Abstract
Purpose
To study the learning curve and outcomes of the first 100 cases of minimally invasive cardiac surgery (MICS) coronary artery bypass grafting (CABG) performed at our center.
Methods
From January 2017 to November 2019, a total of 100 patients underwent CABG via left anterior thoracotomy approach. We have studied the operative times within the MICS CABG patients to analyze our learning curve. We also studied the postoperative outcomes and compared these with those of patients who underwent sternotomy during the same period.
Results
The mean age was 59.33 ± 9.95 (range 37–82) years. The numbers of males and females were 72 and 28 respectively. The preoperative average ejection fraction (EF) was 51.08 ± 9.75%. All these patients underwent CABG via left thoracotomy approach, after satisfying the exclusion criteria. All patients received left internal mammary artery (LIMA) to left anterior descending (LAD) as a standard graft, with the radial artery and saphenous vein being the next alternative conduits. The average length of the incision was 6.06 ± 0.45 cm. Only 2 cases were done on pump. The average number of grafts per patient was 2.33 ± 0.92. The mean operative time was 132.40 ± 11.56 min. The mean duration of ventilation was 4.79 ± 1.90 h and average intensive care unit (ICU) stay was 2.62 ± 0.84 days. There was one conversion and no mortalities in our study. We had analyzed our operative times and noticed a significant reduction after the first 20 cases, which was our learning curve.
Conclusion
MICS CABG can be performed for multivessel disease with the same comfort as for a single or a double vessel disease, once the learning curve has been achieved. Only significant difference from the sternotomy approach was noted in the longer operative times for MICS CABG during the learning curve, and not thereafter. Significant benefits of MICS over sternotomy were noticed in the immediate postoperative parameters like duration of ventilation, mean drainage, postoperative pain, ICU stay, and hospital stay, with no difference in postoperative adverse events.
Keywords: MICS CABG, Multivessel bypass, Learning curve
Introduction
We are in the era of minimally invasive cardiac surgery (MICS) [1, 2]. There has been a considerable rise in general awareness on MICS in the patient population and is being preferred wherever suitable. Surgeons are constantly involved in the process of upgrading the techniques of MICS, robotic telemanipulations, and transcatheter interventions. There are now studies that have put forth the benefits of MICS multivessel coronary artery bypass grafting (CABG) performed via left anterior thoracotomy and their non-inferiority to sternotomy approach [3]. In this study, we have presented our journey of MICS CABG, which began with left internal mammary artery (LIMA) to left anterior descending (LAD) grafting and graduated to multivessel CABG via left anterior thoracotomy with acceptable results.
Material and methods
In this retrospective, observational, non-randomized study, we included all patients undergoing MICS CABG at our center between January 2017 and November 2019. The study was approved by the institutional ethics committee and need for individual consent was waived off. All of these patients were operated on by a single surgeon during the above mentioned time frame. Even though the main intention of the study was to describe our experience with MICS, we also performed an unmatched comparison with patients undergoing CABG through a median sternotomy during the same time period. Of all the coronary revascularizations performed during the study period, 100 (21.73%) were operated through a left anterior thoracotomy approach. We have studied the operative times and analyzed the learning curve at our institute. Operative time discussed in the study was the duration from skin incision until skin closure (skin to skin). A comparison of operative times during the learning curve (the first 20 cases) and in the later part of the study was carried out. For this comparison, data was analyzed separately for single and double as well as multiple grafts, and operative time was compared with that of sternotomy as well.
The primary end points of the study were to analyze the operative times and immediate postoperative outcomes which included duration of ventilation, pain scoring, mean total drainage, number of blood transfusions, day of mobilization out of bed, and duration of intensive care unit (ICU) and in-hospital stay for the MICS CABG patients. The secondary end points of the study were adverse postoperative events like acute kidney injury (AKI), arrhythmias, stroke, recurrent myocardial infarction (MI), wound infections, readmission into hospital and death. These parameters were compared with those operated on by sternotomy approach to understand the benefits of the MICS technique.
Inclusion and exclusion criteria
All patients with target coronaries that could be grafted via a left thoracotomy approach based on coronary angiography, along with their willingness to undergo MICS CABG, were included in the study. The preoperative workup included a coronary angiography, an electrocardiograph (ECG), chest radiograph, 2D echocardiography, and supportive blood investigations. Patients who were deemed not ideal for a MICS procedure, or who did not consent for MICS procedure, as it was an evolving technique at our institution, were operated on using the conventional median sternotomy approach. In a small cohort of patients, where complete revascularization was not possible through a MICS approach, we counseled the patients to undergo a hybrid coronary revascularization (HCR).
Patients with an associated valvular pathology, congenital abnormality, diffuse coronary artery disease with poor distal runoff, ejection fraction (EF) < 25%, prior cardiac surgery, acute/recent MI within the last 7 days, peripheral vascular disease (PVD), active smokers, history of tuberculosis or interstitial lung diseases, spine deformities like kyphosis and scoliosis, and recent history of stroke (6 weeks prior to surgery) were excluded from the study. Relative contra-indications like obesity and females with large breasts were accepted for MICS. Because of the high prevalence of chronic kidney disease (CKD) in our patient population, we had a higher threshold for excluding these patients. These patients were included for MICS if the serum creatinine was < 1.8 mg/dL, with no rise in the value over 3 consecutive preoperative days. Smokers were accepted only after cessation for a minimum period of 4 weeks prior to surgery. Patients with a history of smoking were subjected to pulmonary function testing (PFT) and peripheral vascular (arterial and venous) Doppler study. A ratio of forced expiratory volume in the first 1 s to the forced vital capacity (FEV1/FVC) < 0.75 and a PaCO2 > 50 mmHg and PaO2 < 65 mmHg on room air were not accepted for one lung ventilation.
Procedural aspects
After satisfying the exclusion criteria mentioned above, patients were taken up for MICS CABG. The anesthesiologist assessed the patient suitability for one lung ventilation and the required values, i.e., PaCO2 < 50 mmHg and PaO2 > 65 mmHg on room air, were checked by a preoperative arterial blood gas (ABG) analysis [4]. A total of 6 patients were excluded from the study who did not meet the above criteria on an ABG.
The positioning of the patient was a key step to avoid later difficulties during the procedure related to visualization. A 30° elevation of the left chest with both the upper limbs in adduction was the routine position. Once draped, a few landmarks were marked using a sterile marker pen, which included the midline and intercostal spaces (ICS) (especially 3rd until the 5th) (Fig. 1). The thorax was usually entered through the 5th ICS based on the level of cardiac apex on chest radiograph. The site of incision was one fingerbreadth below the left nipple in males and half an inch below the left sub-mammary crease in females. A change in ICS was considered, if there was an issue with either accessibility or visualization.
Fig. 1.
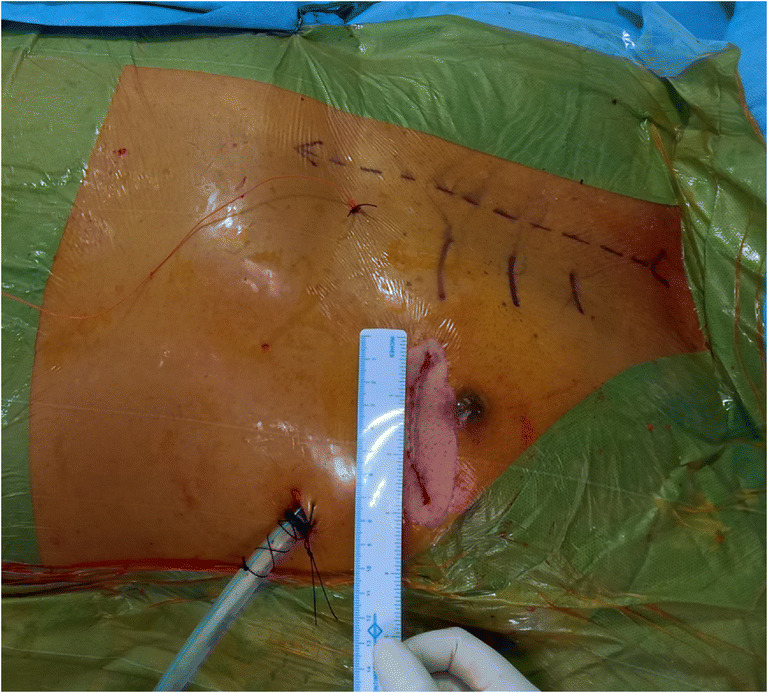
Postoperative image wherein the scar is being measured. On the medial aspect of the chest, midline (dashed line) is marked as well as the 2nd, 3rd, and 4th intercostal spaces (horizontal lines). The epicardial pacing wire and the intercostal drainage tube are also seen
Fehling MICS instruments (Fehling instruments GmbH & Co., Germany) including chest spreader and internal mammary artery (IMA) arc retractor were used at our center. Harvesting of LIMA was done with monopolar cautery during the initial days of the program, but later changed over to harmonic scalpel with a hook tip. The first intercostal branch was dissected and clipped without fail. Cautious clipping and adequate hemostasis were necessary as bleeding in this scenario was found to be troublesome. The adjunct conduits used were the left radial artery (47%) and the great saphenous vein (GSV) (9%). Radial artery was used as the second conduit of choice. This allowed LIMA-radial Y anastomosis and avoided the need for constructing proximal aortic anastomosis. The LIMA-radial Y anastomosis was done prior to distal coronary anastomosis using 8–0 polypropylene suture in a continuous fashion. Wherever GSV was used, the proximal anastomosis was done first on the ascending aorta with a partial clamp using 6–0 polypropylene suture in a continuous fashion.
Myocardial stabilizers used were Nuvo Octopus® (TSMICS1, Medtronic Inc., USA), conventional Octopus® Evolution AS (TS2500, Medtronic Inc., USA), and Starfish® Evo cardiac positioner (Medtronic Inc., USA). The choice of stabilization devices was dependent on the visualization and position of the target vessel. The conventional Octopus, wherever used, was fixed either to the rib spreader or to the manubrium hook of the arc retractor. The sequence of grafting was LAD followed by diagonal, ramus, obtuse marginal (OM), and posterior descending artery (PDA). In sequential grafts using the LIMA, diagonal was the first to be grafted as side to side anastomosis was followed by end to side LAD anastomosis. All the distal anastomoses were done using 8–0 polypropylene sutures with an 8-mm needle.
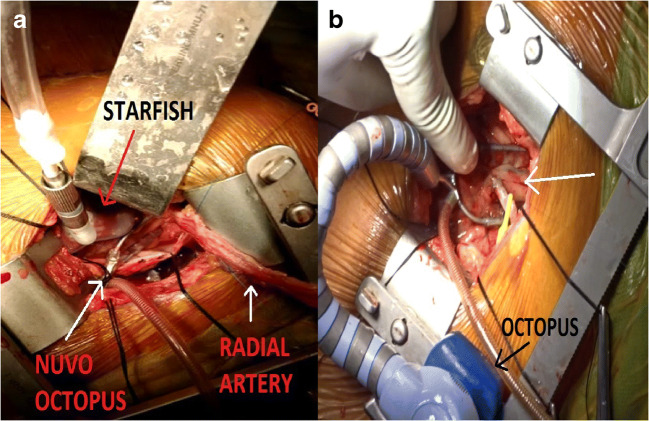
For proper positioning of the heart, pericardial stay sutures were taken using a “0” silk suture along the pericardial margins and fixed to the drape sheets. The heart was maneuvered using the handle of the Langenbeck retractor and sponges were placed behind to maintain the position. We found difficulty exposing the PDA more frequently than the OM artery. We waited for a couple of minutes after positioning the heart to check for cardiac stability before proceeding to grafting. For LAD and diagonal anastomoses, Nuvo Octopus was used via a stab incision, two ICS below the thoracotomy incision, along the anterior axillary line. For the remaining grafts, we utilized a Starfish suction device to lift the apex, followed by the use of either Nuvo Octopus or the conventional Octopus to continue with grafting (Fig. 2a). In tight spaces with poor visualization, Nuvo Octopus gave better visualization along with more operating space. If the space and visualization were found to be adequate, we used the conventional Octopus (Fig. 2b).
Fig. 2.
a Intraoperative image showing the usage of Starfish cardiac positioner to lift the apex while the Nuvo Octopus myocardial stabilization device is used to position for grafting the obtuse marginal (OM) artery. b Intraoperative image where SVG was used to graft the OM (white arrow). In this case, the conventional Octopus was utilized and was fixed to the rib spreader
Off-pump beating heart CABG was our routine practice. Only 2 out of 100 patients were operated on with cardiopulmonary bypass (CPB) due to intraoperative hemodynamic instability. The right groin was draped and kept ready in all of our patients. Cannulation was done after an open cut down, exposing the femoral artery and vein. Both the cases were done by on-pump beating heart technique. The femoral artery and vein were repaired, after decannulation, in two layers with a 6–0 polypropylene suture. Those patients, in whom the intended grafts could not be done via left thoracotomy, were taken for percutaneous transluminal coronary angioplasty (PTCA) with a stent to the target vessel the following day. PTCA was done in the cardiac catheterization laboratory, as we did not have a hybrid operating room (OR).
Thoracotomy closure was done by approximating the rib space with a pericostal number 5 polyester suture. Chest wall muscles were closed in layers using number 1 vicryl intermittent sutures. One chest drainage tube along with one ventricular epicardial pacing wire was our practice (Fig. 1). Chest drain was also introduced via the same port which was utilized for the introduction of Nuvo Octopus stabilization device.
Postoperative management and follow-up
In patients who received radial arterial grafts, intravenous (IV) infusion of diltiazem was started at 10 mg/h in the OR and continued for the next 24 h. Later, it was bridged to oral diltiazem 30 mg thrice a day dosage. The protocol for pain management at our institution was to shift the patient from the OR on IV fentanyl infusion at a dosage of 0.75–1 mcg/h until extubation. Once extubated, they received IV paracetamol 1 g every 6 h for next 24 h and bridged to oral 650 mg every 6 h. During this period, the pain was assessed using Wong–Baker Faces pain rating scale with scores between 0 (no hurt) and 10 (hurts worst). We monitored this pain score over the first 3 postoperative days. Intercostal drainage (ICD) tube was removed if the total drain was < 100 ml over 24 h.
Our discharge medications included dual anti-platelet therapy of aspirin (150 mg) and clopidogrel (75 mg) along with a lipid-lowering drug. Ticagrelor (180 mg per day) was used, instead of clopidogrel, in patients who underwent HCR. Oral Diltiazem of 90 mg/day was advised to those who received radial artery as a conduit and was continued for a period of 12 months post-surgery. Patient follow-ups were reviewed on an outpatient basis. The follow-up included a 2D echocardiography, ECG, and chest radiograph. The follow-up schedule was at 1 week after discharge, at the end of 3 months, and thereafter every 6 months. After a period of 12 months, a computerized tomography (CT) coronary angiography along with 2D echocardiography, ECG, and chest radiograph was advised. But, the decision to undergo a CT coronary angiography was left to the choice of the patient.
Statistical analysis
The data collected was tabulated and analyzed using the IBM SPSS statistics for Windows, version 20.0 (Armonk, NY: IBM Corp.). Parameters such as co-morbidities and complications were expressed as percentages and frequency. Parameters related to echocardiography, age, operative times, etc., were expressed as mean with standard deviation. The comparison between the MICS CABG and the sternotomy groups was done by using a chi-square test for categorical variables and independent sample t test for numeric variables. A p value of < 0.05 was considered significant.
Results
Preoperative patient characteristics
A total of 846 cardiac procedures were performed at our center from January 2017 until November 2019 comprising 468 (55.32%) coronary revascularizations and 378 (44.68%) non-coronary procedures. Out of the total coronary revascularizations, 100 (21.37%) patients were operated on via left anterior thoracotomy approach. Year-wise percentages of MICS coronary revascularizations were 13.82% (n = 17), 18.47% (n = 29), and 28.72% (n = 54) of the total coronary revascularizations for the years 2017, 2018, and 2019, respectively. The percentages of single, double, triple, and 4 grafts were 70.5% (n = 12) and 29.4% (n = 5), and no 3 or more grafts in the first year; 27.5% (n = 8), 37.93% (n = 11), 24.13% (n = 7), and 10.24% (n = 3) in the second year; and 11.1% (n = 6), 24.07% (n = 13), 44.44% (n = 24), and 20.37% (n = 11) in the third year, respectively (Fig. 3).
Fig. 3.
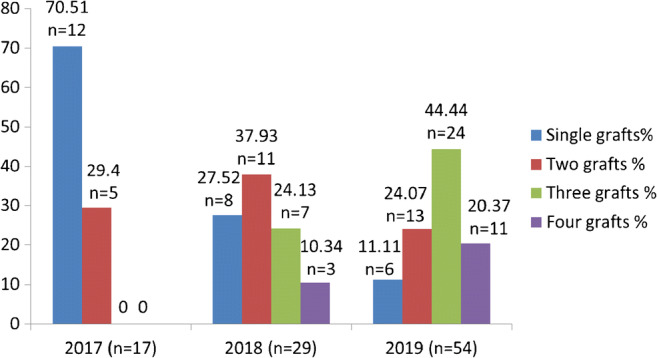
The graphical representation of number of grafts performed over the period of 3 years. The percentage of the number of grafts on Y axis and the year it was performed on X axis
The mean age of the MICS patients was 59.33 ± 9.95 (range 37–82) years. There was a predominance of males in the study, with the numbers of males and females being 72 (72%) and 28 (28%) respectively. The numbers of patients with hypertension and diabetes mellitus were 76 (76%) and 56 (56%) respectively. The preoperative average EF was 51.08 ± 9.75%. Most of the patients were symptomatic in the preoperative period, in the Canadian Cardiovascular Society (CCS) grades III (71%) and IV (21%), while only 8 patients were in grade II. The other comorbidities included CKD in 4 (4%), chronic obstructive pulmonary disease (COPD) 14 (14%), and tobacco use 18 (18%), and 13 (13%) patients had history of hypothyroidism on oral levothyroxine and were confirmed to be euthyroid prior to surgery. Twenty-four (24%) patients had previous MI, 7 (7%) had unstable angina, and 8 (8%) patients had prior PTCA. Nine (9%) patients had left main coronary artery (LMCA) disease, 27 (27%) of them had single vessel disease (SVD), 31 (31%) of them had double vessel disease (DVD), and 42 (42%) had triple vessel disease (TVD). The average risk of mortality, as calculated using the online STS risk score calculator (STS Adult Cardiac Surgery database version 2.9) was 1.3 ± 0.7 (Table 1). Six (6%) patients had presented with cardiogenic shock due to acute MI. They were managed medically and none required intra-aortic balloon pump (IABP). They were accepted for surgery 14 days after medical optimization in the hospital. The mean preoperative EF of these 6 patients was 33.5 ± 1.22%.
Table 1.
Preoperative patient characteristics
| Pre-op patient characteristics (n = 100) | |
|---|---|
| Mean age (in years) | 59.33 ± 9.95 (range 37–82) |
| Male/female | 72 (72%)/28 (28%) |
| Hypertension | 76 (76%) |
| Diabetes mellitus | 56 (56%) |
| Canadian Cardiovascular Society (CCS) grading | |
| Grade I | 0 |
| Grade II | 8 |
| Grade III | 71 |
| Grade IV | 21 |
| Smokers | 18 (18%) |
| Chronic kidney disease | 4 (4%) |
| Thyroid disorder | 13 (13%) |
| Mean pre-op ejection fraction (in %) | 51.08 ± 9.75 |
| COPD | 14 (14%) |
| Cardiogenic shock (recovered) | 6 (6%) |
| Prior PTCA | 8 (8%) |
| Previous MI | 24 (24%) |
| Unstable angina | 7 (7%) |
| LMCA disease | 9 (9%) |
| SVD | 27 (27%) |
| DVD | 31 (31%) |
| TVD | 42 (42%) |
| STS risk scoring (risk of mortality) (mean) | 1.3 ± 0.7 |
COPD, chronic obstructive pulmonary disease; PTCA, percutaneous transluminal coronary angioplasty; LMCA, left main coronary artery; SVD, single vessel disease; DVD, double vessel disease; TVD, triple vessel disease; MI, myocardial infarction; STS, Society of Thoracic Surgeons
Intraoperative details
Ninety-eight patients were operated on beating heart and only 2 needed the support of CPB. The average length of the incision was 6.06 ± 0.45 cm. The average number of grafts per patient was 2.33 ± 0.92. Twenty-six patients received one graft, 29 received 2 grafts, and 31 and 14 patients received 3 and 4 grafts respectively. LAD was grafted in all 100 patients, and 25 patients received a graft to diagonal, of which 19 were in the form of a sequence of LIMA to diagonal and LAD. Thirty-two patients received a graft to the ramus, 58 to the OM, and 18 to the PDA. The ratio of intended grafts to the performed grafts was 259/233, wherein a total of 26 (10.03%) grafts could not be done as planned via left thoracotomy. These patients underwent HCR with a stent to the right coronary artery (RCA) in 22 patients and to PDA in 4 patients the following day. The average time interval between CABG and stenting was 18.84 ± 4.48 h.
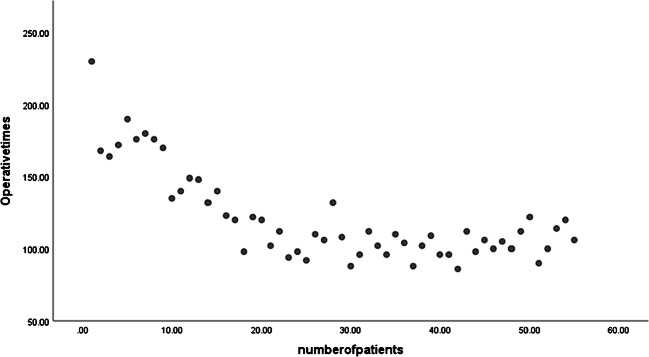
The average operative time of all 100 cases was 132.06 ± 11.56 min. The mean operative times in minutes based on the number grafts were 114.8 ± 37.55, 113.14 ± 21.58, 129.45 ± 8.67, and 145.21 ± 14.6 for 1, 2, 3, and 4 graft cases, respectively (Table 2). We analyzed our findings in a way similar to the study by Une and colleagues [5]. The study population was divided into two groups, those with single and double grafts (n = 55) and the multivessel bypass (> 2 grafts) as the other group (n = 45). We plotted scatter chart of the operative time for the single and double graft group in a sequential order from the beginning of the program (Fig. 4). This graph clearly shows a dip after the 20th case. The mean operative time for the first 20 cases was 164.66 ± 25.61 min, which was reduced to 105.17 ± 10.96 min for the remaining 35 cases, which was our learning curve. But this difference in the operative times was not observed in the multivessel bypass cases, as they were started much later after the MICS program was initiated, in other words, after the learning curve was achieved. In the multivessel bypass group, operative times for the first 20 cases were 133.06 ± 11.10 min and for the remaining 25 cases 135.39 ± 14.20 min respectively. These comparisons between the two groups (Table 3a, b) clearly depict that, apart from the operative times in the first 20 cases in the single and double graft group, there was no significant difference.
Table 2.
Peri-operative patient characteristics
| Intraoperative patient characteristics (n = 100) | |
|---|---|
| On pump | 2 (2%) |
| Off pump | 98 (98%) |
| Average size of incision (centimeters) | 6.06 ± 0.45 |
| Conduits | |
| LIMA | 100 (100%) |
| Radial | 47 (47%) |
| Saphenous vein | 9 (9%) |
| Grafts | |
| LAD | 100 (100%) |
| Diagonal | 25 (25%) |
| Ramus | 32 (31%) |
| OM | 58 (58%) |
| PDA | 18 (18%) |
| Total grafts (average) | 2.33 ± 0.92 |
| Ratio of intended grafts to performed grafts | (259/233) |
| Number of grafts | |
| Single graft | 26 |
| Two grafts | 29 |
| Three grafts | 31 |
| Four grafts | 14 |
| LIMA used as sequence to diagonal and LAD | 19 (19%) |
| Conversion to sternotomy | 1 (1%) |
| Re-explorations | 2 (2%) |
| Graft revision | 1 (1%) |
| Procedure time in min (mean) (n = 100) | 132.40 ± 11.56 |
| Mean operative time for single grafts (min) (n = 27) | 114.8 ± 37.55 |
| Mean operative time for two grafts (min) (n = 29) | 113.14 ± 21.58 |
| Mean operative time for three grafts (min) (n = 31) | 129.45 ± 8.67 |
| Mean operative time for four grafts (min) (n = 14) | 145.21 ± 14.6 |
| HCR (number of patients) | 26 (26%) |
| Stent to RCA | 22 |
| Stent to PDA | 4 |
LIMA, left internal mammary artery; LAD, left anterior descending; OM, obtuse marginal; PDA, posterior descending artery; HCR, hybrid coronary revascularization
Fig. 4.
A scatter plot, in serial order of each patient who underwent single and double vessel disease plotted on X axis with the operative time of each patient plotted on the Y axis
Table 3.
a Comparison of single and double graft cases that were divided into the first 20 and the remaining cases (n = 35). Note the significant difference in the operative times (p value). b Similar comparison between the first 20 and the remaining 25 cases of multivessel bypass. There was no significant difference in any parameters in this group
| a Comparison of single and double graft cases | |||
| First 20 cases | Remaining cases (n = 35) | p value | |
|
Operative time (in minutes) |
164.66 ± 25.61 | 105.17 ± 10.96 | > 0.0001 |
|
ICU stay (in days) |
3.08 ± 0.72 | 2.85 ± 0.48 | 0.175 |
|
In-hospital stay (in days) |
5.56 ± 1.12 | 5.36 ± 0.89 | 0.493 |
| Re-exploration | 1 | 0 | NA |
| Graft revision | 0 | 0 | NA |
| Peri-op MI | 0 | 0 | NA |
| Mortality | 0 | 0 | NA |
| b Comparison of multivessel bypass cases | |||
| First 20 cases | Remaining cases (n = 25) | p value | |
| Operative time (in minutes) | 133.66 ± 11.10 | 135.39 ± 14.20 | 0.584 |
| ICU stay (in days) | 2.45 ± 0.93 | 2.77 ± 0.57 | 0.159 |
| In-hospital stay (in days) | 5.68 ± 0.12 | 5.29 ± 0.88 | 0.096 |
| Re-exploration | 0 | 0 | NA |
| Graft revision | 0 | 1 | NA |
| Peri-op MI | 0 | 0 | NA |
| Mortality | 0 | 0 | NA |
Postoperative data
The mean duration of ventilation was 4.79 ± 1.90 h and average ICU stay was 2.62 ± 0.84 days. The mean postoperative drainage was 173.33 ± 22.39 ml and the mean number of blood and blood product transfusions accounted to 1.15 ± 0.85 units per patient. Our patients were mobilized out of bed on an average of 1.23 ± 0.16 days. The mean pain scores in the 1st, 2nd, and 3rd postoperative days were 3.28 ± 0.69, 2.43 ± 0.28, and 2.22 ± 0.35 respectively. The postoperative complications observed were AKI in 5 patients apart from the 4 patients with prior CKD, though none required renal replacement therapy as they recovered with conservative management. Nine patients had arrhythmias, all of which were atrial fibrillation (AF). They were managed with intravenous as well as oral amiodarone and beta-blockers. The mean in-hospital stay was 5.09 ± 0.96 days. One patient had a stroke due to an infarct causing left hemiparesis on the immediate day following discharge for which she was readmitted under neurology. None of the patients has had recurrent MI. Two re-explorations were documented, of which one was for bleeding and the other was for hemodynamic instability in the immediate postoperative period. In the latter case, LIMA to LAD graft was revised. Both the re-explorations were carried out through the existing thoracotomy incision. One patient required conversion to sternotomy due to dense pleural adhesions, which made visualization of target vessels and one lung ventilation difficult. No deaths were recorded during the study. Three patients had postoperative wound infection, none required admission and was managed with dressings followed by secondary suturing on an outpatient basis (Table 4).
Table 4.
Postoperative patient characteristics
| Postoperative patient characteristics (n 100) | |
|---|---|
| Mean duration of ventilation (hours) | 4.79 ± 1.90 |
| Total drainage in ml (mean) | 173 ± 0.39 |
| Mean no. of blood transfusions | 1.15 ± 0.85 |
| Pain score: Wong–Baker Faces pain rating (0 to 10) (mean) | |
| POD 1 | 3.28 ± 0.69 |
| POD 2 | 2.43 ± 0.28 |
| POD 3 | 2.22 ± 0.35 |
| Mean day of mobilization out of bed | 1.23 ± 0.16 |
| Acute kidney injury | 5 (5%) |
| Arrhythmias | 9 (9%) |
| Stroke | 1 (1%) |
| ICU stay (mean no. of days) | 2.62 ± 0.84 |
| Hospital stay (mean no. of days) | 5.09 ± 0.96 |
| Post-op EF (in %) | 52.44 ± 6.38. |
| Perioperative MI | 0 |
| Readmission into hospital within 30 days | 1 (1%) |
| Deaths | 0 |
| Wound infections | 3 (3%) |
ICU, intensive care unit; EF, ejection fraction; MI, myocardial infarction; POD, postoperative day
The mean postoperative EF measured at the time of discharge was 52.44 ± 6.38%. The longest follow-up was 34 months and the shortest was 3 months. The CT coronary angiography at the end of the 12 months was performed only in 12 patients who had consented for the same. There were no graft-related issues in all the 12 patients who underwent CT coronary angiography (Fig. 5). On follow-up, two patients presented with chest discomfort: one at 6 months and another at 13 months after surgery. Both were subjected to screening coronary angiography which showed patent LIMA to LAD but occluded radial artery graft to OM in both the cases. In the latter case, radial was used to sequentially graft the diagonal and OM, both of which were occluded. Both these patients were managed conservatively as there was no clinical deterioration.
Fig. 5.
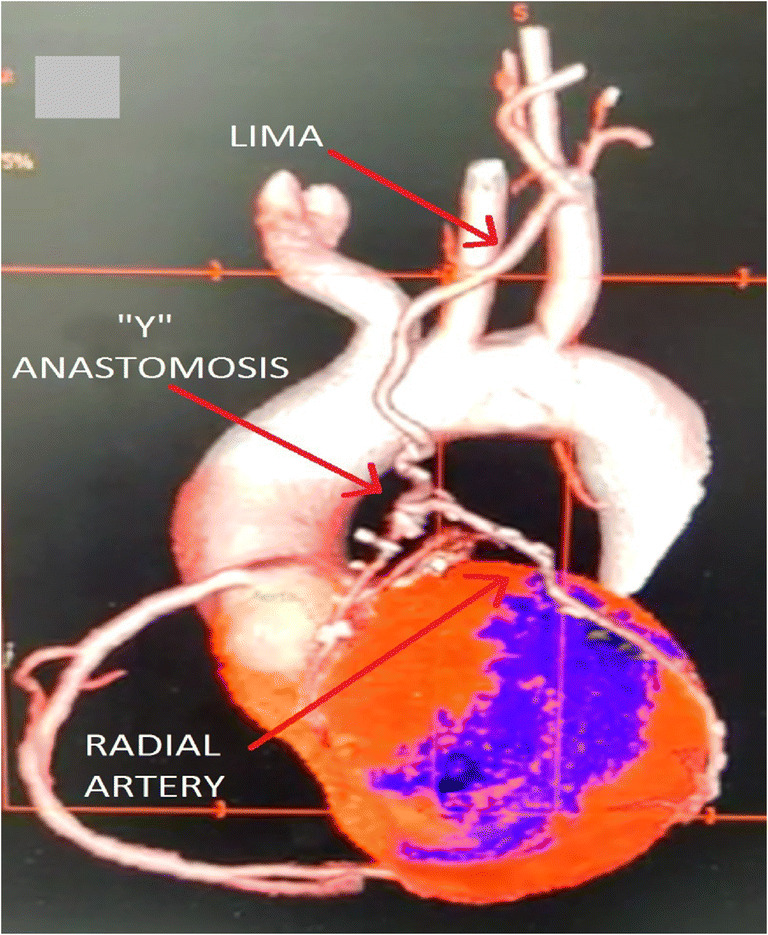
Postoperative CT coronary angiography image showing LIMA-Radial Y anastomosis. LIMA grafted to LAD and radial artery grafted to obtuse marginal artery
Transit time flowmetry (TTFM) was introduced at our center very recently and we have very limited data on the same. Measurements were carried out only in 14 patients so far. We accepted TTFM parameters as published by Kieser and Taggart [6].
Sternotomy patient data
We had compiled data of patients who underwent coronary revascularization via sternotomy approach during the same period. A total of 368 patients underwent coronary revascularization via the sternotomy approach, of which isolated elective CABG was done in 316 (85.87%) patients. Fifty-two (14.13%) patients had associated valvular procedures or were emergency procedures, and hence were not included in this comparison. The number of males in the study was 202 (63.92%) and the mean age of the entire cohort undergoing sternotomy was 63.68 ± 8.68 years (range 32–89). Nineteen (6.01%) had CKD, 33 (10.44%) had thyroid disorders, 29 (9.17%) had COPD, and 21 (6.65%) had history of cardiogenic shock from which they had recovered at the time of surgery. The mean preoperative EF was 45.23 ± 8.56%, while the postoperative EF was 45.86 ± 9.12%. The pre- and postoperative management protocols remained the same between the two groups. The postoperative parameters studied were duration of ventilation, mean total drainage, number of transfusions, pain scoring, duration of ventilation, day of mobilization, and ICU and hospital stay (Table 5a).
Table 5.
a Comparison of baseline, intraoperative, and postoperative parameters between MICS CABG and sternotomy groups. EF, ejection fraction; CKD, chronic kidney disease; COPD, chronic obstructive; IABP, intra-aortic balloon pump, pulmonary disease; POD, postoperative day; AKI, acute kidney injury; MI, myocardial infarction. A p < 0.05 has been considered significant. b A comparison in operative times of MICS CABG and sternotomy cases. In italics is the comparison of single and double graft cases and multivessel bypass cases between the two approaches before and after the learning curve (first 20 cases). A p < 0.05 is considered significant
| a | |||
| Parameter | MICS CABG (n = 100) | Sternotomy (n = 316) | p value |
| Age (mean) | 59.33 ± 9.95 (range 37–82) | 63.68 ± 8.68 (range 32–89) | < 0.0001 |
| Males/females | 72 (72%)/28 (28%) | 202 (63.92%)/114 (36.07%) | 0.138/0.138 |
| Hypertension | 76 (76%) | 259 (81.96%) | 0.190 |
| Diabetes mellitus | 56 (56%) | 236 (74.68%) | 0.0004 |
| CKD | 4 (4%) | 19 (6.01%) | 0.443 |
| Thyroid disorder | 13 (13%) | 33 (10.44%) | 0.477 |
| COPD | 14 (14%) | 29 (9.17%) | 0.167 |
| Cardiogenic shock (recovered) | 6 (6%) | 21 (6.65%) | 0.818 |
| Preop EF (mean) | 51.08 ± 9.75 | 45.23 ± 8.56 | < 0.0001 |
| Average number of grafts | 2.33 ± 0.92 | 3.02 ± 0.63 | < 0.0001 |
| Off pump/on pump | 98 (98%)/2 (2%) | 252 (80.06%)/63 (19.94%) | < 0.0001/< 0.0001 |
| Intraoperative IABP | 0 | 10 (3.16%) | 0.072 |
| Duration of ventilation (mean hours) | 4.79 ± 1.90 | 7.48 ± 2.31 | < 0.0001 |
| ICU stay (mean no. of days) | 2.62 ± 0.84 | 3.18 ± 1.02 | < 0.0001 |
| In-hospital stay (mean no. of days) | 5.09 ± 0.96 | 6.44 ± 1.63 | < 0.0001 |
| Day out of bed (mean day) | 1.23 ± 0.16 | 2.02 ± 1.42 | < 0.0001 |
| Wong–Baker Faces pain rating | |||
| POD 1 | 3.28 ± 0.69 | 3.44 ± 0.64 | 0.033 |
| POD 2 | 2.43 ± 0.28 | 3.02 ± 0.5 | < 0.0001 |
| POD 3 | 2.22 ± 0.35 | 2.87 ± 0.21 | < 0.0001 |
| Total drainage in ml (mean) | 173.33 ± 22.39 | 250.72 ± 37.15 | < 0.0001 |
| Transfusions (mean units) | 1.15 ± 0.85 | 1.89 ± 0.87 | < 0.0001 |
| Re-explorations | 2 (2%) | 11 (3.48%) | 0.459 |
| Graft revisions | 1 (1%) | 4 (1.26%) | 0.835 |
| AKI | 5 (5%) | 17 (5.37%) | 0.885 |
| Arrhythmias | 9 (9%) | 43 (13.06%) | 0.278 |
| Stroke | 1 (1%) | 4 (1.26%) | 0.835 |
| Postop EF (mean) | 52.44 ± 6.38 | 45.86 ± 9.12 | < 0.0001 |
| Death | 0 | 5 (1.58%) | 0.206 |
| Perioperative MI | 0 | 3 (0.94%) | 0.331 |
| Deep sternal wound infections | 0 | 6 (1.89%) | 0.166 |
| b | |||
| MICS CABG (n = 100) | Sternotomy (n = 316) | p value | |
| Number of grafts | |||
| One graft | 26 (26%) | 27 (8.69%) | < 0.0001 |
| Two grafts | 29(29%) | 63 (20.11%) | 0.062 |
| Three grafts | 31 (31%) | 101 (32.06%) | 0.842 |
| Four grafts | 14 (14%) | 125 (39.67%) | < 0.0001 |
| Operative time in min (mean) | |||
| One grafts | 114.8 ± 37.55 | 96.45 ± 14.77 | 0.022 |
| Two grafts | 113.14 ± 21.58 | 109.26 ± 21.2 | 0.419 |
| Three grafts | 129.45 ± 8.67 | 123.78 ± 18.43 | 0.1 |
| Four grafts | 145.21 ± 14.6 | 141.6 ± 32.51 | 0.682 |
| During the learning curve | |||
| MICS CABG: single and double graft (first 20 cases)/all single and double graft cases of sternotomy (n = 90) | 164.66 ± 25.61 | 102.85 ± 24.72 | < 0.0001 |
| After the learning curve | |||
| MICS CABG: single and double graft (remaining cases 35)/all single and double graft cases of sternotomy (n = 90) | 105.17 ± 10.96 | 102.85 ± 24.72 | 0.594 |
| MICS CABG: multivessel bypass (all cases n = 45)/all multivessel bypass cases of sternotomy (n 226) | 134.52 ± 17.35 | 132.69 ± 37.46 | 0.748 |
Comparison between MICS and sternotomy groups
The operative time of single vessel sternotomy group (96.45 ± 14.77 min) was significantly less compared with that of single graft MICS CABG group (114.8 ± 37.55 min); p 0.022. During the learning curve, the time for MICS CABG for single and double grafts was significantly longer compared with that of the sternotomy group (164.66 ± 25.61 min vs. 102.85 ± 24.72 min; p < 0.0001). However, after the learning curve, there was no significant difference in the operating time between the groups, either for single and double grafts (105.17 ± 10.96 min and 102.85 ± 24.72 min; p 0.594) or for multivessel bypass grafting (134.52 ± 17.35 min vs. 132.69 ± 37.46 min; p 0.748) (Table 5b).
Comparisons were made between the two groups for the abovementioned postoperative parameters. The parameters which were found significantly lower in the MICS CABG group versus the sternotomy group included mean duration of ventilation (4.79 ± 1.90 h vs. 7.48 ± 2.31 h; p < 0.0001), average of total drainage (173.33 ± 22.39 ml vs. 250.72 ± 37.15 ml; p < 0.0001), mean units of transfusion (1.15 ± 0.85 vs. 1.89 ± 0.87; p < 0.0001), pain scoring, postoperative day of mobilization (1.23 ± 0.16 vs. 2.02 ± 1.42; p < 0.0001), ICU stay (2.62 ± 0.84 days vs. 3.18 ± 1.02 days; p < 0.0001), and hospital stay (5.09 ± 0.96 days vs. 6.44 ± 1.63 days; p < 0.0001), respectively. We noticed no significant difference in postoperative adverse events like incidence of AKI, arrhythmias, stroke, recurrent MI, re-explorations, graft revision, deep sternal wound infection, and deaths between the two groups (Table 5a).
Discussion
Minimally invasive cardiac surgery has advanced with leaps and bounds in the last decade. Instrumentation has improved to a large extent for coronary as well as for valvular and intracardiac repair procedures. The only catch in the MICS technique seems to be the learning curve [7]. Indeed, it is a long and strenuous process, as the surgeon has to perform every step via a small opening with limited visibility, and most undeniably important is the lack of an assistant’s hand.
Everyone begins with a single graft of LIMA to LAD in their path of MICS CABG journey. It has been well established that the initial few cases might be time consuming and challenging, but that changes quickly to shorter operative times, once the learning curve is achieved [5, 8]. In our study, we found a significant reduction in the operative times after the 20th case, which was our learning curve. After the learning curve, we have noticed no significant difference in the operative time of MICS and sternotomy patients, either for single and double vessel group or the multivessel group.
Ventricular arrhythmias can lead to hemodynamic instability during the procedure. We apply external defibrillation pads over the back of the patient prior to surgery for all MICS cases, as it is extremely cumbersome to shock a patient with handheld paddles (pediatric paddles may be used if needed) via thoracotomy. We made it a routine to keep the right groin draped and ready to access femoral vessels for CPB cannulation. If CPB is required, it has been shown that on-pump beating heart technique was not inferior to a complete bypass with cardioplegic arrest in this scenario [8]. Our practice was to cut open the femoral vessels for cannulation.
Changing the intercostal space after the incision was a useful maneuver to improve visibility, especially while grafting the inferior territory targets. Going a space above might be required to clip the first intercostal branch of LIMA. We avoided spreading the retractor too much to prevent fracture of the ribs. Proper stay sutures and placement of sponges to lift the heart will allow visualization of any target vessel, but too much maneuvering can cause cardiac instability and needs to be avoided. The long-term benefits of LIMA to LAD have already been well established, even in MICS-CABG [9]. Careful harvest of LIMA is necessary as it is a vital conduit [10]. As described by Nambala S, we found that LIMA could be harvested with ease and safety using a harmonic scalpel [11]. The harvest time was longer for the first few cases. We have not attempted use of bilateral IMA in any of our patients yet. Radial artery was our second conduit of choice followed by GSV. The LIMA-radial Y composite anastomosis was done to avoid touching the aorta and was done prior to any distal anastomosis [12]. No endarterectomies were done in any of the patients. The benefits of MICS, especially avoiding the probability of a sternal wound infection, are of great advantage to patients of any age or sex. In our sternotomy group, we had 6 cases of sternal wound infection, including two cases of complete sternal dehiscence. All of them required hospital readmission, along with a proper debridement of the wound in the OR. We lost both patients with complete sternal dehiscence, as they had already developed extensive osteomyelitis and mediastinitis. Three patients in the MICS group developed postoperative wound infection, which required dressings and secondary suturing on an outpatient basis. The benefits of MICS over sternotomy in elderly population above the age of 70 years have also been studied [13]. The postoperative pain in the MICS group was found to be less than that of the sternotomy patients. Early mobilization due to well-maintained sternal integrity is yet another benefit of MICS [14]. The ICU and in-hospital stay in the MICS group was found to be shorter, but without any significant difference from that of the sternotomy group.
The use of TTFM for the graft flow assessment can be adopted in the OR. Graft revision, if at all required, can be done immediately rather than in the postoperative period, if the graft flow does not meet the set values of TTFM [6]. We have recently introduced this flowmetry and now measure the flow in all our grafts, be it MICS or sternotomy approach. On table transesophageal echocardiography (TEE) is also a key tool which gives immediate outcomes in terms of regional wall motion abnormalities. TEE was performed in the OR post procedure in all our patients [4, 15]. In the early part of our MICS program, where we attempted multivessel grafting less often, the adoption of HCR was more frequent. Our strategy of HCR was always stent following surgery as we did not have a hybrid OR [16].
It would be reasonable to say that CABG can now be performed, be it a single vessel or a multivessel disease pattern, via the left thoracotomy approach. Proper selection and evaluation of patients prior to the procedure is the key to good outcomes.
Limitations
The study was a retrospective observational study and not a randomized study. Our follow-up duration was short of up to 34 months only. The angiographic evidence in the postoperative period was restricted to a few cases (12 CT coronary angiographies performed at the end of 12 months and two angiographies done in the two symptomatic patients at 6 and 13 months). We had to rely on postoperative screening echocardiography, ECG, and clinical examination for graft patency assessment. The comparison with patients undergoing sternotomy was inevitably prone to bias where cases deemed not suitable for MICS were operated on using sternotomy and the results have to be interpreted with caution. Intraoperative TTFM was utilized only in 14 patients.
Conclusion
The fraction of MICS CABG procedures still remains low in comparison with the sternotomy approach. There is a short learning curve after which even a multivessel bypass can be comfortably performed using the MICS technique. MICS CABG seems beneficial in terms of early recovery and hospital stay, with no difference in the operative times or postoperative adverse events in comparison with sternotomy approach.
Funding
Nil
Compliance with ethical standards
Ethical committee approval
This study was approved by the institutional ethical committee.
Conflict of interest
The authors declare that they have no conflict of interest.
Informed consent
Individual consents were waived off by the ethics committee.
Human and animal rights statement
All stipulations under the Helsinki declaration 1964 were complied with. No animal studies were carried out.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Nitin Kumar Rajput, Email: drnitinrajput@gmail.com.
Tej Kumar Varma Kalangi, Email: tejkumarvarma87@gmail.com.
Arun Andappan, Email: arunandappan@yahoo.com.
Alok Kumar Swain, Email: dralokswain@yahoo.com.
References
- 1.Dieberg G, Smart NA, King N. Minimally invasive cardiac surgery: a systematic review and meta-analysis. Int J Cardiol. 2016;223:554–560. doi: 10.1016/j.ijcard.2016.08.227. [DOI] [PubMed] [Google Scholar]
- 2.Langer NB, Argenziano M. Minimally invasive cardiovascular surgery: incisions and approaches. Methodist Debakey Cardiovasc J. 2016;12:4–9. doi: 10.14797/mdcj-12-1-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rodriguez M, Ruel M. Minimally invasive multivessel coronary surgery and hybrid coronary revascularization : can we routinely achieve less invasive coronary surgery ? Methodist DeBakey Cardiovasc J. 2016;12:14–19. doi: 10.14797/mdcj-12-1-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Malik V, Jha AK, Kapoor PM. Anesthetic challenges in minimally invasive cardiac surgery : are we moving in a right direction ? Ann Card Anaesth. 2016;19:489–497. doi: 10.4103/0971-9784.185539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Une D, Lapierre H, Sohmer B, Rai V, Ruel M. Can minimally invasive coronary artery bypass grafting be initiated and practiced safely? A Learning Curve Analysis. Innovations. 2013;8:403–409. doi: 10.1097/imi.0000000000000019. [DOI] [PubMed] [Google Scholar]
- 6.Kieser TM, Taggart DP. The use of intraoperative graft assessment in guiding graft revision. Ann Cardiothorac Surg. 2018;7:652–662. doi: 10.21037/acs.2018.07.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lapierre H, Chan V, Sohmer B, Mesana TG, Ruel M. Minimally invasive coronary artery bypass grafting via a small thoracotomy versus off-pump : a case-matched study. Eur J Cardiothorac Surg. 2011;40:804–810. doi: 10.1016/j.ejcts.2011.01.066. [DOI] [PubMed] [Google Scholar]
- 8.Andrawes PA, Shariff MA, Nabagiez JP, et al. Evolution of minimally invasive coronary artery bypass grafting learning curve. Innovations. 2018;13:81–90. doi: 10.1097/imi.0000000000000483. [DOI] [PubMed] [Google Scholar]
- 9.Farid S, Ali JM, Stohlner V, Alam R, Schofield P, Nashef S, de Silva R. Long-term outcome of patients undergoing minimally invasive direct coronary artery bypass surgery, a single-center experience. Innovations. 2018;13:23–28. doi: 10.1097/imi.0000000000000466. [DOI] [PubMed] [Google Scholar]
- 10.Zeff RH, Kongtahworn C, Iannone LA, et al. Internal mammary artery versus saphenous vein graft to the left anterior descending coronary artery : prospective randomized study with 10-year follow-up. Ann Thorac Surg. 1988;45:533–536. doi: 10.1016/S0003-4975(10)64526-2. [DOI] [PubMed] [Google Scholar]
- 11.Nambala S. Minimally invasive total arterial off-pump coronary revascularization: a reproducible technique. Turk J Thorac Cardiovasc Surg. 2019;27:455–457. doi: 10.5606/tgkdc.dergisi.2019.01905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lemma M, Atanasiou T, Contino M. Minimally invasive cardiac surgery-coronary artery bypass graft. Multimedia manual of cardiothoracic surgery: MMCTS / European Association for Cardio-Thoracic Surgery. 10.1093/mmcts/mmt007 published online 29 June 2013. [DOI] [PubMed]
- 13.Barsoum EA, Azab B, Shah N, et al. Long-term mortality in minimally invasive compared with sternotomy coronary artery bypass surgery in the geriatric population (75 years and older patients) Eur J Cardiothorac Surg. 2015;47:862–867. doi: 10.1093/ejcts/ezu267. [DOI] [PubMed] [Google Scholar]
- 14.Walther T, Falk V, Metz S, et al. Pain and quality of life after minimally invasive versus conventional cardiac surgery. Ann Thorac Surg. 1999;67:1643–1647. doi: 10.1016/S0003-4975(99)00284-2. [DOI] [PubMed] [Google Scholar]
- 15.Hillis LD, Smith PK, Anderson JL, et al. 2011 ACCF / AHA guideline for coronary artery bypass graft surgery : a report of the American College of Cardiology Foundation / American Heart Association Task Force on Practice Guidelines. Circulation. 2011;124:e652–e735. doi: 10.1161/CIR.0b013e31823c074e. [DOI] [PubMed] [Google Scholar]
- 16.Puskas JD, Halkos ME, DeRose JJ, et al. Hybrid coronary revascularization for the treatment of multivessel coronary artery disease: a multicenter observational study. J Am Coll Cardiol. 2016;68:356–365. doi: 10.1016/j.jacc.2016.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]