Abstract
Aims
To determine the extent of shared decision-making (SDM), during selection of oral anticoagulant (OAC) and rhythm control treatments, in patients with newly diagnosed atrial fibrillation (AF).
Methods and results
We evaluated survey data from 1006 patients with new-onset AF enrolled at 56 US sites participating in the SATELLITE substudy of the Outcomes Registry for Better Informed Treatment of Atrial Fibrillation (ORBIT II). Patients completed surveys at enrolment and at 6-month follow-up. Patients were asked about who made their AF treatment decisions. Shared decision-making was classified as one that the patient felt was an autonomous decision or a shared decision with their healthcare provider (HCP). Approximately half of patients reported that their OAC treatment decisions were made entirely by their HCP. Compared with those reporting no SDM, patients reporting SDM for OAC were more often female (47.2% vs. 38.4%), while patients reporting SDM for rhythm control were more often male (62.2% vs. 57.6%). The most important factors cited by patients during decision-making for OAC were reducing stroke and bleeding risk, and their HCP’s recommendations. After adjustment, patients with self-reported understanding of OAC, and rhythm control options, had higher odds of having participated in SDM [odds ratio (OR) 2.54, confidence interval (CI): 1.75–3.68 and OR 2.36, CI: 1.50–3.71, both P ≤ 0.001, respectively].
Conclusion
Shared decision-making is not widely implemented in contemporary AF practice. Patient understanding about available therapeutic options is associated with a more than a two-fold higher likelihood of SDM, and may be a potential target for future interventions.
Keywords: Atrial Fibrillation, Patient-reported involvement in treatment decisions, Quality of care, Shared decision-making, Stroke prevention
Introduction
Atrial fibrillation (AF) is the most common sustained cardiac arrhythmia, affecting more than 33 million individuals globally.1 Atrial fibrillation is associated with increased risk of heart failure, cognitive impairment, and reduced quality of life.2 The most important and catastrophic sequela of AF is thromboembolic stroke, which can lead to significant disability or death.3,4 Oral anticoagulation (OAC) effectively reduces the risk of stroke and improves survival in patients with AF, and rhythm control strategies (including cardioversion, antiarrhythmic drugs, or ablation) may improve symptoms and quality of life.5 Each strategy has a unique risk-benefit profile that needs to be considered prior to therapy selection.
There is increasing agreement that patients should be more involved in this process of treatment decision-making, which can enhance therapeutic adherence and satisfaction.6 The goal of shared decision-making (SDM) is to ensure that treatment strategies align with individual patient values, health goals, and treatment preferences.7 The European Society of Cardiology (ESC), American Heart Association (AHA), American College of Cardiology (ACC) guidelines likewise recommend SDM for the management of AF.8,9 In particular, the ESC states that SDM, including patients’ partners and relatives, is ‘indispensable’ for integrated AF management,9 while prior work suggests that patient perception of personal involvement may increase confidence in clinical decisions.10 In fact, all such guidelines recommend multidisciplinary approaches, and universal adoption of SDM.
Widespread SDM adoption faces important barriers, including limited time for clinical interaction and clinician training.11 Patient characteristics, including older age, lower education level, and language barriers,11 may further contribute to suboptimal SDM. Better understanding of the most salient barriers to SDM may help inform strategies to improve persistence on guideline-recommended therapy in AF. However, few studies, to date, have characterized contemporary patterns and predictors of SDM in a national AF population.
To address these knowledge gaps, we conducted the Survey of Patient Knowledge and Personal Priorities for Treatment (SATELLITE), a substudy within the national Outcomes Registry for Better Informed Treatment of Atrial Fibrillation II (ORBIT II) registry. The survey was constructed to elucidate patterns of patient-reported risk perception and understanding of the roles, options, and benefits of existing and novel AF therapies.12 In this analysis, we focused on SDM addressing OAC treatment and antiarrhythmic therapy, and characterized the association between patient understanding of therapeutic options and SDM, at baseline and 6 months after initiating treatment.
Methods
Design and setting
The rationale and design of ORBIT-AF II have been previously described.13 In brief, ORBIT-AF II enrolled patients aged 21 years or older with electrocardiographically confirmed AF who were (i) diagnosed with AF within the 6 months preceding the baseline visit; or (ii) AF patients who initiated or transitioned to a factor Xa inhibitor, or a direct thrombin inhibitor, within the preceding 3 months. Patients in the SATELLITE substudy were enrolled at 56 ORBIT-AF II sites between 20 February 2013 and 12 July 2016. Follow-up data was collected at 6-month intervals, with a minimum follow-up duration of 12 months [median 12 months; interquartile range (IQR) 12–18 months], and included demographics, medical history, cardiovascular risk factors, treatments (pharmacotherapies and procedures), clinical events, and provider information. A representative sample of cardiology, electrophysiology, and primary care practices were selected for participation in the SATELLITE survey substudy. In addition to ORBIT II eligibility criteria, we required SATELLITE patients to have a new AF diagnosis within 6 months of enrolment, and the ability to complete surveys in English. Each site enrolled a convenience sample of patients meeting eligibility criteria until the 1000-patient enrolment target was reached. Substudy participants completed self-administered paper surveys at their baseline visit, and a follow-up survey at a 6-month clinic visit (Supplementary material online A and B). All SATELLITE study participants gave written informed consent before enrolment. The Duke Institutional Review Board (IRB) approved the ORBIT II Registry, and all participating sites obtained approval from local IRBs, before patient consent.
Survey metric development
As numerous guidelines advocate multidisciplinary approaches for SDM,8,9 we assembled a committee of cardiologists, epidemiologists, and electrophysiologists, with subject matter expertise in observational research in AF populations, to draft the SATELLITE survey questions. Content validity was confirmed through review by other ORBIT-AF investigators. Prior to implementation, survey content was beta-tested by 10 laypersons for readability and clarity. Such feedback resulted in the combination of several questions for parsimony and minor revisions to question stem wording. The total number of questions was 10, for the baseline survey, and 9, for the follow-up survey.
Likert scales were used to capture self-reported understanding of three disease concepts, including: (i) disease understanding; (ii) stroke risk understanding; (iii) SDM for OAC and rhythm control; and (iv) understanding of AF treatments, including rhythm control, ablation, warfarin, direct oral anticoagulants (DOACs), and cardioversion. Of these four topics, stroke risk and general AF understanding were only assessed at baseline, while comprehension of AF treatments was examined both at baseline and 6 months.
Questionnaires were provided to patients in clinic by the enrolling site staff at the time of enrolment and during the 6-month follow-up appointment. Instructions were provided in a cover sheet with the questionnaire and patients were encouraged to ask questions of site staff if needed. Because the clinical research workflow varies from site to site, we did not require specific timing of survey completion during the visit. Survey responses were then compared at baseline and at 6 months, with five response categories used to define three levels of comprehension of clinical concepts, for each question (Supplementary material online, Table S1). For queries offering the range of response choices from strongly agree to strongly disagree, high understanding was specified by strongly or somewhat agree; neutral was specified by a reply of neutral; and low understanding was specified by somewhat or strongly disagree.
Patient-reported understanding of atrial fibrillation and stroke risk
High understanding of AF was defined as a response of ‘strongly or somewhat agree’ to the statement ‘I feel I understand what AF is’.
High understanding of stroke risk was defined as a response of ‘strongly or somewhat agree’ to the statement ‘The major risk of atrial fibrillation is stroke’.
Patient-reported shared decision-making for oral anticoagulant and rhythm control
Shared decision-making for OAC was defined as a response of entirely me, mostly me, or equally me and my healthcare provider to the question ‘When choosing your blood thinner(s), who made this treatment decision?’ SDM for rhythm control was similarly defined as a response of entirely me, mostly me, or equally me and my healthcare provider to the question ‘When choosing your rhythm control strategy, who made this treatment decision?’.
Patient-reported understanding of atrial fibrillation treatment options
High understanding of OACs or rhythm control were each defined as a response of ‘strongly or somewhat agree’ to the statements ‘I understand the various options for blood thinners in the treatment of AF’ and ‘I understand the various options for rhythm control in the treatment of AF’. Additionally, a high understanding for the risk and benefits of AF treatment was defined as a response of completely or mostly understand to the following questions ‘How well would you say you understand the risk of using the following therapies for AF’ and ‘How well would you say you understand the benefits of using the following therapies for AF?’ A low understanding was categorized by a response of somewhat understand or understand very little; and no understanding was categorized by never heard of the therapy.
Patient-prioritized factors influencing treatment
To describe factors influencing treatment strategy at baseline, patients prioritized factors (e.g. cost, convenience, risk of bleeding, etc.) on a scale of 1–5, with 1 defined as ‘not at all important’ and 5 as ‘extremely important’.
Patient-prioritized source of information
To describe sources of information regarding AF by SDM responses (e.g. books, family/friends, academic journals, etc.) at baseline and 6 months by decision-making category, patients prioritized sources of information on a 1–9 scale with 1 defined as ‘most important’ and 9 as ‘least important’.
Current atrial fibrillation treatment
Atrial fibrillation treatment was defined using data from case report forms completed by the sites at baseline and 6 months. For each time period, OAC use was defined as use of warfarin, dabigatran, rivaroxaban, apixaban, or edoxaban. Rhythm control use was defined as use of any antiarrhythmic medication; cardioversion was defined as any cardioversion procedure; and ablation was defined as utilization of a catheter ablation for AF, specifically pulmonary vein isolation.
Statistical analysis
Baseline characteristics of those with shared and non-SDM are presented as frequencies and percentages for categorical variables, and medians (IQR) for continuous variables. The items in the questions on the importance of features in choosing treatment decisions and the sources of information a patient prioritized in understanding AF are scaled from 1–5 to 1–9, respectively. The data are summarized for each item, with means and standard deviations. For the outcome of SDM for OAC, three nested logistic models were sequentially constructed: baseline clinical characteristics; plus insurance and education information; plus patient AF understanding scores (Supplementary material online, Table S1A for the lists of variables). The covariates were prespecified, based on clinical input. For each covariate, the unadjusted and adjusted P-values, odds ratios (ORs) and corresponding 95% confidence intervals (CI) were provided. Patients with missing values (incomplete surveys) for the response or any covariate, within a given model, were excluded from the corresponding analysis. This strategy was repeated in models for SDM in rhythm control. To determine the effect that the definition of SDM has on the main analysis, we conducted a sensitivity analysis; whereby we define SDM as a response of entirely me, mostly me, equally me and my healthcare provider, or mostly my healthcare provider to the question ‘When choosing your blood thinner(s)/rhythm control, who made this treatment decision?’ Non-SDM was defined as a response of entirely my healthcare provider. These results were similar to the main analyses, and thus not reported.
Results
Patient characteristics
Of 13 415 patients enrolled in ORBIT II, 1006 (13.3%) patients at 56 US sites participated in SATELLITE, with 39.4% (n = 396) patients only on OACs, 54.5% (n = 548) patients on both OACs and rhythm control, 2.6% (n = 27) patients only on rhythm control, and 3.4% (n = 35) patients on neither OACs nor rhythm control or did not respond. Patients in SATELLITE were slightly younger (69 and 67 vs. 71 years), more likely white (91.0% and 89.9% vs. 85.4%), had private insurance (59.0% and 58.4% vs. 51.4%), lower CHA2DS2-VASc stroke risk, and lower bleeding risk, compared to non-SATELLITE patients. Medical comorbidities, between SATELLITE and ORBIT patients, were similar (Table 1).
Table 1.
SATELLITE vs. Non-SATELLITE patient characteristics
| Characteristics | SATELLITE patients |
Non-SATELLITE patientsa | |
|---|---|---|---|
| OAC (N = 944) | Received rhythm treatment strategies N = 575 | N = 12 388 | |
| Demographics, n (%) | |||
| Age (years), median (IQR) | 69.0 (61.0–76.0) | 67.0 (59.0–75.0) | 71.0 (63.0–78.0) |
| Male | 553 (58.6) | 337 (58.6) | 7259 (58.6) |
| Race | |||
| White | 857 (90.8) | 517 (89.9) | 10 570 (85.4) |
| Black/African American | 35 (3.7) | 21 (3.7) | 637 (5.1) |
| Other | 52 (5.5) | 37 (6.4) | 1168 (9.4) |
| Insurance | |||
| Private | 557 (59.0) | 336 (58.4) | 6362 (51.4) |
| Medicare | 333 (35.3) | 207 (36.0) | 4753 (38.4) |
| Other | 54 (5.7) | 32 (5.6) | 1273 (10.3) |
| Risk factors, n (%) | |||
| Congestive heart failure | 203 (21.5) | 123 (21.4) | 2664 (21.5) |
| Non-ischaemic cardiomyopathy | 150 (15.9) | 86 (15.0) | 1700 (13.8) |
| Functional status: NYHA Class I | 68 (7.2) | 38 (6.6) | 855 (6.9) |
| Stroke | 42 (4.4) | 19 (3.3) | 790 (6.4) |
| AF at most recent 12-lead ECG | 483 (51.2) | 270 (47.0) | 6141 (49.6) |
| Prior MI | 96 (44.2) | 55 (43.0) | 1261 (38.1) |
| Prior CABG | 69 (31.8) | 43 (33.6) | 1107 (33.5) |
| Hypertension | 711 (75.3) | 417 (72.5) | 9886 (79.8) |
| Diabetes | 248 (26.3) | 147 (25.6) | 3237 (26.1) |
| COPD | 112 (11.9) | 67 (11.7) | 1394 (11.3) |
| Hyperlipidaemia | 573 (60.7) | 340 (59.1) | 8007 (64.6) |
| CHA2DS2-VASc score, n (%) | |||
| Low: 0 | 49 (5.2) | 37 (6.4) | 503 (4.1) |
| Medium: 1 | 131 (13.9) | 91 (15.8) | 1327 (10.7) |
| High: 2+ | 764 (80.9) | 447 (77.7) | 10 557 (85.2) |
| ORBIT score, n (%) | |||
| Low: 0–2 | 679 (75.9) | 414 (76.7) | 7967 (69.8) |
| Moderate: 3 | 111 (12.4) | 66 (12.2) | 1685 (14.8) |
| High: 4+ | 105 (11.7) | 60 (11.1) | 1759 (15.4) |
| ATRIA score, n (%) | |||
| =0 | 143 (15.1) | 105 (18.3) | 1515 (12.2) |
| =1 | 365 (38.7) | 221 (38.4) | 4269 (34.5) |
| =2 | 50 (5.3) | 24 (4.2) | 612 (4.9) |
| ≥3 | 386 (40.9) | 225 (39.1) | 5992 (48.4) |
No SATELLITE information provided at both baseline and follow-up periods.
SATELLITE sites were located throughout the USA, with 15.0% of patients enrolled from the West region, 23.9% from the Northeast, 25.1% from Midwest, and 36.0% from the South. The majority of healthcare providers (HCPs) were cardiologists (80.5%) followed by electrophysiologists (14.5%) and internal medicine/primary care practitioners (5.0%).
Shared decision-making
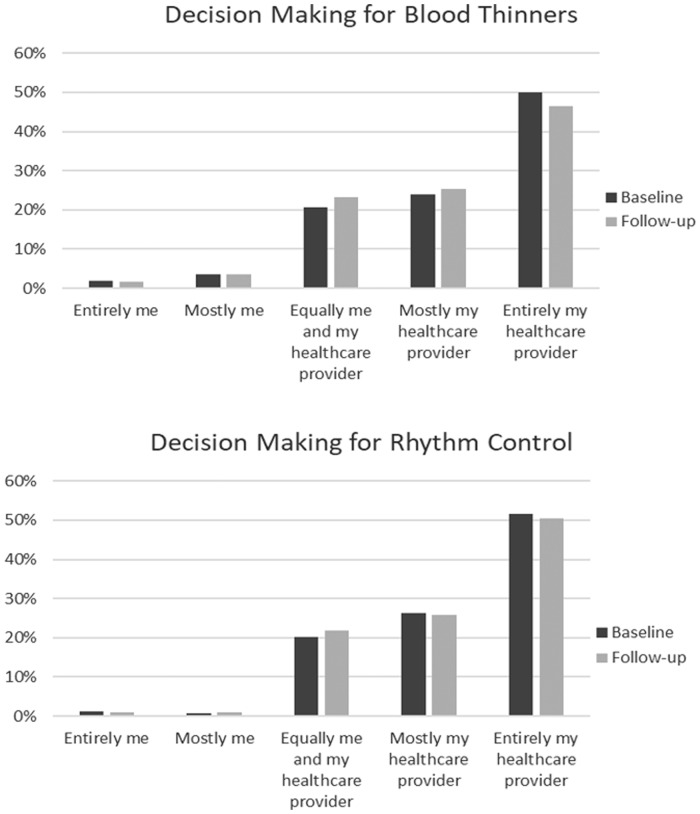
Among the 944 patients on OACs, 246 (26.0%) reported participating in SDM when choosing OAC therapy. In patients managed with rhythm control (n = 575), 127 (22.0%) reported SDM in the selection of their rhythm control strategy. Approximately 50% of patients reported providing no input into their treatment decision, with OAC or rhythm control strategies made entirely by their healthcare provider. At 6-month follow-up, there was little change from baseline in both groups (Figure 1).
Figure 1.
Decision-making for blood thinners (upper panel) and rhythm control (lower panel), at baseline and 6-month follow-up, by degree of self-participation.
Patient-reported understanding of atrial fibrillation and treatment options
Among the 26% of patients participating in SDM for OAC therapy at baseline, 81.8%, 88.1%, and 79.4% reported high understanding of AF, stroke risk, and blood thinner options, respectively. Non-SDM patients had a similar high understanding of AF and stroke risk (82.0%, 87.5%), but less so (61.6%) for blood thinners.
Of the 22% of patients who reported SDM for rhythm control at baseline, 86.2%, 90.2%, and 66.9% reported high understanding of AF, stroke risk and rhythm control options. Non-SDM patients reported a similar high understanding of AF and stroke risk (81.3%, 86.1%), but a lower proportion (47.7%) reported high understanding of rhythm control options (Table 2). At 6-month follow-up, SDM patients continued to have a higher rate of understanding of rhythm control options, compared to non-SDM patients (69.4 vs. 49.0%, P = 0.0004).
Table 2.
Patients with understanding of oral anticoagulant options, stroke risk, and atrial fibrillation by shared decision-making in oral anticoagulant and rhythm control group
| Features | Shared (N = 246) | Not shared (N = 698) |
|---|---|---|
| Blood thinner options in OAC group | ||
| Understand OAC options | 193 (79.42%) | 428 (61.58%) |
| Understand stroke risk | 214 (88.07%) | 600 (87.46%) |
| Understand AF | 198 (81.82%) | 565 (82.00%) |
| Rhythm control options in rhythm control group | ||
| Understand rhythm control options | 83 (66.94%) | 210 (47.73%) |
| Understand stroke risk | 111 (90.24%) | 383 (86.07%) |
| Understand AF | 106 (86.18%) | 360 (81.26%) |
Patient-reported understanding of risk and benefits of atrial fibrillation therapies
The majority of patients who participated in SDM for OAC had a higher level of understanding of risk and benefits of most AF therapies. In contrast, patients who did not participate in SDM had a low to no understanding of the risk and benefits of most AF therapies. Similar trends were reported at 6-month Follow-up although more non-SDM patients stated a high understanding at this later visit than baseline (Supplementary material online, Table S2).
Sources of information for patients
Table 3 displays the source of information about AF therapies categorized by SDM in OAC. At baseline, the most important reported source of information for both SDM and non-SDM patients was the healthcare provider. At follow-up, the healthcare provider remained the most important source of information for patients with non-SDM, while academic journal articles became more important for both groups, compared with baseline, and was the most important source of information in the SDM groups. Similar sources of information were reported for rhythm control (Supplementary material online, Table S3).
Table 3.
Sources of information regarding atrial fibrillation by shared decision-making in oral anticoagulant
| Baseline |
Follow-up |
|||
|---|---|---|---|---|
| Item | Shared | Not shared | Shared | Not shared |
| Family/friends | 3.2 ± 2.8 (236) | 3.0 ± 2.7 (666) | 2.4 ± 2.6 (200) | 2.4 ± 2.6 (497) |
| Television | 3.2 ± 3.1 (236) | 2.9 ± 3.1 (661) | 2.5 ± 3.1 (204) | 2.3 ± 2.9 (498) |
| Radio | 3.1 ± 3.6 (234) | 2.7 ± 3.5 (658) | 1.9 ± 3.2 (199) | 1.7 ± 3.1 (490) |
| Books | 2.8 ± 2.9 (236) | 2.4 ± 2.9 (660) | 2.1 ± 2.9 (203) | 1.8 ± 2.7 (499) |
| Internet | 2.7 ± 2.7 (237) | 2.4 ± 2.5 (668) | 2.0 ± 2.1 (202) | 2.0 ± 2.3 (495) |
| Other people with AF | 2.6 ± 2.6 (231) | 2.7 ± 2.6 (663) | 2.2 ± 2.4 (200) | 2.3 ± 2.5 (493) |
| Academic journal articles | 2.6 ± 2.9 (236) | 2.4 ± 3.0 (660) | 1.7 ± 2.5 (201) | 1.8 ± 2.7 (492) |
| A healthcare provider | 1.9 ± 2.3 (244) | 1.9 ± 2.2 (692) | 1.8 ± 1.9 (210) | 1.6 ± 1.8 (516) |
| Othera | 1.6 ± 3.3 (144) | 1.4 ± 3.0 (396) | 1.7 ± 3.0 (62) | 1.9 ± 3.5 (132) |
The responses are on a 1–9 scale; 1 = most important, 9 = least important.
Unable to report due to uninformative responses.
Factors associated with shared decision-making
In the OAC group, the median age was slightly higher for patients who participated in SDM vs. those who did not (70 vs. 68 years). Patients reporting SDM for OAC were more often female and were more frequently insured by Medicare compared with patients not reporting SDM for OAC. Patients reporting SDM for OAC had higher rates of non-ischaemic cardiomyopathy (18.7% vs. 14.9%), New York Heart Association (NYHA) Class I (9.3% vs. 6.4%), stroke (6.1% vs. 3.9%), and AF on their most recent electrocardiogram (ECG) (57% vs. 48.9%).
In the rhythm control group, the median age was similar between patients who participated in SDM verse those who did not (67.0 years). Patients reporting SDM for rhythm control were more often male and were more frequently insured by Medicare compared with patients not reporting rhythm control SDM. Compared with those not reporting SDM for rhythm control, patients reporting SDM had higher rates of NYHA Class I (7.9% vs. 6.3%), AF at their most recent ECG (54.3% vs. 44.9%), and a prior history of coronary artery bypass grafting (CABG) (48.1% vs. 29.7%) (Table 4).
Table 4.
SATELLITE patient characteristics by shared decision-making at baseline
| OAC |
Rhythm control |
|||
|---|---|---|---|---|
| Characteristics | Shared (N = 246) | Not shared (N = 698) | Shared (N = 127) | Not shared (N = 448) |
| Demographics, n (%) | ||||
| Age (years), median (IQR) | 70.0 (62.0–76.0) | 68.0 (60.0–76.0) | 67.0 (60.0–75.0) | 67.0 (59.0–75.0) |
| Male | 130 (52.8) | 423 (60.6) | 79 (62.2) | 258 (57.6) |
| Race | ||||
| White | 224 (91.1) | 633 (90.7) | 111 (87.4) | 406 (90.6) |
| Black/African American | 12 (4.9) | 23 (3.3) | 8 (6.3) | 13 (2.9) |
| Other | 10 (4.1) | 42 (6.0) | 8 (6.3) | 29 (6.5) |
| Insurance | ||||
| Private | 133 (54.1) | 424 (60.7) | 67 (52.8) | 269 (60.0) |
| Medicare | 99 (40.2) | 234 (33.5) | 53 (41.7) | 154 (34.4) |
| Other | 14 (5.7) | 40 (5.7) | 7 (5.5) | 25 (5.6) |
| Risk factors, n (%) | ||||
| Congestive heart failure | 55 (22.4) | 148 (21.2) | 26 (20.5) | 97 (21.7) |
| Non-ischaemic cardiomyopathy | 46 (18.7) | 104 (14.9) | 18 (14.2) | 68 (15.2) |
| Functional status: NYHA Class I | 23 (9.3) | 45 (6.4) | 10 (7.9) | 28 (6.3) |
| Stroke | 15 (6.1) | 27 (3.9) | 5 (3.9) | 14 (3.1) |
| AF at most recent 12-lead ECG | 142 (57.7) | 341 (48.9) | 69 (54.3) | 201 (44.9) |
| Prior MI | 20 (37.0) | 76 (46.6) | 12 (44.4) | 43 (42.6) |
| Prior CABG | 17 (31.5) | 52 (31.9) | 13 (48.1) | 30 (29.7) |
| Hypertension | 189 (76.8) | 522 (74.8) | 96 (75.6) | 321 (71.7) |
| Diabetes | 66 (26.8) | 182 (26.1) | 37 (29.1) | 110 (24.6) |
| COPD | 24 (9.8) | 88 (12.6) | 18 (14.2) | 49 (10.9) |
| Hyperlipidaemia | 158 (64.2) | 415 (59.5) | 88 (69.3) | 252 (56.3) |
| CHA2DS2-VASc score, n (%) | ||||
| Low: 0 | 12 (4.9) | 37 (5.3) | 10 (7.9) | 27 (6.0) |
| Medium: 1 | 28 (11.4) | 103 (14.8) | 17 (13.4) | 74 (16.5) |
| High: 2+ | 206 (83.7) | 558 (79.9) | 100 (78.7) | 347 (77.5) |
| ORBIT score, n (%) | ||||
| Low: 0–2 | 181 (77.7) | 498 (75.2) | 90 (75.6) | 324 (77.0) |
| Moderate: 3 | 24 (10.3) | 87 (13.1) | 16 (13.4) | 50 (11.9) |
| High: 4+ | 28 (12.0) | 77 (11.6) | 13 (10.9) | 47 (11.2) |
| ATRIA score, n (%) | ||||
| =0 | 30 (12.2) | 113 (16.2) | 19 (15.0) | 86 (19.2) |
| =1 | 102 (41.5) | 263 (37.7) | 54 (42.5) | 167 (37.3) |
| =2 | 14 (5.7) | 36 (5.2) | 5 (3.9) | 19 (4.2) |
| ≥3 | 100 (40.7) | 286 (41.0) | 49 (38.6) | 176 (39.3) |
Continuous variables are presented as median (IQR). Categorical variables are presented as frequencies (percentages).
In a univariate analysis, patients with a self-reported understanding of blood thinner options and private insurance were at higher odds of participating in SDM for OAC (OR 2.51, CI: 1.76–3.58, P < 0.001; OR 0.73, CI: 0.54–0.99, P < 0.04). However, in a multivariable analysis, only self-reported understanding of blood thinner options was associated with higher odds of participating in SDM for OAC (OR 2.54, CI: 1.75–3.68, P < 0.001). There was no association between SDM and insurance type, stroke risk understanding, or level of education (Table 5).
Table 5.
Factors associated with shared decision-making for oral anticoagulant (N = 888)
| Multivariable |
Univariate |
||||
|---|---|---|---|---|---|
| Effect | df | P-value | OR (95% CI) | P-value | OR (95% CI) |
| Age (10 years) | 1 | 0.673 | 0.96 (0.79–1.17) | 0.377 | 1.06 (0.93–1.22) |
| Race: White | 1 | 0.241 | 0.69 (0.38–1.28) | 0.261 | 0.72 (0.40–1.28) |
| Sex | 1 | 0.365 | 1.17 (0.84–1.63) | 0.054 | 1.34 (0.99–1.82) |
| CHADSVASc score | 2 | 0.303 | . | 0.087 | . |
| CHADSVASc (0,1) vs. CHADSVASc >4 | 0.55 (0.23–1.32) | 0.59 (0.37–0.96) | |||
| CHADSVASc (2,3,4) vs. CHADSVASc >4 | 0.64 (0.36–1.13) | 0.72 (0.50–1.05) | |||
| Diabetes | 1 | 0.443 | 0.85 (0.56–1.28) | 0.924 | 1.02 (0.73–1.42) |
| Cancer | 1 | 0.948 | 0.99 (0.64–1.51) | 0.769 | 1.06 (0.71–1.58) |
| Congestive heart failure | 1 | 0.912 | 1.02 (0.67–1.57) | 0.559 | 1.11 (0.78–1.60) |
| Prior MI | 1 | 0.101 | 0.62 (0.35–1.10) | 0.181 | 0.70 (0.41–1.18) |
| COPD | 1 | 0.096 | 0.64 (0.38–1.08) | 0.141 | 0.69 (0.42–1.13) |
| Peripheral vascular disease | 1 | 0.766 | 0.91 (0.48–1.72) | 0.946 | 0.98 (0.53–1.80) |
| Prior cerebrovascular events | 1 | 0.880 | 1.05 (0.57–1.91) | 0.169 | 1.42 (0.86–2.36) |
| Insurance (private vs. not private) | 1 | 0.078 | 0.74 (0.53–1.03) | 0.041 | 0.73 (0.54–0.99) |
| Level of education | 3 | 0.973 | . | 0.898 | . |
| College graduate vs. some school | 0.89 (0.46–1.71) | 0.86 (0.47–1.60) | |||
| High school graduate vs. some school | 0.87 (0.46–1.63) | 0.90 (0.49–1.64) | |||
| Post graduate vs. some school | 0.84 (0.38–1.88) | 0.76 (0.35–1.62) | |||
| Understand blood thinner options | 1 | 0.000 | 2.54 (1.75–3.68) | 0.000 | 2.51 (1.76–3.58) |
| Agree stroke is risk of AF | 1 | 0.500 | 0.84 (0.51–1.39) | 0.710 | 1.09 (0.69–1.73) |
Similarly, only self-reported understanding of rhythm control options was associated with higher odds of SDM for antiarrhythmic therapy (OR 2.36, CI: 1.50–3.71, P ≤ 0.001) SDM was not associated with insurance type, stroke risk understanding, or level of education (Supplementary material online, Table S4).
Patient-reported factors associated with treatment selection
Among the 944 patients on OACs, patients participating in SDM were more likely to be taking aspirin (35.4% vs. 29.5%) or warfarin (15.9% vs. 12.2%) compared with those not participating in SDM. Conversely, DOAC treatment rates were higher in the non-SDM group (76.4%), compared to the SDM group (67.5%). Similar patterns were observed at the 6-month follow-up visit (Supplementary material online, Table S5).
Patients using warfarin reported that the most important factors were a reduction in stroke risk, bleeding risk, provider recommendation, and the availability of antidote reversal agents. Patients using DOACs or antiplatelets reported that the most important factors included reduction in stroke risk or bleeding and provider recommendation. In parallel, cost and insurance coverage were considered moderately important for all three therapeutic classes, while convenience or fewer visits was the least important factor for patients taking warfarin (Supplementary material online, Table S6).
For patients participating in SDM for rhythm control selection, there were no major difference in rhythm control at baseline: SDM (55.9%) vs. non-SDM groups (53.8%); however, at 6-month follow-up, rhythm control rates were marginally higher among non-SDM patients (59.4%) than in SDM (54.1%) (Supplementary material online, Table S7).
Data availability
The data underlying this article are available in the article and in its online supplementary material.
Discussion
Among patients with new-onset AF in a national registry, only one in four reported that their current stroke prevention treatment strategy was the result of a shared decision with their HCP. Similarly, one in five AF patients reported that they participated in SDM when choosing their current rhythm control strategy. Perhaps even more striking, approximately half of patients said decisions about OAC and rhythm control were made entirely by their HCPs, with no input from the patient. Patients with self-reported understanding of OAC and rhythm control options had substantially higher odds of participating in SDM (OR 2.54 and OR 2.36, both P ≤ 0.001). There was no association between SDM and the level of education, understanding stroke as a risk of AF, or insurance type, for either OAC or rhythm control therapy. Patient-reported levels of involvement in treatment decisions were not associated with major differences in treatment.
Shared decision-making can be generally defined as an open exchange of clinical knowledge wherein the practitioner shares the risks and benefits of available therapies, and patients respond regarding their values and preferences concerning the presented options. Together, via cycles of ‘reflection and discussion,’ clinicians and patients come to an agreement.14 However, while SDM has been associated with reduced costs and improved outcomes, and despite its being a major provision of the US Affordable Care Act, its implementation has been sluggish.15 Specifically, one systematic review found three major SDM barriers reported by HCPs: time restrictions, lack of pertinence based on patients’ attributes, and the clinical situation.16 However, while these (and several other) perceived barriers have now been dispelled,17 uptake remains slow, perhaps for other (unresolved) reasons.
Since the treatment options for AF have greatly increased in recent years, this has augmented opportunities for SDM. Patients’ understanding of diseases and therapies is a cornerstone for engaging in SDM. In this regard, we found approximately half of patients said decisions about OAC and rhythm control were made entirely by their HCPs with no input from the patient. Such a finding may be due to the process by which HCPs and patients engage in clinical decision-making. There are HCPs who routinely take the time to have fact-based discussion with their patients, while others may simply give the diagnosis, treatment choices and recommendations with minimal discussion. Similarly, there are patients who prefer an active role (autonomous), shared role (agreement with HCP), or a passive role (leaving the decision to the HCP). However, even in patients who prefer a passive role, they should still be involved in the SDM process, eliciting their concerns and views.17
Prior work suggests that patient engagement (‘patient activation’, e.g. lifestyle changes, prevention measures, etc.) is a strong determinant of positive clinical outcomes, in addition to lower costs (fewer hospitalizations, emergency room visits, etc.).12,18 However, evidence from clinical practice reveals persistent deficiencies in AF understanding, and engagement, among affected patients. In a survey of 180 patients with AF who attended three anticoagulation clinics in the UK, only 54% of patients understood why they were taking warfarin, or their warfarin dose.19 Similar rates of suboptimal disease knowledge were reported in a population of 119 patients with chronic AF, with only 63% of patients reporting that they were aware of their cardiac condition and 61% reporting that AF was ‘not serious’.20 In a larger study of 12 517 AF patients throughout the state of California, 14.5% were unaware of their AF diagnosis, and 20.4% had inadequate health literacy (as measured by a validated three-item instrument).21 It is possible that decision aids and patient educational resources could be utilized to address many of these deficiencies, thus increasing the efficacy of SDM.
Studies have demonstrated that the SDM process confers higher patient knowledge level of disease understanding, increasing patients’ willingness to be involved in SDM,6,22 while one meta-analysis concluded that patients’ knowledge alone was insufficient for their participation in SDM, and that they must specifically be provided the power to do so.22 Indeed, there remains significant debate regarding this topic.23 In our study, while self-reported understanding of OAC options and rhythm control options correlated with higher odds of participating in SDM, there was no association between SDM and higher understanding of stroke risk. However, this may be a limitation of the study, in which patients were asked questions well after the decision, and could develop a better stroke risk understanding as a direct result of the SDM process itself. This phenomenon has been hypothesized to increase compliance of patients with chronic obstructive pulmonary disease (COPD),24 and enhance diabetes management via telemedicine (mobile phone technologies, etc.).25
European Heart Rhythm Association (EHRA) guidelines advocate for AF-related education performed by the HCPs.26 Novel methods, including specialized, nurse-led AF outpatient clinics27 hold promise for promoting AF-related education that could support SDM.
Likewise, emerging evidence suggests that innovative technologies, including mobile telephone applications (mAFA II trial),28 photoplethysmography detection of blood volume changes (Huawei Heart Study),29 and ‘smart watch’ deep neural network analysis30 or may support AF31 detection and self-management. Future work should explore how use of these technologies may support SDM through enhanced self-efficacy and patient engagement.
In addition to adequately understanding the need for treatment and the risk/benefit profiles of available AF therapies, optimal disease management relies upon open communication and accord between clinicians’ medical expertise and patients’ values, goals, and preferences; indeed, we contend that such accord (values/choice concordance) is the actual essence of high quality medical decisions. However, results from the Awareness and Risk Education (AWARE) AF study demonstrated that nearly one-quarter of AF patients rated their own ability to explain AF as poor, and 23% reported that they did not know where to look for more information on their disorder.32 Thus, decision aids provide evidence-based information about the risks, benefits, and treatment options, as part of the SDM process. To that end, one systematic review, including more than 30 000 patients, showed that decision aids (compared to traditional care) improved patient knowledge about their options, while also reducing decisional conflict, making them more likely to make choices consistent with their preferences, values, and goals.33
We observed that AF patients, at 6-month follow-up, reported journal articles as a significant source of information. While poor-quality health information on the internet has been widely portrayed as harmful, this view has been largely dispelled,33 and access to primary medical literature [e.g. PubMed, Public Library of Science (PLOS)] could be quite valuable to SDM.34 Unfortunately, excessive subscription or individual purchase fees considerably limit this avenue of patient gain of knowledge.
Our findings have significant implications for the practice of AF management. The overall finding of over one-half of patients reporting no SDM at all underscores the current lack of patient involvement in treatment decisions, even in a well-treated population of patients. While the evidence base for specific SDM strategies is evolving, past work suggests that SDM may be most successfully implemented using a tripartite framework comprising: (i) introducing choices; (ii) describing options; and (iii) assisting patients in making decisions, based on preferences.35 Adaptation of this simple model to specific clinical populations may enhance the likelihood of meaningful patient engagement in treatment decisions. However, another study showed that, while only 40% of patients desired involvement in decisions, patient confidence increased when they perceived a significant role in decision-making.36 Therefore, interventions targeting enhanced involvement of patients in decision-making hold promise even in scenarios where patients may be initially reluctant to assume an active role in treatment choice.37
Limitations
Several limitations exist in our study. First, this a descriptive analysis; therefore, the indicated associations between patient understanding and treatment decisions may be confounded by other factors, including HCP practices, clinical presentation, and patient socioeconomic factors, determinants not captured by SATELLITE assessments. Also, the possibility of selection bias must be taken into account, since this was a selected population within ORBIT-AF II. Second, while the survey was developed by a multidisciplinary group of investigators and vetted by non-clinicians, the final content may not represent the perspectives of every clinical specialty or AF subpopulation. Further, eventhough the SATELLITE survey was pre-tested for readability and clarity, it was not previously validated. Moreover, as these survey results were self-reports of patient understanding, they should be interpreted with more caution than previously validated measures of disease knowledge (i.e. ‘health literacy’).21 Third, we were unable to measure AF patient adherence to their shared decisions, although such therapeutic behavioural responses have been previously demonstrated in SDM for asthma38 and mental illness.39 Nonetheless, future work should examine whether AF SDM participation influences patient compliance, and improved outcomes. Fourth, the relationship between SDM and treatment satisfaction was not captured by our SATELLITE survey. Future work examining the impact of SDM on patient treatment satisfaction and associations with long-term adherence are needed.
Another limitation includes the US-based nature of the registry, which may limit generalizability of findings to other countries or cultural contexts where SDM processes differ.
Moreover, our SDM survey did not capture all the granularity of the HCP-patient interaction, including the HCP’s and patient’s inherent nature and SDM process per se, to fully understand the value of or limitations of the process. However, we did extensively query patient perception of their engagement in the decision-making process, as AHA and ESC guidelines advocate universal implementation of SDM for AF treatment.8,9 While these guidelines provide lofty goals for structuring SDM approaches, we recognize the difficulties and barriers in implementing these core concepts. Notably, we did find that both SDM and non-SDM patients reported their most important source of information to be their healthcare provider.
Conclusion
Despite the potential benefits, and recommendations by numerous medical communities, SDM is not widely implemented in AF clinical practice, with fewer than one in four patients reporting having participated in SDM for OAC and rhythm control treatments, and approximately half of patients reporting no input, whatsoever, in these treatment decisions. Patient understanding about available therapeutic options was associated with a more than a two-fold higher likelihood of SDM, thus representing a feasible and potential target for increasing rates of SDM in patients with AF.
Supplementary material
Supplementary material is available at European Heart Journal – Quality of Care and Clinical Outcomes online.
Funding
The Outcomes Registry for Better Informed Treatment of Atrial Fibrillation is sponsored by Janssen Scientific Affairs LLC.
Conflict of interest: F.A.A., K.P., A.S.G. have no financial disclosures. R.N.: Funded by the T32 NIH Grant HL079896. L.A.A.: Consultant for Janssen and Novartis. P.S.C.: Consultant for Optum Rx. M.D.E.: Consultant/Advisory Board for Boehringer Ingelheim, Diachi Sanko, Pfizer, Bristol Myers Squibb, and Janssen Scientific Affairs. G.C.F.: Consultant/Advisory Board support from Janssen Pharmaceuticals. J.V.F.: Consultant/Advisory Board for Janssen Scientific, Medtronic, Biosense Webster, Boston Scientific. Salary support from the American College of Cardiology. B.J.G.: Member of a Data Safety Monitoring Board for Mount Sinai St. Lukes, Boston Scientific Corporation, Teva Pharmaceutical Industries, St. Jude Medical, Janssen Research & Development, Baxter Healthcare Corporation, and Cardiovascular Research Foundation. Consultant/Advisory Board for Janssen Scientific Affairs, Cipla Limited, Armetheon Inc., and Medtronic. P.R.K.: Consultant for Johnson and Johnson. K.W.M.: Financial disclosures can be viewed at http://med.stanford.edu/profiles/kenneth-mahaffey (accessed 20 May 2020). G.V.N.: Research grant from Janssen. Consultant/Advisory Board for Janssen and Daiichi Sankyo. S.D.P.: Research grants from the Food and Drug Administration, Bristol-Myers Squibb, Janssen Pharmaceuticals, Gilead, and Boston Scientific and consulting for Boston Scientific, Medtronic, and Bristol-Myers Squibb. J.A.R.: Research grant from Janssen Pharmaceuticals. Research support from Boehringer Ingelheim Pharmaceuticals Inc. and GlaxoSmithKline. Consultancies with Sanofi, Gilead Sciences Inc., CV Therapeutics, GlaxoSmithKline, Merck & Co Inc., Cardiome Pharma Corp, Boehringer Ingelheim Pharmaceuticals Inc., and Medtronic Inc. Speakers’ bureau income from Sanofi and Boehringer Ingelheim Pharmaceuticals Inc. D.E.S.: Consultant/Advisory Board for Boehringer Ingelheim, Bristol-Myers Squibb, Merck, Johnson and Johnson, Pfizer, and Medtronic. Research Grants from Boehringer Ingelheim and Bristol-Myers Squibb. B.A.S.: Research support from Boston Scientific and Janssen. Consult for Janssen. Speakers’ bureau income Biosense Webster. E.D.P.: Research grant from Janssen Pharmaceuticals and Eli Lilly. Consultant for Janssen Pharmaceuticals and Boehringer Ingelheim. J.P.P.: Research grant from Agency for Healthcare Research and Quality, ARCA biopharma, Boston Scientific, Gilead Sciences, Janssen Pharmaceuticals, Johnson &Johnson, ResMed, Spectranetics, and St Jude Medical. Consultant/Advisory Board for BMS/Pfizer, GlaxoSmithK-line, Janssen Pharmaceuticals, Johnson & Johnson, Medtronic, and Spectranetics. E.C.O.B.: Research grants from Janssen Scientific Affairs, BMS, Novartis, and Sanofi.
Supplementary Material
References
- 1. Chamberlain AM, Gersh BJ, Alonso A, Chen LY, Berardi C, Manemann SM et al. Decade-long trends in atrial fibrillation incidence and survival: a community study. Am J Med 2015;128:260–267.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Schnabel RB, Yin X, Gona P, Larson MG, Beiser AS, McManus DD et al. 50 year trends in atrial fibrillation prevalence, incidence, risk factors, and mortality in the Framingham Heart Study: a cohort study. Lancet 2015;386:154–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Go AS, Hylek EM, Phillips KA, Chang Y, Henault LE, Selby JV et al. Prevalence of diagnosed atrial fibrillation in adults. JAMA 2001;285:2370. [DOI] [PubMed] [Google Scholar]
- 4. Plitt A, McGuire DK, Giugliano RP. Atrial fibrillation, type 2 diabetes, and non–vitamin K antagonist oral anticoagulants. JAMA Cardiol 2017;2:442. [DOI] [PubMed] [Google Scholar]
- 5. Ogawa S, Yamashita T, Yamazaki T, Aizawa Y, Atarashi H, Inoue H et al. Optimal treatment strategy for patients with paroxysmal atrial fibrillation. Circ J 2009;73:242–248. [DOI] [PubMed] [Google Scholar]
- 6. Aronis KN, Edgar B, Lin W, Martins MAP, Paasche-Orlow MK, Magnani JW. Health literacy and atrial fibrillation: relevance and future directions for patient-centred care. Eur Cardiol 2017;12:52–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Quill TE, Brody H. Physician recommendations and patient autonomy: finding a balance between physician power and patient choice. Ann Intern Med 1996;125:763–769. [DOI] [PubMed] [Google Scholar]
- 8. January CT, Wann LS, Calkins H, Chen LY, Cigarroa JE, Cleveland JC et al. ACC/HRS Guideline for the management of patients with atrial fibrillation circulation. Circulation 2019;140:125–151. [DOI] [PubMed] [Google Scholar]
- 9. Kirchhof P, Benussi S, Kotecha D, Ahlsson A, Atar D, Casadei B et al. 2016 ESC Guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Rev Esp Cardiol (Engl Ed ) 2017;70:50. [DOI] [PubMed] [Google Scholar]
- 10. Murray E, Pollack L, White M, Lo B. Clinical decision-making: patients’ preferences and experiences. Patient Educ Couns 2007;65:189–196. [DOI] [PubMed] [Google Scholar]
- 11. Légaré F, Witteman HO. Shared decision making: examining key elements and barriers to adoption into routine clinical practice. Health Aff 2013;32:276–284. [DOI] [PubMed] [Google Scholar]
- 12. Steinberg BA, Blanco RG, Ollis D, Kim S, Holmes DN, Kowey PR et al. Outcomes registry for better informed treatment of atrial fibrillation II: rationale and design of the ORBIT-AF II registry. Am Heart J 2014;168:160–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Piccini JP, Fraulo ES, Ansell JE, Fonarow GC, Gersh BJ, Go AS et al. Outcomes registry for better informed treatment of atrial fibrillation: rationale and design of ORBIT-AF. Am Heart J 2011;162:606–612.e1. [DOI] [PubMed] [Google Scholar]
- 14. O'Neill ES, Grande SW, Sherman A, Elwyn G, Coylewright M. Availability of patient decision aids for stroke prevention in atrial fibrillation: a systematic review. Am Heart J 2017;191:1–11. [DOI] [PubMed] [Google Scholar]
- 15. Oshima Lee E, Emanuel EJ. Shared decision making to improve care and reduce costs. N Engl J Med 2013;368:6–8. [DOI] [PubMed] [Google Scholar]
- 16. Légaré F, Ratté S, Gravel K, Graham ID. Barriers and facilitators to implementing shared decision-making in clinical practice: update of a systematic review of health professionals’ perceptions. Patient Educ Couns 2008;73:526–535. [DOI] [PubMed] [Google Scholar]
- 17. Légaré F, Thompson-Leduc P. Twelve myths about shared decision making. Patient Educ Couns 2014;96:281–286. [DOI] [PubMed] [Google Scholar]
- 18. Hibbard JH, Greene J. What the evidence shows about patient activation: better health outcomes and care experiences; fewer data on costs. Health Aff (Millwood) 2013;32:207–214. [DOI] [PubMed] [Google Scholar]
- 19. Nadar S, Begum N, Kaur B, Sandhu S, Lip G. Patients’ understanding of anticoagulant therapy in a multiethnic population. J R Soc Med 2003;96:175–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lip GY, Kamath S, Jafri M, Mohammed A, Bareford D. Ethnic differences in patient perceptions of atrial fibrillation and anticoagulation therapy: the west birmingham atrial fibrillation project. Stroke 2002;33:238–242. [DOI] [PubMed]
- 21. Reading SR, Go AS, Fang MC, Singer DE, Liu IA, Black MH. Health literacy and awareness of atrial fibrillation. J Am Heart Assoc 2017;6:e005128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Joseph-Williams N, Elwyn G, Edwards A. Knowledge is not power for patients: a systematic review and thematic synthesis of patient-reported barriers and facilitators to shared decision making. Patient Educ Couns 2014;94:291–309. [DOI] [PubMed] [Google Scholar]
- 23. Montori VM, Kunneman M, Brito JP. Shared decision making and improving health care: the answer is not in. JAMA 2017;318:617–618. [DOI] [PubMed] [Google Scholar]
- 24. Sciurba F, Rennard SI. Rationale for a redundant formulary. The Hawthorne effect and the art of medicine. Am J Respir Crit Care Med 2015;191:1224–1225. [DOI] [PubMed] [Google Scholar]
- 25. Zhai Y, Zhu W, Cai Y, Sun D, Zhao J. Clinical- and cost-effectiveness of telemedicine in type 2 diabetes mellitus: a systematic review and meta-analysis. Medicine (Baltimore) 2014;93:e312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Mairesse GH, Moran P, Van Gelder IC, Elsner C, Rosenqvist M, Mant J et al. ; ESC Scientific Document Group. Screening for atrial fibrillation: a European Heart Rhythm Association (EHRA) consensus document endorsed by the Heart Rhythm Society (HRS), Asia Pacific Heart Rhythm Society (APHRS), and Sociedad Latinoamericana de Estimulación Cardíaca y Electrofisiología (SOLAECE). Europace 2017;19:1589–1623. [DOI] [PubMed] [Google Scholar]
- 27. Hendriks JML, Crijns H, Vrijhoef H. Integrated chronic care management for patients with atrial fibrillation: a rationale for redesigning atrial fibrillation care. J Atr Fibrillation 2015;7:45–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Guo Y, Lane DA, Wang L, Chen Y, Lip GYH, Eckstein J et al. ; mAF‐App II Trial Investigators. Mobile Health (mHealth) technology for improved screening, patient involvement and optimising integrated care in atrial fibrillation: the mAFA (mAF-App) II randomised trial. Int J Clin Pract 2019;73. [DOI] [PubMed] [Google Scholar]
- 29. Guo Y, Wang H, Zhang H, Liu T, Liang Z, Xia Y et al. Mobile photoplethysmographic technology to detect atrial fibrillation. J Am Coll Cardiol 2019;74:2365–2375. [DOI] [PubMed] [Google Scholar]
- 30. Tison GH, Sanchez JM, Ballinger B, Singh A, Olgin JE, Pletcher MJ et al. Passive detection of atrial fibrillation using a commercially available smartwatch. JAMA Cardiol 2018;3:409–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Perez MV, Mahaffey KW, Hedlin H, Rumsfeld JS, Garcia A, Ferris T et al. Large-scale assessment of a smartwatch to identify atrial fibrillation. N Engl J Med 2019;381:1909–1917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Aliot E, Breithardt G, Brugada J, Camm J, Lip GYH, Vardas PE et al. ; for the Atrial Fibrillation AWareness And Risk Education (AF AWARE) group [comprising the Atrial Fibrillation Association (AFA), the European Heart Rhythm Association (EHRA), Stroke Alliance for Europe (SAFE), and the World Heart Federation (WHF)]. An international survey of physician and patient understanding, perception, and attitudes to atrial fibrillation and its contribution to cardiovascular disease morbidity and mortality. Europace 2010;12:626–633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Stacey D, Légaré F, Col NF, Bennett CL, Barry MJ, Eden KB et al. Decision aids for people facing health treatment or screening decisions In: Stacey D, ed. Cochrane Database of Systematic Reviews 2014;1:CD001431. [DOI] [PubMed] [Google Scholar]
- 34. Elwyn G, Frosch DL, Kobrin S. Implementing shared decision-making: consider all the consequences. Implement Sci 2015;11:114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Elwyn G, Frosch D, Thomson R, Joseph-Williams N, Lloyd A, Kinnersley P et al. Shared decision making: a model for clinical practice. J Gen Intern Med 2012;27:1361–1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Burton D, Blundell N, Jones M, Fraser A, Elwyn G. Shared decision-making in cardiology: do patients want it and do doctors provide it? Patient Educ Couns 2010;80:173–179. [DOI] [PubMed] [Google Scholar]
- 37. Pritchett RV, Bem D, Turner GM, Thomas GN, Clarke JL, Fellows R et al. Improving the prescription of oral anticoagulants in atrial fibrillation: a systematic review. Thromb Haemost 2019;119:294–307. [DOI] [PubMed] [Google Scholar]
- 38. Wilson SR, Strub P, Buist AS, Knowles SB, Lavori PW, Lapidus J et al. Shared treatment decision making improves adherence and outcomes in poorly controlled asthma. Am J Respir Crit Care Med 2010;181:566–577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Dixon LB, Holoshitz Y, Nossel I. Treatment engagement of individuals experiencing mental illness: review and update. World Psychiatry 2016;15:13–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article are available in the article and in its online supplementary material.