel macrophage activation terminology makes cell descriptions more granular, its inherently necessary simplification does not fully capture macrophage phenotypes in vivo either. In tissue, macrophage differentiation may be based on whether macrophages are resident or monocyte derived. Resident macrophages from different tissues may exhibit fundamental functional differences, in part driven by local input emanating from the specific organ of residence.13 , 19 Further, a distinction depending on pro-inflammatory vs. reparatory macrophage functions appears quite useful, in particular in the context of myocardial healing.
Cardiac resident macrophages
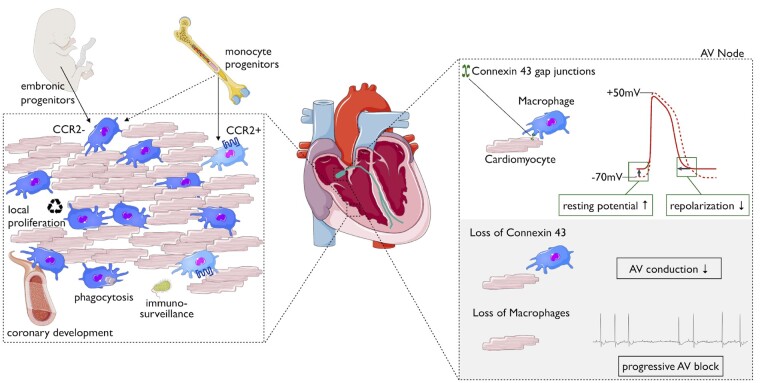
In the murine heart, macrophages constitute 7–8% of the cells that are noncardiomyocytes.20 , 21 Macrophages intersperse the entire heart, where they closely associate with vessels and are enriched in the conduction system.22 Spindle-shaped cardiac resident macrophages can be distinguished by their expression of major histocompatibility complex (MHC) II and CCR2. During murine embryonic development, the first macrophages seed in the epicardium (around E11.5).23 They are derived from primitive yolk sac progenitors, express low levels of MHC II and CCR2 and may impact later stages of coronary vasculature development.24 From E14.5 on, MHC IIlow CCR2high macrophages infiltrate the endocardial surface.24 This macrophage subset’s function in the heart is currently unidentified. Both described cardiac macrophage populations postnatally up-regulate their MHC II expression, and with age, the number of macrophages that express high levels of MHC II further increases. Shortly after birth, an additional CCR2- population accumulates in the heart. This Flt3-Cre+ population definitively originates haematopoietically and presumably descends from foetal monocytes.15 The healthy adult mouse heart contains at least three distinct macrophage populations: (i) CCR2- MHC IIlow, (ii) CCR2- MHC IIhigh, and (iii) CCR2high MHC IIhigh. Monocytes, meanwhile, are identified as CCR2high MHC IIlow.25 The two CCR2-negative macrophage subpopulations stem from embryonic origins and are maintained throughout life by local proliferation, independent from blood monocytes.15 , 20 In contrast, the CCR2-positive subset detectable in the adult heart is derived from and maintained by the influx of blood monocytes (Figure 2). The CCR2-positive subpopulation is small (5–15%) in healthy hearts. Elegant work has recently confirmed that analogous CCR2-positive and CCR2-negative macrophage populations are also present in human hearts.26
Figure 2.
Macrophage populations and functions in the healthy adult heart. Schematic depicting cardiac resident macrophages within the myocardium. Their developmental origin and proposed functions are illustrated during development and homeostasis (left). The interplay between cardiomyocytes and macrophages in the atrioventricular (AV) node in electrical conduction of the heart (right).
Cardiac macrophages facilitate electrical conduction
Our knowledge of cardiac tissue macrophages’ remains limited. In the steady state, cardiac resident macrophages are thought to serve as sentinels for injury and circulating infectious agents, such as bacteria.27 MHC IIhigh macrophages are considered particularly crucial for cardiac immune surveillance and adaptive immune responses, while MHC IIlow macrophages support tissue homeostasis by removing material shed by surrounding cardiomyocytes and fibroblasts and adapting to altered tissue strain.
Gene expression analysis comparing cardiac macrophages to their counterparts from the spleen and brain revealed enriched genes involved in angiogenesis and immune quiescence.21 However, we still lack functional in vivo data that clearly define cardiac macrophages’ role in these processes for the steady state heart. It is increasingly clear that macrophages can endorse tissue-specific functions in other organs, such as modulating norepinephrine levels in adipose tissue28 or alveolar macrophages removing surfactant in the lungs.29 Brain macrophages (microglia) actively contact synaptic clefts and other neural components.30 Direct cell–cell interaction was found in both human and murine hearts where macrophages connect with cardiomyocytes by forming connexin 43 (CX43; also known as GJA1)-containing gap junctions.22 This enables electrical coupling and impacts both cell types: patch clamp analysis revealed that macrophages rhythmically depolarize when they are coupled to cardiomyocytes and cardiomyocytes show a more positive depolarized resting membrane potential in co-culture with macrophages. Further, a coupled cardiomyocyte’s action potential has lower upstroke and overshoot, which leads to earlier repolarization and a shorter action potential and refractory period. Histological analyses show that one cardiomyocyte may couple with up to four macrophages, and mathematical modelling suggests that macrophages’ influence on conducting cells increases with their ratio to cardiomyocytes.22 Optical clearing of hearts showed more macrophages in the atrioventricular (AV) node and around other conduction system structures. Macrophage-specific genetic ablation of connexin 43 delayed conduction through the AV node, and depletion of CD11-positive cells in CD11b DTR mice resulted in progressive AV block. These data demonstrate that macrophages are required for normal conduction in the AV node. Thus far, we do not know if a specific cardiac macrophage subpopulation is enriched for connexin 43, nor whether stress, inflammatory conditions or aging may impact macrophage function in conduction.
Another intriguing question is if resident macrophages are causally involved in the pathophysiology of atrial fibrillation (AF). Several studies in humans have described an association between inflammation and AF. Inflammatory conditions, such as rheumatoid arthritis or sepsis, may trigger AF.31 , 32 In small autopsy series, histological analyses of atria from patients with AF showed increased numbers of monocytes and macrophages compared with control samples. In addition, levels of interleukin (IL)-6, IL-8, and tumour necrosis factor (TNF) were increased in tissue of AF patients.33 , 34 In a prospective, placebo-controlled clinical study with 104 patients, administration of glucocorticoids reduced the recurrence of AF.35 Other clinical trials have reported conflicting results and mechanistic insights are still scarce. Therefore, further research is warranted. In a murine model of lipopolysaccharide-induced AF, depletion of macrophages with clodronate liposomes reduced the inducibility of AF.36 The description of spontaneous AF in certain mouse strains and in particular the increased AF inducibility in certain transgenic mice may fuel further investigation in this area.37
Events that perturb the heart’s leucocyte niche
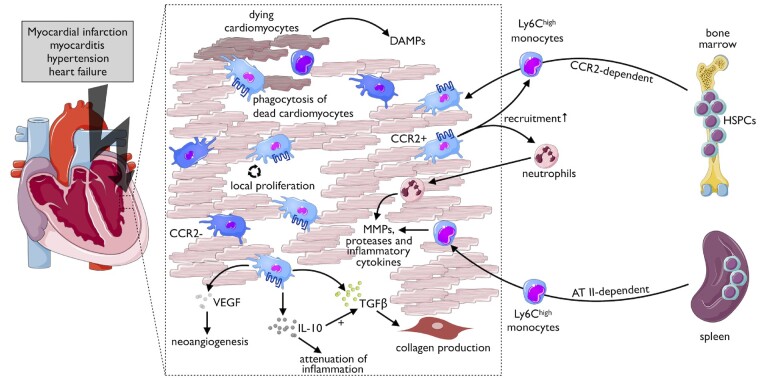
The composition of resident cells changes as the heart ages. Over time, monocyte-derived cardiac macrophages’ contribution increases, and it has been suggested that CCR2high monocytes may differentiate into CCR2- macrophages.38 General immune system changes that come with age—also termed ‘inflammageing’—may result in myeloid bias, impaired tolerance, or cytokine dysregulation, which all can impact cardiac macrophage homeostasis. In addition, drastic alterations in cardiac cell balance follows events such as injury, infection, and haemodynamic stress (Figure 3).
Figure 3.
Cardiac macrophages in the context of stress conditions. Various stressors can activate macrophage subpopulations in heart (left). These stress conditions lead to an increase in macrophages by local proliferation and by recruitment of monocytes from bone marrow and spleen (right). Tissue-resident CCR2+ macrophages may further spur the recruitment of myeloid cells, remove tissue debris, and modulate the cardiac microenvironment by the release of matrix metalloproteinases (MMPs), proteases, and cytokines. The latter may exert beneficial or harmful effects—as illustrated for IL-10—depending on disease context and timing. DAMP, damage-associated molecular pattern.
Myocardial infarction
Ischaemic injury induces forceful changes in cardiac cell composition. Upon coronary occlusion and subsequent cell death, cardiac resident macrophages and cardiomyocytes produce and secrete pro-inflammatory cytokines and chemokines that trigger myeloid cell production and recruitment of these cells to the infarcted heart.39 CCR2+ macrophages mediate neutrophil extravasation in mice by producing the chemoattractants CXCL (C-X-C motif chemokine ligand) 2 and CXCL5.40 Intravital 2-photon microscopy of beating hearts revealed that monocyte recruitment occurs as early as 30 min after the onset of ischaemia.41 The primary monocyte subset recruited to the mouse heart are Ly-6Chigh cells that infiltrate in response to up-regulation of monocyte-chemoattractant protein-1 (MCP-1, CCL2).42–45 Macrophages already present in the heart greatly impact monocyte accumulation: while tissue-resident CCR2+ macrophages promote recruitment via a MYD88 dependent mechanism, resident CCR2- macrophages can hinder monocyte invasion.46 Shortly after ischaemia, cardiac fibroblasts locally produce granulocyte/macrophage colony-stimulating factor (MSCF) that activates neighbouring myeloid cells, which further enhance the neutrophil and monocyte recruitment.47 During the first days, the accumulating leucocytes’ main function is removing necrotic tissue in an active process that depends on MertK, other scavenger receptors and the production of proteolytic enzymes.48 , 49 Exposed DNA in the infarcted heart is sensed by interferon regulatory factor 3 (IRF3)-positive macrophages50 that enhance the inflammatory process by locally releasing pro-inflammatory cytokines, such as IL-1 and IL-6, among others. Ly6Chigh monocytes rapidly turn over during ongoing inflammation and can differentiate locally into Ly6Clow macrophages.7 , 51 Those macrophages are considered to be reparative, may reside in the injured heart for several days and can undergo local proliferation.51 At later stages, lower numbers of Ly-6Clow monocytes are also recruited via chemokine (C–X3–C motif) receptor-1 (CX3CR1) with currently unclear functional consequences. During the reparative phase, macrophages reduce IL-6, TNF, and matrix metalloproteinase 9 (MMP9) expression levels while tissue levels of IL-10 increase.51 , 52 Macrophages secrete both transforming growth factor (TGF)-α and -β, thereby inducing neighbouring fibroblasts to convert into myofibroblasts, and macrophage-derived vascular endothelial growth factor impacts endothelial cells and stimulates angiogenesis.43
Taken together, monocytes and macrophages play vital roles in infarct healing and may represent a promising therapeutic target in patients with acute MI and exaggerated systemic inflammatory activity, as discussed in detail elsewhere.53
Myocarditis
Myocarditis in patients is diagnostically heterogeneous and treatment options are limited.54 Cardiac inflammation is among the most common causes of non-congenital, non-ischaemic sudden death in otherwise normal, healthy young adults.55 The best studied and characterized models of myocarditis are viral infection with the enterovirus Coxsackie B3 (CB3) and autoimmune mediated inflammation in mice.56 In both of these models, cells of the monocyte and macrophage lineages comprise the majority of infiltrating inflammatory cells.57 CB3 infiltrates cardiomyocytes via the coxsackievirus and adenovirus receptor and triggers the release of damage-associated molecular patterns (DAMPs) from infected cells. Such release results in the recruitment and activation of monocytes and dendritic cells.58 The latter impact cardiac inflammation and subsequent heart failure (HF) by generating antigen-specific lymphocytes.59 Measuring viral titres from patient endomyocardial biopsies to optimize the treatment regime is a matter of ongoing debate. A retrospective analysis from a large single-centre study indicates that the number of detectable leucocytes—rather than the virus load—in the patient’s biopsy sample predicts the outcome.60 Selectively immunomodulating myeloid-derived MSCF in CB3-induced myocarditis reduced cardiac monocyte and macrophage numbers without impacting the virus titre.61 In certain susceptible strains of mice, cardiac inflammation lingers beyond the clearance of infectious virus and leads to dilated cardiomyopathy. This is caused by autoreactive adaptive responses, for example, due to congruent epitopes of CB3 and cardiac myosin or production of autoantibodies against Troponin I.62 , 63 During the acute phase of autoimmune cardiac inflammation, monocytes can differentiate into TNF-α- and nitric oxide synthase 2-producing dendritic cells (TipDCs) that may limit antigen-specific T-cell expansion.64 Blocking monocyte accumulation with nanoparticle-encapsulated siRNA against CCR2 reduced cardiac fibrosis and resulted in improved left ventricular function.65 A thorough understanding of the involved pathways and timing must precede any translational therapeutic approach. This is intriguingly illustrated by work using an IL-17A-deficient mouse autoimmune myocarditis model. While immunization with a myocarditogenic peptide showed similar disease severity compared with control mice during acute myocarditis, the absence of IL-17A led to reduced monocyte infiltration and prevented post-myocarditis remodelling, thus demonstrating IL-17A’s role in further disease progression.66
Hypertension and heart failure
While MI and myocarditis both induce fundamental changes in the heart’s macrophage populations, other common conditions cause more subtle changes. For example, the inflammatory response is crucial to the progression of both pressure overload and hypertensive cardiac stress, but the intensity of that response is orders of magnitude lower than after acute ischaemic injury.67 The exact mechanisms that initiate the inflammatory response in cardiac pressure overload are still poorly understood but may involve angiotensin-mediated pro-inflammatory effects, reactive oxygen species production or cardiomyocyte death.68 Commonly used models of cardiac pressure overload include transverse aortic constriction (resembling cardiac stress in aortic stenosis) and administering angiotensin II or aldosterone. If the stimulus is continued long enough (or the constriction remains in place), fibrotic tissue develops, and adverse remodelling leads to impaired cardiac function.
Similarly, angiotensin II–induced hypertension raises resident macrophage proliferation in the heart.69 Prolonged exposure to angiotensin II also increases the HSC proliferation in the spleen and augments monocyte recruitment to the heart.24 , 69 During hypertensive stress, monocyte-derived macrophages show up-regulation of genes associated with the NLRP3 inflammasome. This pathway is also activated in diabetic mice’s cardiac macrophages. This results in the production of IL-1β,70 which increases the risk for arrhythmias, systolic dysfunction, and HF.15 Heart failure affects around 26 million people worldwide and can be considered a global pandemic.71 Failing hearts host elevated numbers of macrophages that multiply by either local proliferation or differentiation from accumulating monocytes.20 , 69 generated by the bone marrow but also extramedullary haematopoiesis in the spleen. Splenectomy resulted in ameliorated cardiac function in a model of ischaemic cardiomyopathy.72
Macrophages not only impact disease progression in cardiac pathologies with impaired systolic function but also play an important role in HF with preserved ejection fraction (HFpEF). Nearly half of all HF patients suffer from HFpEF, in which impaired cardiac performance is thought to be a consequence of increased left ventricular filling pressure caused by diastolic dysfunction.73 While there is a paucity of specific treatment options, known risk factors for HFpEF include hypertension and aging.74 In blood from patients with HFpEF, inflammatory markers75 and circulating monocytes are elevated.76 To mimic HFpEF in mice, a combination of salty drinking water, unilateral nephrectomy, and aldosterone infusion with osmotic minipumps (SAUNA) is administered.77 This regimen resulted in increased haematopoiesis in bone marrow and spleen and elevated numbers of monocyte-derived macrophages in the heart. Analysis of hearts from ageing mice corroborated these findings. Cardiac macrophages from SAUNA-treated mice were predominantly MHC IIhigh and produced more IL-10 than macrophages from healthy hearts. Monocyte- and macrophage-specific deletion of IL-10 using CX3CR1-Cre and IL-10-floxed mice resulted in improved diastolic function after SAUNA treatment. Macrophage-derived IL-10 does not act directly on cardiac fibroblasts but rather indirectly, in collaboration with TGF, induces collagen deposition by myofibroblasts.77 Interleukin-10 is known for its potent anti-inflammatory activity and its administration prevents adverse cardiac remodelling after MI and pressure overload,52 , 78 which illustrates that timing and context is crucial for any therapeutic translation. The same holds true for modulating macrophage biology to prevent atherosclerotic plaque progression and rupture, as reviewed in detail elsewhere.79 , 80
Conclusions
In recent years, we have gathered fundamental new insight into the ontogeny, dynamics, and function of cardiac macrophages. Given these observations we must be selective and precise in any manipulative approaches targeting cardiac macrophages in the context of HF or conduction abnormalities. These discoveries, however, also show the tremendous potential and force of this heterogenous cell population and the potential therapeutic possibilities arising from better understanding of their roles. Future studies will need to decipher harmful and beneficial functions of monocyte/macrophage subsets and their involvement in cardiac (patho-)physiology.
Acknowledgements
We gratefully acknowledge Kaley Joyes for editing the manuscript.
Funding
This work was funded in part by federal funds from the National Institutes of Health Grants HL139598, HL128264, HL125428, and HL131495; the Massachusetts General Hospital Research Scholar Program; the German Research Foundation (DFG, Heisenberg Programme); the DZHK (German Centre for Cardiovascular Research), and the BMBF (German Ministry of Education and Research; Projects DeCaRe and coNfirm).
Conflict of interest: M.N. has been a payed consultant or received research support from Novartis, GSK, Medtronic, Verseaux, Sigilon, Alnylam, IFM therapeutics, and Molecular Imaging, Inc. The authors declare no conflicts of interest, financial or otherwise.
Contributor Information
Florian Leuschner, Department of Cardiology, Angiology and Pneumology, University Hospital Heidelberg, Im Neuenheimer Feld 410, 69120 Heidelberg, Germany; Partner site Heidelberg, DZHK (German Centre for Cardiovascular Research), Im Neuenheimer Feld 410, 69120 Heidelberg, Germany.
Matthias Nahrendorf, Center for Systems Biology, Massachusetts General Hospital, Harvard Medical School, 185 Cambridge Street, Boston, MA 02114, USA; Cardiovascular Research Center, Massachusetts General Hospital and Harvard Medical School, 185 Cambridge Street, Boston, MA 02114, USA.
References
- 1. Wynn TA, Chawla A, Pollard JW. Macrophage biology in development, homeostasis and disease. Nature 2013;496:445–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. van Furth R, Cohn ZA. The origin and kinetics of mononuclear phagocytes. J Exp Med 1968;128:415–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Akashi K, Traver D, Miyamoto T, Weissman IL. A clonogenic common myeloid progenitor that gives rise to all myeloid lineages. Nature 2000;404:193–197. [DOI] [PubMed] [Google Scholar]
- 4. Fogg DK, Sibon C, Miled C, Jung S, Aucouturier P, Littman DR, Cumano A, Geissmann F. A clonogenic bone marrow progenitor specific for macrophages and dendritic cells. Science 2006;311:83–87. [DOI] [PubMed] [Google Scholar]
- 5. Hettinger J, Richards DM, Hansson J, Barra MM, Joschko AC, Krijgsveld J, Feuerer M. Origin of monocytes and macrophages in a committed progenitor. Nat Immunol 2013;14:821–830. [DOI] [PubMed] [Google Scholar]
- 6. Perie L, Duffy KR, Kok L, de Boer RJ, Schumacher TN. The branching point in erythro-myeloid differentiation. Cell 2015;163:1655–1662. [DOI] [PubMed] [Google Scholar]
- 7. Leuschner F, Rauch PJ, Ueno T, Gorbatov R, Marinelli B, Lee WW, Dutta P, Wei Y, Robbins C, Iwamoto Y, Sena B, Chudnovskiy A, Panizzi P, Keliher E, Higgins JM, Libby P, Moskowitz MA, Pittet MJ, Swirski FK, Weissleder R, Nahrendorf M. Rapid monocyte kinetics in acute myocardial infarction are sustained by extramedullary monocytopoiesis. J Exp Med 2012;209:123–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Robbins CS, Chudnovskiy A, Rauch PJ, Figueiredo JL, Iwamoto Y, Gorbatov R, Etzrodt M, Weber GF, Ueno T, van Rooijen N, Mulligan-Kehoe MJ, Libby P, Nahrendorf M, Pittet MJ, Weissleder R, Swirski FK. Extramedullary hematopoiesis generates Ly-6C(high) monocytes that infiltrate atherosclerotic lesions. Circulation 2012;125:364–U415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ziegler-Heitbrock L, Ancuta P, Crowe S, Dalod M, Grau V, Hart DN, Leenen PJ, Liu YJ, MacPherson G, Randolph GJ, Scherberich J, Schmitz J, Shortman K, Sozzani S, Strobl H, Zembala M, Austyn JM, Lutz MB. Nomenclature of monocytes and dendritic cells in blood. Blood 2010;116:e74–e80. [DOI] [PubMed] [Google Scholar]
- 10. Thomas GD, Hamers AAJ, Nakao C, Marcovecchio P, Taylor AM, McSkimming C, Nguyen AT, McNamara CA, Hedrick CC. Human blood monocyte subsets: a new gating strategy defined using cell surface markers identified by mass cytometry. Arterioscler Thromb Vasc Biol 2017;37:1548–1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Villani AC, Satija R, Reynolds G, Sarkizova S, Shekhar K, Fletcher J, Griesbeck M, Butler A, Zheng SW, Lazo S, Jardine L, Dixon D, Stephenson E, Nilsson E, Grundberg I, McDonald D, Filby A, Li WB, De Jager PL, Rozenblatt-Rosen O, Lane AA, Haniffa M, Regev A, Hacohen N. Single-cell RNA-seq reveals new types of human blood dendritic cells, monocytes, and progenitors. Science 2017;356:eaah4573.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hanna RN, Carlin LM, Hubbeling HG, Nackiewicz D, Green AM, Punt JA, Geissmann F, Hedrick CC. The transcription factor NR4A1 (Nur77) controls bone marrow differentiation and the survival of Ly6C(-) monocytes. Nat Immunol 2011;12:778–785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Gautier EL, Shay T, Miller J, Greter M, Jakubzick C, Ivanov S, Helft J, Chow A, Elpek KG, Gordonov S, Mazloom AR, Ma'ayan A, Chua WJ, Hansen TH, Turley SJ, Merad M, Randolph GJ; Immunological Genome Consortium. Gene-expression profiles and transcriptional regulatory pathways that underlie the identity and diversity of mouse tissue macrophages. Nat Immunol 2012;13:1118–1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hoeffel G, Chen J, Lavin Y, Low D, Almeida FF, See P, Beaudin AE, Lum J, Low I, Forsberg EC, Poidinger M, Zolezzi F, Larbi A, Ng LG, Chan JK, Greter M, Becher B, Samokhvalov IM, Merad M, Ginhoux F. C-Myb(+) erythro-myeloid progenitor-derived fetal monocytes give rise to adult tissue-resident macrophages. Immunity 2015;42:665–678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Epelman S, Lavine KJ, Beaudin AE, Sojka DK, Carrero JA, Calderon B, Brija T, Gautier EL, Ivanov S, Satpathy AT, Schilling JD, Schwendener R, Sergin I, Razani B, Forsberg EC, Yokoyama WM, Unanue ER, Colonna M, Randolph GJ, Mann DL. Embryonic and adult-derived resident cardiac macrophages are maintained through distinct mechanisms at steady state and during inflammation. Immunity 2014;40:91–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Mass E, Ballesteros I, Farlik M, Halbritter F, Gunther P, Crozet L, Jacome-Galarza CE, Handler K, Klughammer J, Kobayashi Y, Gomez-Perdiguero E, Schultze JL, Beyer M, Bock C, Geissmann F. Specification of tissue-resident macrophages during organogenesis. Science 2016;353:aaf4238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Frodermann V, Nahrendorf M. Neutrophil-macrophage cross-talk in acute myocardial infarction. Eur Heart J 2017;38:198–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Murray PJ, Allen JE, Biswas SK, Fisher EA, Gilroy DW, Goerdt S, Gordon S, Hamilton JA, Ivashkiv LB, Lawrence T, Locati M, Mantovani A, Martinez FO, Mege JL, Mosser DM, Natoli G, Saeij JP, Schultze JL, Shirey KA, Sica A, Suttles J, Udalova I, van Ginderachter JA, Vogel SN, Wynn TA. Macrophage activation and polarization: nomenclature and experimental guidelines. Immunity 2014;41:14–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lavin Y, Winter D, Blecher-Gonen R, David E, Keren-Shaul H, Merad M, Jung S, Amit I. Tissue-resident macrophage enhancer landscapes are shaped by the local microenvironment. Cell 2014;159:1312–1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Heidt T, Courties G, Dutta P, Sager HB, Sebas M, Iwamoto Y, Sun Y, Da Silva N, Panizzi P, van der Laan AM, van der Lahn AM, Swirski FK, Weissleder R, Nahrendorf M. Differential contribution of monocytes to heart macrophages in steady-state and after myocardial infarction. Circ Res 2014;115:284–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Pinto AR, Ilinykh A, Ivey MJ, Kuwabara JT, D’Antoni ML, Debuque R, Chandran A, Wang L, Arora K, Rosenthal NA, Tallquist MD. Revisiting cardiac cellular composition. Circ Res 2016;118:400–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hulsmans M, Clauss S, Xiao L, Aguirre AD, King KR, Hanley A, Hucker WJ, Wulfers EM, Seemann G, Courties G, Iwamoto Y, Sun Y, Savol AJ, Sager HB, Lavine KJ, Fishbein GA, Capen DE, Da Silva N, Miquerol L, Wakimoto H, Seidman CE, Seidman JG, Sadreyev RI, Naxerova K, Mitchell RN, Brown D, Libby P, Weissleder R, Swirski FK, Kohl P, Vinegoni C, Milan DJ, Ellinor PT, Nahrendorf M. Macrophages facilitate electrical conduction in the heart. Cell 2017;169:510–522.e20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lavine KJ, Pinto AR, Epelman S, Kopecky BJ, Clemente-Casares X, Godwin J, Rosenthal N, Kovacic JC. The macrophage in cardiac homeostasis and disease JACC macrophage in CVD series (Part 4). J Am Coll Cardiol 2018;72:2213–2230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Leid J, Carrelha J, Boukarabila H, Epelman S, Jacobsen SEW, Lavine KJ. Primitive embryonic macrophages are required for coronary development and maturation. Circ Res 2016;118:1498–1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Epelman S, Lavine KJ, Randolph GJ. Origin and functions of tissue macrophages. Immunity 2014;41:21–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Bajpai G, Schneider C, Wong N, Bredemeyer A, Hulsmans M, Nahrendorf M, Epelman S, Kreisel D, Liu YJ, Itoh A, Shankar TS, Selzman CH, Drakos SG, Lavine KJ. The human heart contains distinct macrophage subsets with divergent origins and functions. Nat Med 2018;24:1234–1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Swirski FK, Nahrendorf M. Cardioimmunology: the immune system in cardiac homeostasis and disease. Nat Rev Immunol 2018;18:733–744. [DOI] [PubMed] [Google Scholar]
- 28. Pirzgalska RM, Seixas E, Seidman JS, Link VM, Sanchez NM, Mahu I, Mendes R, Gres V, Kubasova N, Morris I, Arus BA, Larabee CM, Vasques M, Tortosa F, Sousa AL, Anandan S, Tranfield E, Hahn MK, Iannacone M, Spann NJ, Glass CK, Domingos AI. Sympathetic neuron-associated macrophages contribute to obesity by importing and metabolizing norepinephrine. Nat Med 2017;23:1309–1318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Shibata Y, Berclaz PY, Chroneos ZC, Yoshida M, Whitsett JA, Trapnell BC. GM-CSF regulates alveolar macrophage differentiation and innate immunity in the lung through PU.1. Immunity 2001;15:557–567. [DOI] [PubMed] [Google Scholar]
- 30. Li QY, Barres BA. Microglia and macrophages in brain homeostasis and disease. Nat Rev Immunol 2018;18:225–242. [DOI] [PubMed] [Google Scholar]
- 31. Lazzerini PE, Capecchi PL, Laghi-Pasini F. Systemic inflammation and arrhythmic risk: lessons from rheumatoid arthritis. Eur Heart J 2017;38:1717–1727. [DOI] [PubMed] [Google Scholar]
- 32. Shahreyar M, Fahhoum R, Akinseye O, Bhandari S, Dang G, Khouzam RN. Severe sepsis and cardiac arrhythmias. Ann Transl Med 2018;6:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Boos CJ, Anderson RA, Lip G. Is atrial fibrillation an inflammatory disorder?. Eur Heart J 2006;27:136–149. [DOI] [PubMed] [Google Scholar]
- 34. Guo YT, Lip GYH, Apostolakis S. Inflammation in atrial fibrillation. J Am Coll Cardiol 2012;60:2263–2270. [DOI] [PubMed] [Google Scholar]
- 35. Dernellis J, Panaretou M. Relationship between C-reactive protein concentrations during glucocorticoid therapy and recurrent atrial fibrillation. Eur Heart J 2004;25:1100–1107. [DOI] [PubMed] [Google Scholar]
- 36. Sun Z, Zhou D, Xie X, Wang S, Wang Z, Zhao W, H X, Zheng L. Cross-talk between macrophages and atrial myocytes in atrial fibrillation. Basic Res Cardiol 2016;111:63.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Riley G, Syeda F, Kirchhof P, Fabritz L. An introduction to murine models of atrial fibrillation. Front Physiol 2012;3:296.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Lavine KJ, Epelman S, Uchida K, Weber KJ, Nichols CG, Schilling JD, Ornitz DM, Randolph GJ, Mann DL. Distinct macrophage lineages contribute to disparate patterns of cardiac recovery and remodeling in the neonatal and adult heart. Proc Natl Acad Sci USA 2014;111:16029–16034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Frangogiannis NG. The inflammatory response in myocardial injury, repair, and remodelling. Nat Rev Cardiol 2014;11:255–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Li W, Hsiao HM, Higashikubo R, Saunders BT, Bharat A, Goldstein DR, Krupnick AS, Gelman AE, Lavine KJ, Kreisel D. Heart-resident CCR2(+) macrophages promote neutrophil extravasation through TLR9/MyD88/CXCL5 signaling. JCI Insight 2016;1:e87315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Jung K, Kim P, Leuschner F, Gorbatov R, Kim JK, Ueno T, Nahrendorf M, Yun SH. Endoscopic time-lapse imaging of immune cells in infarcted mouse hearts. Circ Res 2013;112:891–899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Frangogiannis NG, Dewald O, Xia Y, Ren GF, Haudek S, Leucker T, Kraemer D, Taffet G, Rollins BJ, Entman ML. Critical role of monocyte chemoattractant protein-1/CC chemokine ligand 2 in the pathogenesis of ischemic cardiomyopathy. Circulation 2007;115:584–592. [DOI] [PubMed] [Google Scholar]
- 43. Nahrendorf M, Swirski FK, Aikawa E, Stangenberg L, Wurdinger T, Figueiredo JL, Libby P, Weissleder R, Pittet MJ. The healing myocardium sequentially mobilizes two monocyte subsets with divergent and complementary functions. J Exp Med 2007;204:3037–3047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Leuschner F, Dutta P, Gorbatov R, Novobrantseva TI, Donahoe JS, Courties G, Lee KM, Kim JI, Markmann JF, Marinelli B, Panizzi P, Lee WW, Iwamoto Y, Milstein S, Epstein-Barash H, Cantley W, Wong J, Cortez-Retamozo V, Newton A, Love K, Libby P, Pittet MJ, Swirski FK, Koteliansky V, Langer R, Weissleder R, Anderson DG, Nahrendorf M. Therapeutic siRNA silencing in inflammatory monocytes in mice. Nat Biotechnol 2011;29:1005–1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Meyer IS, Jungmann A, Dieterich C, Zhang M, Lasitschka F, Werkmeister S, Haas J, Muller OJ, Boutros M, Nahrendorf M, Katus HA, Hardt SE, Leuschner F. The cardiac microenvironment uses non-canonical WNT signaling to activate monocytes after myocardial infarction. Embo Mol Med 2017;9:1279–1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Bajpai G, Bredemeyer AL, Li W, Zaitsev K, Koenig AL, Lokshina IV, Mohan J, Ivey B, Hsiao H-M, Weinheimer CJ, Kovacs A, Epelman S, Artyomov MN, Kreisel D, Lavine KJ. Tissue resident CCR2- and CCR2+ cardiac macrophages differentially orchestrate monocyte recruitment and fate specification following myocardial injury. Circ Res 2019;124:263–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Anzai A, Choi JL, He S, Fenn AM, Nairz M, Rattik S, McAlpine CS, Mindur JE, Chan CT, Iwamoto Y, Tricot B, Wojtkiewicz GR, Weissleder R, Libby P, Nahrendorf M, Stone JR, Becher B, Swirski FK. The infarcted myocardium solicits GM-CSF for the detrimental oversupply of inflammatory leukocytes. J Exp Med 2017;214:3293–3310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Wan E, Yeap XY, Dehn S, Terry R, Novak M, Zhang S, Iwata S, Han X, Homma S, Drosatos K, Lomasney J, Engman DM, Miller SD, Vaughan DE, Morrow JP, Kishore R, Thorp EB. Enhanced efferocytosis of apoptotic cardiomyocytes through myeloid-epithelial-reproductive tyrosine kinase links acute inflammation resolution to cardiac repair after infarction. Circ Res 2013;113:1004–1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Prabhu SD, Frangogiannis NG. The biological basis for cardiac repair after myocardial infarction: from inflammation to fibrosis. Circ Res 2016;119:91–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. King KR, Aguirre AD, Ye YX, Sun Y, Roh JD, Ng RP Jr, Kohler RH, Arlauckas SP, Iwamoto Y, Savol A, Sadreyev RI, Kelly M, Fitzgibbons TP, Fitzgerald KA, Mitchison T, Libby P, Nahrendorf M, Weissleder R. IRF3 and type I interferons fuel a fatal response to myocardial infarction. Nat Med 2017;23:1481–1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Hilgendorf I, Gerhardt LM, Tan TC, Winter C, Holderried TA, Chousterman BG, Iwamoto Y, Liao R, Zirlik A, Scherer-Crosbie M, Hedrick CC, Libby P, Nahrendorf M, Weissleder R, Swirski FK. Ly-6Chigh monocytes depend on Nr4a1 to balance both inflammatory and reparative phases in the infarcted myocardium. Circ Res 2014;114:1611–1622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Krishnamurthy P, Rajasingh J, Lambers E, Qin G, Losordo DW, Kishore R. IL-10 inhibits inflammation and attenuates left ventricular remodeling after myocardial infarction via activation of STAT3 and suppression of HuR. Circ Res 2009;104:e9–e18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Nahrendorf M, Pittet MJ, Swirski FK. Monocytes: protagonists of infarct inflammation and repair after myocardial infarction. Circulation 2010;121:2437–2445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Leuschner F, Katus HA, Kaya Z. Autoimmune myocarditis: past, present and future. J Autoimmun 2009;33:282–289. [DOI] [PubMed] [Google Scholar]
- 55. Cooper LT Jr. Myocarditis. N Engl J Med 2009;360:1526–1538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Barin JG, Rose NR, Cihakova D. Macrophage diversity in cardiac inflammation: a review. Immunobiology 2012;217:468–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Afanasyeva M, Georgakopoulos D, Belardi DF, Ramsundar AC, Barin JG, Kass DA, Rose NR. Quantitative analysis of myocardial inflammation by flow cytometry in murine autoimmune myocarditis—correlation with cardiac function. Am J Pathol 2004;164:807–815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Coyne CB, Bergelson JM. Virus-induced Abl and Fyn kinase signals permit coxsackievirus entry through epithelial tight junctions. Cell 2006;124:119–131. [DOI] [PubMed] [Google Scholar]
- 59. Clemente-Casares X, Hosseinzadeh S, Barbu I, Dick SA, Macklin JA, Wang Y, Momen A, Kantores C, Aronoff L, Farno M, Lucas TM, Avery J, Zarrin-Khat D, Elsaesser HJ, Razani B, Lavine KJ, Husain M, Brooks DG, Robbins CS, Cybulsky M, Epelman SA. CD103(+) conventional dendritic cell surveillance system prevents development of overt heart failure during subclinical viral myocarditis. Immunity 2017;47:974–989.e8. [DOI] [PubMed] [Google Scholar]
- 60. Escher F, Kuhl U, Lassner D, Stroux A, Westermann D, Skurk C, Tschope C, Poller W, Schultheiss HP. Presence of perforin in endomyocardial biopsies of patients with inflammatory cardiomyopathy predicts poor outcome. Eur J Heart Fail 2014;16:1066–1072. [DOI] [PubMed] [Google Scholar]
- 61. Meyer IS, Goetzke CC, Kespohl M, Sauter M, Heuser A, Eckstein V, Vornlocher HP, Anderson DG, Haas J, Meder B, Katus HA, Klingel K, Beling A, Leuschner F. Silencing the CSF-1 axis using nanoparticle encapsulated sirna mitigates viral and autoimmune myocarditis. Front Immunol 2018;9:2303.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Li Y, Heuser JS, Cunningham LC, Kosanke SD, Cunningham MW. Mimicry and antibody-mediated cell signaling in autoimmune myocarditis. J Immunol 2006;177:8234–8240. [DOI] [PubMed] [Google Scholar]
- 63. Goser S, Andrassy M, Buss SJ, Leuschner F, Volz CH, Ottl R, Zittrich S, Blaudeck N, Hardt SE, Pfitzer G, Rose NR, Katus HA, Kaya Z. Cardiac troponin I but not cardiac troponin T induces severe autoimmune inflammation in the myocardium. Circulation 2006;114:1693–1702. [DOI] [PubMed] [Google Scholar]
- 64. Kania G, Siegert S, Behnke S, Prados-Rosales R, Casadevall A, Luscher TF, Luther SA, Kopf M, Eriksson U, Blyszczuk P. Innate signaling promotes formation of regulatory nitric oxide-producing dendritic cells limiting T-cell expansion in experimental autoimmune myocarditis. Circulation 2013;127:2285–2294. [DOI] [PubMed] [Google Scholar]
- 65. Leuschner F, Courties G, Dutta P, Mortensen LJ, Gorbatov R, Sena B, Novobrantseva TI, Borodovsky A, Fitzgerald K, Koteliansky V, Iwamoto Y, Bohlender M, Meyer S, Lasitschka F, Meder B, Katus HA, Lin C, Libby P, Swirski FK, Anderson DG, Weissleder R, Nahrendorf M. Silencing of CCR2 in myocarditis. Eur Heart J 2015;36:1478–1488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Baldeviano GC, Barin JG, Talor MV, Srinivasan S, Bedja D, Zheng D, Gabrielson K, Iwakura Y, Rose NR, Cihakova D. Interleukin-17A is dispensable for myocarditis but essential for the progression to dilated cardiomyopathy. Circ Res 2010;106:1646–1655. [DOI] [PubMed] [Google Scholar]
- 67. Xia Y, Lee K, Li N, Corbett D, Mendoza L, Frangogiannis NG. Characterization of the inflammatory and fibrotic response in a mouse model of cardiac pressure overload. Histochem Cell Biol 2009;131:471–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Kong P, Christia P, Frangogiannis NG. The pathogenesis of cardiac fibrosis. Cell Mol Life Sci 2014;71:549–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Sager HB, Hulsmans M, Lavine KJ, Moreira MB, Heidt T, Courties G, Sun Y, Iwamoto Y, Tricot B, Khan OF, Dahlman JE, Borodovsky A, Fitzgerald K, Anderson DG, Weissleder R, Libby P, Swirski FK, Nahrendorf M. Proliferation and recruitment contribute to myocardial macrophage expansion in chronic heart failure. Circ Res 2016;119:853–864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Monnerat G, Alarcon ML, Vasconcellos LR, Hochman-Mendez C, Brasil G, Bassani RA, Casis O, Malan D, Travassos LH, Sepulveda M, Burgos JI, Vila-Petroff M, Dutra FF, Bozza MT, Paiva CN, Carvalho AB, Bonomo A, Fleischmann BK, de Carvalho AC, Medei E. Macrophage-dependent IL-1beta production induces cardiac arrhythmias in diabetic mice. Nat Commun 2016;7:13344.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Ponikowski P, Anker SD, AlHabib KF, Cowie MR, Force TL, Hu S, Jaarsma T, Krum H, Rastogi V, Rohde LE, Samal UC, Shimokawa H, Budi Siswanto B, Sliwa K, Filippatos G. Heart failure: preventing disease and death worldwide. ESC Heart Fail 2014;1:4–25. [DOI] [PubMed] [Google Scholar]
- 72. Ismahil MA, Hamid T, Bansal SS, Patel B, Kingery JR, Prabhu SD. Remodeling of the mononuclear phagocyte network underlies chronic inflammation and disease progression in heart failure: critical importance of the cardiosplenic axis. Circ Res 2014;114:266–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Borlaug BA. The pathophysiology of heart failure with preserved ejection fraction. Nat Rev Cardiol 2014;11:507–515. [DOI] [PubMed] [Google Scholar]
- 74. Paulus WJ, Tschope C, Sanderson JE, Rusconi C, Flachskampf FA, Rademakers FE, Marino P, Smiseth OA, De Keulenaer G, Leite-Moreira AF, Borbely A, Edes I, Handoko ML, Heymans S, Pezzali N, Pieske B, Dickstein K, Fraser AG, Brutsaert DL. How to diagnose diastolic heart failure: a consensus statement on the diagnosis of heart failure with normal left ventricular ejection fraction by the Heart Failure and Echocardiography Associations of the European Society of Cardiology. Eur Heart J 2007;28:2539–2550. [DOI] [PubMed] [Google Scholar]
- 75. Niethammer M, Sieber M, von Haehling S, Anker SD, Munzel T, Horstick G, Genth-Zotz S. Inflammatory pathways in patients with heart failure and preserved ejection fraction. Int J Cardiol 2008;129:111–117. [DOI] [PubMed] [Google Scholar]
- 76. Glezeva N, Voon V, Watson C, Horgan S, McDonald K, Ledwidge M, Baugh J. Exaggerated inflammation and monocytosis associate with diastolic dysfunction in heart failure with preserved ejection fraction: evidence of M2 macrophage activation in disease pathogenesis. J Card Fail 2015;21:167–177. [DOI] [PubMed] [Google Scholar]
- 77. Hulsmans M, Sager HB, Roh JD, Valero-Munoz M, Houstis NE, Iwamoto Y, Sun Y, Wilson RM, Wojtkiewicz G, Tricot B, Osborne MT, Hung J, Vinegoni C, Naxerova K, Sosnovik DE, Zile MR, Bradshaw AD, Liao R, Tawakol A, Weissleder R, Rosenzweig A, Swirski FK, Sam F, Nahrendorf M. Cardiac macrophages promote diastolic dysfunction. J Exp Med 2018;215:423–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Verma SK, Krishnamurthy P, Barefield D, Singh N, Gupta R, Lambers E, Thal M, Mackie A, Hoxha E, Ramirez V, Qin G, Sadayappan S, Ghosh AK, Kishore R. Interleukin-10 treatment attenuates pressure overload-induced hypertrophic remodeling and improves heart function via signal transducers and activators of transcription 3-dependent inhibition of nuclear factor-kappaB. Circulation 2012;126:418–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Fayad ZA, Swirski FK, Calcagno C, Robbins CS, Mulder W, Kovacic JC. Monocyte and macrophage dynamics in the cardiovascular system: JACC macrophage in CVD series (part 3). J Am Coll Cardiol 2018;72:2198–2212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Tabas I, Lichtman AH. Monocyte-macrophages and T cells in atherosclerosis. Immunity 2017;47:621–634. [DOI] [PMC free article] [PubMed] [Google Scholar]