Abstract
Traditionally, Tinospora cordifolia is commonly used in the treatment of diabetes and obesity; has been evaluated for their anti-diabetic and anti-obese potency in experimental animal models. However, the binding affinity of multiple bioactives with various proteins involved in the pathogenesis of diabetes and obesity has not been reported yet. Hence, the present study aimed to assess the binding affinity of multiple bioactives from T. cordifolia with various targets involved in the pathogenesis of diabetes and obesity. The ligands and targets were retrieved from the PubChem and Protein Data Bank respectively and docked using autodock4.0. Druglikeness and absorption, distribution, metabolism, excretion, and toxicity profile were predicted using Molsoft and admetSAR1 respectively. The multiple bioactives from T. cordifolia were identified to interact with multiple proteins involved in the pathogenesis of diabetes/obesity, i.e., isocolumbin (− 9 kcal/mol) with adiponectin (PDB: 4DOU), β-sitosterol (− 10.9 kcal/mol) with cholesteryl ester transfer protein (PDB: 2OBD), tinocordiside (− 6.9 kcal/mol) with lamin A/C (PDB: 3GEF), berberine (− 9.5 kcal/mol) with JNK1 (PDB:3ELJ), β-sitosterol & isocolumbin (− 10.1 kcal/mol) with peroxisome proliferator-activated receptor-γ (PDB:4CI5), berberine (− 7.5 kcal/mol) with suppressor of cytokine signaling 3 (PDB: 2BBU), isocolumbin (− 9.6 kcal/mol) with pancreatic α-amylase (PDB: 1B2Y), isocolumbin (− 9 kcal/mol) with α-glucosidase (PDB: 3TOP), and β-sitosterol (− 10.8 kcal/mol) with aldose reductase (PDB: 3RX2). Similarly, among the selected bioactives, tembetarine scored highest druglikeness score, i.e., 1.21. In contrast, isocolumbin scored lowest drug-likeness character i.e. − 0.52. The predicted result of phytochemicals from T. cordifolia for acute oral toxicity, rat acute toxicity, fish toxicity, drug-likeness score, and aqueous solubility showed the probability of lower side/adverse effects in human consumption. The study suggests processing for bioactives from T. cordifolia against diabetes and obesity via in-vitro and in-vivo approaches.
Keywords: Diabetes mellitus, Guduchi, Gurjo, Molecular docking, Obesity, Tinospora cordifolia
Introduction
Obesity, amplification of normal adiposity is a key player in the pathophysiology of type 2 diabetes mellitus (T2DM), insulin resistance, hypertension, atherosclerosis, and dyslipidemia; occurs due to excessive secretion of adipokines (Redinger 2007), is increasing rapidly (Golay and Ybarra 2005). The velocity of obesity increment is excessively greater than the progress of the development of efficient therapies to control and treat adiposity and associated metabolic disorders (Ramírez et al. 2017). Frequently as time proceeds, obesity and T2DM may act as head and tail of a single coin. Statistics reports 60–90% of total patients with T2DM are obese (Golay and Ybarra 2005). Further, diabetes is considered a common endocrinal disorder that occurs due to low secretion of the insulin from the islets of Langerhans of the pancreas; known as Type 1 diabetes mellitus or due to insulin resistance; known as T2DM (Prabhu and Vijayakumar 2014).
Presently, the major portion of prescription for the pharmacotherapy of T2DM is covered by synthetic or single-targeted oral-hypoglycemic agents. Although these molecules are effective in the management of T2DM, they are associated with serious side effects in special conditions like pregnancy and poly-pharmacy. Hence, it is important to identify/develop new therapeutic agents with minimum side effects and high therapeutic efficacy (Zhang et al. 2015) which can be identified from the various secondary metabolites of traditional medicines. Further, the World Health Organization (WHO) has also recommended investigating newer hypoglycemic agents for T2DM from traditional medicinal plants (WHO 1965). Previously, approaches have been made to identify the lead hit molecules as anti-diabetic agents from traditional medicines via the experimental and computational approaches (Khanal and Patil 2019, 2020a, b).
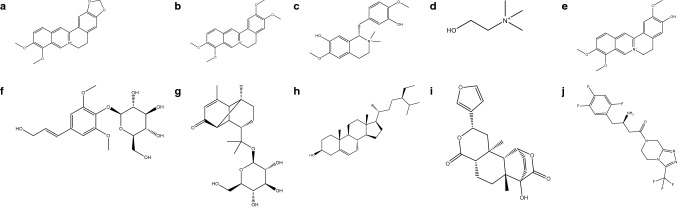
Gurjo or Guduchi; scientifically recognized as Tinospora cordifolia (Willd.) Miers is used as a traditional medicine for various ailments viz. fever, jaundice, diarrhea, cough, asthma, skin diseases, eye disorders, bites of poisonous snakes and venomous snake and modulator of Insulin signaling pathway via the regulation of PTPN1 and ACACB (Khanal et al. 2019a, c). Further, the utilization of T. cordifolia as an anti-diabetic has been recorded in the Ayurvedic Pharmacopoeia of India (Government of India 2001). T. cordifolia has been reported for the reduction of oxidative stress, unclear carbohydrate metabolism, hypoglycemic activity, and modulators of multiple proteins involved in diabetes via experimental and computational approaches (Puranik et al. 2010; Patel and Mishra 2011; Khanal et al. 2019a, b, c). However, as per the review of literature, the binding affinity of phytoconstituents from T. cordifolia with proteins involved in the progression of diabetes and obesity has not been reported yet. Hence, the present study aimed to screen secondary metabolites from T. cordifolia, i.e., berberine, palmatine, tembetarine, choline, jatrorrhizine, syringin, tinocordiside, β-sitosterol, and isocolumbin (Fig. 1) as anti-diabetic/obese agents via in silico molecular docking, predict their druglikeness and absorption distribution, metabolism, excretion and toxicity profile. The obtained results were compared with one of the clinically used anti-diabetic agents, sitagliptin.
Fig. 1.
Chemical structure of a berberine, b palmatine, c tembetarine, d choline, e jatrorrhizine, f syringin, g tinocordiside, h β-sitosterol, i isocolumbin and j sitagliptin
Materials and methods
Ligands and energy minimization
The chemical structures of selected ligands were retrieved from PubChem (https://pubchem.ncbi.nlm.nih.gov/) in.sdf format and then converted into.pdb format using Discovery studio. All the ligands were minimized using mmff94 (Halgren 1996) forcefield and steepest descent as an optimization algorithm. The energy minimized ligands were then converted to autodock ligand in the format of.pdbqt.
Protein preparation
Nine protein molecules were selected that are involved in the pathogenesis of diabetes and obesity. The selected proteins, i.e., suppressor of cytokine signaling 3(Socs3), cholesteryl ester transfer protein (CETP), C-Jun N-terminal kinases-1(JNK1), lamin A/C, peroxisome proliferator-activated receptor γ (PPAR-γ), adiponectin, α-amylase, aldose reductase, and α-glucosidase were retrieved from Research Collaboratory for Structural Bioinformatics (https://www.rcsb.org/) protein data bank. The retrieved 3D proteins were combined with hetero-atoms and water molecules. Hence, Discovery studio 2019 (https://www.3dsbiovia.com/) was used to remove water molecules, heteroatoms, and other chains from the retrieved protein to avoid docking interference. These modified proteins were then saved in the.pdb format.
Absorption, distribution, metabolism, excretion, and toxicity profile and druglikeness
Pharmacokinetic parameters of a compound like absorption, distribution, metabolism, excretion, and toxicity (ADMET) play a vital role in the drug discovery process. Hence, an online server admetSAR (http://lmmd.ecust.edu.cn/admetsar1) was used to predict the ADMET properties of each ligand. We predicted blood–brain barrier permeability, human intestinal absorptivity, caco-2 permeability, ames toxicity, acute oral toxicity, rat acute toxicity, and fish toxicity using of each ligand. Further, MolSoft (http://www.molsoft.com/plosone.html), an online server was used to calculate the drug-likeness properties of each selected ligand.
Ligand protein docking
Docking was performed using autodock4 (Morris et al. 2009) at PyRx (https://pyrx.sourceforge.io/) 0.8 platform. After docking ten different poses of ligand molecules were obtained; the pose scoring lowest binding energy was chosen to visualize the ligand–protein interaction using Discovery studio 2019.
Results
Drug likeness properties of selected ligand
Among the selected ligands, tembetarine scored the highest druglikeness score, i.e., 1.21 with the molecular weight of 344.19, 4 hydrogen bond acceptors, 2 hydrogen bond donors, and 2.63 MolLogP. Similarly, isocolumbin scored lowest druglikeness score i.e. − 0.52 with a molecular weight of 358.14, 6 hydrogen bond acceptors, 1 hydrogen bond donor, and 2.38 MolLogP. The druglikeness score of each bioactive including their molecular weight, number of hydrogen bond donor/acceptor, MolLogP, and aqueous solubility is summarized in Table 1.
Table 1.
Ligand molecules with molecular formula, Lipinski rule of five, drug likeness score
| Compounds | Molecular formula | Molecular weight | NHBA | NHBD | MolLogP | MolLogS | MolPSA (A2) | MolVol (A3) | DLS | |
|---|---|---|---|---|---|---|---|---|---|---|
| mg/L | Log(moles/L) | |||||||||
| Berberine | C20H18NO4 | 336.12 | 4 | 0 | 4.39 | 12.56 | − 4.43 | 33.45 | 332.12 | 0.77 |
| Palmatine | C21H22NO4 | 352.15 | 4 | 0 | 3.96 | 19.09 | − 4.27 | 31.60 | 355.56 | 0.69 |
| Tembetarine | C20H26NO4 | 344.19 | 4 | 2 | 2.63 | 1102.82 | − 2.49 | 47.44 | 360.00 | 1.21 |
| Choline | C5H14NO | 104.11 | 1 | 1 | − 0.42 | 532,925.11 | 0.71 | 16.73 | 124.09 | 0.02 |
| Jatrorrhizine | C20H20NO4 | 338.14 | 4 | 1 | 3.82 | 43.89 | − 3.89 | 40.52 | 335.56 | 0.84 |
| Syringin | C17H24O9 | 372.14 | 9 | 5 | − 0.85 | 52,811.16 | − 0.85 | 112.19 | 350.63 | 0.05 |
| Tinocordiside | C21H32O7 | 396.21 | 7 | 4 | 0.50 | 1892.48 | − 2.32 | 92.91 | 429.04 | 0.49 |
| Β-sitosterol | C29H50O | 414.39 | 1 | 1 | 8.45 (> 5) | 0.19 | − 6.34 | 16.28 | 519.36 | 0.78 |
| Isocolumbin | C20H22O6 | 358.14 | 6 | 1 | 2.38 | 449.34 | − 2.90 | 64.85 | 397.65 | − 0.52 |
| Sitagliptin | C16H15F6N5O | 407.12 | 4 | 2 | 1.34 | 7358.01 | − 1.74 | 61.67 | 329.55 | 0.52 |
NHBA number of hydrogen bond acceptor, NHBD number of hydrogen bond donor, DLS Druglikeness score
ADMET prediction of ligands
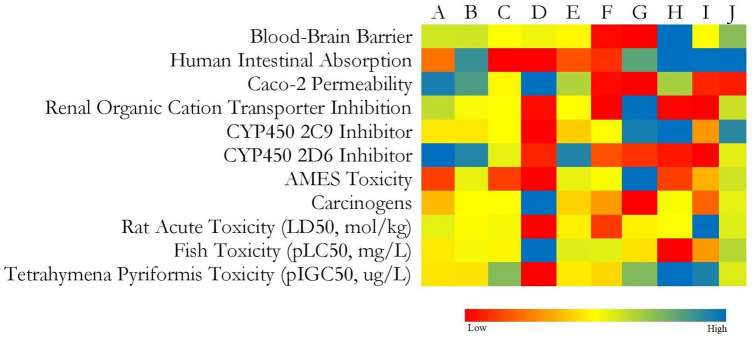
We studied probable physiological properties of each selected ligands to cross the blood–brain barrier, human intestinal absorptivity, caco-2 permeability, ames toxicity, carcinogen character, acute oral toxicity, rat acute toxicity, and fish toxicity. The heatmap (Fig. 2) represents the ADMET profile of individual bioactives which are comparable with sitagliptin.
Fig. 2.
Heatmap representing ADMET profile of a berberine, b palmatine, c tembetarine, d choline, e jatrorrhizine, f syringin, g tinocordiside, h β-sitosterol, i isocolumbin, and j sitagliptin
Ligand Protein Docking
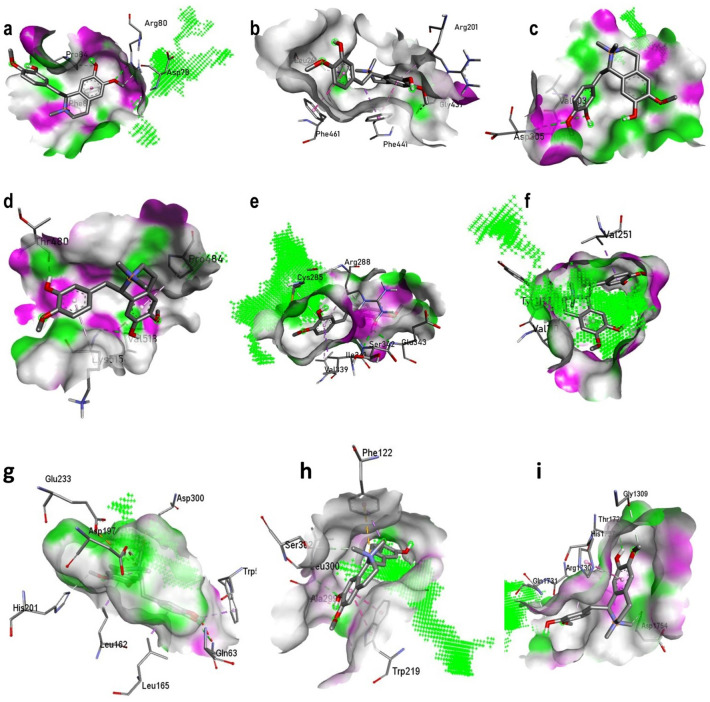
β-sitosterol showed the highest binding affinity with CETP via − 10.9 kcal/mol binding energy. However, there was no hydrogen bond interaction. Choline had the lowest binding affinity with Socs3 and JNK1 with via − 3.6 kcal/mol binding energy. However, three hydrogen bond interactions were found with Soc3, i.e., ARG80, ASP78, and GLN76. Isocolumbin showed the highest binding affinity with PPAR-γ with − 10.1 kcal/mol binding energy with 1 hydrogen bond interaction, i.e., ARG288. Jatrorrhizine showed the binding affinity with JNK1 with two hydrogen bond interactions i,e, VAL219, and HIS221. Similarly, palmatine showed the binding affinity with JNK1 with − 8.9 kcal/mol by interacting with 1 hydrogen bond, i.e., SER179. Syringin was predicted to have the binding affinity with CETP with − 5.9 kcal/mol by interacting with two hydrogen bond interactions, i.e., CYS325 and SER 342. Tembetarine showed the binding affinity with PPAR-γ by two hydrogen bond interactions, i.e., GLU343 and SER342 with − 8.2 kcal/mol binding energy. Tinocordiside showed the binding affinity with PPAR-γ by two hydrogen bond interactions, i.e., GLU343 and SER342 with − 9.5 kcal/mol binding energy. Similarly, isocolumbin showed the highest binding affinity with pancreatic α-amylase and α-glucosidase. Likewise, β-sitosterol showed the highest binding affinity with aldose reductase with the binding energy of − 10.8 kcal/mol via 1 hydrogen bond interaction with SER302. Energy dissimilarity of the binding energies of each compound with an individual target is represented in Table 2. Similarly, the interaction of tembetarine with individual proteins is represented in Fig. 3; predicted for the highest druglikeness score.
Table 2.
Binding energy and mode of interaction of each ligands with each targets
| Ligand | Parameters predicted | Targets | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Suppressor of cytokine signaling 3 (PDB:2BBU) | C-Jun N-terminal kinases (PDB:3ELJ) | Lamin A/C (PDB:3GEF) | Peroxisome proliferator-activated receptor-γ (PDB:4CI5) | Adiponectin (PDB:4DOU) | Cholesteryl ester transfer protein (PDB:2OBD) | α-amylase (PDB:1B2Y) | Human aldose reductase (PDB:3RX2) | α-glucosidase (PDB:3TOP) | ||
| β-sitosterol | Binding energy | − 7.3 | − 7.5 | − 6.8 | − 10.1 | − 8 | − 10.9 | − 9.3 | − 10.8 | − 8.5 |
| NHB | 2 | – | – | – | – | – | – | 1 | – | |
| HBR | ASP78, ARG80 | – | – | – | – | – | – | SER302 | – | |
| Choline | Binding Energy | − 3.6 | − 3.6 | − 2.9 | − 3.5 | − 3.3 | − 3.4 | − 3.4 | − 4 | − 3.4 |
| NHB | 3 | – | 1 | – | – | 1 | 1 | 1 | – | |
| HBR | ARG80, ASP78, GLN76 | – | GLY438 | – | – | THR27 | ASP300 | SER214 | – | |
| Isocolumbin | Binding Energy | − 8 | − 8.2 | − 7 | − 10.1 | − 9 | − 9 | − 9.6 | − 8.6 | − 9 |
| NHB | 2 | 2 | 5 | 1 | 2 | − | 1 | 2 | 3 | |
| HBR | ASP78, ARG80 | ARG72 | GLY465, SER533, THR534, SER437, SRG439 | ARG288 | HIS382, TYR111 | − | GLN63 | TYR48, TRP111 | GLY992, THR1150, ARG1206 | |
| Jatrorrhizine | Binding Energy | − 7.1 | − 9 | − 6.2 | − 8.9 | − 7.4 | − 8.2 | − 8.3 | − 8 | − 8.9 |
| NHB | 2 | 2 | 1 | 2 | 1 | – | 1 | – | 2 | |
| HBR | ARG80, GLN83 | VAL219, HIS221 | ARG455 | LEU228 | TYR391 | – | LYS200 | – | TRP1749 | |
| Palmatine | Binding Energy | − 7.2 | − 8.9 | − 6.1 | − 8.6 | − 7.1 | − 8.6 | − 8.2 | − 8.8 | − 8.1 |
| NHB | 2 | 1 | 1 | – | – | – | – | 2 | 3 | |
| HBR | ARG80 | SER179 | VAL513 | – | – | – | – | SER302, TYR48 | ARG1311, ASN1792 | |
| Syringin | Binding Energy | − 5.8 | − 7.3 | − 5.4 | − 7.2 | − 7.1 | − 5.9 | − 7.3 | − 6.9 | − 6.7 |
| NHB | 5 | 2 | 3 | 4 | 1 | 2 | 5 | 2 | 6 | |
| HBR | GLU69, ASP78, ARG80 | ASP151, ARG69 | PHE483, TRP482, VAL513 | HIS323, ARG288, GLU343, SER342 | PRO386 | CYS325, SER342 | ILE235, GLU233, HIS305, GLN63, TRP59 | TRP219, ARG296 | GLY1309, ARG1311, MET1310, HIS1727, SER1757 | |
| Tembetarine | Binding Energy | − 7.1 | − 6.8 | − 6.3 | − 8.2 | − 8 | − 7.3 | − 7.8 | − 8.3 | − 7.7 |
| NHB | 1 | 3 | 3 | 2 | – | 1 | 1 | 1 | 1 | |
| HBR | ARG80 | ILE304, ASP305 | VAL513, LYS515, THR480 | GLU343, SER342 | – | ARG201 | GLN63 | LEU300 | THR1726 | |
| Tinocordiside | Binding Energy | − 7 | − 8.5 | − 6.9 | − 9.5 | − 8.9 | − 8.1 | − 8.5 | − 7.8 | − 8.2 |
| NHB | 4 | 2 | 4 | 2 | 5 | – | 1 | 5 | 1 | |
| HBR | ASP78, ARG80, THR82 | ARG72, ILE148 | LYS515, TRP482, GLN517, TRP514 | GLU343, SER342 | HIS241, TYR391, LEU520, LEU521 | – | ASP300 | SER302, LEU301 | ASP1526 | |
| Berberine | Binding Energy | − 7.5 | − 9.5 | − 6.8 | − 9.3 | − 7.4 | − 7.6 | − 8.6 | − 9.6 | − 8 |
| NHB | – | 2 | 1 | – | – | – | – | 1 | 3 | |
| HBR | – | SER179, LYS153 | VAL513 | – | – | – | – | TYR48 | ARG1730, TYR1787 | |
| Sitagliptin F | Binding Energy | − 7.5 | − 9.3 | − 7 | − 9.5 | − 9.1 | − 9.1 | − 9.3 | − 10.2 | − 8.5 |
| NHB | 6 | 8 | 2 | 1 | 6 | – | 4 | 3 | 4 | |
| HBR | PHE73, GLU69, SER81, THR82, GLN83 | ARG150, TYR202, ILE148, GLY146, ARG345, GLU344 | VAL513, TRP482 | ILE326 | LEU379, TYR252, LEU521, HIS523 | – | LYS200, ILE235, ASP197, GLN63 | VAL47, SER210, HIS110 | ARG1311, ARG1730, HIS1727, LYS1795 | |
Binding energy is represented in kcal/mol
NHB number of Hydrogen bonds, HBR hydrogen bond residues
Fig. 3.
Interaction of tembetarine with a suppressor of cytokine signaling 3, b cholesteryl ester transfer protein, c C-Jun N-terminal kinase-1, d lamin A/C, e peroxisome proliferator-activated receptor-γ, f adiponectin, g α-amylase, h aldose reductase and i α-glucosidase
Discussion
The current study was designed to understand the interaction of active biomolecules from T. cordifolia with proteins involved in diabetes and obesity. Although X-ray crystallography and nuclear magnetic resonance techniques are utilized to understand the ligand–protein interaction; techniques are limited by the complexity in crystallization techniques of proteins, a requirement of experienced personnel for data interpretation and being time-consuming (Yano and Yachandra 2009; Chatham and Blackband 2001). To overcome these limitations, various bioinformatics tools have been utilized previously to understand ligand–protein interaction (Duyu et al. 2020; Khanal et al. 2019b; Patil et al. 2019).
Lipinski’s rule of five explains the druglikeness character of any organic moiety. Druglikeness character of a molecule depends on its molecular weight (< 500), number of hydrogen bond acceptors (< 10), number of hydrogen bond donors (< 5), and MolLogP (< 5). The molecule can be predicted to possess poor intestinal absorptivity if it violates more than two rules (Lipinski 2004). Similarly, physiochemical properties of drug molecules depend on molecular weight, hydrophobic and hydrophilic characters (Savjani et al. 2012). This prediction of druglikeness character is important because most of the active biomolecules fail in clinical trials due to their lower bioavailability (Kennedy 1997). In the current study, eight bioactives from T. cordifolia were predicted for positive drug-likeness score except for isocolumbin; suggests their oral bioavailability so that the bioactives get absorbed into the systemic circulation and interact with multiple proteins involved in the disease pathogenesis as explained previously (Khanal et al. 2019a, c).
ADMET profiling reflected syringin and tinocordiside were predicted to possess the minimum blood–brain permeability. Likewise, berberine, palmatine, tembetarine, choline, jatrorrhizine, and syringin were predicted to have less human intestinal absorptivity. Further, syringin, tinocordiside, β-sitosterol, and isocolumbin were predicted for minimum caco-2 permeability. The heatmap (Fig. 2) provides a glance over the ADMET profile of lead phytoconstituents from T. cordifolia and was comparable with Sitagliptin.
In the current study, nine macromolecules were targeted which are involved in diabetes and obesity; binding of ligands to these proteins may alter the function of these receptors. Hence it will be helpful to control diabetes from the rational usage of phytochemicals from T. cordifolia. Adiponectin, collagen-like circulating protein, produced by adipocytes has itself link to diabetes mellitus, insulin resistance, and cardiovascular diseases. Adiponectin binding results in increased glucose uptake viz. glucose transporter type 4 translocation and promoting the oxidation of fatty acids (Kawano and Arora 2009) which was majorly targeted by isocolumbin with the binding energy of − 9 kcal/mol with two hydrogen bond interactions with HIS382 and TYR111. CETP possesses the role as the mediator between neutral lipid transport of cholesteryl esters and triglycerides in between lipoproteins;occurs via the reversible binding of neutral lipids to CETP (Dallinga-Thie et al. 2007). In the current study β-sitosterol showed the highest binding affinity with CETP; could contribute to the management of obesity and lipidaemia with the binding energy of − 10.9 kcal/mol; however, there was no hydrogen bond interaction of this ligand with CETP. Lamin A/C is involved in the regulation of insulin resistance, loss of subcutaneous adipose tissue, dyslipidemia, and T2DM and also it shares many features related to metabolic syndrome (Mesa et al. 2007); was majorly targeted by tinocordiside with the binding energy of − 6.9 kcal/mol via 4 hydrogen bond interactions with LYS515, TRP482, GLN517, and TRP514. JNKs are identified as a signaling molecule that links in inflammation and insulin resistance. JNK1 is activated via inflammatory cytokines forwarding towards insulin resistance through the increment in the level of serine phosphorylation of insulin receptor substrate-1 leading to impair insulin signaling (Yang and Trevillyan 2008). Targeting this protein could be helpful in the treatment of obesity mediated inflammation and insulin resistance. In the present study, JNK1 was majorly targeted by berberine with the binding energy of − 9.5 kcal/mol and two hydrogen bond interactions with SER179, and LYS153. PPAR family is associated with glucose metabolism and insulin sensitization. Further, this family plays an important role in metabolic and energy homeostatic regulation providing the protective effects towards diabetes mellitus, obesity, dyslipidemia, inflammation, pain, lung diseases including cancer (Tyagi et al. 2011). Experimental reports have been found regarding the action of phytochemicals from T. cordifolia to act on PPAR family receptors (Puranik et al. 2010). In the present study, PPAR was majorly targeted by β-sitosterol and isocolumbin with the binding energy of − 10.1 kcal/mol; however, the hydrogen bond interaction was formed only by isocolumbin with ARG288. However, phytochemicals from the T. cordifolia may show the anti-obese and anti-diabetic activity as PPAR-γ receptor agonist which needs to be further investigated. Hypothalamic leptin resistance is obesity-induced (can be presented via diet-induced obesity) which could be the outcome of increased expression of Socs3 in the hypothalamus in diet-induced obese animals (Reed et al. 2010); was majorly targeted by berberine; however, no hydrogen bond interactions were found. Pancreatic α-amylase is involved in the hydrolysis of starch (polysaccharides) into smaller oligosaccharides (maltose, sucrose, etc.) and multiple α-(l–6 and 1–4) oligoglucans (de Souza and de Oliveira Magalhães 2010) which is further hydrolyzed into monosaccharides for the systemic absorption; contributes in post-prandial hyperglycemia (Khanal and Patil 2020b). In the present study, isocolumbin was predicted to possess the highest binding affinity with α-amylase with − 9.6 kcal/mol via 1 hydrogen bond interaction with GLN63 and α-glucosidase with − 9 kcal/mol and three hydrogen bond interactions with GLY992, THR1150, ARG1206; could contribute in the management of postprandial hyperglycemia. Similarly, aldose reductase acts as a rate-limiting step for polyol pathway and is involved in diabetic retinopathy (Kinoshita et al. 1979) which was majorly targeted by β-sitosterol with the binding energy of − 10.8 kcal/mol and one hydrogen bond interaction with SER302.
Further, it is to be understood that a single compound can modulate multiple proteins and can regulate multiple pathways (Khanal et al. 2020). Hence, the present study also suggests assessing the lead hits from T. cordifolia to identify probable modulation of multiple pathways in diabetes and obesity.
Conclusion
The present study represents the possible role of phytochemicals from T. cordifolia as a ligand against nine different targets that are involved in obesity and diabetes. Results from acute oral toxicity, rat acute toxicity, and fish toxicity predict the molecules are non-toxic and are absorbable via the human intestine. All the selected ligands have scored well drug-likeness scores which affect the physicochemical property of the compound either towards pharmacological action or towards the drug development steps. The obtained Log S value represents the solubility of the drug molecules which would further help in the formulation steps in the pharmaceutical industry. This study should encourage further investigation of the properties of the phytochemicals from T. cordifolia followed by a pharmacological investigation of these in-silico findings in suitable models. Further, most of the bioactives identified to interact with proteins involved in diabetes were alkaloids (basic) suggesting the performance of wet-lab experiments in both role-based and pH based ionization.
Acknowledgements
The authors are thankful to Principal KLE College of Pharmacy, Belagavi for providing necessary facilities and Head of Department of Pharmacology and Toxicology, KLE College of Pharmacy, Belagavi, for supporting to complete the work.
Funding
This work has not received any funds from national and international agencies.
Data availability
Data will be provided in case of a request.
Compliance with ethical standards
Conflict of interest
There are no conflicts of interest to declare.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Pukar Khanal, Email: pukarkhanal58@gmail.com.
B. M. Patil, Email: drbmpatil@klepharm.edu, Email: bmpatil59@hotmail.com
References
- Chatham JC, Blackband SJ. Nuclear magnetic resonance spectroscopy and imaging in animal research. ILAR J. 2001;42(3):189–208. doi: 10.1093/ilar.42.3.189. [DOI] [PubMed] [Google Scholar]
- Dallinga-Thie GM, Dullaart RP, van Tol A. Concerted actions of cholesteryl ester transfer protein and phospholipid transfer protein in type 2 diabetes: effects of apolipoproteins. Curr Opin Lipidol. 2007;18(3):251–257. doi: 10.1097/MOL.0b013e3280e12685. [DOI] [PubMed] [Google Scholar]
- de Souza PM, de Oliveira MP. Application of microbial αamylase in industry—a review. Braz J Microbiol. 2010;41(4):850–861. doi: 10.1590/S1517-83822010000400004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duyu T, Khanal P, Khatib NA, Patil BM. Mimosa pudica modulates neuroactive ligand-receptor interaction in Parkinson’s disease. Indian J Pharm Educ. 2020;54(3):732–739. doi: 10.5530/ijper.54.3.124. [DOI] [Google Scholar]
- Golay A, Ybarra J. Link between obesity and type 2 diabetes. Best Pract Res Clin Endocrinol Metab. 2005;19(4):649–663. doi: 10.1016/j.beem.2005.07.010. [DOI] [PubMed] [Google Scholar]
- Government of India (2001) The Ayurvedic pharmacopoeia of India. Ministry Health Fam Welf 1:53
- Halgren TA. Merck molecular force field. I. Basis, form, scope, parameterization, and performance of MMFF94. J Comput Chem. 1996;17:490–519. doi: 10.1002/(SICI)1096-987X(199604)17:5/6<490::AID-JCC1>3.0.CO;2-P. [DOI] [Google Scholar]
- Kawano J, Arora R. The role of adiponectin in obesity, diabetes, and cardiovascular disease. J Cardiometab Syndr. 2009;4(1):44–49. doi: 10.1111/j.1559-4572.2008.00030.x. [DOI] [PubMed] [Google Scholar]
- Kennedy T. Managing the drug discovery/development interface. Drug Discov Today. 1997;2(10):436–444. doi: 10.1016/S1359-6446(97)01099-4. [DOI] [Google Scholar]
- Khanal P, Patil BM. Gene set enrichment analysis of alpha-glucosidase inhibitors from Ficus benghalensis. Asian Pac J Trop Biomed. 2019;9(6):263–270. doi: 10.4103/2221-1691.260399. [DOI] [Google Scholar]
- Khanal P, Patil BM. Gene ontology enrichment analysis of α-amylase inhibitors from Duranta repens in diabetes mellitus. J Diabetes Metab Disord Available at: 2020 doi: 10.1007/s40200-020-00554-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khanal P, Patil BM. α-Glucosidase inhibitors from Duranta repens modulate p53 signaling pathway in diabetes mellitus. Adv Tradit Med. 2020 doi: 10.1007/s13596-020-00426-w. [DOI] [Google Scholar]
- Khanal P, Mandar BK, Magadum P, Patil BM, Hullatti KK. In silico docking study of limonoids from Azadirachta indica with pfpk5: a novel target for Plasmodium falciparum. Indian J Pharm Sci. 2019;81(2):326–332. doi: 10.36468/pharmaceutical-sciences.514. [DOI] [Google Scholar]
- Khanal P, Mandar BK, Patil BM, Hullatti KK. In silico antidiabetic screening of borapetoside C, cordifolioside A and magnoflorine. Indian J Pharm Sci. 2019;81(3):550–555. doi: 10.36468/pharmaceutical-sciences.543. [DOI] [Google Scholar]
- Khanal P, Patil BM, Mandar BK, Dey YN, Duyu T. Network pharmacology-based assessment to elucidate the molecular mechanism ofanti-diabetic action of Tinospora cordifolia. Clin Phytosci. 2019;5:35. doi: 10.1186/s40816-019-0131-1. [DOI] [Google Scholar]
- Khanal P, Patil BM, Chand J, Naaz Y. Anthraquinone derivatives as an immune booster and their therapeutic option against COVID-19. Nat Prod Bioprospect. 2020 doi: 10.1007/s13659-020-00260-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinoshita JH, Fukushi S, Kador P, Merola LO. Aldose reductase in diabetic complications of the eye. Metabolism. 1979;28(4 Suppl 1):462–469. doi: 10.1016/0026-0495(79)90057-x. [DOI] [PubMed] [Google Scholar]
- Lipinski CA. Lead- and drug-like compounds: the rule-of-five revolution. Drug Discov Today Technol. 2004;1(4):337–341. doi: 10.1016/j.ddtec.2004.11.007. [DOI] [PubMed] [Google Scholar]
- Mesa JL, Loos RJ, Franks PW, et al. Lamin A/C polymorphisms, type 2 diabetes, and the metabolic syndrome: case-control and quantitative trait studies. Diabetes. 2007;56(3):884–889. doi: 10.2337/db06-1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris GM, Huey R, Lindstrom W, Sanner MF, Belew RK, Goodsell DS, Olson AJ. AutoDock4 and AutoDockTools4: automated docking with selective receptor flexibility. J Comput Chem. 2009;30:2785–2791. doi: 10.1002/jcc.21256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel MB, Mishra S. Hypoglycemic activity of alkaloidal fraction of Tinospora cordifolia. Phytomedicine. 2011;18(12):1045–1052. doi: 10.1016/j.phymed.2011.05.006. [DOI] [PubMed] [Google Scholar]
- Patil VS, Biradar PR, Attar V, Khanal P. In silico docking analysis of active biomolecules from Cissus quadrangularis L. against PPAR-γ. Indian J Pharm Educ. 2019;53(3):S332–S3377. doi: 10.5530/ijper.53.3s.103. [DOI] [Google Scholar]
- Prabhu S, Vijayakumar S. Antidiabetic, hypolipidemic and histopathological analysis of Gymnema sylvestre (R. Br) leaves extract on streptozotocin induced diabetic rats. Biomed Prev Nutr. 2014;4(3):425–430. doi: 10.1016/j.bionut.2014.03.008. [DOI] [Google Scholar]
- Puranik N, Kammar KF, Devi S. Anti-diabetic activity of Tinospora cordifolia (Willd.) in streptozotocin diabetic rats; does it act like sulfonylureas? Turk J Med Sci. 2010;40(2):265–270. doi: 10.3906/sag-0802-40. [DOI] [Google Scholar]
- Ramírez NM, Toledo RCL, Moreira MEC, et al. Anti-obesity effects of tea from Mangifera indica L. leaves of the Ubá variety in high-fat diet-induced obese rats. Biomed Pharmacother. 2017;91:938–945. doi: 10.1016/j.biopha.2017.05.015. [DOI] [PubMed] [Google Scholar]
- Redinger RN (2007) The pathophysiology of obesity and its clinical manifestations. Gastroenterol Hepatol (N Y) 3(11):856–863. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3104148/pdf/GH-03-856.pdf [PMC free article] [PubMed]
- Reed AS, Unger EK, Olofsson LE, Piper ML, Myers MG, Jr, Xu AW. Functional role of suppressor of cytokine signaling 3 upregulation in hypothalamic leptin resistance and long-term energy homeostasis. Diabetes. 2010;59(4):894–906. doi: 10.2337/db09-1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savjani KT, Gajjar AK, Savjani JK. Drug solubility: importance and enhancement techniques. ISRN Pharm. 2012;2012:195727. doi: 10.5402/2012/195727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyagi S, Gupta P, Saini AS, Kaushal C, Sharma S. The peroxisome proliferator-activated receptor: a family of nuclear receptors role in various diseases. J Adv Pharm Technol Res. 2011;2(4):236–240. doi: 10.4103/2231-4040.90879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO (1965) Diabetes mellitus. Report of a WHO expert committee. World Health Organization technical report series, pp 1–44. https://apps.who.int/iris/bitstream/handle/10665/38442/WHO_TRS_310.pdf;jsessionid=2C4D0847578CDB3E30D98B6B54F12241?sequence=1 [PubMed]
- Yang R, Trevillyan JM. c-Jun N-terminal kinase pathways in diabetes. Int J Biochem Cell Biol. 2008;40(12):2702–2706. doi: 10.1016/j.biocel.2008.06.012. [DOI] [PubMed] [Google Scholar]
- Yano J, Yachandra VK. X-ray absorption spectroscopy. Photosynth Res. 2009;102(2–3):241–254. doi: 10.1007/s11120-009-9473-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Onakpoya IJ, Posadzki P, Eddouks M. The safety of herbal medicine: from prejudice to evidence. Evid Based Complement Altern Med. 2015;2015:316706. doi: 10.1155/2015/316706. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be provided in case of a request.