Abstract
The aim was to investigate the effect of lily bulbs on the microecological characteristics of intestinal microbiota and enzyme activities in normal mice. Thirty SPF Kunming mice were randomly divided into the control group, Lilium lancifolium (LL) group and Lilium davidii var. unicolor (LDU) group. Mice of the latter two groups were given 0.15 g·mL−1 lily bulb solution, respectively, by gavage twice a day, while the control group was given the same volume of sterilized water. After 49 days, intestinal contents and mucosa of all mice were collected and the characteristics of intestinal microbiota and enzyme activities were analyzed. Results showed that the number of Lactobacillus spp. and Bifidobacteria spp. in the LL group was significantly higher than that in the control group (t = 2.68 × 107, P = 0.000; t = 5.96 × 107; P = 0.000) and the LDU group (t = 6.12 × 107, P = 0.000; t = 2.71 × 107, P = 0.000), while the number of total bacteria was significantly lower (P = 0.040). Microbial activity in intestinal contents and mucosa of the LDU group (t = 0.43, P = 0.001; t = 0.69, P = 0.000) decreased, and microbial activity in intestinal mucosa of the LL group decreased significantly (t = 0.89, P = 0.000) but increased significantly in intestinal contents of the LL group (t = 0.81, P = 0.000). The activities of amylase (t = 455.73, P = 0.000; t = 206.56, P = 0.000) and protease (t = 52.32, P = 0.000) increased but the activities of lactase (t = 443.51, P = 0.000; t = 15.71, P = 0.000) and sucrase (t = 5.82, P = 0.000; t = 366.82, P = 0.000) decreased significantly in contents from the LL group and LDU group. Except for the sucrase activity, enzyme activities in mucosa of the LL group were completely opposite to those in contents (t = 44.15, P = 0.000; t = 1.25, P = 0.007; t = 14.64, P = 0.011). In conclusion, dietary lily bulbs increased intestinal contents amylase activities and mucosa lactase activity significantly. Lily bulbs, especially Lilium lancifolium, can promote the growth of Lactobacillus spp. and Bifidobacteria spp., and inhibit the growth of total bacteria in the intestines of normal mice. Lilium lancifolium bulbs have the potential to be a functional food.
Keywords: Lily bulb, Intestinal microbiota, Enzyme activity, Functional food
Introduction
The intestinal tract is an important digestive organ and immune organ of the human body, in which intestinal digestive enzymes and intestinal microbiota play a vital role. Intestinal microbiota, distributed in contents and intestinal mucosa, has a total of 1014 colony-forming units (CFU), weighing about 1.0–1.5 kg, and participates in the physiological, pathological and toxicological process (Sekirov et al. 2010). Under physiological conditions, the microecosystem is in dynamic balance (Gilbert et al. 2018). For example, in bacterial membrane barriers, Bifidobacteria spp. can inhibit colonization and growth of pathogenic bacteria by occupying and competing nutrients, thus maintaining human health. Lactobacillus can inhibit Enterohemorrhagic, Escherichia coli, Salmonella, Shigella and Vibrio cholerae by secreting rhodobactin (Spinler et al. 2008) . However, once this balance is destroyed, more diseases will occur, especially chronic diseases (Kalliomäki and Walker 2005) . Some studies have shown that the occurrence of basic gastrointestinal diseases and nervous system diseases is related to the imbalance of intestinal microecosystem (Han et al. 2009; Kim et al. 2019).
Intestinal digestive enzymes reflect the digestive function of monogastric animals to a certain extent, and play an important role in nutrient absorption and immunity. According to the sources, intestinal digestive enzymes can be divided into endogenous digestive enzymes and exogenous digestive enzymes. The former is secreted by the small intestinal mucosa, such as amylase and lipase. Substances, such as plant polysaccharides, disaccharides, oligosaccharides and lignin, cannot be metabolized by the host, but are digested by digestive enzymes released by the intestinal microbiota. After being decomposed by enzymes released by intestinal bacteria, these substances are metabolized into butyric acid and methane, amino acids, etc., which are also called intestinal microbial metabolites and further participate in the physiological and pathological processes. However, the contribution of intestinal bacteria and intestinal mucosal bacteria to digestive enzyme activity and immunity may be different. Bacteria in intestinal mucosa are closer to the host epithelium than contents. Long et al. found that the bacterial composition of the intestinal mucosa may be different from the intestinal contents (Long et al. 2018). Zeng et al. discovered mucosa microbiota may play a more important role in the occurrence and development of gastrointestinal diseases (Zeng et al. 2019). Therefore, our study focuses not only on changes in enzyme activity in intestinal contents but also on the changes of enzyme activity in the intestinal mucosa.
Lily bulb is the earliest food that can be used as both food and medicine, with a potent ability to enhance immunity and antioxidation, anti-inflammatory, anti-depressant (Chang et al. 2007; Pan et al. 2017; Hui et al. 2020), and is very popular in East Asia. It was reported that it contains starch, protein, amino acids and trace elements, and also contains active ingredients such as polyphenols, flavonoids, alkaloids and saponins (Liu et al. 2019). The compounds in the lily bulbs may vary from region to region. We used two common varieties of lily in China—Lilium lancifolium and Lilium davidii var. Unicol (Fig. 1) in the study. There are few studies on the intestinal microbiota of lily bulb, but the conclusions are significant. Okazaki et al. found dextran sulfate sodium (DSS)-induced colitis in rats showed a lower fecal abundance of bifidobacteria, but it was recovered by lily bulbs intake (Okazaki et al. 2016a, b). In another of his articles, eating lily can reduce the fecal Firmicutes/Bacteroidetes ratio, and thus resist obesity (Okazaki et al. 2016a, b).
Fig. 1.
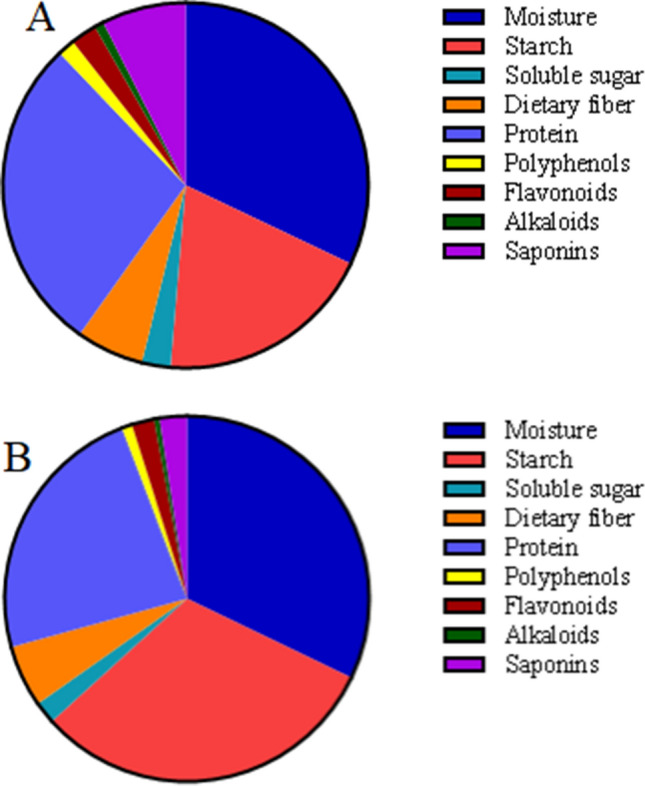
Main components of Lilium lancifolium (a) and Lilium davidii var. unicolor (b)
In our research, we use two species of lily bulbs and investigate the effect of lily bulbs on the microecological of normal mice from three indicators: main intestinal bacteria communities, enzyme activity, and microbial activity.
Materials and methods
Animals and procedures
Thirty 4-week-old-specific pathogen-free (SPF) Kunming mice (All mice are male), weighting 20 ± 2 g, were purchased from Hunan Slaccas Jingda Laboratory Animal Co., Ltd (SCXK (Xiang) 2019-0004). All procedures involving animals were reviewed and approved by the Animal Ethics and Welfare Committee of Hunan University of Chinese Medicine, Animal license SCXK (Xiang) 2019-0009. Mice were reared in a stable environment (temperature 23–25 °C, relative humidity 50–70%, 12 h light/dark cycles, free access to diet and water). According to Pharmacopoeia of the People’s Republic of China (2015), mice in Lilium lancifolium (LL) group and Lilium davidii var. unicolor (LDU) group were given 0.15 g·mL−1 lily bulb solution at a dose of 3 g·kg−1·day−1, respectively, by gavage, twice a day for 49 days, while the control group was given equal sterile water by gavage.
Reagents
Four point five grams ultrafine powders of LL and LDU (offered by Hunan Academy of TCM) was dissolved and in boiling water, respectively, to make the final concentration was 0.15 g·mL−1. Foline-phenol was purchased from Hefei Bomei Biotechnology Co., Ltd (Anhui China). DNS reagent, o-Nitrophenyl β-D-galactopyranoside (ONPG) reagent, and four substrate solutions were prepared in the lab. Acetone was purchased from Hunan Huihong reagent Co., Ltd (Hunan China). Fluorescein diacetate (FDA) was purchased from Shanghai Yuanye Biotechnology Co., Ltd (Shanghai China), and sodium phosphate buffer was prepared in the lab.
Extraction of intestinal contents
All mice were sacrificed by cervical dislocation after being gavaged for 49 days. The intestinal contents (from the jejunum to the rectum) of all mice in each group were collected, respectively, in a sterile environment and cooled to 4 °C refrigerator (Guo et al. 2015).
Intestinal mucosa extraction
Intestinal mucosa in each group was collected according to the collection method of sterile intestinal mucosa established by our research group (He et al. 2017) . Under sterile conditions, the contents on the wall of the small intestine (the jejunum to the rectum) were washed with sterile saline, and then the intestinal mucosa was scraped off using coverslips and cooled to – 80 °C liquid nitrogen tank.
Determination of microbiota in intestinal contents
A certain amount of intestinal contents from each group were weighed aseptically and transferred into the corresponding group’s triangle bottle filled with sterile water and glass beads. Triangle bottles were shaken on a bed temperature incubator at 120 rpm for 30 min to release microorganisms from intestinal contents completely. The number of microorganisms was counted by dilution plate culture counting. The experiment was performed in triplicate. Total numbers of bacteria were detected after being cultured at 37 °C for 24 h, the numbers of Lactobacillus spp. and Bifidobacteria spp. were determined after being anaerobic cultured at 37 °C for 48 h.
Analysis of enzyme activities of intestinal in mice
The intestinal mucosa and contents were transferred into the corresponding centrifuge tubes filled with glass beads and added an appropriate amount of sterile water. Then, the intestinal mucosa and contents were shaken on a vortex mixer for 10 min to release enzymes completely and centrifuged at 3000 rpm for 8 min. Enzyme activities were analyzed by UV spectrophotometer. Protease activity was determined by the Foline-phenol method, the activities of amylase and sucrase were determined by the DNS colorimeter method and lactase by the ONPG method. The unit of enzyme activity is defined according to the reference (Tan et al. 2012).
Determination of microbial activity (Schnürer and Rosswall 1982; Tang et al. 2020)
FDA was dissolved in acetone and stored as a stock solution (2 mg/mL) at − 20 °C, the sample (intestinal mucosa and contents) was simultaneously added with FDA (final concentration, 10 μg/mL) to sterile sodium phosphate buffer, pH 7.6, and the mixture was incubated at 24 °C on a rotary shaker. Mixture was incubated at 24 °C on a rotary shaker. After 90 min, 2 mL of acetone was added to stop the reaction, and the value of A490 was determined. A group of blank control group was established, the sample (intestinal mucosa and contents) was simultaneously added with acetone, FDA (final concentration, 10 μg/mL) to sterile sodium phosphate buffer. Three repeated tests were performed on each sample.
Statistical analysis
Measurement data of each group were expressed by the form of mean ± standard deviation and analyzed using a single factor of variance (one-way ANOVA) in SPSS 21.0 software. LSD-t method was performed for multiple comparisons. Differences among groups were considered significant when P values less than 0.05.
Results
Effects of different lily bulbs on mice body weight
The weight of the mice can be seen from Fig. 2. Generally speaking, the weight of each group of mice increased with an increase in feeding time. Specifically, compared with the control group, the weight of mice in the lily bulb group gradually decreased with time, but there was no significant difference between the control group (P > 0.05). The weight of the mice in the LDU group was gradually lower than that in the LL group, but there was no significant difference between the two lily bulb groups (P > 0.05).
Fig. 2.
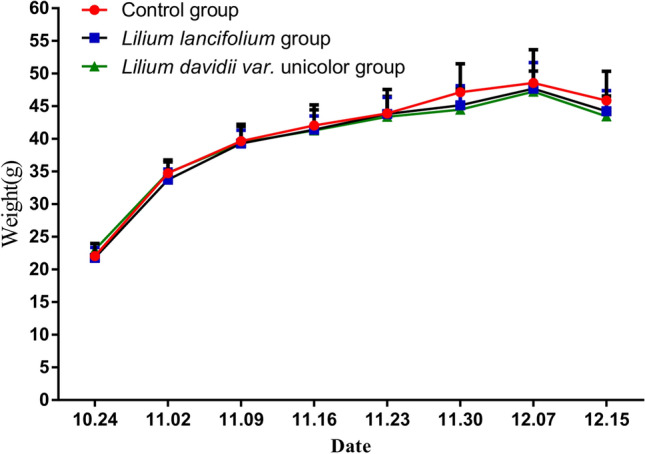
Body weight changes of control group (●), LL group (□), LDU group (△). Each value is the mean ± SD of five replicates (n = 5)
Effects of lily bulbs on microbiota in mice contents
Intestinal microbiota plays an important role in the human body. It can be seen from Fig. 3, the total numbers of bacteria in the LL group was significantly lower than that in the control group (t = 2.21 × 106, P = 0 0.040), while the number of Lactobacillus spp. and Bifidobacteria spp. was significantly higher than those in the control group (t = 2.68 × 107, P = 0.000; t = 5.96 × 107; P = 0.000). Different from the LL group, the changes of the number of three culturable microbiota in the LDU group was not significant difference from that in the control group (P > 0.05). Similar to the control group, the number of bacteria in the LDU group was significantly higher than that in the LL group (t = 4.0 × 106, P = 0.003), while the number of Lactobacillus spp. and Bifidobacteria spp. was significantly lower than that in the LL group (t = 6.12 × 107, P = 0.000; t = 2.71 × 107, P = 0.000).
Fig. 3.
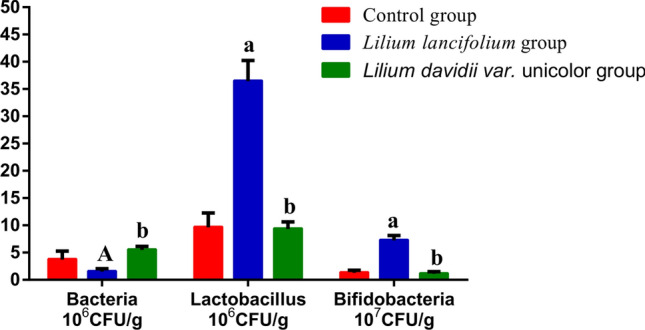
They represented the number of total bacteria, Bifidobacteria spp and Lactobacillus spp. colony between different groups. The colony numbers of the three strains in the LL group and other groups (the control group and the LDU group) were significantly different. Compared with the control group, AP < 0.05, aP < 0.01; Compared with the Lilium lancifolium group, bP < 0.01
Effects of lily bulbs on enzyme activities in mice contents
Table 1 demonstrates the effects of two kinds of lilies on the enzyme activity in contents. The activities of four digestive enzymes in the LL group were significantly different from those in the control group. The activities of amylase and protease were significantly higher than those in the control group (t = 455.73, P = 0.000; t = 52.32, P = 0.000), while the activities of sucrase and lactase were significantly lower than those of the control group (t = 5.82, P = 0.000; t = 443.51, P = 0.000). The changes in the four digestive enzyme activities of the LDU group were similar to those of the LL group. Except for protease, the other three enzyme activities were significantly different from the control group (t = 206.56, P = 0.000; t = 366.82, P = 0.000; t = 15.71, P = 0.000). The activities of amylase, protease and sucrase in the LDU group were significantly lower than those of the LL group (t = 249.18, P = 0.000; t = 52.15, P = 0.000; t = 9.89, P = 0.000), while lactase activity was significantly higher than that in the LL group (t = 76.69, P = 0.000).
Table 1.
Effects of different lilies on enzyme activity in feces
| Groups | Amylase (U/g) | Protease (U/g) | Sucrase (U/g) | Lactase (U/g) |
|---|---|---|---|---|
| Control group | 271.52 ± 24.55 | 27.49 ± 2.88 | 41.03 ± 0.69 | 451.04 ± 9.83 |
| Lilium lancifolium group | 727.26 ± 48.38a | 79.81 ± 2.89a | 35.39 ± 0.49a | 7.53 ± 5.38a |
| Lilium davidii var. unicolor group | 478.08 ± 7.8ab | 26.66 ± 1.27b | 25.33 ± 0.62ab | 84.21 ± 19.56ab |
Each value is the mean ± standard deviation, N = 5. Compared with the control group, aP < 0.01; compared with the Lilium lancifolium group, bP < 0.01
Effects of lily bulbs on mucosa enzyme activity in mice
As shown in Table 2, contrary to the changes of enzyme activity in mice contents, the activities of amylase and protease in the intestinal mucosa of the LL group (t = 44.15, P = 0.000; t = 1.25, P = 0.007) and the LDU group (t = 15.41, P = 0.012; t = 0.867, P = 0.031) were obviously lower than that of the control group. In addition, the amylase and protease activities in the LL group were lower than those in the LDU group, and the amylase activity was significantly different (t = 28.74, P = 0.001). There was no significant difference between the LL group and the control group in the activity of sucrase in the intestinal mucosa, but the activity of sucrase in the LDU group was significantly higher than that of the control group (t = 3.49, P = 0.015). Compared with the control group and the LL group, lactase activity in intestinal mucosa of the LDU group increased significantly (t = 34.45, P = 0.000; t = 19.81, P = 0.003). The lactase activity of the intestinal mucosa in the LL group was higher than that in the control group, with a significant difference (t = 14.64, P = 0.011).
Table 2.
Effects of different lilies on enzyme activity in mucosa
| Groups | Amylase (U/g) | Protease (U/g) | Sucrase (U/g) | Lactase (U/g) |
|---|---|---|---|---|
| Control group | 70.17 ± 8.78 | 2.23 ± 0.33 | 10.61 ± 0.38 | 198.76 ± 3.65 |
| Lilium lancifolium group | 26.02 ± 0.32a | 0.98 ± 0.53a | 12.41 ± 1.99 | 213.40 ± 3.71A |
| Lilium davidii var. unicolor group | 54.76 ± 2.58Ab | 1.36 ± 0.20A | 14.10 ± 0.85A | 233.21 ± 6.83ab |
Each value is the mean ± standard deviation, N = 5. Compared with the control group, AP < 0.05, aP < 0.01; compared with the Lilium lancifolium group, bP < 0.01
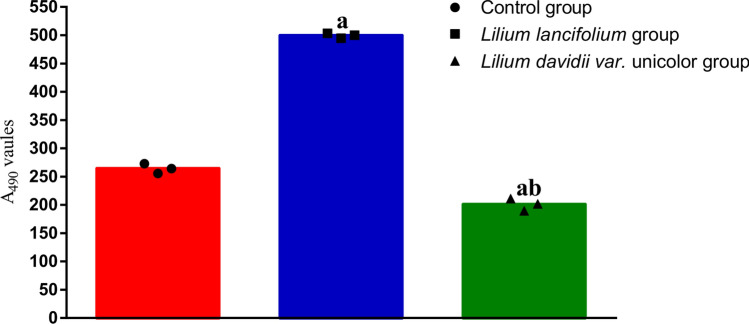
Effects of lily bulbs on microbial activity in mice contents
Figure 4 demonstrates the effects of two lilies on the microbial activity in mice contents. Microbial activity of the LL group was significantly higher than that of the control group (t = 0.81, P = 0 0.000), while that of the LDU group was significantly lower than that of the control group (t = 0.43, P = 0.001). Compared with the LL group, the microbial activity of the LDU group decreased significantly (t = 1.23, P = 0.000).
Fig. 4.
They represented microbial activity in feces of control group (●), LL group (□), LDU group (△). The microbial activity of LL group was the strongest, followed by the normal group, and finally the LL group. Compared with the control group, aP < 0.01; Compared with the Lilium lancifolium group, bP < 0.01
Effects of lily bulbs on microbial activity in mice mucosa
The results of the microbial activity in mucosa are shown in Fig. 5. Compared with the control group, the mucosa microbial activity of the LL group and the LDU group was significantly decreased (t = 0.89, P = 0.000; t = 0.69, P = 0.000). There was no significant difference in the mucosa microbial activity between the LL group and the LDU group (P > 0.05).
Fig. 5.
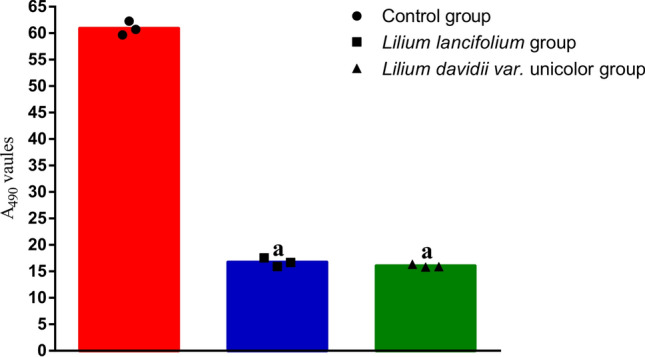
They represented microbial activity in mucosa of control group (●), LL group (□), LDU group (△). The microbial activity in the normal group was the strongest, and there was no difference between the LL group and the LDU group. Compared with the control group, aP < 0.01
Discussion and conclusion
Lily bulbs can regulate the digestive enzyme activity and microbial activity of the intestinal contents in normal mice
The composition of lily bulbs is complex, and intestinal digestive enzymes are easily affected by various active substances in lily. The regulatory mechanism may include the regulation of the intestinal mucosa, intestinal microbiota and intestinal microenvironment (Long et al. 2017). The digestive enzymes in the intestinal contents are mainly endogenous digestive enzymes, which are determined by the enzyme-producing microbial community. The larger number of enzyme-producing microbial communities, the higher digestive enzyme activity, the more complete absorption and utilization of foods, and the more intestinal microbial metabolites. Fluorescein diacetate, also known as FDA, can be hydrolyzed by esterases, proteases, lipases, etc. (Schnürer and Rosswall 1982). The changes in intestinal microbial enzyme activity can be reflected using the method of FDA. Combined with changes in intestinal microbiota and digestive enzyme activity, it can help us to better understand changes in the intestinal microenvironment (Tang et al. 2020).
The study has shown that lily bulbs, especially Lilium lancifolium, increase the activity of microbial in intestinal contents. At the same time, amylase and protease activities in intestinal contents are increased significantly by lily. Amylase and protease are secreted by the intestinal tract and intestinal microorganisms, which are closely related to the digestion and absorption function of the small intestine. Our research shows that eating lily bulbs can improve the activity of amylase and protease in the small intestine, and enhance the digestion and absorption of food. Amylase is the main enzyme for digesting carbohydrates, studies showed that when weaned pigs consume more carbohydrates, the amylase activity will increase (Makkink et al. 1994). Probiotics can stimulate the activity of digestive enzymes. Studies have shown that adding probiotics to aquatic feed can increase the digestion and absorption of fish, and increase the activity of protease and amylase (Zhang et al. 2020). Long-term consumption of lily bulbs in mice and bacteria will stimulate the production of amylase. At the same time, the growth of Lactobacillus spp. and Bifidobacteria spp. in the intestinal contents of normal mice may also stimulate amylase activity to some extent.
Lily bulbs can regulate the digestive enzyme activity and microbial activity in the intestinal mucosa of normal mice
Maintain the normal morphology and structure of the intestinal mucosa so that intestinal digestive enzymes can digest and absorb nutrients. Normal intestinal mucosa can secrete digestive enzymes to digest and absorb nutrients. Once the structure of intestinal mucosa is destroyed, it will reduce the activity of digestive enzymes. According to reports, the chemical components in traditional Chinese medicine can thicken and lengthen intestinal villi, making it more compact and orderly, which has a positive impact on the production and secretion of digestive enzymes (Long et al. 2017). In other words, changes in enzyme activity of intestinal mucosa can reflect changes in the intestinal mucosa structure. Jiang et al. found that the addition of 1.0% glutamine to the feed increased the activities of sucrase, maltase, and lactase significantly in the jejunum of 14-day-old pigs (Jiang et al. 2010). Investigations demonstrated that probiotics can increase the activity of diabylase (referring to lactase, sucrase, and maltase) and intestinal lipase in the small intestinal mucosa, and regulate the intestinal microbiota (Yang et al. 2005).
Eating lily enhanced the activity of lactase in the intestinal mucosa, which was mainly produced by small intestinal epithelial cells and decomposed lactose into galactose. Galactose is an oligosaccharide that provides nutrients for probiotics. In the studies, the microbial activity of the LDU group was lower than that of the normal group, so were the activity of mucosa amylase and protease. However, lily bulbs increased the activity of lactase in the mucosa, which indicated that lily bulb mainly promote the secretion of lactase in the intestine. Galactose is the product of lactase decomposition, which can promote the growth of probiotics, and probiotics can stimulate the activity of amylase and sucrose (Zhang et al. 2020). All the above can promote the digestion and absorption of the body and enhance the intestinal immune function.
Lily bulbs can promote the growth of Lactobacillus spp. and Bifidobacteria spp.
Our experimental results show that eating lily bulbs promote the growth of Lactobacillus spp. and Bifidobacteria spp. The experiment of Gong et al. confirmed that lily bulbs extract has a positive influence on the growth of Bifidobacteria spp. and even higher than that of Isomalto-oligosaccharide. Gong thinks that several oligosaccharides of lily bulbs promote the growth of Bifidobacteria spp. and put forward that lily bulbs have the potential as a bifidus factor or functional food (Gong et al. 2009). Zhao et al. found that purified lily bulb polysaccharide can restore the microbiota of mice with intestinal microbiota disorders (Zhao et al. 2020) . The research by Sim et al. revealed that Korean L. lancifolium bulbs are more anti-inflammatory and anti-aging than Japanese Lily (Sim et al. 2020). In our study, we also found that L. lancifolium bulbs increase the number of Lactobacillus spp. and Bifidobacteria spp. more than Lilium davidii var. Unicol bulbs.
Our experiments have proven that lily bulbs increased amylase activities in intestine contents significantly and improved digestion and absorption of foods. In addition, lily bulbs stimulate intestinal epithelial cells to secrete lactase, increase mucosa lactase activity, and maintain intestinal mucosa immunity. Lily bulbs promote the growth of Lactobacillus spp. and Bifidobacteria spp. and inhibit the growth of total bacteria in the intestines of normal mice, indicating that lily may maintain the intestinal mucosa barrier. This may be related to the oligosaccharides (such as glucomannan) of lily. Above all, the study might contribute to improving the current understanding the interaction between lily bulbs and intestinal digestive enzymes and microbiota, and is worthy of further study. Lily bulbs, especially L. lancifolium bulb has the potential to be functional foods, and it is necessary to figure out which ingredients of lily bulbs can improve intestinal microecology.
Acknowledgements
Thanks for the ethical certification of Hunan University of Chinese Medicine.
Author contribution
ZT designed the study; HS and CZ performed the experiments; YW analyzed the data; YW wrote the paper; ZT and HL checked the paper. The decision to submit the manuscript for publication was made by all the authors.
Funding
No funded projects for this experiment.
Data availability
Material and raw data are available.
Compliance with ethical standards
Conflict of interest
The authors declare that there is no conflict of interests regarding the publication of this paper.
Institutional animal care and use committee statement
The study was approved by the Animal Ethics and Welfare Committee of Hunan University of Chinese Medicine.
Contributor Information
Huaihao Luo, Email: 980195904@qq.com.
Zhoujin Tan, Email: tanzhjin@sohu.com.
References
- Chang YZ, Zhong SM, Cao YC, Wang YX. Study on the function of lily dietary fiber on the moderating hyperlipaemia and weight lose in the SD. Food Sci Technol. 2007;09:245–247. doi: 10.3969/j.issn.1005-9989.2007.09.080. [DOI] [Google Scholar]
- Gilbert JA, Blaser MJ, Caporaso JG, Jansson JK, Lynch SV, Knight R. Current understanding of the human microbiome. Nat Med. 2018;24:392–400. doi: 10.1038/nm.4517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong FH, He S, Zhang DC, Guo Preliminary study on growth promotion of lily bulbs extract on bifidobacterium. Food Ferment Ind. 2009;35(11):64–67. doi: 10.1007/978-3-540-85168-4_52. [DOI] [Google Scholar]
- Guo KX, Tan ZJ, Xie MZ, She Y, Wang YH. The synergic effect of ultra-micro powder Qiweibaizhusan combined with yeast on dysbacteriotic diarrhea mice. Chin J Appl Environ Biol. 2015;21(01):61–67. doi: 10.3724/SP.J.1145.2013.10002. [DOI] [Google Scholar]
- Han XY, Deng H, Cai Y, Fu JH, Yu Z, Ren X, Yin XL, Xie C (2009) Intestinal microbes and chronic diseases. Chin J Micro Ecol 21(11):1039–1042, 1046. https://doi.org/CNKI:SUN:ZGWS.0.2009-11-023
- He L, Long C, Liu Y, Guo Y, Xiao N, Tan Z. Effects of Debaryomyces hansenii treatment on intestinal microorganisms in mice with antibiotics-induced diarrhea. 3 Biotech. 2017;7:347. doi: 10.1007/s13205-017-0953-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hui H, Xin A, Cui H, Jin H, Yang X, Liu H, Qin B. Anti-aging effects on Caenorhabditis elegans of a polysaccharide, O-acetyl glucomannan, from roots of Lilium davidii var. unicolor Cotton. Int J Biol Macromol. 2020;155:846–852. doi: 10.1016/j.ijbiomac.2020.03.206. [DOI] [PubMed] [Google Scholar]
- Jiang ZY, Zheng WC, Lin YC, Zheng CT, Huang L, Chen F. Effects of glutamine on growth performance, serum hormone levels and intestinal mucosal enzyme activity in newborn piglets. Chin J Anim Nutr. 2010;22(1):125–131. doi: 10.3969/j.issn.1006-267x.2010.01.019. [DOI] [Google Scholar]
- Kalliomäki MA, Walker WA. Physiologic and pathologic interactions of bacteria with gastrointestinal epithelium. Gastroenterol Clin North Am. 2005;34(383–399):vii. doi: 10.1016/j.gtc.2005.05.007. [DOI] [PubMed] [Google Scholar]
- Kim S, Kwon SH, Kam TI, Panicker N, Karuppagounder SS, Lee S, Lee JH, Kim WR, Kook M, Foss CA, Shen C, Lee H, Kulkarni S, Pasricha PJ, Lee G, Pomper MG, Dawson VL, Dawson TM, Ko HS. Transneuronal propagation of pathologic α-synuclein from the gut to the brain models Parkinson’s disease. Neuron. 2019;103:627–641.e7. doi: 10.1016/j.neuron.2019.05.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu P, Lin ZJ, Zhang B. Research progress on chemical constituents and pharmacological effect of Lilii Bulbus. Chin J Exp Trad Med Formulae. 2019;23(23):201–211. doi: 10.13422/j.cnki.syfjx.2017230201. [DOI] [Google Scholar]
- Long CX, Guo YF, Liu YW, Peng XX, Tan ZJ. Immunoprotective effect of traditional Chinese medicine on intestinal mucosa. World Chin J Digest. 2017;25(35):3115–3122. doi: 10.11569/wcjd.v25.i35.3115. [DOI] [Google Scholar]
- Long C, Liu Y, He L, Yu R, Li D, Tan Z, Hui H. Bacterial lactase genes diversity in intestinal mucosa of dysbacterial diarrhea mice treated with Qiweibaizhu powder. 3 Biotech. 2018;8:423. doi: 10.1007/s13205-018-1460-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makkink CA, Berntsen PJ, den Kamp op BM, Kemp B, Verstegen MW. Gastric protein breakdown and pancreatic enzyme activities in response to two different dietary protein sources in newly weaned pigs. J Anim Sci. 1994;72:2843–2850. doi: 10.2527/1994.72112843x. [DOI] [PubMed] [Google Scholar]
- Okazaki Y, Chiji H, Kato N. Protective effect of dietary lily bulb on dextran sulfate sodium-induced colitis in rats fed a high-fat diet. J Nutr Sci Vitaminol (Tokyo) 2016;62:206–212. doi: 10.3177/jnsv.62.206. [DOI] [PubMed] [Google Scholar]
- Okazaki Y, Sekita A, Chiji H, Kato N. Consumption of lily bulb modulates fecal ratios of firmicutes and bacteroidetes phyla in rats fed a high-fat diet. Food Sci Biotechnol. 2016;25:153–156. doi: 10.1007/s10068-016-0112-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan G, Xie Z, Huang S, Tai Y, Cai Q, Jiang W, Sun J, Yuan Y. Immune-enhancing effects of polysaccharides extracted from Lilium lancifolium Thunb. Int Immunopharmacol. 2017;52:119–126. doi: 10.1016/j.intimp.2017.08.030. [DOI] [PubMed] [Google Scholar]
- Schnürer J, Rosswall T. Fluorescein diacetate hydrolysis as a measure of total microbial activity in soil and litter. Appl Environ Microbiol. 1982;43:1256–1261. doi: 10.1128/AEM.43.6.1256-1261.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekirov I, Russell SL, Antunes LC, Finlay BB. Gut microbiota in health and disease. Physiol Rev. 2010;90:859–904. doi: 10.1152/physrev.00045.2009. [DOI] [PubMed] [Google Scholar]
- Sim WS, Choi SI, Jung TD, Cho BY, Choi SH, Park SM, Lee OH. Antioxidant and anti-inflammatory effects of Lilium lancifolium bulbs extract. J Food Biochem. 2020;44:e13176. doi: 10.1111/jfbc.13176. [DOI] [PubMed] [Google Scholar]
- Spinler JK, Taweechotipatr M, Rognerud CL, Ou CN, Tumwasorn S, Versalovic J. Human-derived probiotic Lactobacillus reuteri demonstrate antimicrobial activities targeting diverse enteric bacterial pathogens. Anaerobe. 2008;14:166–171. doi: 10.1016/j.anaerobe.2008.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan ZJ, Wu H, Liu FL, Cai Y, Cai GX, Zeng A. Effect of ultra-micro powder qiweibaizhusan on the intestinal microbiota and enzyme activities in mice. Acta Ecol Sin. 2012;32(21):6856–6863. doi: 10.5846/stxb201109271422. [DOI] [Google Scholar]
- Tang Y, Wu Y, Hui HY, Tan ZJ. Effet of Tongxieyaofang prescription on intestinal microbia lactivity in mice with Ganqichenpi diarrhea. Chin J Micro Ecol. 2020;32(01):17–20. doi: 10.13381/j.cnki.cjm.202001004. [DOI] [Google Scholar]
- Yang SC, Chen JY, Shang HF, Cheng TY, Tsou SC, Chen JR. Effect of synbiotics on intestinal microflora and digestive enzyme activities in rats. World J Gastroenterol. 2005;11:7413–7417. doi: 10.3748/wjg.v11.i47.7413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng A, Peng M, Liu H, Guo Z, Xu J, Wang S, He L, Tan Z. Effects of Debaryomyces hansenii treatment on intestinal mucosa microecology in mice with antibiotic-associated diarrhea. PLoS ONE. 2019;14(11):e0224730. doi: 10.1371/journal.pone.0224730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang JJ, Yang HL, Yan YY, Zhang CX, Ye JD, Sun YZ. Effects of fish origin probiotics on growth performance, immune response and intestinal health of shrimp (Litopenaeus vannamei) fed diets with fish meal partially replaced by soybean meal. Aquacult Nutr. 2020;26:1255–1265. doi: 10.1111/anu.13081. [DOI] [Google Scholar]
- Zhao ZM, Zhao H, Wang YL, Shen Y, Wang CX, Meng FL, Zhang M, ZhangY (2020) Purification of lily polysaccharide and its regulatory effect on intestinal flora dysregulation mice. Science and Technology of Food Industry 41(08):295–300, 306. http://kns.cnki.net/kcms/detail/11.1759.TS.20191104.1456.022.html
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Material and raw data are available.