Abstract
Objectives:
To estimate the impact of goal-directed therapy on outcome after traumatic brain injury, our team applied goal-directed therapy to standardize care in patients with moderate to severe traumatic brain injury, who were enrolled in a large multicenter clinical trial.
Design:
Planned secondary analysis of data from Progesterone for the Treatment of Traumatic Brain Injury III, a large, prospective, multicenter clinical trial.
Setting:
Forty-two trauma centers within the Neurologic Emergencies Treatment Trials network.
Patients:
Eight-hundred eighty-two patients were enrolled within 4 hours of injury after nonpenetrating traumatic brain injury characterized by Glasgow Coma Scale score of 4–12.
Measurements and Main Results:
Physiologic goals were defined a priori in order to standardize care across 42 sites participating in Progesterone for the Treatment of Traumatic Brain Injury III. Physiologic data collection occurred hourly; laboratory data were collected according to local ICU protocols and at a minimum of once per day. Physiologic transgressions were predefined as substantial deviations from the normal range of goal-directed therapy. Each hour where goal-directed therapy was not achieved was classified as a “transgression.” Data were adjudicated electronically and via expert review. Six-month outcomes included mortality and the stratified dichotomy of the Glasgow Outcome Scale-Extended. For each variable, the association between outcome and either: 1) the occurrence of a transgression or 2) the proportion of time spent in transgression was estimated via logistic regression model.
Results:
For the 882 patients enrolled in Progesterone for the Treatment of Traumatic Brain Injury III, mortality was 12.5%. Prolonged time spent in transgression was associated with increased mortality in the full cohort for hemoglobin less than 8 gm/dL (p = 0.0006), international normalized ratio greater than 1.4 (p < 0.0001), glucose greater than 180 mg/dL (p = 0.0003), and systolic blood pressure less than 90 mm Hg (p < 0.0001). In the patient subgroup with intracranial pressure monitoring, prolonged time spent in transgression was associated with increased mortality for intracranial pressure greater than or equal to 20 mm Hg (p < 0.0001), glucose greater than 180 mg/dL (p = 0.0293), hemoglobin less than 8 gm/dL (p = 0.0220), or systolic blood pressure less than 90 mm Hg (p = 0.0114). Covariates inversely related to mortality included: a single occurrence of mean arterial pressure less than 65 mm Hg (p = 0.0051) or systolic blood pressure greater than 180 mm Hg (p = 0.0002).
Conclusions:
The Progesterone for the Treatment of Traumatic Brain Injury III clinical trial rigorously monitored compliance with goal-directed therapy after traumatic brain injury. Multiple significant associations between physiologic transgressions, morbidity, and mortality were observed. These data suggest that effective goal-directed therapy in traumatic brain injury may provide an opportunity to improve patient outcomes.
Keywords: anemia, hypotension, hypoxia, increased intracranial pressure, progesterone, traumatic brain injury
Traumatic brain injury (TBI) is a devastating public health problem, resulting in 56,000 patient deaths and 282,000 hospitalizations in the U.S. annually (1–4). Difficulty in improving care through clinical trials is compounded by the complexity of optimizing physiologic management after brain injury. Despite the staggering impact of TBI on society, little progress has been made to identify successful clinical interventions (4–8). Prior work has documented the effect of physiologic variability on outcome after TBI. Data include studies on optimal values for glycemic control, hemoglobin concentration, temperature, systolic blood pressure (SBP), mean arterial pressure (MAP), oxygenation, brain tissue oxygen tension (Pbto2), coagulation status, intracranial pressure (ICP), and cerebral perfusion pressure (CPP) (6, 9–18). Goal-directory therapy (GDT) attempts to target each of these variables and improve outcomes by mitigating the secondary injury cascade (17, 19). Targeting goal-directed values, such as threshold for hemoglobin or CPP, enhances standardization of care across the study population while highlighting opportunities for patient-specific treatment of underlying pathophysiology (4, 18, 20).
For the Progesterone for the Treatment of Traumatic Brain Injury (ProTECT) III trial, our research team designed an approach to apply GDT across institutions. A clinical standardization team (CST) defined guidelines for physiologic goals of therapy, a priori, to standardize care (Appendix I, Supplemental Digital Content 1, http://links.lww.com/CCM/E404). Failure to meet target variables for hemoglobin, platelet count, international normalized ratio (INR), glucose, temperature, Pao2, oxygen saturation (Sao2), MAP, SBP, ICP, Pbto2, or CPP was defined as a physiologic “transgression.”
Transgression data were compiled hourly from patient enrollment through death, withdrawal of care, or hospital discharge. The database contains prospectively derived data on the occurrence and time spent in physiologic transgressions after TBI. The objective of this analysis is to evaluate the association between physiologic variability and outcome after TBI.
MATERIALS AND METHODS
Study Design and Subject Population
The ProTECT III trial was a National Institutes of Health/National Institute on Neurological Disorders and Stroke (NINDS)-funded, randomized, double-blind, multicenter, phase 3 clinical trial designed to test the neuroprotective effect of IV progesterone versus placebo in patients with moderate to severe acute TBI. Patients with nonpenetrating TBI and Glasgow Coma Scale (GCS) 4–12 were enrolled if treatment could be initiated within 4 hours of injury. Each site’s institutional review board reviewed and approved the protocol, including procedures related to 21CFR50.24 regulations on Exception from Informed Consent for emergency research. In 2013, after 882 of the planned 1,140 subjects had been randomized, the NINDS-appointed Data and Safety Monitoring Board advised ending the trial due to futility. Details of the study protocol and results have been previously published (5).
To standardize and monitor the management of subjects, a multidisciplinary team of experts in TBI management created the ProTECT III Guidelines for the Management of TBI (CST Guidelines, Appendix I, Supplemental Digital Content 1, http://links.lww.com/CCM/E404). These guidelines were designed to augment those of the Brain Trauma Foundation by providing a template to standardize care across institutions (19). Failure to meet a physiologic goal was considered a transgression, independent of whether this inability was associated with a modifiable factor. Table 1 lists physiologic goals and transgression definitions. The CST guidelines also describe expected interventions in response to transgressions, for example, transfusion for anemia.
TABLE 1.
Initial Definitions of Transgressions in Physiologic Goals of Therapy
| Transgression Variable | Definition |
|---|---|
| Mean arterial pressure | < 80 mm Hg |
| Systolic blood pressure | |
| Low | < 100 mm Hg |
| High | > 180 mm Hg |
| Intracranial pressure | ≥ 20 mm Hg |
| Hemoglobin | < 8 gm/dL |
| International normalized ratio | > 1.4 |
| Platelets | <75 × 103/mm3 |
| Oxygen saturation | < 90% |
| Temperature | |
| Low | < 36.0°C (96.8°F) |
| High | > 38.3°C (101°F) |
| Brain tissue oxygen tension | < 15 mm Hg |
| Cerebral perfusion pressure | < 60 mm Hg |
| Pao2 | < 100 mm Hg |
| Glucose | |
| Low | < 80 mg/dL |
| High | >180 mg/dL |
In-person training on the CST guidelines was completed at each site prior to study initiation. Transgression data were reported daily via case report form (CRF) and reviewed by a central monitoring team. Feedback was provided regularly in order to facilitate standardization of medical management. On-call response to questions about patient management was available via a 24/7 telephone hotline. Physiologic data were collected on electronic CRFs and included but were not limited to hourly annotations. Data were collected throughout each patient’s initial hospitalization. Continuous physiologic variables, such as CPP, SBP, Pao2, ICP, MAP, Sao2, and Pbto2 were monitored according to the local ICU protocol at each site. If the recorded physiologic variable did not meet its specified goal during a particular hour, the study team initiated treatment and completed a transgression specific CRF. Each CRF noted the total duration of the transgression, as well as all interventions applied to correct the transgression. Laboratory values, such as INR and hemoglobin were collected according to each ICU protocol (at a minimum of daily intervals). A transgression of hemoglobin, for example, resulted in an action to transfuse to hemoglobin greater than 8 gm/dL, as well as documentation of resolution of the transgression. The total number of hours spent in transgression were calculated. Data were derived according to local ICU protocols using both laboratory draws and i-STAT (Abbott, Chicago, IL) values according to local protocols. All data underwent manual expert review and annotation, as well as electronic adjudication.
Data Analysis
Favorable outcome was defined using a stratified dichotomy of the Glasgow Outcome Scale-Extended (GOS-E) at 6 months post-injury (5). GOS-E measures global neurologic recovery; scores range from 1 (death) to 8 (good recovery). Using a prespecified stratified dichotomy, favorable outcome was defined according to severity of initial injury (the highest reliable GCS score documented prior to subject randomization).
The effect of physiologic transgressions on patient outcome was explored via logistic regression analyses for each variable as a predefined secondary statistical analysis. Analysis considered: 1) a binary indicator that reflected the occurrence of at least a single transgression hour and 2) the proportion of total observed time spent in transgression, during the first 2 weeks of patient enrollment. This proportion was calculated as the number of hours for which a transgression was reported, divided by the number of hours monitored for that variable. Because time was found to violate the linearity assumption, time was divided into five categories. Subjects who experienced the transgression were divided into quartiles based upon the proportion of time that they spent in transgression. A fifth category represents subjects who did not experience the transgression.
No significant differences were found between outcomes for the treatment (progesterone) and control (placebo) group when controlling for physiologic transgressions. The control arm was used to determine the relationship between outcome and physiologic transgressions. Coefficients derived from the control model were applied to all subjects to obtain a predicted probability of outcome. Outcome was then modeled using the predicted probability of outcome, treatment, and the corresponding interaction. The treatment arms were combined for analysis. The analysis assesses the effect of each clinical covariate on outcome for the entire subject population.
The association between outcome and either: 1) occurrence of transgression or 2) proportion of observed time spent in transgression was estimated via logistic regression model. Simple models included physiologic variables with adjustments for severity, sex, age, and treatment. Among the full cohort of subjects, a full multivariable model was derived and backward selection applied to identify the physiologic variables associated with outcome. A significance level of 0.05 was required for inclusion in the model. Within each analysis, a Holm correction was applied to control the type I error rate associated with the multiple hypothesis tests conducted. The full model did not include CPP, ICP, and Pbto2 because fewer than 60% of subjects received this monitoring. An analysis of these transgressions was conducted in the ICP monitored group (tICP; n = 480). Two variables, Pco2 and temperature, were excluded from the analyses.
RESULTS
A total of 882 of the planned 1,140 subject sample were randomized. The overall mean Injury Severity Score (ISS) was 24.4 (± 11.4) on a scale from 0 to 75 (higher scores indicate greater severity). Four-hundred eighty subjects (54.4%) received an ICP monitor and this group demonstrated a higher mean ISS (28.3; SD, 10.6). Subjects receiving progesterone versus placebo had similar baseline characteristics and outcomes. Supplemental Table 1 (Supplemental Digital Content 2, http://links.lww.com/CCM/E405) lists the descriptive statistics for all subjects within the ProTECT III study.
GDT Transgression Monitoring
For each physiologic variable described in the CST guidelines, Table 2 presents the number/percentage of subjects monitored who experienced a transgression of that variable. Transgressions were common in both occurrence and duration. The total hours of subject observation during the initial 2 weeks of care was 209,417. The total transgression hours across the first 2 weeks of the study was 52,702. The full cohort is defined as the 763 subjects who were monitored for all transgressions.
TABLE 2.
Number of Subjects With Monitoring of Each Variable and With Greater Than or Equal to 1 Occurrence of Transgression of Each Variable
| Subjects With Monitoring of Variable, n (%) |
Subjects With ≥ 1 Occurrence of a Transgression, n (%) |
||||||
|---|---|---|---|---|---|---|---|
| Transgression Variable | Progesterone (n = 442) | Placebo (n = 440) | Total (n = 882) | Progesterone (n = 442) | Placebo (n = 440) | Total (n = 882) | Subjects Not Monitored, n (%) |
| Pao2 | 415 (93.9) | 410 (93.2) | 825 (93.5) | 257 (58.1) | 245 (55.7) | 502 (56.9) | 57 (6.5) |
| Glucose (high) | 437 (98.9) | 437 (99.3) | 874 (99.1) | 219 (49.5) | 223 (50.7) | 442 (50.1) | 8 (0.9) |
| Glucose (low) | 437 (98.9) | 437 (99.3) | 874 (99.1) | 88 (19.9) | 115 (26.1) | 203 (23.0) | 8 (0.9) |
| Cerebral perfusion pressure | 242 (54.8) | 222 (50.5) | 464 (52.6) | 176 (39.8) | 176 (40.0) | 352 (39.9) | 418 (474) |
| International normalized ratio | 404 (91.4) | 408 (92.7) | 812 (92.1) | 85 (19.2) | 84 (19.1) | 169 (19.2) | 70 (7.9) |
| Platelets | 437 (98.9) | 437 (99.3) | 874 (99.1) | 25 (5.7) | 25 (5.7) | 50 (5.7) | 8 (0.9) |
| O2 | 440 (99.5) | 440 (100.0) | 880 (99.8) | 160 (36.2) | 162 (36.8) | 322 (36.5) | 2 (0.2) |
| Hemoglobin | 435 (98.4) | 438 (99.5) | 873 (99.0) | 165 (37.3) | 158 (35.9) | 323 (36.6) | 9 (1.0) |
| Intracranial pressure | 238 (53.8) | 228 (51.8) | 466 (52.8) | 189 (4.8) | 167 (38.0) | 356 (40.4) | 416 (472) |
| Mean arterial pressure | 435 (98.4) | 434 (98.6) | 869 (98.5) | 302 (68.3) | 318 (72.3) | 620 (70.3) | 13 (1.5) |
| Brain tissue oxygen tension | 80 (18.1) | 75 (17.0) | 155 (17.6) | 29 (6.6) | 27 (6.1) | 56 (6.3) | 727 (82.4) |
| SBP (high) | 441 (99.8) | 440 (100.0) | 881 (99.9) | 200 (45.2) | 206 (46.8) | 406 (46.0) | 1 (0.1) |
| SBP (low) | 441 (99.8) | 440 (100.0) | 881 (99.9) | 216 (48.9) | 221 (50.2) | 437 (49.5) | 1 (0.1) |
| Temperature (high) | 440 (99.5) | 440 (100.0) | 880 (99.8) | 311 (70.4) | 315 (71.6) | 626 (71.0) | 2 (0.2) |
| Temperature (low) | 440 (99.5) | 440 (100.0) | 880 (99.8) | 161 (36.4) | 161 (36.6) | 322 (36.5) | 2 (0.2) |
SBP = systolic blood pressure.
Mortality
Two-week mortality was 12.5% in the full cohort and 16.9% in the tICP subgroup. Table 3 describes the relationship between mortality and single transgression occurrence in the full cohort. In the full cohort, a single occurrence of INR greater than 1.4 (odds ratio [OR], 5.1; 95% CI, 2.93–8.75; p < 0.0001) was associated with increased mortality. In the tICP subgroup, mortality increased with a single occurrence of either CPP less than 60 mm Hg (OR, 3.8; 95% CI, 1.54–9.45; p = 0.0038) or INR greater than 1.4 (OR, 2.8; 95% CI, 1.53–5.25; p = 0.0009) and decreased with a single occurrence of MAP less than 65 mm Hg (OR, 0.3; 95% CI, 0.16–0.73; p = 0.0051) or SBP greater than 180 mm Hg (OR, 0.3; 95% CI, 0.17–0.57; p = 0.0002).
TABLE 3.
Mortality According to Transgression Occurrence
| Simple Models Include Only One Transgression, Adjusted for Treatment, Severity, Gender, and Age |
Full Model Includes All Transgressions Except CPP, ICP, Pbto2, and Temperature; n = 752 |
Final Model After Backward Selection, Using α = 0.05 As Criteria for Removal |
|||||
|---|---|---|---|---|---|---|---|
| Transgression Variable | n Used | P | OR (95% CI) | P | OR (95% CI) | P | OR (95% CI) |
| Pao2 | 811 | 0.1924 | 0.741 (0.472–1.163) | 0.0923 | 0.623 (0.359–1.081) | 0.0253 | 0.546 (0.321–0.928) |
| Glucose (high) | 856 | 0.0033a | 2.070 (1.274–3.363) | 0.0226 | 1.940 (1.098–3.428) | 0.0168 | 1.970 (1.130–3.433) |
| Glucose (low) | 856 | 0.0478 | 0.557 (0.312–0.994) | 0.0254 | 0.472 (0.244–0.912) | 0.0222 | 0.472 (0.248–0.898) |
| CPP | 457 | 0.0059 | 2.936 (1.364–6.321) | ||||
| International nor- malized ratio | 798 | < 0.0001a | 5.233 (3.176–8.622) | < 0.0001a | 4.585 (2.533–8.300) | < 0.0001a | 5.063 (2.929–8.750) |
| Platelets | 856 | < 0.0001a | 4.389 (2.211–8.714) | 0.1217 | 1.914 (0.841–4.353) | ||
| O2 | 862 | 0.3802 | 0.818 (0.522–1.282) | 0.3642 | 0.777 (0.451–1.340) | ||
| Hemoglobin | 855 | 0.2810 | 1.280 (0.817–2.007) | 0.6863 | 0.889 (0.501–1.576) | ||
| ICP | 460 | 0.0702 | 1.839 (0.951–3.556) | ||||
| Mean arterial pressure (65) | 853 | 0.4130 | 0.817 (0.503–1.327) | 0.1353 | 0.608 (0.316–1.168) | ||
| Pbto2 | 152 | Quasi-complete separation | |||||
| SBP (high) | 863 | 0.0012a | 0.466 (0.293–0.741) | 0.0172 | 0.526 (0.310–0.892) | 0.0127 | 0.518 (0.309–0.869) |
| SBP (low, 90) | 863 | 0.2688 | 1.280 (0.826–1.984) | 0.4008 | 1.293 (0.710–2.355) | ||
| Temperature (high) | 862 | 0.0308 | 0.598 (0.375–0.954) | ||||
| Temperature (low) | 862 | < 0.0001a | 2.841 (1.819–4.435) | ||||
CPP = cerebral perfusion pressure, ICP = intracranial pressure, OR = odds ratio, Pbto2 = brain tissue oxygen tension, SBP = systolic blood pressure.
Significant after Holm correction for multiple comparisons.
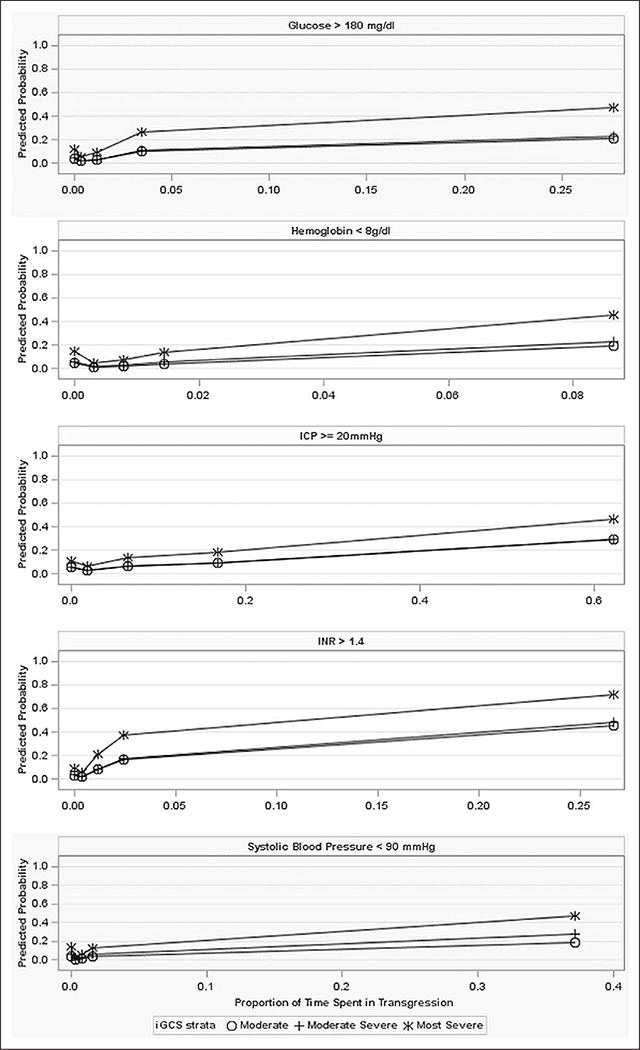
In the full cohort, the final model indicated that prolonged time spent in transgression was associated with increased mortality when hemoglobin less than 8 gm/dL (p = 0.0006), INR greater than 1.4 (p = 0.0001), glucose greater than 180 mg/dL (p = 0.0003), and SBP less than 90 mm Hg (p < 0.0001). In the tICP subgroup, prolonged time spent in transgression was associated with increased mortality for ICP greater than or equal to 20 mm Hg (p < 0.0001), glucose greater than 180 mg/dL (p = 0.0293), hemoglobin less than 8 gm/dL (p = 0.0220), or SBP less than 90 mm Hg (p = 0.0114). Also in the tICP subgroup, a short duration of time spent with SBP greater than 180 mm Hg (p = 0.0071) or MAP less than 65 mm Hg (p = 0.0133) was associated with decreased mortality; however, prolonged time spent with SBP greater than 180 mm Hg or MAP less than 65 mm Hg was not significantly associated with mortality. Associations are presented graphically in Figure 1. Complete results illustrating the relationship between mortality, proportion of time spent in transgression, and incident transgression are illustrated for the full and tICP cohorts in Supplemental Table 2 (Supplemental Digital Content 3, http://links.lww.com/CCM/E406), Supplemental Table 3 (Supplemental Digital Content 4, http://links.lww.com/CCM/E407), and Supplemental Table 4 (Supplemental Digital Content 5, http://links.lww.com/CCM/E408).
Figure 1.
The relationship between time spent in transgression and mortality. The proportion of observed hours spent in transgression is represented on the x-axis. An indicator located at x = 0, represents the class of subjects who did not experience the transgression. An indicator at the midpoint of each quartile represents increasing proportions of time spent in transgression. The y-axis represents the predicted outcome probability; a separate line is presented for each severity strata. Although the simple models from which these graphs are derived include adjustments for treatment and gender, these effects were not significant; the plots have therefore smoothed over treatment and gender to focus on the effect of duration. ICP = intracranial pressure, iGCS = initial Glasgow Coma Scale, INR = international normalized ratio.
Functional Outcome
Poor outcome was found in 343 of all subjects (45.0%) as defined by stratified GOS-E. In the tICP subgroup, 237 (52.9%) demonstrated poor outcome. Table 4 describes the relationship between transgression occurrence and functional outcome in the full cohort. In the full cohort, the odds of poor outcome were increased with a single occurrence of either glucose greater than 180 mg/dL (OR, 1.7; 95% CI, 1.25–2.42; p = 0.0010) or INR greater than 1.4 (OR, 1.8; 95% CI, 1.19–2.61; p = 0.0046). In the tICP subgroup, the odds of poor outcome were increased with a single occurrence of ICP greater than or equal to 20 mm Hg (OR, 2.1; 95% CI, 1.24–3.71; p = 0.0064) or SBP less than 90 mm Hg (OR, 1.7; 95% CI, 1.06–2.74; p = 0.0286) and decreased with a single occurrence of MAP less than 65 mm Hg (OR, 0.488; 95% CI, 0.27–0.89; p = 0.0185). Prolonged time spent in transgression was associated with poor outcome when glucose greater than 180 mg/dL (p < 0.0001); hemoglobin less than 8 gm/dL (p = 0.0035); and oxygen less than 90% (p = 0.0031). In the tICP subgroup, poor outcome was associated with prolonged time spent with ICP greater than or equal to 20 mm Hg (p = 0.0075), oxygen less than 90% (p = 0.0180), or glucose greater than 180 mg/dL (p = 0.0167). Short duration of time spent with MAP less than 65 mm Hg was associated with decreased poor outcome (p = 0.0047). The relationship between duration of transgression and functional outcome is illustrated in Supplemental Figure 1 (Supplemental Digital Content 6, http://links.lww.com/CCM/E409; legend: the relationship between time spent in transgression and mortality). Complete results illustrating the relationship between functional outcome, proportion of time spent in transgression, and incident transgression are illustrated for the full and tICP cohorts in Supplemental Table 5 (Supplemental Digital Content 7, http://links.lww.com/CCM/E410), Supplemental Table 6 (Supplemental Digital Content 8, http://links.lww.com/CCM/E411), and Supplemental Table 7 (Supplemental Digital Content 9, http://links.lww.com/CCM/E412).
TABLE 4.
Functional Outcome According to Transgression Occurrence in the Full Cohort
| Simple Models Include Only One Transgression, Adjusted for Treatment, Severity, Gender, and Age | Full Model Includes All Transgressions Except CPP, ICP, Pbto2, and Temperature; n = 726 | Final Model After Backward Selection, Using a = 0.05 As Criteria for Removal | |||||
|---|---|---|---|---|---|---|---|
| Transgression Variable | n Used | P | OR (95% CI) | P | OR (95% CI) | P | OR (95% CI) |
| Pao2 | 782 | 0.0008a | 1.724 (1.254–2.372) | 0.1344 | 1.326 (0.916–1.920) | ||
| Glucose (high) | 823 | < 0.0001a | 2.046 (1.505–2.782) | 0.0080 | 1.613 (1.133–2.296) | 0.0010a | 1.740 (1.250–2.423) |
| Glucose (low) | 823 | 0.8954 | 0.977 (0.692–1.380) | 0.2795 | 0.810 (0.553–1.187) | ||
| CPP | 444 | 0.0515 | 1.612 (0.997–2.606) | ||||
| International normalized ratio | 769 | 0.0002a | 2.051 (1.404–2.994) | 0.0231 | 1.638 (1.070–2.507) | 0.0046a | 1.761 (1.190–2.605) |
| Platelets | 823 | 0.8621 | 1.056 (0.570–1.955) | 0.0791 | 0.538 (0.270–1.075) | ||
| O2 | 829 | 0.2957 | 1.175 (0.868–1.591) | 0.2150 | 0.802 (0.566–1.137) | ||
| Hemoglobin | 822 | 0.0001a | 1.856 (1.358–2.537) | 0.0276 | 1.518 (1.047–2.201) | ||
| ICP | 448 | 0.0005a | 2.486 (1.493–4.140) | ||||
| Mean arterial pressure (65) | 821 | 0.7350 | 1.058 (0.763–1.468) | 0.1127 | 0.716 (0.474–1.082) | ||
| Pbto2 | 143 | 0.3467 | 1.442 (0.673–3.088) | ||||
| SBP (high) | 830 | 0.0190 | 1.446 (1.062–1.967) | 0.5009 | 1.127 (0.795–1.598) | ||
| SBP (low, 90) | 830 | 0.0235 | 1.411 (1.047–1.900) | 0.2455 | 1.249 (0.858–1.816) | ||
| Temperature (high) | 829 | 0.0009a | 1.788 (1.270–2.516) | ||||
| Temperature (low) | 829 | 0.0033a | 1.583 (1.165–2.150) | ||||
CPP = cerebral perfusion pressure, ICP = intracranial pressure, OR = odds ratio, Pbto2 = brain tissue oxygen tension, SBP = systolic blood pressure.
Significant after Holm correction for multiple comparisons.
DISCUSSION
ProTECT III was a multicenter phase III randomized controlled trial to examine the effect of progesterone in moderate-severe TBI. Guidelines were developed a priori for physiologic goals of therapy in order to standardize treatment across sites. Clinical compliance was rigorously monitored. Among the 882 patients, we identified transgressions in 25% of monitored hours. Association between patient outcomes and specific transgressions were observed. These data provide a foundation for future studies to evaluate whether GDT in TBI has the potential to improve patient care.
Methodological Limitations
Our study has several potential limitations. First, the data represent post hoc analysis and rely upon investigator documentation, introducing the possibility of measurement and verification error. However, the analysis was prespecified, and data were collected prospectively on standardized CRFs. Second, although analyses adjusted for disease severity, future study should examine the effect of disease phenotype. A further limitation is that we did not examine how goals of care, for example, strategies to prevent ventilator-associated pneumonia, influenced results. Such potential influence of non-TBI specific variables requires further study. Additionally, in the ProTECT III analyses, there were not significant differences in outcome between patients receiving progesterone and placebo. For this reason, the groups are pooled together in the transgression analyses. Progesterone may have unidentified/previously unknown effects on outcome, and therefore this pooling may present a potential limitation to the analyses. GDT also has its limitations; it is constructed from population-based targets. Recent advances in precision medicine advocate for more patient-specific approaches to care (21). In large national clinical trials on new clinical interventions within the ICU, it is likely that a combination of precision-based medicine and GDT is the best approach to optimizing individualized patient care. GDT in the ProTECT III trial served to provide basic guidelines in the treatment of moderate and severe TBI. These guidelines were adapted at both general trauma ICUs and neurocritical care specialty units. Future research will assess how GDT guidelines are integrated with precision-based medicine in large scale multicenter clinical trials to study novel therapies in TBI, such as in the “Brain Oxygen Optimization in Severe TBI III” clinical trial. Despite the limitations of population-based GDT, our findings are robust. The ProTECT III study has compiled the largest prospectively collected database reported on physiologic variability after TBI.
GDT
GDT is a technique used in critical care that involves intensive monitoring and aggressive management of physiologic variables. GDT is used in multiple clinical settings; however, consensus on how best to apply GDT is undefined (22–25). The relationship between guideline adherence and outcome remains difficult to study after TBI due to many methodological limitations (10, 26–30). Although previous studies indicate that the use of GDT after TBI is associated with improved outcome, causation remains undefined.
In this study, we examined how specific physiologic goals in TBI influenced outcome. Our data demonstrate that inability to achieve targets, even intermittently, is associated with an adverse effect on mortality and morbidity. This is an important new finding in the critical care of TBI and suggests that efforts to achieve specific physiologic targets may help improve outcomes.
Physiologic Targets
We examined a variety of physiologic variables and the exclusion of three warrants discussion: 1) The CST did not recommend the use of prophylactic hypothermia; however, several centers implemented hypothermia to treat refractory ICP. Since this intervention was applied in a subset of severe TBI subjects, it was not possible to independently extract effect of temperature on outcome. Temperature was thus excluded from multivariable analysis, since it may represent a transgression or a treatment effect; 2) We excluded analysis of Paco2, as it may represent a goal of therapy or a clinical manifestation of metabolic/respiratory pathology; and 3) The cohort of patients who had Pbto2 monitoring was small, and therefore this variable will be explored in future analyses.
We identified several physiologic targets that were associated with outcome. First, we found that there was a significant correlation between proportion of time spent in hyperglycemia (glucose > 180 mg/dL) and patient morbidity/mortality. There have previously been mixed findings related to glycemic control (9, 21, 22). There are no current level I guidelines for target thresholds of hyperglycemia (17). Second, after adjusting for covariates, we found that proportion of time spent in anemia (hemoglobin < 8 gm/dL) adversely affected outcome. This is consistent with other studies that find that hemoglobin greater than or equal to 9 g/L is associated with improved functional outcome and that hemoglobin concentration less than 9 g/L is associated with mortality (15, 31). This is an important finding since transfusion thresholds in TBI have yet to be clearly defined. Hemoglobin, INR, and platelet goals after TBI are complex and are influenced by both polytrauma physiology and cerebral pathophysiology. Boutin et al (32) identified trauma severity and coagulopathy as significant determinants of blood transfusion of packed RBCs after TBI. However, the authors were unable to define a relationship between transfusion and outcome due to multiple physiologic confounders, as well as their use of a single admission measurement of hemoglobin (32). Ngwenya et al (33) assessed a convenience sample of 1,565 patients treated with either restrictive (hemoglobin > 7 g/dL) or liberal (hemoglobin > 10 g/dL) transfusion protocols during a period between 2011 and 2014. During these years, the ICU protocols shifted to a restrictive threshold at their institution. These researchers found that liberal transfusion was significantly associated with increased frequency of fever, but not associated with any difference in ICU length of stay, ventilation days, lung injury events, or thromboembolism (33). Such retrospective analyses, do not adequately assess for the effect of confounders, such as duration of anemia, duration of concurrent coagulopathy, or additional polytrauma physiology. In our data set, we analyze the relationships between multiple physiologic confounders and assess the effect of each individual variable on outcome. We observed that a single occurrence of INR greater than 1.4 was associated with a fivefold increase in the odds of mortality. The importance of coagulopathy in TBI is well defined and can influence resuscitation, fluid administration, need for transfusion, and progression of intracranial hemorrhage (34). Additionally, we found that both a single occurrence and prolonged time with ICP greater than or equal to 20 mm Hg was associated with increased morbidity and mortality. This is important since previous studies describe a relationship between increased ICP and mortality but not functional outcome (35). In addition, they call into question the latest Brain Trauma Foundation’s guidelines that suggest the recommended ICP threshold should be 22 mm Hg (36). Our findings are consistent with prior analyses that show decreased mortality with adherence to GDT for ICP and CPP variables (22, 26, 27). It is important to recognize that a single threshold for ICP may be an oversimplification of a complex problem and that patient-specific thresholds, as well as the effect of cerebral autoregulation needs further study (37). Our data also illustrated that a single occurrence of CPP less than 60 mm Hg was associated with increased mortality. Although we were unable to examine cerebral autoregulation, our data support previous observations that suggest a CPP of 60 mm Hg is reasonable. However, optimization of individual patient CPP should be studied in future research (33). We observed that prolonged time spent with oxygen less than 90% was associated with poor outcome in the tICP group, this is consistent with the existing literature. Finally, we observed that prolonged duration of SBP less than 90 mm Hg was associated with increased mortality and a single occurrence of SBP less than 90 mm Hg was associated with increased morbidity. Hypotension is a well-known risk factor for adverse outcome after TBI. Our results are consistent with others that confirm a U-shaped relationship between SBP and outcome (35). We observed that a single occurrence of SBP greater than 180 mm Hg or MAP less than 65 mm Hg was inversely associated with mortality. The inverse relationship between one episode of MAP less than 65 mm Hg and mortality is unexpected. It is possible that this relationship may be attributed to treatment effect, that is, that nonsustained hypotension was in fact—hypotension responsive to treatment. Further analyses would be necessary to quantify the treatment of hypotension within the study sample in order to evaluate this hypothesis.
Limitations to this research include the inability to examine cerebral autoregulation via continuous data. Therefore, the bimodal effects of SBP/MAP are not captured in our statistical model. An additional limitation is that methods to zero a transducer (e.g., tragus vs phlebostatic axis) varied across sites, and that data integrity (e.g., events related to transduction of arterial line) introduced variability in measured values. Nevertheless, the results suggest that the BTF guideline of a SBP greater than 90 mm Hg is reasonable, although there are no level I or II recommendations to support this (17, 34). Further study will be required to elucidate the role of patient-specific goals (11, 21).
CONCLUSIONS
Robust standardization of care and data management were implemented across the ProTECT III clinical trial. Adherence to the guidelines was quantified hourly at participating centers, and daily feedback about compliance were provided to each center. We found a significant association between GDT and outcome across multiple variables. The findings provide a foundation for future research to prospectively assess the relationships between physiologic targets of therapy and outcomes after TBI.
Supplementary Material
Acknowledgments
Work by the authors on the Progesterone for the Treatment of Traumatic Brain Injury III clinical trial was supported, in part, by grants from the National Institute of Neurological Disorders and Stroke of the National Institutes of Health (NS062778, 5U10NS059032, and U01NS056975) and was conducted through the Neurological Emergencies Treatment Trials network.
Drs. Merck’s, Yeatts’s, Pauls’s, Palesch’s, Miller’s, and Wright’s institutions received funding from the National Institutes of Health (NIH) National Institute on Neurological Disorders and Stroke (NINDS). Drs. Merck, Yeatts, Silbergleit, Manley, Pauls, Palesch, Conwit, Le Roux, Miller, Frankel, and Wright received support for article research from the NIH. Dr. Yeatts’ institution received funding from Bard (for role on Data and Safety Monitoring Board), and she received funding from Genetech (for role on Potential of rTPA for Ischemic Strokes with Mild Symptoms [PRISMS] Trial Steering Committee) and the University of Michigan (for faculty role on the NINDS-funded Clinical Trials Methodology course). Drs. Silbergleit’s, Le Roux’s, and Frankel’s institutions received funding from the NIH. Drs. Silbergleit and Frankel disclosed off-label product use of progesterone for neuroprotection after traumatic brain injury (TBI). Dr. Pauls disclosed work for hire. Dr. Conwit disclosed government work. Dr. Wright’s institution received funding from the NIH/National Heart, Lung, and Blood Institute, the Department of Defense, and the National Football League; he received funding as an expert witness; and he disclosed that he is listed on a patent held by Emory University of progesterone for the treatment of TBI.
Footnotes
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s website (http://journals.lww.com/ccmjournal).
REFERENCES
- 1.Taylor CA, Bell JM, Breiding MJ, et al. : Traumatic brain injury-related emergency department visits, hospitalizations, and deaths - United States, 2007 and 2013. MMWR Surveill Summ 2017; 66:1–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Finkelstein E, Corso PS, Miller TR: The Incidence and Economic Burden of Injuries in the United States. New York, Oxford University Press, 2006 [Google Scholar]
- 3.Bruns J Jr, Hauser WA: The epidemiology of traumatic brain injury: A review. Epilepsia 2003; 44:2–10 [DOI] [PubMed] [Google Scholar]
- 4.Narayan RK, Michel ME, Ansell B, et al. : Clinical trials in head injury. J Neurotrauma 2002; 19:503–557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wright DW, Yeatts SD, Silbergleit R, et al. ; NETT Investigators: Very early administration of progesterone for acute traumatic brain injury. N Engl J Med 2014; 371:2457–2466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Clifton GL, Valadka A, Zygun D, et al. : Very early hypothermia induction in patients with severe brain injury (the National Acute Brain Injury Study: Hypothermia II): A randomised trial. Lancet Neurol 2011; 10:131–139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dewan Y, Komolafe EO, Mejía-Mantilla JH, et al. ; CRASH-3 Collaborators: CRASH-3 - tranexamic acid for the treatment of significant traumatic brain injury: Study protocol for an international randomized, double-blind, placebo-controlled trial. Trials 2012; 13:87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Edwards P, Arango M, Balica L, et al. ; CRASH Trial Collaborators: Final results of MRC CRASH, a randomised placebo-controlled trial of intravenous corticosteroid in adults with head injury—outcomes at 6 months. Lancet 2005; 365:1957–1959 [DOI] [PubMed] [Google Scholar]
- 9.Coester A, Neumann CR, Schmidt MI: Intensive insulin therapy in severe traumatic brain injury: A randomized trial. J Trauma 2010; 68:904–911 [DOI] [PubMed] [Google Scholar]
- 10.Talving P, Karamanos E, Teixeira PG, et al. : Intracranial pressure monitoring in severe head injury: Compliance with Brain Trauma Foundation guidelines and effect on outcomes: A prospective study. J Neurosurg 2013; 119:1248–1254 [DOI] [PubMed] [Google Scholar]
- 11.Berry C, Ley EJ, Bukur M, et al. : Redefining hypotension in traumatic brain injury. Injury 2012; 43:1833–1837 [DOI] [PubMed] [Google Scholar]
- 12.Butcher I, Maas AI, Lu J, et al. : Prognostic value of admission blood pressure in traumatic brain injury: Results from the IMPACT study. J Neurotrauma 2007; 24:294–302 [DOI] [PubMed] [Google Scholar]
- 13.Manley G, Knudson MM, Morabito D, et al. : Hypotension, hypoxia, and head injury: Frequency, duration, and consequences. Arch Surg 2001; 136:1118–1123 [DOI] [PubMed] [Google Scholar]
- 14.Bershad EM, Farhadi S, Suri MFK, et al. : Coagulopathy and Inhospital Deaths in Patients With Acute Subdural Hematoma. 2008. Available at: http://thejns.org/doi/full/10.3171/JNS/2008/109/10/0664 Accessed November 4, 2016 [DOI] [PubMed]
- 15.Griesdale DE, Sekhon MS, Menon DK, et al. : Hemoglobin area and time index above 90 g/L are associated with improved 6-month functional outcomes in patients with severe traumatic brain injury. Neurocrit Care 2015; 23:78–84 [DOI] [PubMed] [Google Scholar]
- 16.Steiner LA, Czosnyka M, Piechnik SK, et al. : Continuous monitoring of cerebrovascular pressure reactivity allows determination of optimal cerebral perfusion pressure in patients with traumatic brain injury. Crit Care Med 2002; 30:733–738 [DOI] [PubMed] [Google Scholar]
- 17.Carney N, Totten AM, O’Reilly C, et al. : Guidelines for the management of severe traumatic brain injury, fourth edition. Neurosurgery 2017; 80:6–15 [DOI] [PubMed] [Google Scholar]
- 18.Cryer HG, Manley GT, Adelson D, et al. : ACS TQIP Best Practices in the Management of Traumatic Brain Injury - Traumatic Brain Injury Guidelines. Ashx. Available at: https://www.facs.org/~/media/files/quality%20programs/trauma/tqip/traumatic%20brain%20injury%20guidelines.ashx Accessed October 25, 2016
- 19.Brain Trauma Foundation, American Association of Neurological Surgeons, Congress of Neurological Surgeons: Guidelines for the management of severe traumatic brain injury. J Neurotrauma 2007; 24(Suppl 1):S1–S106 [DOI] [PubMed] [Google Scholar]
- 20.Maas AI, Marmarou A, Murray GD, et al. : Prognosis and clinical trial design in traumatic brain injury: The IMPACT study. J Neurotrauma 2007; 24:232–238 [DOI] [PubMed] [Google Scholar]
- 21.Spaite DW, Hu C, Bobrow BJ, et al. : Mortality and prehospital blood pressure in patients with major traumatic brain injury: Implications for the hypotension threshold. JAMA Surg 2017; 152:360–368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rowan KM, Angus DC, et al. ; PRISM Investigators: Early, goal-directed therapy for septic shock - a patient-level meta-analysis. N Engl J Med 2017; 376:2223–2234 [DOI] [PubMed] [Google Scholar]
- 23.Rossi AF, Khan DM, Hannan R, et al. : Goal-directed medical therapy and point-of-care testing improve outcomes after congenital heart surgery. Intensive Care Med 2005; 31:98–104 [DOI] [PubMed] [Google Scholar]
- 24.Pearse RM, Harrison DA, MacDonald N, et al. ; OPTIMISE Study Group: Effect of a perioperative, cardiac output-guided hemodynamic therapy algorithm on outcomes following major gastrointestinal surgery: A randomized clinical trial and systematic review. JAMA 2014; 311:2181–2190 [DOI] [PubMed] [Google Scholar]
- 25.Wilms H, Mittal A, Haydock MD, et al. : A systematic review of goal directed fluid therapy: Rating of evidence for goals and monitoring methods. J Crit Care 2014; 29:204–209 [DOI] [PubMed] [Google Scholar]
- 26.Gerber LM, Chiu YL, Carney N, et al. : Marked reduction in mortality in patients with severe traumatic brain injury. J Neurosurg 2013; 119:1583–1590 [DOI] [PubMed] [Google Scholar]
- 27.Alali AS, Fowler RA, Mainprize TG, et al. : Intracranial pressure monitoring in severe traumatic brain injury: Results from the American College of Surgeons Trauma Quality Improvement Program. J Neurotrauma 2013; 30:1737–1746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shafi S, Diaz-Arrastia R, Madden C, et al. : Intracranial pressure monitoring in brain-injured patients is associated with worsening of survival. J Trauma 2008; 64:335–340 [DOI] [PubMed] [Google Scholar]
- 29.Cnossen MC, Scholten AC, Lingsma HF, et al. : Adherence to guidelines in adult patients with traumatic brain injury: A living systematic review. J Neurotrauma 2016. August 25 [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.English SW, Turgeon AF, Owen E, et al. : Protocol management of severe traumatic brain injury in intensive care units: A systematic review. Neurocrit Care 2013; 18:131–142 [DOI] [PubMed] [Google Scholar]
- 31.Sekhon MS, McLean N, Henderson WR, et al. : Association of hemoglobin concentration and mortality in critically ill patients with severe traumatic brain injury. Crit Care 2012; 16:R128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Boutin A, Moore L, Lauzier F, et al. : Transfusion of red blood cells in patients with traumatic brain injuries admitted to Canadian trauma health centres: A multicentre cohort study. BMJ Open 2017; 7:e014472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ngwenya LB, Suen CG, Tarapore PE, et al. : Safety and cost efficiency of a restrictive transfusion protocol in patients with traumatic brain injury. J Neurosurg 2018; 128:1530–1537 [DOI] [PubMed] [Google Scholar]
- 34.Maegele M, Schöchl H, Menovsky T, et al. : Coagulopathy and haemorrhagic progression in traumatic brain injury: Advances in mechanisms, diagnosis, and management. Lancet Neurol 2017; 16:630–647 [DOI] [PubMed] [Google Scholar]
- 35.Treggiari MM, Schutz N, Yanez ND, et al. : Role of intracranial pressure values and patterns in predicting outcome in traumatic brain injury: A systematic review. Neurocrit Care 2007; 6:104–112 [DOI] [PubMed] [Google Scholar]
- 36.Marmarou A, Anderson RL, Ward JD, et al. : Impact of ICP instability and hypotension on outcome in patients with severe head trauma. Spec Suppl 1991; 75(1s):S59–S66 [Google Scholar]
- 37.Chesnut RM, Bleck TP, Citerio G, et al. : A consensus-based interpretation of the benchmark evidence from South American trials: Treatment of intracranial pressure trial. J Neurotrauma 2015; 32:1722–1724 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.