Abstract
Purpose
To collate evidence and evaluate the effects of physical activity interventions on physical activity level among pediatric cancer survivors who had completed active cancer treatment.
Methods
Relevant published studies were identified in May 2020 via five databases and reference checking. Searches were limited to randomized controlled trials or controlled clinical trials, published in English involving pediatric cancer survivors aged 18 years or below. Interventions were related to promote physical activity among the survivors. Included studies were assessed using the revised version of the Cochrane’s Risk of Bias Tool.
Results
Eight randomized controlled trials (620 pediatric cancer survivors and 53 caregivers of pediatric cancer survivors) were included. All studies investigated interventions for pediatric cancer survivors to increase their physical activity level. The interventions used varied across the eight included studies: three mHealth—medical and public health practice supported by mobile devices; two eHealth—the use of information and communication technologies to improve health care; two adventure-based training; and one educational program. Measures of physical activity level also varied: five used various objective measurements (i.e., accelerometer, pedometer, multisensory activity monitor); three used different self-reported questionnaires. Owing to high variability of the interventions and measures, it was impossible to perform meta-analysis. Overall, eHealth and mHealth interventions showed effectiveness and feasibility to promote physical activity among pediatric cancer survivors.
Conclusions
eHealth and mHealth interventions appear to be increasingly important strategies to promote physical activity among pediatric cancer survivors.
Implications for Cancer Survivors
Future larger-scale studies using a core-set of assessment tools are warranted to further promote regular physical activity in pediatric cancer survivors.
Keywords: Intervention strategies, Pediatric oncology, Pediatric cancer survivors, Physical activity
Introduction
The 5-year survival rate for pediatric cancer has now reached to nearly 85% due to remarkable advances in cancer treatment regimens [1]. There is growing population of pediatric cancer survivors, yet about two third of the pediatric cancer survivors are contending with a host of cancer or treatment-related late effects throughout their survivorship [2]. Decline in physical fitness (i.e., cardiopulmonary dysfunction), reduced functional capacity (i.e., impaired musculoskeletal function), and cancer-related fatigue are common tangible late effects experienced by pediatric cancer survivors, all of which consequently compromise their quality of life [3–6]. Additionally, pediatric cancer survivors are at ten times increased risk of developing significant chronic diseases, including obesity, hypertension, type 2 diabetes mellitus, and secondary malignancies [7].
Regular physical activity has been shown to have beneficial effect on improving physical fitness, ameliorating adverse late effects, and preventing future treatment-related morbidity among children with cancer [8, 9]. Owing to the pivotal role played by physical activity in pediatric oncology, increasing concern has been attached to the issue of physical inactivity among pediatric cancer survivors. Previous studies have shown that more than 50% of pediatric cancer survivors in Western countries pediatric cancer survivors did not meet the Centre for Disease Control and Prevention (CDC) recommended 60 min of moderate-to-vigorous physical activity per day [10, 11]. The situation is even worse in Hong Kong, where approximately 92.2% of Hong Kong Chinese pediatric cancer survivors did not adhere to the CDC recommended physical activity [12]. Pediatric cancer survivors are less physically active, in term of the amount of time spent in performing physical activity and the level of intensity, than the healthy children in general population [13, 14]. Decreased levels of physical activity have been identified as a leading cause of the diminished physical fitness in pediatric cancer survivors [15]. Evidence also suggests that physical inactivity is a risk factor for non-communicable diseases, including cardiovascular disease, hypertension, obesity, diabetes, and cancer [16], thereby further aggravating the adverse late effects resulting from the cancer and its treatment [11].
Extensive research has examined the effectiveness of interventions to promote physical activity in children with cancer. To date, six prior systematic reviews have addressed the effect of the physical activity interventions in pediatric oncology, targeting on pediatric oncology inpatients undergoing active or maintenance treatment [17–22]. Among the six existing reviews, three reviews mainly focused on homogenous pediatric patients with acute lymphoblastic leukemia, which are diagnosis-specific [17, 18, 22]. Moreover, most of the studies included in these six reviews were delivered in hospital setting as they targeted children with pediatric cancer during cancer treatment [17–22]. Findings from the previous reviews cannot be generalized to the pediatric cancer survivors population owing to the differential differences in disease experience and health conditions and behaviors between children undergoing active cancer treatment and children surviving from cancer as well as significant differences in clinical characteristics and prognosis between children with acute lymphoblastic leukemia and children with solid tumors [6, 23]. To our knowledge, only one existing review included RCTs and controlled clinical trials (CCTs) to evaluate the effect of physical activity interventions for children who were undergoing active cancer treatment for acute lymphoblastic leukemia [18]. Yet, no published review has collated high levels of evidence (i.e., RCTs, CCTs) of the effect of all physical activity interventions solely for pediatric cancer survivors with mixed types of cancer diagnosis as well as had completed active cancer treatment. Hence, this review aimed to evaluate the effect of physical activity intervention specifically on pediatric cancer survivors, focusing on this heterogeneous group helps to enhance the generalizability of the findings. The primary objective of this review is to identify and synthesize the current evidence on the effectiveness of physical activity interventions on promoting physical activity level for pediatric cancer survivors who were diagnosed of any types of cancer and had completed their cancer treatment. The secondary objective was to determine the effect of physical activity interventions on cancer-related fatigue, physical functioning, quality of life, and the feasibility (i.e., retention, adherence) of the intervention.
Methods
The reporting of this systematic review and its procedure follow the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) statement guidelines [24].
Search strategy
A comprehensive search strategy was developed to identify relevant studies that included physical activity interventions in pediatric cancer survivors. A total of five electronic databases, including MEDLINE, CINAHL, Embase, PsycINFO, and Cochrane central register of controlled trials were systematically searched. The following search terms were used: (child OR pediatric OR youth OR adolescent) AND (cancer OR carcinoma OR neoplasm OR tumour OR tumor OR oncology) AND (exercise OR exercise therapy OR physical activity OR physical training OR physical education) AND (intervention OR randomised controlled trial OR randomized OR controlled clinical trial OR clinical trial OR randomly OR placebo OR comparative study). A manual review was then performed to identify additional relevant studies from the reference lists of the included studies and published systematic reviews on physical activity intervention. All searches were conducted in May 2020.
Eligibility criteria
The PICOS format was used to clearly define the inclusion and exclusion criteria of the studies [25]. Inclusion criteria applied in the selection process are (1) population: pediatric cancer survivors (aged 18 years or below), diagnosed with any types of cancer, and had completed cancer treatment or on remission phase; (2) intervention: any types of interventions that aimed to promote the physical activity among pediatric cancer survivors; (3) comparison: compares the intervention to an alternative intervention or usual care; (4) outcome measurements: the primary outcome variable was physical activity level. Secondary outcomes of interests were physical function, cancer-related fatigue, quality of life, and the feasibility of the intervention, and (5) study type: only RCTs and CCTs published within 10 years were included. We excluded studies written in a language other than English or in which full texts were not available.
Study selection
Two reviewers (ATC and WHCL) independently screened the title and abstract of the articles for eligibility. The full texts of the selected studies were then retrieved for further assessing the eligibility. Eligible studies were included for data extraction and quality assessment. A standardized form was developed and used for the data extraction from the included studies by two reviewers. Any disagreements between authors were resolved by discussing the issues with the third reviewer (OKJC).
Data extraction and methodological quality assessment
We extracted and summarized data for all included studies, including study design, number of participants in each group characteristics of participants, intervention characteristics (i.e., intervention content, intervention provider, delivery mode and setting, duration, frequency and total numbers of sessions), details of comparators, outcome measures (i.e., physical activity levels, fatigue, physical fitness, cancer-related fatigue, quality of life, physical activity stage of change, physical activity self-efficacy, cardio-metabolic assessments, weight status, health behaviors, neurocognitive function, and psychological well-being), and relevant findings (i.e., feasibility, retention, and adherence). We used the revised version of the Cochrane’s Risk of Bias Tool to assess the methodological rigor of each study [26]. This tool is a domain-based evaluation that consists five domains, which included bias arising from the randomization process, bias due to deviations from intended interventions, bias due to missing outcome data, bias in measurement of the outcome, and bias in selection of the reported result. Each domain was ranked as low risk of bias, some concerns of bias or high risk of bias. The overall risk-of-bias judgement (low risk of bias, some concerns, high risk of bias) of each study was reached by the following criteria, (i) low risk of bias (if all domains are evaluated as low risk of bias), (ii) some concerns (if at least one domain is/are evaluated as some concerns of bias, but not to be at high risk of bias for any domain), and (iii) high risk of bias (if at least one domain is/are evaluated as high risk of bias or if multiple domains are evaluated as some concerns of bias). Two independent reviewers conducted the methodological quality assessment and then compared the results for each study, and any discrepancies and disagreement were discussed and resolved upon by the team.
Results
Search results
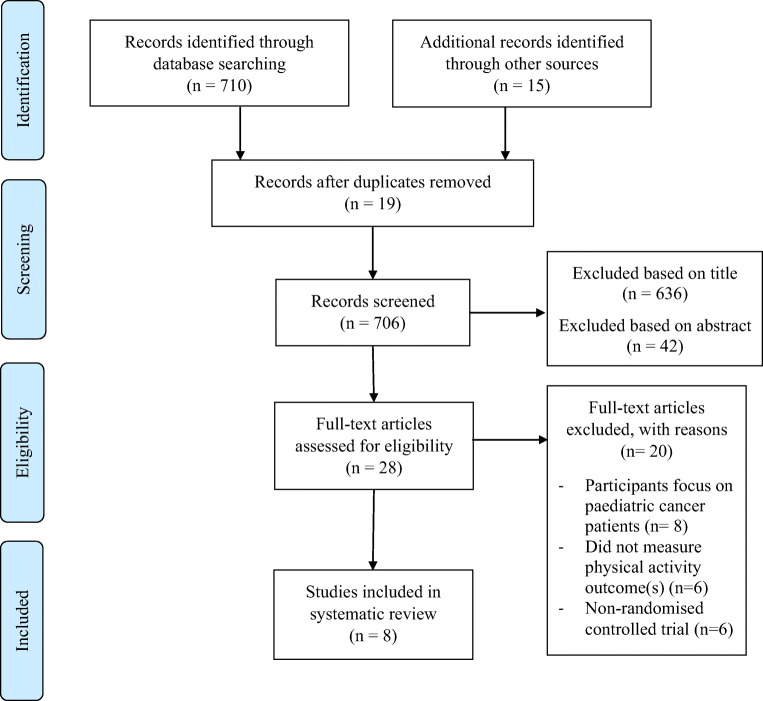
The search strategy retrieved a total of 725 records. After removing duplicate records (n = 19), 706 records were identified. The title and abstract were then screened and reviewed, 678 recorded were excluded, leaving 28 articles for full text review. After full review, eight studies met the eligibility criteria and were included in this systematic review. The study selection process and results are presented in the PRISMA flow chart (see Fig. 1).
Fig. 1.
PRISMA 2009 flowchart
Study characteristics
The publication period of the included studies was between 2013 and 2019. Four studies were conducted in the USA [27–30], one in Sweden [31], two in Hong Kong [32, 33], and the remaining one in Taiwan [34]. All studies were RCTs. A summary of the characteristics of the included studies is presented in Table 1.
Table 1.
Characteristics of the studies and results (n = 8)
| Author, year, country | Study design/study duration | Characteristics of participants/ sample size N | Mode of delivery and setting | Intervention, duration, and frequency, intervention provider |
Comparator | Outcome measures/ measurement | Main findings | Feasibility Retention, and adherence |
|---|---|---|---|---|---|---|---|---|
| Howell et al., 2018, USA |
RCT; 6-month |
Adolescent survivors of pediatric cancer with mixed types of cancer aged 11-15 years (mean age 12.7 years) N = 94 47 females; 47 males |
eHealth; interactive and rewards-based Setting: home- and web-based |
n = 63 Intervention: Educational materials, an activity monitor and access to an interactive website designed to motivate increased physical activity via rewards Intervention period: 6 months Duration: NR Frequency: NR Intervention provider: Self-administered Theory guided: NR |
n = 31 Educational materials and activity monitor |
- Physical activity (Accelerometer) - Fitness - Hand grip strength (Hand-held dynamometer) - Proximal muscle strength (Sit-ups and push-ups) - Neurocognitive function (Wechsler Abbreviated Scale of Intelligence) - Health Related Quality of Life (Pediatric Quality of Life Inventory v4) |
↑ Fitness (hand grip strength, number of sit-ups and push-ups) ↑Neurocognitive function ↑ Health Related Quality of Life |
Retention: 80.4% Adherence: NR |
| Huang et al., 2014, USA |
RCT; 4-month |
Pediatric survivors of acute lymphoblastic leukemia aged 8-18 years (mean age 13 years) N = 38 23 females; 15 males |
mHealth; web, phone and text message-based Setting: web-based, home |
n = 19 Fit4Life intervention: A web-and text- and phone counselling-based tailored weight management intervention, in which participants received written materials about weight management topics and skills and lifestyle tips via an Internet program weekly. Tailored short message service messages were delivered twice per day to the participants. A Health Coach provided counselling calls weekly during the first month and biweekly in months 2-4. Parents received printed materials regarding the information on behavioral and parenting strategies to facilitate their child lose weight and become healthy together. Intervention period: 4 months Duration: at least 1 h/day (physical activity goal) Frequency: Weekly Intervention provider: Health Coach Theory guided: Bandura's Social Cognitive Theory |
n = 19 General weight management intervention delivered via phone and mail. Survivors and parents received printed weight management materials on nutrition, physical activity and general tips on weight management. Survivors received a biweekly call from a Health Coach in month 1 and monthly in months 2-4 months to ensure they received the monthly material. |
- Weight status (Calibrated digital scale) - Weight-related health behaviors - physical activity (Accelerometer) - dietary intake (Youth Adolescent Questionnaire) - Cardio-metabolic (Blood glucose, hemoglobin A1c, lipids) - Psychological behaviors- depressive symptoms (Children’s Depression Inventory) |
↑ Moderate-to-vigorous physical activity |
Retention: 92.1% Average Adherence for experimental/ control group: 80%/50% |
| Li et al., 2013, Hong Kong |
RCT; 9-month |
Pediatric cancer survivors with mixed types of cancer aged 9-16 years (mean age 12.7 years) N = 71 34 females; 37 males |
Face-to-face; adventure-based Setting: Community (an adventure camp) |
n = 34 A 4-day integrated adventure-based training and health education program with adventure-based training activities and other activities, such as four educational talks (each around 40 min) and a workshop (90-min on day 4) to develop a feasible individual action plan for regular physical activity Intervention period: 6 months Duration: 1-day Frequency: 1-day camp/3 months Intervention provider: Two qualified adventure-based training instructors & healthcare professionals Theory guided: Kolb’s experiential learning theory |
n = 37 Placebo control intervention 4 days of leisure activities (film shows, handicraft workshops, chess games, health talks on the prevention of influenza and healthy diet, organized by a community center and a day visit to a museum and theme park over a 6-month period |
- Physical activity (The Chinese University of Hong Kong: Physical Activity Rating for Children and Youth) - Physical activity Stages of change (Physical Activity Stages of Change Questionnaire) - Physical activity self-efficacy (Physical Activity Self-Efficacy) - Quality of life (Pediatric Quality of Life Inventory) |
↑ Physical activity level ↑ Self-efficacy ↑ Physical activity Stages of change |
Retention: 93.0% Adherence: 85.3% of participants in the experimental group attended all sessions; 78.4% of participants in the attention placebo control group attended all sessions |
| Li et al., 2018, Hong Kong |
RCT; 12-month |
Pediatric cancer survivors with mixed types of cancer aged 9-16 years (mean age 12.6 years) N = 222 104 females; 118 males |
Face-to-face; adventure-based Setting: Community (at a campsite) |
n = 117 A 4-day adventure-based training program, which comprised a 40-min briefing session covers brief health education components; and adventure activities with increasing levels of difficulty Intervention period: 6 months Duration: 1-day, around 7 h 45 min Frequency: 1-day camp/2 months Intervention provider: Two adventure-based trainers and a registered nurse Theory guided: Kolb’s experiential learning theory |
n = 105 Placebo control intervention: 4 days of different leisure activities (cartoon film shows, chess games, handcraft workshops, health education talks) organized by a community center over a 6-month period |
- Fatigue (Chinese version of the fatigue scale-children and adolescents) - Physical activity (The Chinese University of Hong Kong: Physical Activity Rating for Children and Youth) - Physical activity self-efficacy (Physical Activity Self-Efficacy) - Quality of life (Pediatric Quality of Life Inventory) |
↓ Fatigue ↑ Physical activity level ↑ Self-efficacy ↑ Quality of life |
Retention: 86.5% Adherence: 91.5% of participants in the experimental group attended all sessions; 89.5% of participants in the attention placebo control group attended all sessions |
| Mendoza et al., 2017, USA |
RCT; 10-week |
Pediatric cancer survivors with mixed types of cancer aged 14-18 years (mean age 16.6 years) N = 60 35 females; 24 males |
mHealth; wearable technology with social media component Setting: home- and web-based |
n = 29 Participants wore a physical activity tracking device sync with the Fitbit mHealth app, as well as joined a peer-based virtual support Facebook group. Contact via text message or telephone once per week from week 2 by research staff to help set a daily step goal. Intervention period: 10 weeks Duration: NR (achieve daily step goal at least 10,000–11,700 steps/day) Frequency: 7 days/week Intervention provider: Self-administered and research staff Theory guided: Self-determination theory |
n = 30 Usual care |
- Physical activity (ActiGraph GT3X+) - Quality of life (Pediatric Quality of Life Inventory 4.0 Generic Core and Cancer Module Scales) - Psychological needs (Psychological Need Satisfaction in Exercise Scale) |
No major training effects |
Retention: 90% Adherence: - An average of 71.5% of intervention participants wore Fitbit Flex - 100% of participants at Time 1 and 90% of participants at Time 2 completed the online questionnaires - 89.7% intervention participants joined the intervention’s private Facebook group |
| Sabel et al., 2016, Sweden | RCT; 10-12 weeks |
Pediatric brain tumor survivors aged 7-17 years (mean age of 12.5 years) N = 13 7 females; 6 males |
eHealth; active video gaming Setting: home- based; |
n = 7 Each participant received an off-the-shelf motion-controlled video console, Nintendo Wii and instructed to perform at least 30-min active video gaming per day Intervention period: 10–12 weeks Duration: NR Frequency: at least 5 days/week (target active video gaming frequency) Intervention provider: Self-administered and weekly coaching sessions provided by a research nurse via video conferencing Theory guided: NR |
n = 6 Wait-list control |
- Physical activity (<ultisensory activity monitor SenseWear Pro 2 Armband and SenseWear Professional Software) - Physical functioning (Bruininks-Osteretsky Test of Motor Performance, Second Edition) |
↑ Body Coordination score by 15% |
Retention: 100% Adherence: AVG sessions (mean duration 47 min) were performed on 72% of all days |
| Stern et al., 2018, USA |
RCT; 4 months |
Pediatric cancer survivors/caregivers dyads with mixed types of cancer aged 5–13 years (mean age 9.9 years) N = 53 Survivors: 28 females; 25 males |
mHealth; caregiver-based; psycho-educational Setting: Face-to-face (first and last sessions), Web- and phone-based (4 sessions) |
n = 27 NOURISH-T intervention: A parent-focused 6 manualized phone psycho-educational sessions with each about topics on changing eating and physical activity behaviors. The families also received relevant print and web-based resources throughout the program. Caregivers received a booster phone call 2 months post-intervention and additional educational mailings regarding nutrition and physical activity Intervention period: 6 weeks Duration: less than 1 h/session Frequency: 1 session/week Intervention provider: Group leaders supervised by licensed psychologists Theory guided: Social Cognitive and Cognitive Behavioral Theories |
n = 26 Enhanced usual care: One-hour wellness session addressing the role of diet and physical activity in pediatric overweight using material from the publicly available We Can! Manual. Participants also received nationally available web-based information on wellness issues at two additional times 6-weeks study period via mail. |
For both survivors and caregivers: - Anthropometric Measures (height, weight, waist and hip circumferences) - Dietary Recall (Automated Self-administered 24-h Dietary Recall-2011) - Step Counts (Pedometer) For caregivers only: - Approaches to and attitudes about feeding their children (Child Feeding Questionnaire) - Family’s eating, exercise, and weight-related habits (Family Eating and Exercise Behaviors) - Satisfaction/Exit Surveys For survivors: - Child Sugar Sweet Beverage and Fast Food Intake - Physical Activity Questionnaire for Children - Rating of Medical Late Effects |
Caregivers outcomes: ↓ Average BMI ↓ Daily caloric intake ↓ Pressuring of their child to eat ↓ Restriction of eating over time Survivors outcomes: ↓ BMI percentile ↑ Daily steps ↓ Sugared beverage consumption |
Retention: 69.8% Adherence: NR |
| Wu et al., 2019, Taiwan |
RCT; 4 months |
Pediatric cancer survivors with mixed types of cancer aged 8–20 years (mean age: 11.89 years) N = 69 27 females; 37 males |
Face-to-face; Educational-based Setting: Pediatric hematology/oncology wards or clinics |
n = 34 Participants received (a) Six individual education sessions (each 40-60 mins) within 1 week (b) A handbook provided guidance and educational information regarding self-management, delayed effects and complications of cancer treatments, individual exposure-related risks and long-term follow-up (c) Follow-up telephone counselling to each participant at 1- and 4-month post-intervention Duration: 4 months Frequency: 6 sessions (each 45-60 min) within 1 week Intervention provider: A research assistant under the supervision of the first author Theory guided: self-efficacy theory |
n = 35 Educational intervention upon completing the 4-month post-intervention follow-up. |
- Health behavior self-efficacy (healthy diet, exercise, well-being and health accountability) - Health promotion lifestyle (nutrition, exercise behaviors, stress adaption, interpersonal support, self-achievement and healthy behaviors) |
↑ Health self-efficacy (healthy diet, well-being and healthy accountability with the exception in exercise) |
Retention: 92.8% Adherence: NR |
NR not reported
Table 2 presents a summary of the risk of bias of the included studies. Among all studies, three studies were rated as low risk of bias [31–33], two as some concerns of bias [28, 29], and three as high risks of bias [27, 30, 34]. Specifically, three studies were rated as presenting some concerns of bias arising from the randomization process [27–29]. Two studies were rated as presenting some concerns of bias due to deviations from intended interventions [27, 28]. Three studies were rated as presenting high risk of bias due to missing outcome data [27, 33, 34].
Table 2.
Assessment of methodological quality of the studies
| Overall risk-of-bias judgement | Bias arising from the randomization process | Bias due to deviations from intended interventions | Bias due to missing outcome data | Bias in measurement of the outcome | Bias in selection of the reported result | |
|---|---|---|---|---|---|---|
| Howell et al., 2018 | High | Some concerns | Some concerns | High | Low | Low |
| Huang et al., 2014 | Some concerns | Some concerns | Some concerns | Low | Low | Low |
| Li et al., 2013 | Low | Low | Low | Low | Low | Low |
| Li et al., 2018 | Low | Low | Low | Low | Low | Low |
| Mendoza et al., 2017 | Some concerns | Some concerns | Low | Low | Low | Low |
| Sabel et al., 2016 | Low | Low | Low | Low | Low | Low |
| Stern et al., 2018 | High | Low | Low | High | Low | Low |
| Wu et al., 2019 | High | Low | Low | High | Low | Low |
Each domain assigned a judgement of low risk of bias, high risk of bias, or unclear risk of bias
Characteristics of participants
A total of 620 pediatric cancer survivors and 53 caregivers of pediatric cancer survivors were included across the eight studies. There was variability in the sample sizes of the included studies ranging from 13 to 222 participants. In general, the sample size of each study was small. The largest sample size was 222 pediatric cancer survivors, with 117 children in the experimental group and 105 in placebo control group [33]. Six studies focused on pediatric cancer survivors with mixed types of cancer, which included acute lymphoblastic leukemia, acute myeloid leukemia, lymphoma, brain tumors, sarcoma, bone tumors, neuroblastoma, and Langerhans cell histiocytosis [27, 29, 30, 32–34], one specifically on pediatric brain tumor survivors [31] and the remaining one on pediatric survivors of acute lymphoblastic leukemia [28]. Seven included studies focused on pediatric cancer survivors aged 7 to 20 years [27–29, 31–34]. Only one study involved both pediatric cancer survivors who aged 5 to 13 years and their caregivers [30]. The mean age of pediatric cancer survivors in the included studies ranged between 9.9 years old and 16.6 years old. All studies included both males (N = 315, 50.8%) and females (N = 305, 49.2%).
Characteristics of interventions
All interventions were performed after the completion of cancer treatment. Three studies included pediatric cancer survivors who had completed cancer treatment for at least 6 months [30, 32, 33]. Two studies included children who had off all treatment for at least 1 year [29, 31]. One study included children who had completed treatment for at least 2 years without any relapse [28]. Another study included pediatric cancer survivors currently in remission and within ± 2 months of completing treatment at the time of enrollment [34]. Only one study did not specify the exact timing of treatment completion, and just stated that children who were not currently undergoing active cancer treatment were eligible [27].
All interventions were aimed to promote physical activity and improve health behaviors among pediatric cancer survivors through different strategies. Five studies employed mobile health interventions (mHealth) [28–30] and electronic health (eHealth) [27, 31]. For the two mobile health interventions, one employed a web- and text- and phone counselling-based tailored weight management intervention for pediatric cancer survivors [28]. Another one was a mobile health intervention consisting of a wearable physical activity tracking device that sync with its mobile phone-based app, as well as a peer-based virtual support Facebook group to promote physical activity among adolescents and young adult cancer survivors [29]. The remaining one provided manualized phone psycho-educational sessions and web-based resources to the survivors and their families to improve health behaviors in pediatric cancer survivors with obesity [30]. For the two eHealth interventions, one was interactive and rewards-based, in which participants received educational materials, an activity monitor and access to an interactive website designed to encourage physical activity via rewards, such as t-shirts, stickers, and/or gift cards [27]. Another study employed an active video gaming as an eHealth intervention to motivate participants to engage in a minimum of 30-min active video gaming daily, at least 5 days per week [31]. Two interventions used adventure-based training were carried out in community setting, which was at an adventure campsite [32, 33]. Only one intervention employed educational approach was delivered in hospital setting, which were at pediatric hematology/oncology wards or clinics [34].
Regarding the intervention provider, three eHealth and mHealth interventions were self-administered by the participants [27, 29, 31], in which two of the studies involved both self-administrated and a research nurse or staff to deliver weekly coaching sessions via video conferencing [31] and to contact participants weekly via text messages or telephone to help set personal daily step goal [29]. Two interventions were delivered by professional adventure-based trainers and registered nurses [32, 33]. Health coach [28], group leaders who were supervised by licensed psychologists [30], and research assistants under the supervision of the research team [34] were the other personnel delivering physical activity interventions.
The intervention period and duration and frequency of each session differed across studies. The intervention period lasted between 6 weeks and 6 months. The duration of each training session ranged from 40 min to 1 day. The duration of the two adventure-based interventions was 1 day [32, 33]. One study delivered psycho-educational session per week and lasted less than 1 h per session. Another study delivered 6 individual education sessions within 1 week and each lasted between 40 and 60 min [34]. Two mobile health interventions did not report the duration and frequency of the interventions, instead they stated the targeted physical activity goals [28, 29]. One included study aimed to motivate children engaging in at least 1 h of moderate-to-vigorous physical activity daily and a 15,000 daily step goal [28]. Another one targeted children to achieve daily step goal at least 10,000–11,700 steps/day [29]. Similarly, one eHealth intervention targeted children to perform active video gaming at least 5 days/week [31]. The remaining one did not report this information [27].
All included studies had control groups. Most of the included studies had control groups receiving usual care or placebo care, which implies no additional physical activity-related care was provided [27–29, 32–34]. Of the other two studies, one had wait-list control group [31]. Another one employed enhanced usual care for the control group, in which 1-h wellness session addressing the role of diet and physical activity was delivered [30]. All participants of the control groups were assessed at the same time points as the intervention group.
Outcome measures
Outcome measures assessed across the included studies included physical activity level [27, 29–33], physical fitness [27, 31], cancer-related fatigue [33], quality of life [27, 29, 32, 33], physical activity stage of change [32], physical activity self-efficacy [32, 33], cardio-metabolic assessments [28], weight status [28], health behaviors [28, 30, 34], psychological well-being [28, 29], and neurocognitive function [27]. One included study has published the findings of neurocognitive outcomes from the trial in a separate publication [31, 35].
Across the included studies, different outcome assessing scales were used to evaluate physical activity level. In three studies, self-reported questionnaires were used to assess participants’ physical activity levels [32–34]. Of which, the Chinese University of Hong Kong: Physical Activity Rating for Children and Youth was used in two studies [32, 33]. Another one used Health behavior self-efficacy to assess the exercise behavior of pediatric cancer survivors [34]. Other studies used objective measurements, including accelerometer [27, 28], pedometer [30], actiGraph GT3X+ [29], and multisensory activity monitor SenseWear Pro 2 Armband [31], to assess the physical activity levels.
Effects of interventions
Primary outcome: physical activity levels
All included studies evaluated the effects of physical activity intervention on physical activity level. Only four studies found an increase in physical activity levels after the interventions [28, 30, 32, 33].
Secondary outcomes
Physical function
Two studies assessed the effect of interventions on physical function [27, 31]. One evaluated the effects of physical activity intervention on hand-grip strength and proximal muscle strength with hand-held dynamometer and sit-ups and push-ups. The results showed a significant improvement in physical fitness in terms offhand-grip strength, number of sit-ups, and push-ups after the intervention [27]. Another study assessed the physical functioning with Bruininks-Osteretsky Test of Motor Performance, Second Edition, and found a significant increase in body coordination score by 15% after the intervention [31].
Cancer-related fatigue
Only one study evaluated the effect of an adventure-based training program on cancer-related fatigue with a self-reported Chinese version of the fatigue scale-children and adolescents. The study found that the program was effective in reducing cancer-related fatigue among Chinese pediatric cancer survivors [33].
Quality of life
Four studies measured the effect of physical activity interventions on quality of life with Pediatric Quality of Life Inventory [27, 29, 32, 33]. Two studies found a significant improvement in quality of life among pediatric cancer survivors after the interventions [27, 33].
Adherence to the intervention and adverse events
Retention of participants in all included studies ranged from 69.8 to 100%. Adherence has been examined within five studies and ranges between 71.5 and 91.5% [29, 31–33]. No major adverse events and health-related issues were reported in any of these eight studies. The findings of all included studies suggest that physical activity interventions are feasible and acceptable to pediatric cancer survivors.
Discussion
This systematic review identified new evidence for the effects of physical activity interventions on promoting physical activity in pediatric cancer survivors. Most studies were found to have methodological limitations that affected their overall quality rating. Only two studies were rated as low risk of bias according to the revised version of the Cochrane’s Risk of Bias Tool [32, 33].
In general, the results of this review support the use of physical activity interventions to promote increased levels of physical activity, with 4 out of 8 included studies reporting statistically significant results for the different interventions tested, echoing results from the prior reviews despite they targeted children with cancer during medical treatment [17, 19–21]. In line with the results of this review, previous reviews have also suggested that physical activity training is a safe and feasible therapeutic intervention, which exert positive effects on physical well-being and quality of life for pediatric cancer populations [17, 21, 22]. One of the key findings of this systematic review is that there is increasing use of eHealth and mHealth interventions to promote physical activity and health behaviors in pediatric oncology research. Notably, these eHealth and mHealth interventions demonstrated effectiveness in promoting the adoption and maintenance of physical activity among pediatric cancer survivors [27, 28, 30]. Many included studies also examined the feasibility of these innovative interventions in pediatric oncology population, results showed that eHealth and mHealth interventions were feasible to be implemented with good adherence and high acceptance [28, 29, 31]. The use of digital health interventions (i.e., eHealth and mHealth) is expanding rapidly worldwide, emerging research has integrated eHealth and mHealth into health care delivery and health promotion [36]. In particular, the World Health Organization (WHO) has advocated the use of mHealth intervention and highly recommended it as a new strategy for health promotion [37]. According to the definition by the WHO, mobile health refers to medical and public health practice which are supported by mobile devices, such as using instant messaging applications on mobile phones, patient monitoring devices, and other wireless devices [38]. There are several special features of mobile technologies, such as instant messaging, that make them particularly appropriate for promoting health behaviors. First, using instant messaging (i.e., WhatsApp or WeChat) allows quick, direct, and continuing professional advice and individualized support tailored for the targeted population to improve their health-related behaviors. Second, instant messaging can be delivered instantaneously that can be accessed at a time that suits recipient and offers mutual communication, in which participants can elicit feedback and interact flexibly [39]. Most importantly, instant messaging is a more feasible, flexible, and efficient intervention than face-to-face interventions, particularly during the COVID-19 pandemic, where the delivery of many face-to-face health care services have been suspended [40]. Hence, mHealth based on information communication technologies has the potential to mitigate the challenges posed by the pandemic on the health care research.
Optimizing the long-term functionality and quality of life of pediatric cancer survivors has been the primary focus in the healthcare paradigm today. Early evidence suggests that face-to-face supervised physical activity programs appear to be more effective than those non-supervised home- or community-based physical activity programs [41, 42]. Yet, considering the cost-effectiveness of intervention, face-to-face supervised physical activity programs, such as adventure-based training program, are often labor-intensive, resource-expensive, and time-consuming [32, 33]. Geographical distance may also be another limitation of such face-to-face supervised program, as it is often impractical for the children and their families who may have to travel long distance to a venue of the program [43]. Notably, cost-effectiveness and sustainability are the crucial factors that have to be taken into account when designing a realistic, sustainable, and ongoing healthcare program for the pediatric cancer survivors [44], with the ultimate goal at transferring the intervention into practice to enable the children and their families to integrate physical activity into their everyday lives. eHealth and mHealth intervention seems to be an alternative and effective strategy to promote regular physical activity among pediatric cancer survivors. Most importantly, these strategies may enhance the sustainability of the intervention by making it transferable to daily practice [45, 46], particularly in busy healthcare settings, where implementation of intensive intervention is impossible. Yet, those included eHealth or mHealth studies had small sample size and lacked sufficient rigor in the study design. More methodologically rigorous studies with larger sample size are needed to confirm the effectiveness of such eHealth and mHealth interventions on promoting physical activity in pediatric oncology.
It is worth noting that the included study that used an educational-based approach to promote physical activity in pediatric cancer survivors showed insignificant results [34]. This finding is in conjunction with those of literature, suggesting that educational alone is ineffective to change people’s health-related behavior [47]. Previous studies suggested that the belief about the role of education in determining and changing patients’ health-related behavior is completely incorrect and unscientific [48]. Merely providing information and knowledge to patients was unlikely to act as a driving force to change their current behavior and practice [48]. Thereby, healthcare professionals should not only provide education to patients, but also explore appropriate and practical strategies to promote physical activity among pediatric cancer survivors.
The main limitation of this review is that meta-analysis was not performed owing to the heterogeneous measurement tools for assessing the primary outcome (physical activity level) of this review. This implies that a core-set of measurement tool for physical activity level is required in pediatric oncology research to generate current best evidence on the effect of physical activity intervention in promoting regular physical activity in pediatric cancer survivors. Moreover, there was considerable heterogeneity on the delivery approaches and intervention period of the physical activity interventions in all included studies, making it difficult to compare the intervention content and make clear conclusions on their effectiveness in promoting physical activity among pediatric cancer survivors.
Conclusions
This systematic review evaluates the evidence on the effect of physical activity interventions on the promotion of physical activity and health behaviors among pediatric cancer survivors. We have collated studies with RCT design, which is the gold-standard for examining causal relationship between an intervention and outcomes, thereby generating a high quality of evidence on the effect of interventions. eHealth and mHealth interventions appear to be an effective strategy to promote the physical activity among pediatric cancer survivors. Our findings highlight the ineffectiveness of the educational approach to elicit positive physical activity behavior change among pediatric cancer survivors. Thus, healthcare professionals should devise and implement novel strategies to promote the adoption and maintenance of regular physical activity in pediatric cancer survivors. It is also vital for the future research to empower the children to acquire essential physical activity skills, help them develop their interests in physical activity and hence facilitate their formation of physical activity habits in their everyday lives. Conducting larger-scale studies that use a core-set of measurement tools to assess physical activity-related variables may foster the evaluation of the effects of physical activity intervention in pediatric oncology research.
Authors’ contributions
ATC, WHCL, LLKH, KYH, GCFC, and JOKC created the concept and design of the study. ATC and WHCL performed the literature search, screened records, and extracted data and data analysis. ATC and WHCL drafted the manuscript. All authors critically revised and approved the final manuscript.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.National Cancer Institute. SEER Cancer Statistics Review (CSR) 1975-2016. Surveillance, Epidemiology, and End Results (SEER) Program. 2019. https://seer.cancer.gov/archive/csr/1975_2016/browse_csr.php?sectionSEL=29&pageSEL=sect_29_table.06. Assessed 10 Mar 2020.
- 2.Castellino SM, Geiger AM, Mertens AC, Leisenring WM, Tooze JA, Goodman P, Stovall M, Robison LL, Hudson MM. Morbidity and mortality in long-term survivors of Hodgkin lymphoma: a report from the Childhood Cancer Survivor Study. Blood. 2011;117(6):1806–1816. doi: 10.1182/blood-2010-04-278796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Armenian SH, Landier W, Hudson MM, Robison LL. Bhatia S; COG Survivorship and Outcomes Committee. Children's Oncology Group's 2013 blueprint for research: survivorship and outcomes. Pediatr Blood Cancer. 2013;60(6):1063–1068. doi: 10.1002/pbc.24422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cheung AT, Li WHC, Ho LLK, Ho KY, Chiu SY, Chan GCF, et al. Impact of brain tumor and its treatment on the physical and psychological well-being, and quality of life amongst pediatric brain tumor survivors. Eur J Oncol Nurs. 2019;41:104–109. doi: 10.1016/j.ejon.2019.06.003. [DOI] [PubMed] [Google Scholar]
- 5.Daniel LC, Wang M, Mulrooney DA, Srivastava DK, Schwartz LA, Edelstein K, Brinkman TM, Zhou ES, Howell RM, Gibson TM, Leisenring W, Oeffinger KC, Neglia J, Robison LL, Armstrong GT, Krull KR. Sleep, emotional distress, and physical health in survivors of childhood cancer: a report from the Childhood Cancer Survivor Study. Psychooncology. 2019;28(4):903–912. doi: 10.1002/pon.5040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ho LLK, Li WHC, Cheung AT, Ho KY, Lam KKW, Chiu SY, et al. Relationships among hope, psychological well-being and health-related quality of life in childhood cancer survivors [published online ahead of print, 2019 Oct 17]. J Health Psychol. 2019:1359105319882742. 10.1177/1359105319882742. [DOI] [PubMed]
- 7.Nathan PC, Greenberg ML, Ness KK, Hudson MM, Mertens AC, Mahoney MC, Gurney JG, Donaldson SS, Leisenring WM, Robison LL, Oeffinger KC. Medical care in long-term survivors of childhood cancer: a report from the childhood cancer survivor study. J Clin Oncol. 2008;26(27):4401–4409. doi: 10.1200/JCO.2008.16.9607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gilliam MB, Schwebel DC. Physical activity in child and adolescent cancer survivors: a review. Health Psychol Rev. 2013;7(1):92–110. doi: 10.1080/17437199.2011.603641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jones LW, Liu Q, Armstrong GT, Ness KK, Yasui Y, Devine K, Tonorezos E, Soares-Miranda L, Sklar CA, Douglas PS, Robison LL, Oeffinger KC. Exercise and risk of major cardiovascular events in adult survivors of childhood hodgkin lymphoma: a report from the childhood cancer survivor study. J Clin Oncol. 2014;32(32):3643–3650. doi: 10.1200/JCO.2014.56.7511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.San Juan AF, Wolin K, Lucía A. Physical activity and pediatric cancer survivorship. Recent Results Cancer Res. 2011;186:319–347. doi: 10.1007/978-3-642-04231-7_14. [DOI] [PubMed] [Google Scholar]
- 11.Schindera C, Weiss A, Hagenbuch N, Otth M, Diesch T, von der Weid N, et al. Physical activity and screen time in children who survived cancer: a report from the Swiss Childhood Cancer Survivor Study. Pediatr Blood Cancer. 2020;67(2):e28046. doi: 10.1002/pbc.28046. [DOI] [PubMed] [Google Scholar]
- 12.Chung OK, Li HC, Chiu SY, Ho KY, Lopez V. The impact of cancer and its treatment on physical activity levels and behavior in Hong Kong Chinese childhood cancer survivors. Cancer Nurs. 2014;37(3):E43–E51. doi: 10.1097/NCC.0b013e3182980255. [DOI] [PubMed] [Google Scholar]
- 13.Kelly AK. Physical activity prescription for childhood cancer survivors. Curr Sports Med Rep. 2011;10(6):352–359. doi: 10.1249/JSR.0b013e318237be40. [DOI] [PubMed] [Google Scholar]
- 14.Winter C, Müller C, Hoffmann C, Boos J, Rosenbaum D. Physical activity and childhood cancer. Pediatr Blood Cancer. 2010;54(4):501–510. doi: 10.1002/pbc.22271. [DOI] [PubMed] [Google Scholar]
- 15.Warner JT. Body composition, exercise and energy expenditure in survivors of acute lymphoblastic leukaemia. Pediatr Blood Cancer. 2008;50(2 Suppl):456–468. doi: 10.1002/pbc.21411. [DOI] [PubMed] [Google Scholar]
- 16.Swaminathan S, Vaz M. Childhood physical activity, sports and exercise and noncommunicable disease: a special focus on India. Indian J Pediatr. 2013;80(Suppl 1):S63–S70. doi: 10.1007/s12098-012-0846-1. [DOI] [PubMed] [Google Scholar]
- 17.Baumann FT, Bloch W, Beulertz J. Clinical exercise interventions in pediatric oncology: a systematic review. Pediatr Res. 2013;74(4):366–374. doi: 10.1038/pr.2013.123. [DOI] [PubMed] [Google Scholar]
- 18.Braam KI, van der Torre P, Takken T, Veening MA, van Dulmen-den Broeder E, Kaspers GJ. Physical exercise training interventions for children and young adults during and after treatment for childhood cancer. Cochrane Database Syst Rev. 2016;3(3):CD008796. doi: 10.1002/14651858.CD008796.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huang TT, Ness KK. Exercise interventions in children with cancer: a review. Int J Pediatr. 2011;2011:461512–461511. doi: 10.1155/2011/461512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Morales JS, Valenzuela PL, Rincón-Castanedo C, Takken T, Fiuza-Luces C, Santos-Lozano A, Lucia A. Exercise training in childhood cancer: A systematic review and meta-analysis of randomized controlled trials. Cancer Treat Rev. 2018;70:154–167. doi: 10.1016/j.ctrv.2018.08.012. [DOI] [PubMed] [Google Scholar]
- 21.Rustler V, Hagerty M, Daeggelmann J, Marjerrison S, Bloch W, Baumann FT. Exercise interventions for patients with pediatric cancer during inpatient acute care: a systematic review of literature. Pediatr Blood Cancer. 2017;64(11). 10.1002/pbc.26567.10.1002/pbc.26567. [DOI] [PubMed]
- 22.Wong J, Fetters L. Effects of exercise intervention for children with acute lymphoblastic leukemia: a systematic review. Rehab Oncol. 2014;32(3):40–51. doi: 10.1097/01893697-201432030-00006. [DOI] [Google Scholar]
- 23.Du H, Chen J, Qin M, Fang J, Li Z, Zhu Y, et al. Pediatric hematopoietic stem cell transplantation in China: Data and trends during 1998-2012. Pediatr Transplant. 2015;19(5):563–570. doi: 10.1111/petr.12525. [DOI] [PubMed] [Google Scholar]
- 24.Moher D, Shamseer L, Clarke M, Ghersi D, Liberati A, Petticrew M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev. 2015;4(1):1. doi: 10.1186/2046-4053-4-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Centre for Reviews and Dissemination (CRD). Systematic Reviews: CRD’s Guidance for Undertaking Reviews in Health Care. University of York; 2009. https://www.york.ac.uk/crd/SysRev/!SSL!/WebHelp/SysRev3.htm. Accessed 18 Nov 2019.
- 26.Higgins JPT, Savović J, Page MJ, Elbers RG, Sterne JAC. Chapter 8: Assessing risk of bias in a randomized trial. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA. Cochrane Handbook for Systematic Reviews of Interventions version 6.0 (updated July 2019). Cochrane; 2019. https://training.cochrane.org/handbook/current/chapter-08. Accessed 18 Nov 2019.
- 27.Howell CR, Krull KR, Partin RE, Kadan-Lottick NS, Robison LL, Hudson MM, Ness KK. Randomized web-based physical activity intervention in adolescent survivors of childhood cancer. Pediatr Blood Cancer. 2018;65(8):e27216. doi: 10.1002/pbc.27216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Huang JS, Dillon L, Terrones L, Schubert L, Roberts W, Finklestein J, Swartz MC, Norman GJ, Patrick K. Fit4Life: a weight loss intervention for children who have survived childhood leukemia. Pediatr Blood Cancer. 2014;61(5):894–900. doi: 10.1002/pbc.24937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mendoza JA, Baker KS, Moreno MA, Whitlock K, Abbey-Lambertz M, Waite A, et al. A Fitbit and Facebook mHealth intervention for promoting physical activity among adolescent and young adult childhood cancer survivors: a pilot study. Pediatr Blood Cancer. 2017;64(12). 10.1002/pbc.26660. [DOI] [PubMed]
- 30.Stern M, Bleck J, Ewing LJ, Davila E, Lynn C, Hale G, Mazzeo S. NOURISH-T: Targeting caregivers to improve health behaviors in pediatric cancer survivors with obesity. Pediatr Blood Cancer. 2018;65(5):e26941. doi: 10.1002/pbc.26941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sabel M, Sjölund A, Broeren J, Arvidsson D, Saury JM, Blomgren K, Lannering B, Emanuelson I. Active video gaming improves body coordination in survivors of childhood brain tumours. Disabil Rehabil. 2016;38(21):2073–2084. doi: 10.3109/09638288.2015.1116619. [DOI] [PubMed] [Google Scholar]
- 32.Li HC, Chung OK, Ho KY, Chiu SY, Lopez V. Effectiveness of an integrated adventure-based training and health education program in promoting regular physical activity among childhood cancer survivors. Psychooncology. 2013;22(11):2601–2610. doi: 10.1002/pon.3326. [DOI] [PubMed] [Google Scholar]
- 33.Li WHC, Ho KY, Lam KKW, Lam HS, Chui SY, Chan GCF, Cheung AT, Ho LLK, Chung OK. Adventure-based training to promote physical activity and reduce fatigue among childhood cancer survivors: a randomized controlled trial. Int J Nurs Stud. 2018;83:65–74. doi: 10.1016/j.ijnurstu.2018.04.007. [DOI] [PubMed] [Google Scholar]
- 34.Wu LM, Chen CM, Hsu HT, Liu Y, Su HL. Tailored education enhances healthy behaviour self-efficacy in childhood cancer survivors: A randomised controlled study with a 4-month follow-up. Eur J Cancer Care (Engl). 2019;28(4):e13063. doi: 10.1111/ecc.13063. [DOI] [PubMed] [Google Scholar]
- 35.Sabel M, Sjölund A, Broeren J, Arvidsson D, Saury JM, Gillenstrand J, Emanuelson I, Blomgren K, Lannering B. Effects of physically active video gaming on cognition and activities of daily living in childhood brain tumor survivors: a randomized pilot study. Neurooncol Pract. 2017;4(2):98–110. doi: 10.1093/nop/npw020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Free C, Phillips G, Galli L, Watson L, Felix L, Edwards P, Patel V, Haines A. The effectiveness of mobile-health technology-based health behaviour change or disease management interventions for health care consumers: a systematic review. PLoS Med. 2013;10(1):e1001362. doi: 10.1371/journal.pmed.1001362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.World Health Organization. mHealth: New horizons for health through mobile technologies. 2011. https://www.who.int/goe/publications/goe_mhealth_web.pdf. Accessed 10 Dec 2019.
- 38.WHO Global Observatory for eHealth. mHealth: New horizons for health through mobile technologies: second global survey on eHealth. 2011. https://apps.who.int/iris/handle/10665/44607. Accessed 10 Dec 2019.
- 39.Kaplan WA. Can the ubiquitous power of mobile phones be used to improve health outcomes in developing countries? Global Health. 2006;2:9. doi: 10.1186/1744-8603-2-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Archer S, Holch P, Armes J, Calman L, Foster C, Gelcich S, et al. "No turning back" Psycho-oncology in the time of COVID-19: Insights from a survey of UK professionals [published online ahead of print, 2020 Jul 20]. Psychooncology. 2020. 10.1002/pon.5486. [DOI] [PMC free article] [PubMed]
- 41.Mutrie N, Campbell AM, Whyte F, McConnachie A, Emslie C, Lee L, Kearney N, Walker A, Ritchie D. Benefits of supervised group exercise programme for women being treated for early stage breast cancer: pragmatic randomised controlled trial. BMJ. 2007;334(7592):517. doi: 10.1136/bmj.39094.648553.AE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Simoes EJ, Hallal P, Pratt M, Ramos L, Munk M, Damascena W, Parra Perez D, Hoehner CM, Gilbertz D, Malta DC, Brownson RC. Effects of a community-based, professionally supervised intervention on physical activity levels among residents of Recife, Brazil. Am J Public Health. 2009;99(1):68–75. doi: 10.2105/AJPH.2008.141978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shields N, Synnot A. Perceived barriers and facilitators to participation in physical activity for children with disability: a qualitative study. BMC Pediatr. 2016;16:9. doi: 10.1186/s12887-016-0544-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Marimuthu M, Paulose H. Emergence of sustainability based approaches in healthcare: expanding research and practice. Procedia Soc Behav Sci. 2016;224:554–561. doi: 10.1016/j.sbspro.2016.05.437. [DOI] [Google Scholar]
- 45.Badawy SM, Barrera L, Sinno MG, Kaviany S, O'Dwyer LC, Kuhns LM. Text messaging and mobile phone apps as interventions to improve adherence in adolescents with chronic health conditions: a systematic review. JMIR Mhealth Uhealth. 2017;5(5):e66. doi: 10.2196/mhealth.7798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Majeed-Ariss R, Baildam E, Campbell M, Chieng A, Fallon D, Hall A, McDonagh JE, Stones SR, Thomson W, Swallow V. Apps and adolescents: a systematic review of adolescents' use of mobile phone and tablet apps that support personal management of their chronic or long-term physical conditions. J Med Internet Res. 2015;17(12):e287. doi: 10.2196/jmir.5043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rosen L, Zucker D, Brody D, Engelhard D, Manor O. The effect of a handwashing intervention on preschool educator beliefs attitudes, knowledge and self-efficacy. Health Educ. Res. 2009;24:686–698. doi: 10.1093/her/cyp004. [DOI] [PubMed] [Google Scholar]
- 48.Kelly MP, Barker M. Why is changing health-related behaviour so difficult? Public Health. 2016;136:109–116. doi: 10.1016/j.puhe.2016.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]