Abstract
Aims
The prognosis of patients with MINOCA (myocardial infarction with non-obstructive coronary arteries) is poorly understood. We examined major adverse cardiac events (MACE) defined as all-cause mortality, re-hospitalization for acute myocardial infarction (AMI), heart failure (HF), or stroke 12-months post-AMI in patients with MINOCA versus AMI patients with obstructive coronary artery disease (MICAD).
Methods and results
Multicentre, observational cohort study of patients with AMI (≥65 years) from the National Cardiovascular Data Registry CathPCI Registry (July 2009–December 2013) who underwent coronary angiography with linkage to the Centers for Medicare and Medicaid (CMS) claims data. Patients were classified as MICAD or MINOCA by the presence or absence of an epicardial vessel with ≥50% stenosis. The primary endpoint was MACE at 12 months, and secondary endpoints included the components of MACE over 12 months. Among 286 780 AMI admissions (276 522 unique patients), 16 849 (5.9%) had MINOCA. The 12-month rates of MACE (18.7% vs. 27.6%), mortality (12.3% vs. 16.7%), and re-hospitalization for AMI (1.3% vs. 6.1%) and HF (5.9% vs. 9.3%) were significantly lower for MINOCA vs. MICAD patients (P < 0.001), but was similar between MINOCA and MICAD patients for re-hospitalization for stroke (1.6% vs. 1.4%, P = 0.128). Following risk-adjustment, MINOCA patients had a 43% lower risk of MACE over 12 months (hazard ratio = 0.57, 95% confidence interval 0.55–0.59), in comparison to MICAD patients. This pattern was similar for adjusted risks of the MACE components.
Conclusion
This study confirms an unfavourable prognosis in elderly patients with MINOCA undergoing coronary angiography, with one in five patients with MINOCA suffering a major adverse event over 12 months.
Keywords: Myocardial infarction with non-obstructive coronary arteries, AMI and obstructive coronary disease, Prognosis, Clinical outcomes
See page 879 for the editorial comment on this article (doi: 10.1093/eurheartj/ehz561)
Introduction
In patients with acute myocardial infarction (AMI), and no evidence of obstructive coronary artery disease (CAD), the underlying cause of the AMI is not always apparent. This ambiguity has prompted clinical researchers to coin the term ‘myocardial infarction with non-obstructive coronary arteries’ (MINOCA) in order to stimulate systematic research into the area.1–3 MINOCA encompasses an intriguing group of patients, usually younger and with a different sex distribution than those with obstructive CAD. Several key questions remain unanswered, in particular, do they have the same prognosis as those patients with AMI and obstructive coronary disease (MICAD) and thus managed with the same therapeutic paradigm despite the angiographic findings? However, if their prognosis differs, is it appropriate to institute the same treatment guidelines when there is no evidence that MINOCA patients derive benefit from these therapies?
In relation to prognosis, a recent systematic review identified only a small number of comparative studies revealing a 12-month all-cause mortality rate of 3.5% in MINOCA compared with 6.7% for patients with MICAD.4 MINOCA is now recognized as a distinct entity,5 and therefore, a comprehensive understanding of prognosis is needed. The majority of prognostic studies report in-hospital and 12-month mortality between MINOCA and MICAD,6,7 but relatively few distinguish cardiac events such as re-infarction,8,9 although a recent Swedish study reports a 1.2% prevalence of non-fatal AMI at 12 months in MINOCA.10
Accordingly, the current study compared outcomes of patients with MINOCA to those with MICAD in relation to: (i) major adverse cardiac events (MACE) over 12 months; and (ii) the components of MACE [all-cause mortality, re-hospitalization for AMI, heart failure (HF), or stroke] over 12 months.
Methods
This study used data from the National Cardiovascular Data Registry (NCDR®) CathPCI Registry linked with corresponding claims data from Centers for Medicare and Medicaid Services (CMS).
Data sources
CathPCI Registry
The NCDR CathPCI Registry is a national quality improvement data registry of the American College of Cardiology and the Society for Cardiovascular Angiography and Interventions. The details and design of the NCDR CathPCI Registry have been previously described.11 In brief, the CathPCI Registry includes over 1500 facilities enrolled in the USA, with data collected for patients undergoing cardiac catheterization and/or percutaneous coronary intervention (PCI) by trained personnel at each participating institution using a standardized case report form.12 For this study, the angiography findings and the AMI diagnosis were sourced from the CathPCI Registry version 4.0. The Human Investigation Committee at Yale University School approved the use of a limited data set from the CathPCI registry, which did not require informed consent.
Centers for Medicare and Medicaid administrative (claims and beneficiary) data
The CMS database is an administrative claims dataset which contains information on all hospitalizations of patients enrolled in fee-for-service Medicare (the primary health insurance programme for people aged ≥65 years) and includes service dates and ICD-9-CM diagnosis codes. The Medicare inpatient claims file data contains anonymous patient identifiers, which enables follow-up of beneficiaries over time. In addition, the Medicare denominator data, which links to the inpatient data, contains information on beneficiary eligibility, demographic characteristics, and date of death. For this study, the ICD-9-CM primary and secondary diagnosis codes, and date of death were sourced, and the beneficiary ID, admission date and discharge date of the claims were used for linkage to the CathPCI Registry data.
Patient selection and acute myocardial infarction definition
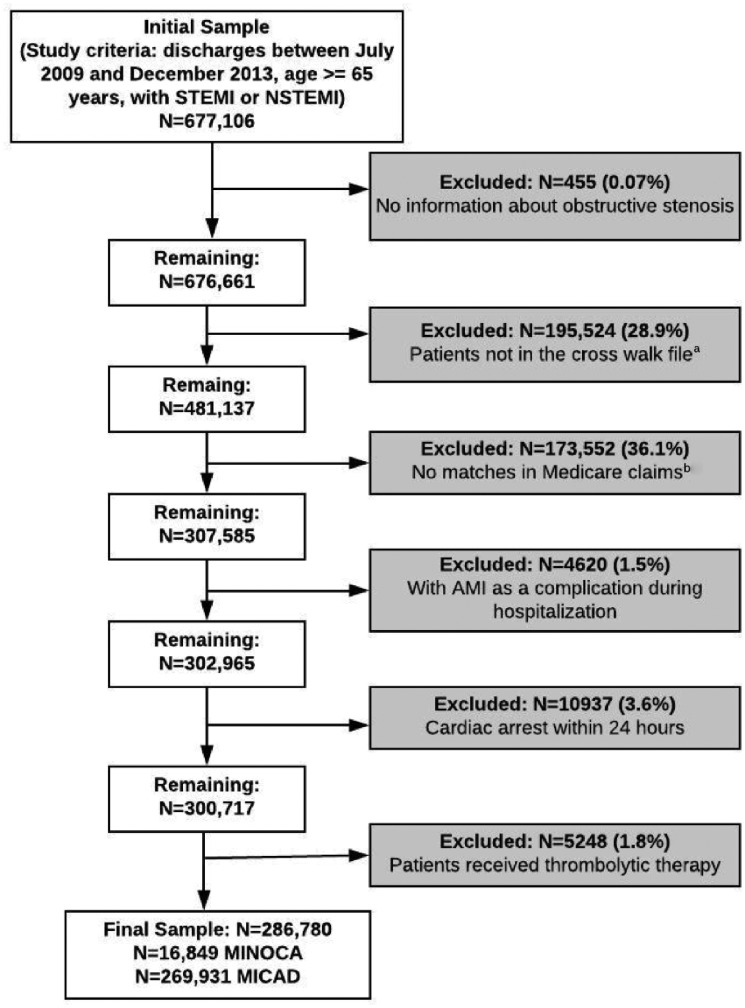
The study cohort included patient admissions in the CathPCI registry between 1 July 2009 and 31 December 2013, who were aged at least 65 years and older to enable linkage to CMS. CathPCI stays where the CAD presentation in the first visit during the stay was either ST-elevation AMI or non-ST-elevation AMI were identified. Subsequently, the patient, discharge diagnosis of AMI, and discharge date in the CathPCI stay were matched to the CMS claims data. CathPCI stays were excluded if they had incomplete data for the estimates of coronary artery stenosis, if patients presented with cardiac arrest within 24 hours of catheterization, if thrombolytics were administered prior to catheterization, and if visits reported AMI as an intra and/or post-procedure complication. The final study sample consisted of CathPCI patients undergoing angiography for AMI with a confirmed AMI discharge diagnosis in the CMS claims data (N = 286 780 total; 276 522 unique patients) (Figure 1).
Figure 1.
Study sample selection flow diagram. aMatching by patients through the National Cardiovascular Data Registry patient ID and Centers for Medicare and Medicaid beneficiary ID in the crosswalk data file. This crosswalk data file was created using the patients’ direct identifiers, while the administrative data was requested for all the patients in all the ACC registries. bMatching by patient beneficiary ID and the discharge date.
The MINOCA admissions were identified as admissions in which patients did not have evidence of obstructive CAD, defined as no stenosis or stenosis <50% and no history of previous revascularization, and admissions with MICAD were defined as admissions in which the patient stenosis ≥50% in any coronary artery or grafts or with previous or current revascularization,4,13 based on the estimates of coronary anatomy available in the CathPCI data. Detailed information on the cardiovascular risk profile and discharge medications was not available in CathPCI as this data were more robustly collected for patients undergoing PCI. Therefore, patient risk factors and cardiovascular/comorbid conditions for the CathPCI stays were derived using the claims data from the linked CMS files (defined according to the ICD-9-CM coding).14–16
Endpoints
The primary endpoint was MACE at 12 months (defined as the first occurrence of an event in the 12-month time period). Secondary endpoints included the components of MACE over 1-, 6-, and 12 months. The mortality outcomes were derived using the CMS data. Re-hospitalizations post-discharge were defined using the CMS inpatient claim file, identified through the primary diagnosis of ICD-9-CM diagnosis codes (Supplementary material online, Table S1).17–20
Data linkage and consolidation
The CathPCI patient stays were linked to records in the CMS inpatient claims data using direct patient identifiers that were present in the both the CMS claims files and the CathPCI Registry.21 To ensure the accuracy of inclusion of true admissions with MINOCA and MICAD patients, CathPCI patient stays included in the study sample required verification of the AMI diagnosis. This was confirmed if the linked CMS record for the CathPCI hospitalization also included AMI as the primary diagnosis (410.X). Matching to the CMS data also allowed: (i) the identification of subsequent inpatient claims for 12 months following discharge for the angiogram; (ii) mortality for any reason (both in-hospital/out of hospital); and (iii) prior inpatient and outpatient claims for 12 months prior to angiography. With the prior inpatient or outpatient claims, we derived the patients’ risk factors for adjustment. For post-inpatient claims, we used ICD-9 codes to identify re-hospitalization for AMI, HF, and stroke.
Statistical analysis
Baseline characteristics were examined for the total sample and compared between admissions with MINOCA and MICAD patients using χ2 tests for categorical variables and t-tests for continuous variables. The unadjusted MACE outcomes over 12 months between admissions with MINOCA and admissions with MICAD patients were also compared using χ2 tests for categorical variables. The cumulative incidence rates for the MACE outcomes over 12 months were calculated for admissions with MINOCA and MICAD and presented as Kaplan–Meir curves.
The MACE outcomes over 1, 6, and 12 months and the components of MACE were examined using Cox proportional-hazard models with competing risks, and sequential adjustment for potential confounders, represented by risk-adjusted hazard ratios (HRs) and 95% confidence intervals (CIs). We used this approach to assess the independent effect of MINOCA on outcomes following AMI. More specifically, to examine whether differences in these outcomes persisted after adjustment for demographics, risk factors, and comorbidities and to identify which patient and/or clinical factors may help explain the difference in outcomes. The proportionality assumption was tested and met for the Cox proportional-hazard models used. No violation on the proportional-hazards assumption was found, the proportional-hazards survival models are appropriate for the evaluation of MINOCA on 12-month outcomes. For computing risk analyses, we used the Fine–Gray approach extension of the Cox regression that models the hazards of the cumulative incidence function.22,23 Variables included in the model correspond to those in the CMS AMI risk-standardized readmission measure.15,24 For all outcomes, the first model included sociodemographics (age, sex, and race), the second model included Model 1 and cardiovascular history and risk factors, and the third model included Model 2 and comorbidities (refer to Supplementary material online, Table S2). For all statistical analyses, the significance level was two-sided with a P value of <0.001. All analyses were performed using SAS statistical software, version 9.4 (SAS Institute Inc., Cary, NC, USA) and version 4.0 of the CathPCI Registry.
Results
Baseline characteristics
Between 1 July 2009 and 31 December 2013, there were 677 106 AMI admissions in CathPCI for patients aged ≥65 years. Figure 1 outlines the inclusion and exclusions for the study cohort. The final study cohort consisted of 286 780 AMI admissions. Of these, 124 900 (43.6%) were female, the mean age was 75.6 years and 259 343 (90.4%) were white. MINOCA was classified in 16 849 (5.9%) visits (Table 1).
Table 1.
Baseline clinical characteristics stratified by MINOCA and MICAD patients
| Characteristics | Total sample (N = 286 780) |
MINOCA (N = 16 849; 5.9%) |
MICAD (N = 269 931; 94.1%) |
|||
|---|---|---|---|---|---|---|
| n | % | n | % | n | % | |
| Sociodemographics | ||||||
| Age (years): Mean, SD | 75.6 | 7.3 | 75.1 | 6.9 | 75.6 | 7.3 |
| Female sex | 124 900 | 43.6 | 12 970 | 77.0 | 111 930 | 41.5 |
| Race | ||||||
| White | 259 343 | 90.4 | 14 637 | 86.9 | 244 706 | 90.7 |
| Black | 19 041 | 6.6 | 1713 | 10.2 | 17 328 | 6.4 |
| Other | 8396 | 2.9 | 499 | 3.0 | 7897 | 2.9 |
| Cardiovascular history | ||||||
| Congestive heart failure | 31 571 | 11.0 | 1568 | 9.3 | 30 003 | 11.1 |
| Angina pectoris/old myocardial infarction | 47 040 | 16.4 | 1315 | 7.8 | 45 725 | 16.9 |
| Valvular/rheumatic heart disease | 47 232 | 16.5 | 3301 | 19.6 | 43 931 | 16.3 |
| Arrhythmias | 30 464 | 10.6 | 1817 | 10.8a | 28 647 | 10.6 |
| Acute coronary syndrome | 25 586 | 8.9 | 468 | 2.7 | 25 118 | 9.3 |
| Anterior myocardial infarction | 52 139 | 18.1 | 2649 | 15.7 | 49 490 | 18.3 |
| Admission presentation | ||||||
| ST-elevation myocardial infarction | 77 305 | 27.0 | 2344 | 13.9 | 74 961 | 27.8 |
| Comorbidities | ||||||
| Cerebrovascular disease | 12 099 | 4.2 | 529 | 3.1 | 11 570 | 4.3 |
| Stroke | 3438 | 1.2 | 207 | 1.2a | 3231 | 1.2 |
| Vascular or circulatory disease | 26 559 | 9.3 | 1168 | 6.9 | 25 391 | 9.4 |
| Diabetes/diabetes complications | 106 623 | 37.2 | 4352 | 25.8 | 102 271 | 37.9 |
| Renal failure | 30 558 | 10.7 | 1289 | 7.7 | 29 269 | 10.8 |
| Chronic obstructive pulmonary disease | 60 092 | 21.0 | 4170 | 24.8 | 55 922 | 20.7 |
| Pneumonia | 29 455 | 10.3 | 2086 | 12.4 | 27 369 | 10.1 |
| Asthma | 8993 | 3.1 | 899 | 5.3 | 8094 | 3.0 |
| Drug/alcohol abuse/dependence/psychosis | 38 478 | 13.4 | 1751 | 10.4 | 36 727 | 13.6 |
| Major psychiatric disorders | 6086 | 2.1 | 541 | 3.2 | 5545 | 2.1 |
Denotes non-significant values >0.001.
Compared to the MICAD patients, MINOCA patients were slightly younger, more likely to be female (77.0% vs. 41.5%; P < 0.001), and were more likely to be black (10.2% vs. 6.4%; P < 0.001). Patients with MINOCA were less likely to have a history of prior AMI/acute coronary syndrome, or HF, compared with MICAD patients (Table 1).
Unadjusted mortality and re-hospitalization outcomes over 12 months
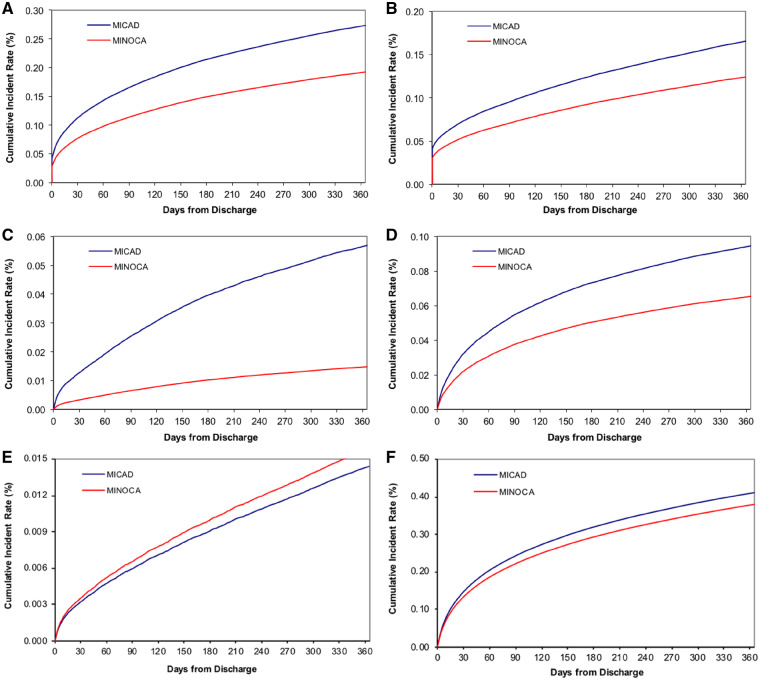
Patients with MINOCA experienced fewer MACE events during the index hospitalization and at 1-, 6-, or 12-month follow-up, compared to those with MICAD (Table 2; Figure 2A–F). Similarly, all-cause mortality was lower in the MINOCA patients at the index hospitalization and at 1-, 6-, and 12-month follow-up. Overall, re-hospitalization rates were slightly lower over the 12-month follow-up period in patients with MINOCA compared with MICAD patients (38.2% vs. 41.1% P < 0.001), with MINOCA patients having fewer re-hospitalizations for AMI or HF, but a similar rate for stroke.
Table 2.
Unadjusted mortality and re-hospitalization rates over 12 months stratified by MINOCA and MICAD
| Characteristics | Total sample (N = 286 780) |
MINOCA (N = 16 849; 5.9%) |
MICAD (N = 269 931; 94.1%) |
|||
|---|---|---|---|---|---|---|
| n | % | n | % | n | % | |
| In-hospital mortality | 11 322 | 4.0 | 348 | 2.1 | 10 974 | 4.1 |
| 1-Month outcomes | ||||||
| MACE (mortality, AMI, HF, stroke) | 30 773 | 10.7 | 1094 | 6.5 | 29 679 | 11.0 |
| Mortality from discharge | 19 313 | 6.7 | 735 | 4.4 | 18 578 | 6.9 |
| Re-hospitalization for AMI | 3572 | 1.2 | 44 | 0.3 | 3528 | 1.3 |
| Re-hospitalization for HF | 8368 | 2.9 | 289 | 1.7 | 8079 | 3.0 |
| Re-hospitalization for Stroke | 932 | 0.3 | 64 | 0.4a | 868 | 0.3 |
| Re-hospitalization | 41 912 | 14.6 | 2135 | 12.7 | 39 777 | 14.7 |
| 6-Month outcomes | ||||||
| MACE (mortality, AMI, HF, stroke) | 60 054 | 20.9 | 2322 | 13.8 | 57 732 | 21.4 |
| Mortality from discharge | 35 004 | 12.2 | 1524 | 9.0 | 33 480 | 12.4 |
| Re-hospitalization for AMI | 11 361 | 4.0 | 134 | 0.8 | 11 227 | 4.2 |
| Re-hospitalization for HF | 19 863 | 6.9 | 735 | 4.4 | 19 128 | 7.1 |
| Re-hospitalization for Stroke | 2646 | 0.9 | 179 | 1.1a | 2467 | 0.9 |
| Re-hospitalization | 91 500 | 31.9 | 4889 | 29.0 | 86 611 | 32.1 |
| 12-Month outcomes | ||||||
| MACE (mortality, AMI, HF, stroke) | 27.0 | 3145 | 18.7 | 74 409 | 27.6 | 27.0 |
| Mortality from discharge | 16.4 | 2080 | 12.3 | 44 958 | 16.7 | 16.4 |
| Re-hospitalization for AMI | 5.8 | 221 | 1.3 | 16 323 | 6.0 | 5.8 |
| Re-hospitalization for HF | 9.1 | 1002 | 5.9 | 25 029 | 9.3 | 9.1 |
| Re-hospitalization for Stroke | 1.4 | 267 | 1.6 | 3887a | 1.4 | 1.4 |
| Re-hospitalization | 40.9 | 6443 | 38.2 | 110 817 | 41.1 | 40.9 |
AMI, acute myocardial infarction; CAD, coronary artery disease; HF, heart failure; MACE, major adverse cardiac events.
Denotes non-significant values >0.001.
Figure 2.
Kaplan–Meier curves for unadjusted incidence of outcomes over 12 months showing the cumulative incidence for (A) major adverse cardiac events, (B) mortality, (C) acute myocardial infarction re-hospitalization, (D) heart failure re-hospitalization, (E) stroke re-hospitalization, and (F) all-cause re-hospitalization (blue line: MICAD; red line: MINOCA).
Independent effect of MINOCA on 12-month outcomes
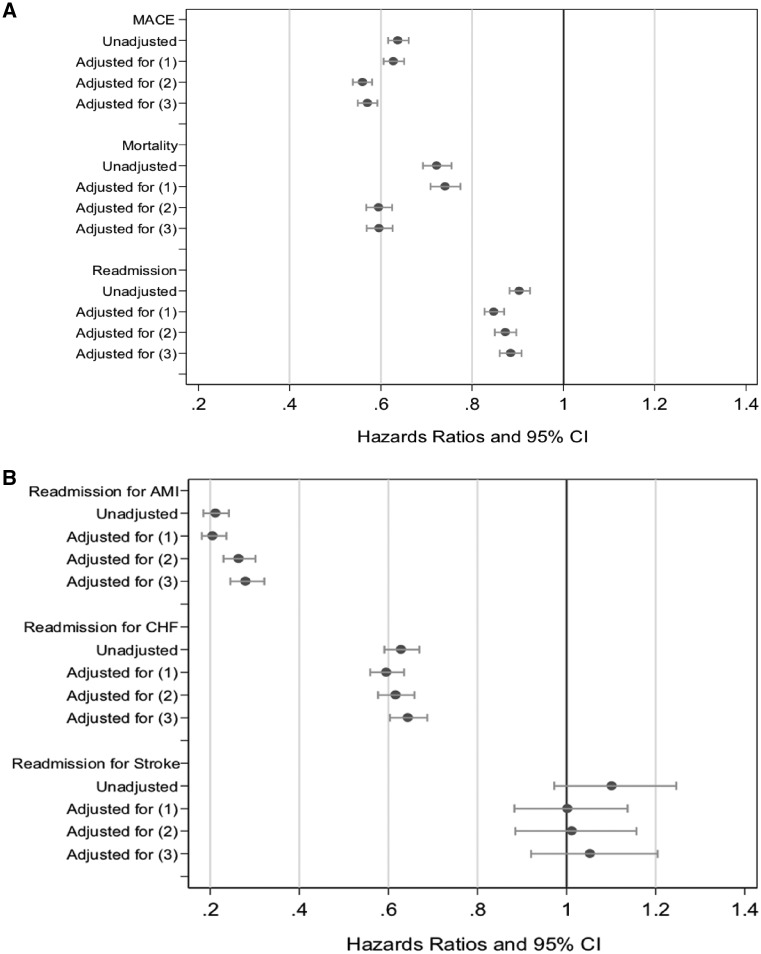
Figure 3A–B shows the association and/or effect of MINOCA on outcomes at 12 months, sequentially adjusted for confounders. Supplementary material online, Figures S1A–S2B show the association and/or effect of MINOCA on outcomes over 1- and 6-month post-AMI.
Figure 3.
A forest plot showing unadjusted/adjusted hazard ratio and 95% confidence interval for the independent effect of MINOCA on (A) 12-month major adverse cardiac events, mortality, and readmission; and (B) readmission for acute myocardial infarction, congestive heart failure, and stroke. Cox models, censored at 12 months; competing risk also considered here.
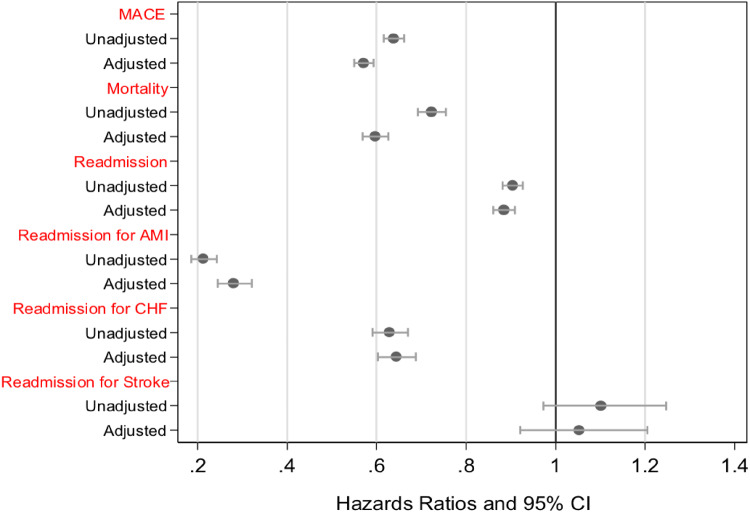
Take home figure.
A forest plot showing unadjusted/adjusted hazard ratio and 95% confidence interval for the independent effect of MINOCA on 12-month outcomes. In the unadjusted model, MINOCA patients had a lower likelihood of major adverse cardiac events, mortality from discharge, and re-hospitalization vs. MICAD patients. After adjusting for confounders, MINOCA patients had a significant but persistently lower hazard of major adverse cardiac events, mortality following discharge, and a lower likelihood of re-hospitalization.
In the unadjusted model, MINOCA patients had a lower likelihood of MACE (HR = 0.64, 95% CI 0.62–0.66), mortality from discharge (HR = 0.72; 95% CI 0.69–0.76), as well as re-hospitalization (HR = 0.90; 95% CI 0.88–0.93), in comparison to MICAD patients over 12 months. After adjusting for sociodemographics, cardiovascular history, and comorbidities MINOCA patients had a significant but persistently lower hazard of MACE (HR = 0.57, 95% CI 0.55–0.59), mortality following discharge (HR = 0.60, 95% CI 0.57–0.63), and a lower likelihood of re-hospitalization (HR = 0.89; 95% CI 0.86–0.91).
Discussion
To our knowledge, this study represents the largest cohort of MINOCA patients with longitudinal follow-up for adverse events. Moreover, it provides comparable data on outcomes from a MICAD cohort. In this elderly US AMI population, we demonstrate that MINOCA accounts for ∼6% of all AMI presentations. Although MACE events over 12 months were lower in patients with MINOCA compared with MICAD, the prognosis amongst MINOCA patients remains of concern. For example, ∼5% of MINOCA patients do not survive the first 30 days post-AMI, almost 40% are re-hospitalized within 12 months and the rate of stroke is the same to the rate observed in MICAD patients. Accordingly, the findings of this study underscore the need to closely consider the on-going management of patients with MINOCA. In particular, these patients warrant further investigation to elucidate the underlying aetiology responsible for their AMI, thereby initiating appropriate mechanistic-targeted therapy to prevent future adverse events.
Prevalence of MINOCA
There is increasing awareness that a proportion of AMI patients do not have evidence of obstructive CAD on angiography; however, the reported prevalence of these patients has varied due to diverse data collection methods and definitions.25 A recent systematic review of the published literature using the conventional <50% stenosis13 threshold for non-obstructive CAD, estimated a prevalence of 6%.4 More contemporary registry data of unselected AMI patients reveals a prevalence of MINOCA as high as 11%,26 possibly reflecting the more widespread use of coronary angiography in acute coronary syndromes. The lower prevalence of MINOCA in the present study may have been influenced by the elderly cohort evaluated (mean age 75 years) since the condition is more prevalent in the young.
Previous studies have consistently demonstrated an over-representation of women in the MINOCA cohort compared to those with MICAD.4 In these studies, which include women of all ages (median age of 55 years), 40% of the MINOCA cohort were women. The VIRGO (Variation in Recovery: Role of Gender on Outcomes of Young AMI Patients) study, which was restricted to AMI patients <55 years, showed that women had five times higher odds of having MINOCA than men.27 In the present study, where the age is restricted to those above 65 years, this sex difference is also exaggerated with almost 80% of the MINOCA cohort being female. The mechanism responsible for this striking sex difference in elderly MINOCA patients requires further investigation but may include plaque-related events, loss of endogenous oestrogen and/or progestins, the use of hormone replacement therapy or microvascular dysfunction and/or vasospasm.28–30 Regarding the latter, studies such from WISE (Women’s ischaemia Syndrome Evaluation study) have demonstrated that microvascular dysfunction is central to the mechanism of non-obstructive coronary disease in women and accounts for their symptoms and prognosis.31
Previous prognostic studies
The prognosis of MINOCA is often presumed to be benign given the absence of obstructive CAD; however, the present study demonstrates a guarded prognosis in relation to all-cause mortality and MACE albeit better than patients with MICAD. The largest study to date exploring in-hospital MACE in MINOCA demonstrated a lower prevalence of events compared to MICAD,32 however, the AMI population was predominately young and supports a guarded prognosis in MINOCA.
Concerning all-cause mortality at 12 months, this was found to be 12.3% in the present study and although lower than patients with MICAD (16.7%), it is considerably higher than reported from a prior meta-analysis (4.7%)4 and a recent French registry (3.3%).33 This may reflect the elderly cohort studied in the present study. Of importance, a prospectively conducted Korean AMI Registry demonstrated that patients with MINOCA had the equivalent 12-month all-cause mortality to those with an AMI with single- or double-vessel CAD.9 In the present study, a post hoc subgroup analysis revealed that, compared to MICAD patients with single- or double-vessel CAD, MINOCA patients had a similar, although statistically lower, 6-month mortality (9.1% vs. 10.1%, P < 0.001) and 12-month mortality (12.3% vs. 13.9%, P < 0.001). In relation to MACE over the 12-month study period, MINOCA patients had a 43% lower risk as compared with MICAD. The 1.3% re-infarction rate at 12 months amongst MINOCA patients observed in this study is consistent with a previous report of 1.4%.34 Overall, MINOCA patients had lower event rates compared to MICAD patients for all outcomes except for stroke, consistent with a recent report from the national Swedish registry.10 Of note, the prevalence of hypertension and arrhythmias was similar between MINOCA and MICAD patients which may account for the similar stroke events. Despite a better prognosis compared to MICAD, MINOCA patients still have a higher risk of mortality and recurrent AMI in comparison to a healthy, age and gender matched population, as recently described by a national New Zealand study (11.1% vs. 3.0% at 2 years).35 This study did not include a control population, however life expectancy at age 65 is 19 years in the USA,36 but 12% of MINOCA patients were deceased at 1 year, suggesting a higher mortality burden in these patients. Further, MINOCA patients with angiographically smooth coronaries still suffer adverse outcomes with 12-month mortality/recurrent AMI reported to be 3.9%, as compared to 6.1% for MINOCA patients with non-obstructive CAD.37
The guarded prognosis in patients with MINOCA beckons the question as to the responsible mechanism. Previous studies have implicated coronary plaque disruption, coronary thrombosis and embolism, epicardial coronary artery spasm, and microvascular dysfunction, as pathophysiological mechanisms.38,39 In addition, psychosocial factors may also play an important role, such as depression, due to the increased risk of ongoing symptoms in patients without obstructive CAD,40 and perceived stress, which is associated with increased long-term mortality in AMI patients.41 Lastly, the differential treatment patterns with cardioprotective medications in patients with MINOCA vs. MICAD is a potential responsible mechanism for their poorer outcomes. This study did not formally evaluate medical management at discharge, however, guideline-recommended therapies are less likely to be prescribed in MINOCA patients,42 and thus, the role of these therapies in influencing outcomes requires further scrutiny.
Study implications
The guarded prognosis evident in this large study has important implications in the management of patients with MINOCA. First, these patients have a clinically important condition associated with significant morbidity and mortality, and should not be dismissed as having ‘minor disease’. Second, the diagnosis of MINOCA should prompt a comprehensive diagnostic work-up to identify the underlying cause of the presentation in each individual patient.43 This is crucial, as although no randomized trials exist to define the optimal management of these patients, the diagnostic workup can aid in identifying the appropriate forms of targeted therapy, since therapies that may be appropriate for one cause, e.g. anticoagulation for thromboembolism or calcium channel blockers for vasospasm, will not be appropriate for all MINOCA patients. Further, prognosis may vary according to the underlying cause43 and this should be explored in future multicentre studies that incorporate data on additional diagnostic investigations following angiography. Third, strategies are needed to improve prognosis, and reduce the re-hospitalization burden. A recent observational study suggests that statins and angiotensin-converting enzyme inhibitors/angiotensin receptor blockers have long-term beneficial effects on MACE in patients with MINOCA.44 However, this study also demonstrated that dual antiplatelet therapy had no impact on MACE in patients with MINOCA. Hence it cannot be assumed that evidence-based cardioprotective therapies for MICAD are equally effective in MINOCA and dedicated clinical trials establishing their efficacy are required. Lastly, angina burden in AMI patients without obstructive CAD may be as high as those with MICAD,45 however, patient-reported outcomes measuring psychosocial factors and health status in MINOCA patients have received limited attention, but should be a focus in future prospective studies assessing treatment and prognosis.
Limitations
Our study has several limitations. First, the CathPCI registry accounts for only in-hospital post-angiogram events and therefore the database was linked to CMS claims data to obtain clinical outcomes over 12 months. Although CMS provides a comprehensive data source, this database has inherent limitations including lack of data on AMI patients <65 years and the potential for a billing coding bias associated with administrative claims datasets. In addition, more than 50% of patients in the initial sample were excluded from the study due to completeness or accuracy of the patients’ identification data (29%) and the data linkage (25% which may due to the non-Medicare insurance), which may limit the generalizability of our findings. Second, we did not have data on cause-specific mortality or outcomes from a population matched control group, and clinically relevant information which may have influenced outcomes including complete discharge medications and extent of necrosis. The CathPCI dataset routinely captures this information only for PCI patients and thus limits our findings. The CathPCI angiographic interpretation was dependent upon the clinician’s angiographic report and thus may be limited in accuracy. Lastly, diagnosis of MINOCA was based upon a discharge diagnosis of AMI. Accordingly, conditions mimicking AMI were clinically excluded (e.g. myocarditis) but whether investigations to actively exclude these non-ischaemic causes (e.g. cardiac magnetic resonance imaging) were undertaken is unknown. Hence it is possible that not all the patients in the study cohort experienced an ischaemic AMI. This equally applies for the MICAD cohort.
Conclusion
In conclusion, although elderly patients with MINOCA have fewer MACE events 12 months after AMI compared to those with MICAD, one in five MINOCA patients will require a cardiovascular-related re-hospitalization and one in ten will not survive by 12 months. Whether conventional cardioprotective therapies shown to improve prognosis in MICAD are also effective in MINOCA necessitates clinical trials specifically targeting this unique condition.
Supplementary Material
Funding
This work was supported by the Center for Cardiovascular Outcomes Research at Yale University (grant 1U01HL105270) from the National Heart, Lung, and Blood Institute to H.M.K. The content is solely the responsibility of the authors and does not represent the official views of the National Heart, Lung, and Blood Institute.
Conflict of interest: H.M.K. and J.P.C. work under contract with the Centers for Medicare and Medicaid Services to develop and maintain performance measures that are publicly reported. H.M.K. was a recipient of a research grant, through Yale, from Medtronic and the U.S. Food and Drug Administration to develop methods for post-market surveillance of medical devices; is was a recipient of research agreements with Medtronic and Johnson & Johnson (Janssen), through Yale, to develop methods of clinical trial data sharing; chairs a Cardiac Scientific Advisory Board for UnitedHealth; is a participant/participant representative of the IBM Watson Health Life Sciences Board; is a member of the Advisory Boards for Element Science and for Facebook, and the Physician Advisory Board for Aetna; was a recipient of a research agreement, through Yale, from the Shenzhen Center for Health Information for work to advance intelligent disease prevention and health promotion; collaborates with the National Center for Cardiovascular Diseases in Beijing; received payment from the Arnold & Porter Law Firm for work related to the Sanofi clopidogrel litigation and from the Ben C. Martin Law Firm for work related to the Cook IVC filter litigation; and is the founder of Hugo, a personal health information platform. J.P.C. has a contract with the American College of Cardiology for his role as Senior Medical Officer and receives salary support from the American College of Cardiology, NCDR. J.P.C. holds equity interest in Medtronic. T.M.M discloses grant funding from the NIH NCATS (1U24TR002306-01: A National Center for Digital Health Informatics Innovation), consulting for Creative Educational Concepts, Inc., employment at BJC HealthCare/Washington University School of Medicine, and honoraria payments from Brown University, Washington State Clinical Outcomes Assessment Program (COAP), Virginia Mason, University of Utah, New York Presbyterian, Westchester Medical Center, Sentara Heart Hospital, and the Henry Ford health system. All other authors have no conflict of interest to declare.
References
- 1. Beltrame JF. Assessing patients with myocardial infarction and nonobstructed coronary arteries (MINOCA). J Intern Med 2013;273:182–185. [DOI] [PubMed] [Google Scholar]
- 2. Agewall S, Beltrame JF, Reynolds HR, Niessner A, Rosano G, Caforio AL, De Caterina R, Zimarino M, Roffi M, Kjeldsen K, Atar D, Kaski JC, Sechtem U, Tornvall P, Pharmacotherapy W. ESC working group position paper on myocardial infarction with non-obstructive coronary arteries. Eur Heart J 2017;38:143–153. [DOI] [PubMed] [Google Scholar]
- 3. Montalescot G, Sechtem U, Achenbach S, Andreotti F, Arden C, Budaj A, Bugiardini R, Crea F, Cuisset T, Di Mario C, Ferreira JR, Gersh BJ, Gitt AK, Hulot JS, Marx N, Opie LH, Pfisterer M, Prescott E, Ruschitzka F, Sabate M, Senior R, Taggart DP, van der Wall EE, Vrints CJ, Zamorano JL, Achenbach S, Baumgartner H, Bax JJ, Bueno H, Dean V, Deaton C, Erol C, Fagard R, Ferrari R, Hasdai D, Hoes AW, Kirchhof P, Knuuti J, Kolh P, Lancellotti P, Linhart A, Nihoyannopoulos P, Piepoli MF, Ponikowski P, Sirnes PA, Tamargo JL, Tendera M, Torbicki A, Wijns W, Windecker S, Knuuti J, Valgimigli M, Bueno H, Claeys MJ, Donner-Banzhoff N, Erol C, Frank H, Funck-Brentano C, Gaemperli O, Gonzalez-Juanatey JR, Hamilos M, Hasdai D, Husted S, James SK, Kervinen K, Kolh P, Kristensen SD, Lancellotti P, Maggioni AP, Piepoli MF, Pries AR, Romeo F, Ryden L, Simoons ML, Sirnes PA, Steg PG, Timmis A, Wijns W, Windecker S, Yildirir A, Zamorano JL. 2013 ESC guidelines on the management of stable coronary artery disease: the Task Force on the management of stable coronary artery disease of the European Society of Cardiology. Eur Heart J 2013;34:2949–3003. [DOI] [PubMed] [Google Scholar]
- 4. Pasupathy S, Air T, Dreyer RP, Tavella R, Beltrame JF. Systematic review of patients presenting with suspected myocardial infarction and nonobstructive coronary arteries. Circulation 2015;131:861–870. [DOI] [PubMed] [Google Scholar]
- 5. Thygesen K, Alpert JS, Jaffe AS, Chaitman BR, Bax JJ, Morrow DA, White HD; ESC Scientific Document Group. Fourth universal definition of myocardial infarction. Eur Heart J 2019;40:237–269. [DOI] [PubMed] [Google Scholar]
- 6. Larsen AI, Galbraith PD, Ghali WA, Norris CM, Graham MM, Knudtson ML, Investigators A. Characteristics and outcomes of patients with acute myocardial infarction and angiographically normal coronary arteries. Am J Cardiol 2005;95:261–263. [DOI] [PubMed] [Google Scholar]
- 7. Frycz-Kurek AM, Gierlotka M, Gąsior M, Wilczek K, Lekston A, Kalarus Z, Poloński L. Patients with no significant lesions in coronary arteries and ST-segment elevation myocardial infarction have worse outcome than patients with non-ST-segment elevation myocardial infarction: analysis from PL-ACS Registry. Kardiol Pol 2010;68:1211–1217. [PubMed] [Google Scholar]
- 8. Rhew SH, Ahn Y, Kim MC, Jang SY, Cho KH, Hwang SH, Lee MG, Ko JS, Park KH, Sim DS, Yoon NS, Yoon HJ, Kim KH, Hong YJ, Park HW, Kim JH, Jeong MH, Cho JG, Park JC, Kang JC. Is myocardial infarction in patients without significant stenosis on a coronary angiogram as benign as believed? Chonnam Med J 2012;48:39–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kang WY, Jeong MH, Ahn YK, Kim JH, Chae SC, Kim YJ, Hur SH, Seong IW, Hong TJ, Choi DH, Cho MC, Kim CJ, Seung KB, Chung WS, Jang YS, Rha SW, Bae JH, Cho JG, Park SJ. Are patients with angiographically near-normal coronary arteries who present as acute myocardial infarction actually safe? Int J Cardiol 2011;146:207–212. [DOI] [PubMed] [Google Scholar]
- 10. Hjort M, Lindahl B, Baron T, Jernberg T, Tornvall P, Eggers KM. Prognosis in relation to high-sensitivity cardiac troponin T levels in patients with myocardial infarction and non-obstructive coronary arteries. Am Heart J 2018;200:60–66. [DOI] [PubMed] [Google Scholar]
- 11. Messenger JC, Ho KK, Young CH, Slattery LE, Draoui JC, Curtis JP, Dehmer GJ, Grover FL, Mirro MJ, Reynolds MR, Rokos IC, Spertus JA, Wang TY, Winston SA, Rumsfeld JS, Masoudi FA, Science N; NCDR Science and Quality Oversight Committee Data Quality Workgroup. The National Cardiovascular Data Registry (NCDR) Data Quality Brief: the NCDR Data Quality Program in 2012. J Am Coll Cardiol 2012;60:1484–1488. [DOI] [PubMed] [Google Scholar]
- 12.What Each Registry Collects—Quality Improvement for Institutions. http://cvquality.acc.org/NCDR-Home/Data-Collection/What-Each-Registry-Collects.aspx (11 January 2016).
- 13. Scanlon PJ, Faxon DP, Audet AM, Carabello B, Dehmer GJ, Eagle KA, Legako RD, Leon DF, Murray JA, Nissen SE, Pepine CJ, Watson RM, Ritchie JL, Gibbons RJ, Cheitlin MD, Gardner TJ, Garson A Jr, Russell RO Jr, Ryan TJ, Smith SC Jr. ACC/AHA guidelines for coronary angiography: executive summary and recommendations. A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee on Coronary Angiography) developed in collaboration with the Society for Cardiac Angiography and Interventions. Circulation 1999;99:2345–2357. [DOI] [PubMed] [Google Scholar]
- 14. McNamara RL, Wang Y, Partovian C, Montague J, Mody P, Eddy E, Krumholz HM, Bernheim SM. Development of a hospital outcome measure intended for use with electronic health records: 30-day risk-standardized mortality after acute myocardial infarction. Med Care 2015;53:818–826. [DOI] [PubMed] [Google Scholar]
- 15.Center or Medicare and Medicaid Services. Planned Readmission Algorithm http://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/HospitalQualityInits/Measure-Methodology (1 March 2016).
- 16. Krumholz HM, Lin Z, Drye EE, Desai MM, Han LF, Rapp MT, Mattera JA, Normand SL. An administrative claims measure suitable for profiling hospital performance based on 30-day all-cause readmission rates among patients with acute myocardial infarction. Circ Cardiovasc Qual Outcomes 2011;4:243–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Krumholz HM, Wang Y, Mattera JA, Wang Y, Han LF, Ingber MJ, Roman S, Normand SL. An administrative claims model suitable for profiling hospital performance based on 30-day mortality rates among patients with an acute myocardial infarction. Circulation 2006;113:1683–1692. [DOI] [PubMed] [Google Scholar]
- 18. Krumholz HM, Wang Y, Mattera JA, Wang Y, Han LF, Ingber MJ, Roman S, Normand SL. An administrative claims model suitable for profiling hospital performance based on 30-day mortality rates among patients with heart failure. Circulation 2006;113:1693–1701. [DOI] [PubMed] [Google Scholar]
- 19. Fonarow GC, Pan W, Saver JL, Smith EE, Reeves MJ, Broderick JP, Kleindorfer DO, Sacco RL, Olson DM, Hernandez AF, Peterson ED, Schwamm LH. Comparison of 30-day mortality models for profiling hospital performance in acute ischemic stroke with vs without adjustment for stroke severity. JAMA 2012;308:257–264. [DOI] [PubMed] [Google Scholar]
- 20. Fonarow GC, Smith EE, Reeves MJ, Pan W, Olson D, Hernandez AF, Peterson ED, Schwamm LH; Get With The Guidelines Steering Committee and Hospitals. Hospital-level variation in mortality and rehospitalization for medicare beneficiaries with acute ischemic stroke. Stroke 2011;42:159–166. [DOI] [PubMed] [Google Scholar]
- 21. Curtis JP, Geary LL, Wang Y, Chen J, Drye EE, Grosso LM, Spertus JA, Rumsfeld JS, Weintraub WS, Masoudi FA, Brindis RG, Krumholz HM. Development of 2 registry-based risk models suitable for characterizing hospital performance on 30-day all-cause mortality rates among patients undergoing percutaneous coronary intervention. Circ Cardiovasc Qual Outcomes 2012;5:628–637. [DOI] [PubMed] [Google Scholar]
- 22. Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Association 1999;94:496–509. [Google Scholar]
- 23. So Y, Lin G, Johnson G. Using the PHREG Procedure to Analyze Competing-Risks Data. Washington, DC: SAS Global Forum; 2014. http://support.sas.com/rnd/app/stat/papers/2014/competingrisk2014.pdf (4 December 2018). [Google Scholar]
- 24.Agency for Healthcare Research and Quality (AHRQ). Acute myocardial infarction (AMI): hospital 30 day, all-cause, risk-standardized mortality rate (RSMR) following AMI hospitalization; 2015. https://content.govdelivery.com/accounts/USAHRQ/bulletins/fe2710 (17 November 2018).
- 25. Pasupathy S, Tavella R, McRae S, Beltrame JF. Myocardial infarction with non-obstructive coronary arteries—diagnosis and management. Eur Cardiol Rev 2015;10:79–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Pasupathy S, Air T, Dreyer RP, Tavella R, Beltrame JF. Response to Letter Regarding Article, “Systematic Review of Patients Presenting With Suspected Myocardial Infarction and Nonobstructive Coronary Arteries”. Circulation 2015;132:e232. [DOI] [PubMed] [Google Scholar]
- 27. Safdar B, Spatz ES, Dreyer RP, Beltrame JF, Lichtman JH, Spertus JA, Reynolds HR, Geda M, Bueno H, Dziura JD, Krumholz HM, D'Onofrio G. Presentation, clinical profile, and prognosis of young patients with myocardial infarction with nonobstructive coronary arteries (MINOCA): results from the VIRGO Study. J Am Heart Assoc 2018;7:e009174.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lansky AJ, Pietras C. Coronary microvascular dysfunction: does sex matter? JACC Cardiovasc Interv 2015;8:1442–1444. [DOI] [PubMed] [Google Scholar]
- 29. Reynolds HR, Srichai MB, Iqbal SN, Slater JN, Mancini GB, Feit F, Pena-Sing I, Axel L, Attubato MJ, Yatskar L, Kalhorn RT, Wood DA, Lobach IV, Hochman JS. Mechanisms of myocardial infarction in women without angiographically obstructive coronary artery disease. Circulation 2011;124:1414–1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Della Rocca DG, Pepine CJ. What causes myocardial infarction in women without obstructive coronary artery disease? Circulation 2011;124:1404–1406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Mehta LS, Beckie TM, DeVon HA, Grines CL, Krumholz HM, Johnson MN, Lindley KJ, Vaccarino V, Wang TY, Watson KE, Wenger NK; American Heart Association Cardiovascular Disease in Women and Special Populations Committee of the Council on Clinical Cardiology, Council on Epidemiology and Prevention, Council on Cardiovascular and Stroke Nursing, and Council on Quality of Care and Outcomes Research. Acute myocardial infarction in women: a scientific statement from the American Heart Association. Circulation 2016;133:916–947. [DOI] [PubMed] [Google Scholar]
- 32. Smilowitz NR, Mahajan AM, Roe MT, Hellkamp AS, Chiswell K, Gulati M, Reynolds HR. Mortality of myocardial infarction by sex, age, and obstructive coronary artery disease status in the ACTION Registry-GWTG (Acute Coronary Treatment and Intervention Outcomes Network Registry-Get With the Guidelines). Circ Cardiovasc Qual Outcomes 2017;10:e003443.. [DOI] [PubMed] [Google Scholar]
- 33. Feldman L, Steg PG, Amsallem M, Puymirat E, Sorbets E, Elbaz M, Ritz B, Hueber A, Cattan S, Piot C, Ferrieres J, Simon T, Danchin N; FAST-MI investigators. Editor's Choice-Medically managed patients with non-ST-elevation acute myocardial infarction have heterogeneous outcomes, based on performance of angiography and extent of coronary artery disease. Eur Heart J Acute Cardiovasc Care 2017;6:262–271. [DOI] [PubMed] [Google Scholar]
- 34. Aldous S, Elliott J, McClean D, Puri A, Richards AM. Outcomes in patients presenting with symptoms suggestive of acute coronary syndrome with elevated cardiac troponin but non-obstructive coronary disease on angiography. Heart Lung Circ 2015;24:869–878. [DOI] [PubMed] [Google Scholar]
- 35. Williams MJA, Barr PR, Lee M, Poppe KK, Kerr AJ. Outcome after myocardial infarction without obstructive coronary artery disease. Heart 2019;105:524–530. [DOI] [PubMed] [Google Scholar]
- 36. Kochanek K, Murphy SL, Xu JQ, Arias E. Mortality in the United States, 2016. NCHS Data Brief, no 293. Hyattsville, MD: National Center for Health Statistics; 2017. https://www.cdc.gov/nchs/data/databriefs/db293.pdf (25 November 2018). [Google Scholar]
- 37. Bainey KR, Welsh RC, Alemayehu W, Westerhout CM, Traboulsi D, Anderson T, Brass N, Armstrong PW, Kaul P. Population-level incidence and outcomes of myocardial infarction with non-obstructive coronary arteries (MINOCA): Insights from the Alberta contemporary acute coronary syndrome patients invasive treatment strategies (COAPT) study. Int J Cardiol 2018;264:12–17. [DOI] [PubMed] [Google Scholar]
- 38. Beltrame JF, Crea F, Camici P. Advances in coronary microvascular dysfunction. Heart Lung Circ 2009;18:19–27. [DOI] [PubMed] [Google Scholar]
- 39. Yetkin E, Turhan H, Erbay AR, Aksoy Y, Senen K. Increased thrombolysis in myocardial infarction frame count in patients with myocardial infarction and normal coronary arteriogram: a possible link between slow coronary flow and myocardial infarction. Atherosclerosis 2005;181:193–199. [DOI] [PubMed] [Google Scholar]
- 40. Arnold SV, Spertus JA, Ciechanowski PS, Soine LA, Jordan-Keith K, Caldwell JH, Sullivan MD. Psychosocial modulators of angina response to myocardial ischemia. Circulation 2009;120:126–133. [DOI] [PubMed] [Google Scholar]
- 41. Arnold SV, Smolderen KG, Buchanan DM, Li Y, Spertus JA. Perceived stress in myocardial infarction: long-term mortality and health status outcomes. J Am Coll Cardiol 2012;60:1756–1763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. De Ferrari GM, Fox KA, White JA, Giugliano RP, Tricoci P, Reynolds HR, Hochman JS, Gibson CM, Theroux P, Harrington RA, Van de Werf F, White HD, Califf RM, Newby LK. Outcomes among non-ST-segment elevation acute coronary syndromes patients with no angiographically obstructive coronary artery disease: observations from 37,101 patients. Eur Heart J Acute Cardiovasc Care 2014;3:37–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Niccoli G, Scalone G, Crea F. Acute myocardial infarction with no obstructive coronary atherosclerosis: mechanisms and management. Eur Heart J 2015;36:475–481. [DOI] [PubMed] [Google Scholar]
- 44. Lindahl B, Baron T, Erlinge D, Hadziosmanovic N, Nordenskjold A, Gard A, Jernberg T. Medical therapy for secondary prevention and long-term outcome in patients with myocardial infarction with nonobstructive coronary artery disease. Circulation 2017;135:1481–1489. [DOI] [PubMed] [Google Scholar]
- 45. Grodzinsky A, Arnold SV, Gosch K, Spertus JA, Foody JM, Beltrame J, Maddox TM, Parashar S, Kosiborod M. Angina frequency after acute myocardial infarction in patients without obstructive coronary artery disease. Eur Heart J Qual Care Clin Outcomes 2015;1:92–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.