Abstract
Bio-inoculants play an important role for sustainable agriculture. Application of nanocompounds in the agriculture sector provides strength and is reported to enhance crop production but the combined effect of nanocompounds and plant growth-promoting rhizobacteria on plants has not been studied much. Therefore, the present study was planned to observe the effect of two plant growth promotory Bacillus spp. along with nanozeolite on maize under field conditions using a randomized block design. Combined treatment of nanozeolite and bio-inoculants promoted plant height, root length, fresh and dry weight of shoot and root, chlorophyll, carotenoids, total sugar, protein and phenol contents in maize significantly over control. Enhanced level of catalase, peroxidase, superoxide dismutase, phenols, alcohols and acid-esters in treated plants over control showed their role in stress management. An increase of 29.80% in maize productivity over control was reported in the combined treatment of Bacillus sp. and nanozeolite. Our results indicate that the application of bio-inoculants with nanozeolite showed a positive response on the health and productivity of maize plants. Hence, these may be used to enhance the productivity of different crops.
Supplementary Information
The online version contains supplementary material available at 10.1007/s13205-020-02561-2.
Keywords: Plant growth-promotory rhizobacteria, Nanozeolite, Zea mays, Antioxidant enzymes, Bacillus spp
Introduction
Maize is mainly grown as a food crop for human and animal consumption, industrial and pharmaceutical purposes worldwide. Nanoparticles ranging in size from 1 to 100 nm possess specific physical properties because of their small size, large surface area, and high reactivity compared to their bulk counterparts (Yadav 2013). To study the interaction of nanoparticles with the biological system is an important researchable issue to figure out their potential effect on plants, soil, animals and humans (Boczkowski and Hoet 2009).
Zeolites are compounds of alumina-silicate and reported to have broad applications in agriculture and environmental engineering (Ramesh et al. 2010). Application of zeolite in soil enhances crop yield by improving nutrient use efficiency of the plants. Properties like water retention due to large internal porosity, easy incorporation due to uniform particle-size distribution and better nutrient retention due to high cation-exchange capacity make this compound desirable for improving soil properties (Ok et al. 2003). Application of zeolite in soil increased tomato (Solanum lycopersicum) yield significantly but did not show a similar effect on sweet corn (Olczyk 2005).
Nanozeolite also enhanced protein expression and supports the growth of PGPR in nutrient broth (Khati et al. 2019a). Nanogypsum (50 mg/L) supported the growth and protein contents of plant growth-promotory Pseudomonas taiwanensis and Pantoea agglomerans in liquid media (Chaudhary and Sharma 2019). Khati et al. (2019b) using metagenomics reported that nanozeolite is better for the survival of soil microorganisms which is involved in nutrient cycling and improved plant growth. Nanozeolite can be used to support the growth of PGPR for a longer time due to the slow release of nutrients and offers an environmentally sustainable approach to increase crop production which is easily degradable and do not affect microbial activity in the soil.
Application of nanocompounds of zinc oxide and silica enhanced the rate of seed germination, root and shoot formation as well as accumulation of vegetative biomass in many crop plants (Nair et al. 2010). Arora et al. (2012), studied the impact of different concentrations of gold NPs on black mustard (Brassica juncea) under field conditions and observed enhanced growth and seed yield. The application of titanium oxide (TiO2 NPs) showed a positive effect on the growth of spinach (Tripathi et al. 2016). Recent studies have shown that chitosan induces mechanisms against various biotic (fungi, bacteria and insects) and abiotic (salinity, drought, heavy metal and cold) stresses in plants and helps in maintaining barriers to enhance plant productivity (Katiyar et al. 2015). The formation of reactive oxygen species during metabolic reactions in living systems is controlled by various enzymatic and non-enzymatic antioxidative systems (Ozyigit et al. 2016).
Application of chitosan nanoparticles (CNP) significantly enhanced seed germination, seedling vigour, induced systemic resistance, and also showed protection against downy mildew in pearl millet (Siddaiah et al. 2018). The CNP treatment also resulted in higher expression of pathogenesis-related proteins (PR1 and PR5) in pearl millet. As per reports, nanoparticles impose positive and negative responses on plant growth and microbial population of soil. A number of studies have suggested the role of nanomaterials at lower doses showed enhanced percent seed germination and growth rate in different crop plants (Zhao et al. 2012, 2014; Mousavi Kouhi et al. 2015; Mukherjee et al. 2016). Aminiyan et al. (2018) reported that the application of nanozeolite (10 and 30%) increased the actinomycetes population in the soil. Chavan et al. (2020) observed that TiO2 (50, 100, 500 and 1000 µg/ml) had a negative effect on soil nitrogen fixers and phosphate solubilizers. Ge et al. (2012) observed the toxic effect of titanium and zinc oxide nanoparticles (1 and 2 mg/L) on nitrogen-fixing bacteria in a microcosm.
Free-living plant growth-promoting rhizobacteria colonize the plant roots and found crucial to maintain soil fertility as they play an important role in recycling the soil nutrients (Glick 2012). PGPR are involved in nitrogen uptake, synthesis of phytohormones, solubilization of minerals such as phosphorus and production of iron chelators (siderophores) and make them available to the plants (Zakry et al. 2012). Species of Azospirillum, Bacillus, Burkholderia, Enterobacter, Klebsiella and Pseudomonas are invariably used as bioinoculants as they improve the growth and yield of different crops (Ghevariya and Desai 2014).
A pot experiment on maize using nanozeolite (50 mg/L) and two Bacillus spp. (PS2 and PS10) was conducted by Khati et al. (2018). They reported a positive effect of Bacillus spp. and nanozeolite on maize growth and soil health. Validation of the experiment at the field level was not conducted. Therefore, the objective of the present study was to evaluate the plant growth-promoting efficacy of Bacillus spp. on maize crop in the presence of nanozeolite under field conditions.
Materials and methods
Site description
A field experiment on maize was conducted at Crop Research Center (CRC) of G.B. Pant University of Agriculture and Technology, Pantnagar. It falls under the subtropical climatic zone and is situated at an altitude of 243.84 above mean sea level, 29°N latitude, and 79.3°E longitude. This area comes under foothills of “Shivalik” ranges of “Himalaya”, a narrow belt called “Tarai”. It is characterized by a humid and subtropical climate with summer being hot and dry while, winter is being cold with fog. The summer is hot with maximum temperature exceeds even 35 °C (June and July) whereas minimum temperature 23 °C during (September and October). Relative humidity was recorded highest in July and lowest in June. During the experimental period, maximum rainfall was received during the month of July. The rainfall distribution, relative humidity and thermal regimes during the cropping season, recorded at the meteorological observatory at CRC of the University.
Experimental design
Bacterial strains and growth conditions
Two species of Bacillus (PS2: Assession number- KX650178 and PS10-KX650179) were isolated from nanocompounds treated soil of an agricultural field of the University where different agriusable nanocompounds are being used since 4 years. Both the bacterial species were routinely grown in nutrient broth at 30 °C. Nanozeolite was purchased from Intelligent Material Pvt. Ltd India, stock number NS6130-09–905. The size of nanozeolite was < 80 nm in size, having pH 7–8, refractive index 1.47, purity 99.9% and bulk density 0.6–0.8 g/cm3 (Khati et al. 2019b). Chemicals for enzyme assays and other experiments were purchased from SRL and Hi media Laboratories Pvt. Ltd. India. Maize seeds (variety- DH296) were provided by the Department of Plant Breeding and Genetics at Govind Ballabh University of Agriculture and Technology, Pantnagar.
Seed bacterization
Maize seeds were sterilized as per the standard protocol described by Tyagi et al. (2017). Carboxy methyl cellulose (CMC) 1% was added to the overnight grown bacterial cultures for proper adherence of bacterial culture to seeds. Seeds were soaked in bacterial culture (2 × 108 cfu seed−1) for 10 min and dried at room temperature. Nanozeolite was applied at the rate of 50 mg/L in the nutrient broth. Treatments without nanozeolite and bio-inoculants served as control. The experiment was planned using a completely randomized block design with three replications and six treatments. Treatments used in the experiment were: control (T1: without bacterial culture and nanozeolite), Bacillus sp. (T2: PS2), Bacillus sp. (T3: PS10), nanozeolite alone (T4), PS2 and PS10 with nanozeolite (T5 and T6).
A plot size of 14.70 m2 was employed for the experiment, where the distance between two rows and two plants was 60 cm and 20 cm respectively. Each plot had seven rows and treated seeds were sown in seven rows. NPK was added at a basal level of 120:60:40 kg/hm2. Irrigation was provided as and when required particularly during different physiological growth stages of the crop. Thinning was done 30–35 days after sowing to ensure uniformity.
The soil of the experimental plot was classified under silty clay loam type (Pachic Ultic Argixerolls) (Soil Survey Staff 2010). Soil samples, collected from a depth of 15 cm were air-dried and mixed to generate a representative composite sample. After sieving through 2 mm mesh, the soil was stored for two days at 4 °C to perform physicochemical analysis. Experimental soil had pH 7.2, Electrical conductivity—0.207 (dS/m) and organic carbon (%) was 0.75. Available nitrogen, phosphorus and potassium of the soil was 212.89 kg/hm2, 25 kg/hm2 and 136 kg/hm2 respectively (Khati et al. 2017b).
Measurement of plant health
Seed germination
Percent seed germination of each treatment from different plots was evaluated using the following formula:
Agronomical parameters
Plant samples from each plot were collected at an interval of 20, 40 and 60 days after sowing (DAS). Twelve plants (four plants from each plot) were chosen at random to study agronomical parameters like plant height, root length, number of leaves, leaf area, fresh and dry weight of shoot and root.
Total chlorophyll
Chlorophyll content of maize leaves was estimated according to the method of Hiscox and Israelstam (1979). For chlorophyll extraction, leaf samples were washed with deionized water to remove any surface contamination. Then 50 mg of leaves were cut into small pieces and placed in test tubes containing 10 ml dimethyl sulfoxide (DMSO). Tubes were kept in water-bath for 3 h at 60 °C till leaves become colorless. After filtration extract was maintained at room temperature. The absorbance of the leaf extract was taken at 663 and 645 nm using visible spectrophotometer (Labtronics Model LT-39). DMSO was used as a blank, and the amount of total chlorophyll in the extract was calculated in mg g−1 of tissue using the following formula:
Carotenoid content
Same leaf extract was used for the estimation of carotenoid content and absorbance was taken at 470 nm using a visible spectrophotometer (Kirk and Allen 1965). Carotenoid content of leaf extract was calculated using the following formula.
Total sugar content
Fresh leaves were dried in a hot air oven at 80 °C for 48 h and then 0.1gm dried leaves were powdered with the help of mortar and pestle. Powdered leaves were added to 3 ml of 80% ethyl alcohol, boiled in a water bath, and then centrifuged for 15 min at 1000 rpm. The supernatant was taken in a test tube and the final volume was made 6 ml with 80% ethyl alcohol. To 1 ml ethanolic leaf extract, 4 ml ice cold Anthrone reagent was added. The mixture was shaken properly and boiled in a water bath for 10 min. After cooling, absorbance was recorded at 620 nm. The amount of total sugar was estimated using a standard graph prepared by taking glucose in the range of 10–100 µg/ml (Dubois et al. 1956).
Protein estimation
One gram of fresh leaves was collected and transferred to a mortar and pestle after thorough washing. After adding 5 ml of 0.2 M Tris–cl (pH-8), leaves were crushed gently for 20 min until a fine slurry was formed. The slurry was centrifuged at 10,000 rpm at 4 °C for 20 min. The supernatant was transferred to a fresh tube and stored at 4 °C for further use. Protein was estimated according to Bradford (1976). From the supernatant, 20 µl was added to 280 µl of extraction buffer to which 3 ml Coomassie brilliant blue (CBB) G-250 was added. The mixture was kept at 37 °C for 5 min and absorbance was read at 595 nm in a spectrophotometer against a reagent blank. The amount of protein was calculated using a standard curve prepared with different concentrations of BSA (10-100 µg/ml).
Estimation of total phenolic content (TPC)
The total phenolic content of the maize leaves was estimated according to the method of Ainsworth and Gillespie (2007). Plant leaves (200 mg) were homogenized in 800 µl ice-cold 95% methanol in a cold mortar and pestle and incubated for 48 h in dark at room temperature and then centrifuged at 10,000 rpm for 5 min. The obtained supernatant was used to determine total phenolic content by Folin-Ciocalteu method. Gallic acid (10–100 µg/ml) was used as a standard for preparing the standard curve and total phenolic contents were expressed as mg/g.
Antioxidant activity
Catalase activity (CAT)
100 μl of enzyme extract was added in 3 ml of reaction mixture containing 100 mM sodium phosphate buffer (pH-7) and 0.1 ml H2O2 (10 mM). A decrease in optical density was monitored at 230 nm for 3 min. The assay mixture without enzyme extract was used as a control. CAT activity was calculated by using extinction coefficient of 39.4 mM −1 cm−1 and enzyme activity was expressed as decomposition of 1 mM of H2O2 min−1 (Chandlee and Scandalios 1984).
Peroxidase activity (POD)
Peroxidase activity was determined by method (Mali et al. 1989). A 3 ml reaction mixture was taken in a cuvette containing 0.4 ml of pyrogallol in phosphate buffer, 0.1 ml of the enzyme extract, and 0.5 ml of H2O2 were added and change in absorbance at 420 nm was noted at an interval of 15 sec for a period of 3 min. Reaction mixture without enzyme extract served as control. POD activity was calculated by using an extinction coefficient of 26.6 mM−1 cm−1 and enzyme activity was expressed in U mg−1 protein.
Superoxide dismutase (SOD) activity
SOD activity was determined on the basis of inhibition of Nitrobluetetrazolium chloride (NBT), which was measured spectrophotometrically at 560 nm (Giannopolitis and Ries 1977). For this reaction mixture was prepared using 100 mM phosphate buffer (pH-7.5), riboflavin (75 mM), EDTA (3 mM), methionine (200 mM) and 100 µl of enzyme extract. Enzyme activity was expressed as units of enzyme g-1 FW.
Gas chromatography–mass spectroscopy (GC–MS)
For GC–MS analysis of plant metabolites, maize leaves from the treated and control samples were shade dried and crushed in methanol for extraction. GC–MS analysis of plant extract was performed using silica column (30 m 90.25 mm) adjusted at a gas flow rate of 1 ml min−1 at 8 °C by Shimadzu GC–MS QP Ver. 2010. Temperature was maintained at 280 °C. The organic compounds present in different samples were identified by comparing with the standards or the mass spectrum matched with the inbuilt library (Wiley 8). The facility for GC–MS analysis was provided by JNU Campus, New Delhi.
Crop yield
Observations for 50% tasselling, 50% silking, cob length, cob weight, grain yield and 100 grain weight per plot were recorded at the time of harvesting after 85 days.
Statistical analysis
Difference in maize yield was estimated using one-way analysis of variance. The statistical analysis of other parameters was carried out using two-way analysis of variance (ANOVA) using SPSS, ver. 16.0 software. Significant differences among means were tested with Duncan’s Multiple Range Test (DMRT) at P < 0.05. The data represented in the tables and figures are expressed as means of three replicates ± standard deviation (SD).
Results
Seed germination
Both the bacterial cultures with nanozeolite showed a significant (P < 0.05) increase in seed germination as compared to their respective controls. PS2, PS10, nanozeolite, PS2 + nanozeolite and PS10 + nanozeolite showed 88.33, 89.99, 90.94, 94.99 and 96.90% seed germination in comparison to control which showed only 70% germination.
Agronomical parameters of maize plants
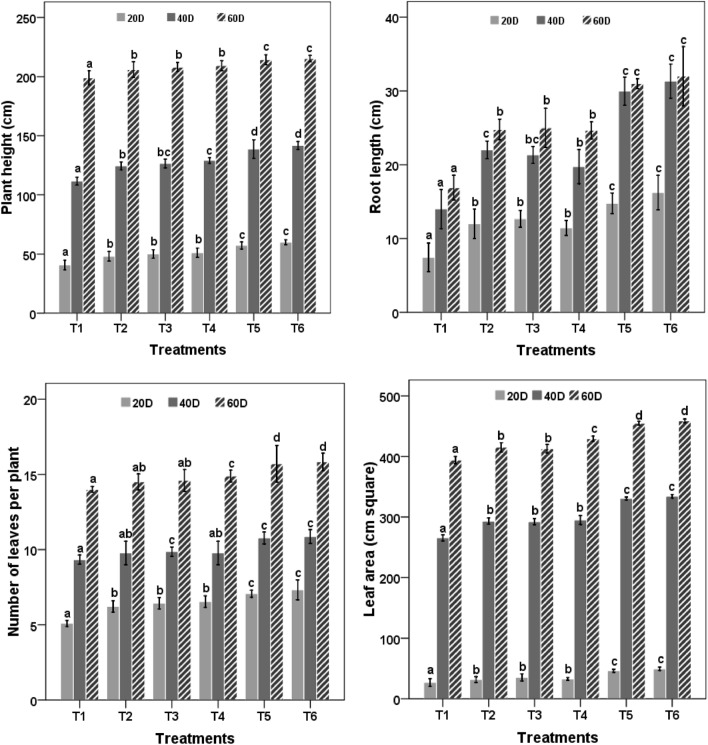
Combined treatment of bacterial cultures with nanozeolite showed significant changes in agronomical parameters in comparison to their respective controls on 20, 40 and 60 DAS (Fig. 1). T5 and T6 treatments performed best throughout the experiment. The data revealed that this treatment performed significantly (P < 0.05) better than the rest of the treatments with respect to plant height and root length. T2, T3, T4, T5 and T6 treatments showed 1.03, 1.04, 1.05, 1.07 and 1.08 fold increase in plant height and 1.46, 1.48, 1.46, 1.83 and 1.89 fold increase in root length as compared to control till the end of the experiment.
Fig. 1.
Effect of nanozeolite and Bacillus spp. on plant height, root length, leaf number and leaf area of Zea mays. Bars followed by different letters mean significant differences (P < 0.05) among the treatments at the same sampling time
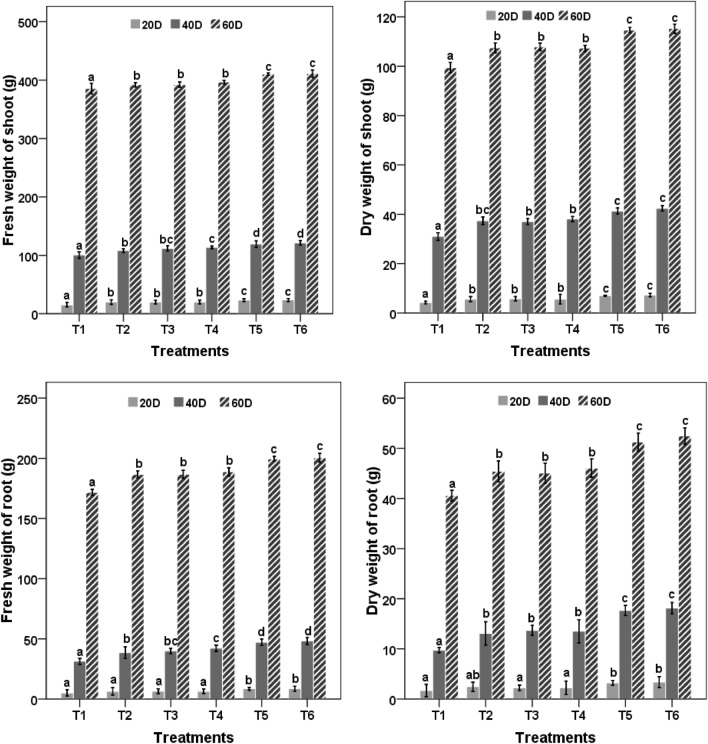
The number of leaves and leaf area was highest in treatment T5 and T6 and was significantly different from the rest of the treatments. Order of the performance was T6 > T5 > T4 > T3 > T2 throughout the experimental period. All the treatments performed statistically significant (P < 0.05) over absolute control. For fresh and dry weight of shoot and root after 20, 40 and 60 DAS, treatments using bacterial culture(s) along with nanozeolite performed comparatively better than PS2, PS10 and nanozeolite but they were statistically better than the control. After 60 days of sowing, T5 and T6 treatments showed highest plant growth with maximum fresh and dry weight of shoot and root. Fresh weights of plant material treated with T5 and T6 were 410 g and 411 g and dry weights were 199 and 200 g, respectively, whereas the least weight was recorded in control (Fig. 2).
Fig. 2.
Effect of nanozeolite and Bacillus spp. on fresh and dry weight of shoot and root of Zea mays. Bars followed by different letters mean significant differences (P < 0.05) among the treatments at the same sampling time
Plant biochemical parameters
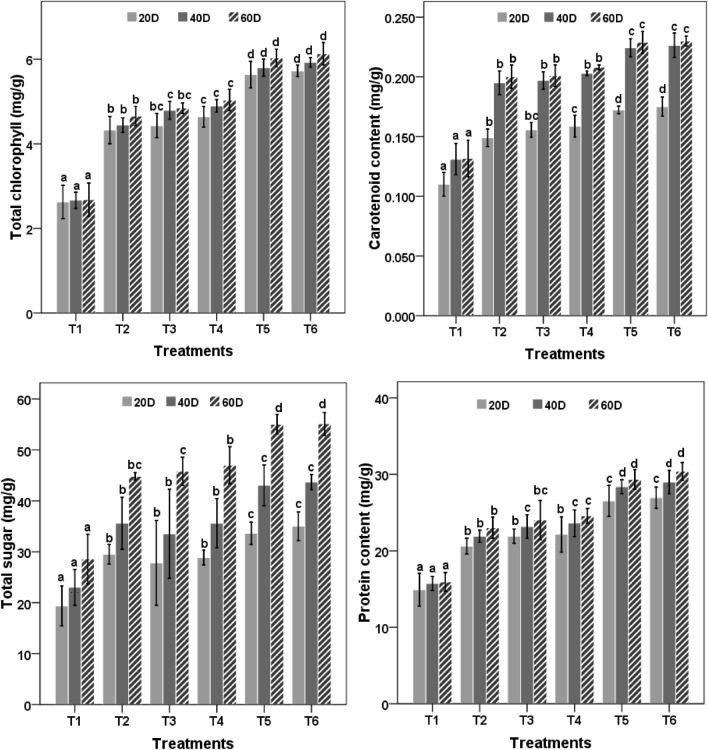
The chlorophyll content of maize leaves under different treatments also showed a similar pattern and was highest in T6 treated plants followed by T5 > T4 > T3 > T2 which showed 2.28, 2.25, 1.87, 1.80 and 1.73 fold increase in chlorophyll content as compared to control. During the first (20 DAS) sampling to the last sampling (60 DAS), treatments having PS2, PS10, nanozeolite with and without Bacillus sp. performed significantly (P < 0.05) different than the control. Pattern of increase in chlorophyll content was 20 DAS < 40 DAS < 60DAS, respectively (Fig. 3).
Fig. 3.
Effect of nanozeolite and Bacillus spp. on biochemical parameters of Zea mays. Bars followed by different letters mean significant differences (P < 0.05) among the treatments at the same sampling time
The increase in carotenoid content in maize leaves was 1.74 and 1.75 fold in T5 and T6 treatments respectively as compared to control. T2, T3 and T4 treatments showed an increase of 1.52, 1.53 and 1.58 fold in carotenoid content with respect to control.
Total sugar content in maize leaves was highest in combined treatment of bacterial culture and nanozeolite as compared to other treatments. Pattern of sugar content was T6 > T5 > T4 > T3 > T2 and had 1.92 > 1.92 > 1.64 > 1.60 > 1.56 fold increase in sugar content in maize leaves as compared to control (Fig. 3).
Results pertaining to protein content in plant leaves after 20, 40 and 60 DAS are presented in Fig. 3. Data revealed that higher protein content was observed in plants treated with bacterial cultures and nanozeolite as compared to their respective controls. Order of protein level was T6 > T5 > T4 > T3 > T2 > T1 which was equivalent to 26.95 > 26.53 > 22.15 > 21.90 > 20.61 > 14.90 mg/g protein at 20 DAS, which gradually increased to 30.40 > 29.35 > 24.58 > 24.02 > 23.02 > 15.93 mg/g at the time of sampling at 60 DAS. T5 and T6 treatments showed the highest protein level which was statistically comparable with all the treatments.
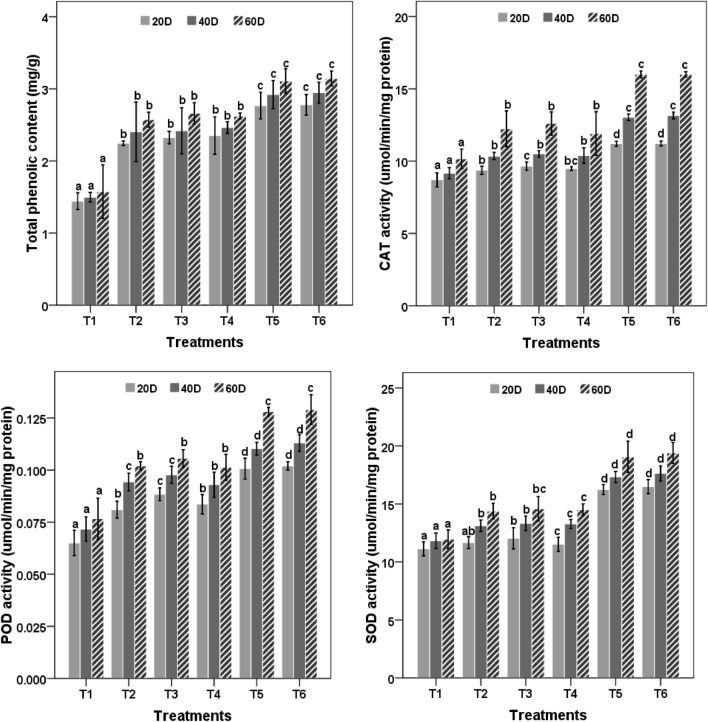
Total phenolic content of plants was highest in T5 and T6 treatments and showed 1.97 and twofold increase than the control. Other treatments (T2, T3 and T4) showed 1.63, 1.69 and 1.66 fold increase in phenolic which was statistically (P < 0.05) different from the control (Fig. 4).
Fig. 4.
Effect of nanozeolite and Bacillus spp. on phenol content and antioxidant enzymes in Zea mays. Bars followed by different letters mean significant differences (P < 0.05) among the treatments at the same sampling time
Analysis of antioxidant enzymes in maize leaves
T2, T3, T4, T5 and T6 treatments showed 1.20, 1.24, 1.17, 1.57 and 1.57 fold increase of CAT activity respectively as compared to control after 60 days. A gradual increase in CAT activity in all the treatments with time was observed till the end of the field experiment.
A similar pattern was found for peroxidase activity. T2, T3, T4, T5 and T6 treatments showed 1.24, 1.35, 1.27, 1.53 and 1.56 fold increases in POD activity respectively at 20 DAS as compared to the control. T2, T3, T4, T5, and T6 treatments showed 1.2, 1.22, 1.21, 1.59 and 1.62 fold increase in SOD activity respectively after 60 days as compared to control Combined treatment of nanozeolite and bacterial culture in maize plants showed the highest activity of CAT, POD and SOD after 60 days of sowing (Fig. 4).
GC–MS analysis
The relative abundance of volatile compounds in maize leaf extract of control, PS2 + nanozeolite and PS10 + nanozeolite treatments is presented in the supplementary material (SM1). GC–MS results revealed an increased level of phenols, acid esters and sugar in the treated maize plants over control.
Maize yield
A difference of one day in 50% tasseling and 50% silking was observed in treated and control maize plants. Significant variation in cob length was observed in treated plants. Cob length and weight were highest in T5 and T6 treatments. After harvesting, grain yield of control and various treatments with PS2, PS10 and nanozeolite was 3.59, 4.30, 4.35 and 4.33 kg/plot, respectively. Use of PS2 and PS10 with nanozeolite had maximum grain yield of 4.63 and 4.66 kg/plot which shows 28.96% and 29.80% increase in grain yield over absolute control. Treatments like PS2, PS10, nanozeolite, PS2 + nanozeolite, PS10 + nanozeoilte showed 10.93, 11.92, 12.13, 21.23 and 21.52% increase respectively in 100 g grain weight compared to control (Table 1).
Table 1.
Effect of nanozeolite and Bacillus spp. on the yield of maize
| Treatments | Days to 50% tasseling | Days to 50% silking | Cob length (cm) | Cob weight (Kg) | Grain yield (Kg/plot) | 100 grains weight (g) |
|---|---|---|---|---|---|---|
| T1 | 53.99 ± 0.87c | 55.99 ± 0.33b | 10.02 ± 0.43a | 4.01 ± 0.08a | 3.59 ± 0.13a | 24.06 ± 0.15a |
| T2 | 53.33 ± 0.33bc | 55.44 ± 0.50b | 14.81 ± 0.94b | 4.75 ± 0.18b | 4.30 ± 0.12b | 26.69 ± 0.34b |
| T3 | 53.00 ± 1.00abc | 55.33 ± 0.57b | 15.44 ± 0.49b | 4.85 ± 0.14b | 4.35 ± 0.10b | 26.93 ± 0.10b |
| T4 | 52.88 ± 0.19ab | 55.44 ± 0.50b | 14.77 ± 0.57b | 4.73 ± 0.02b | 4.33 ± 0.04b | 26.98 ± 0.12b |
| T5 | 52.22 ± 0.19a | 54.07 ± 0.12a | 17.18 ± 0.74c | 5.11 ± 0.13c | 4.63 ± 0.10c | 29.17 ± 0.15c |
| T6 | 52.11 ± 0.19a | 54.22 ± 0.38a | 17.66 ± 0.61c | 5.20 ± 0.13c | 4.66 ± 0.08c | 29.24 ± 0.25c |
Values in each column followed by the same letter were not significantly different (P < 0.05)
Discussion
Agronomical parameters of the plants are influenced by a variety of biotic and abiotic factors. Seed germination, the first stage of plant’s life cycle is exposed to different environmental conditions and affected by various factors. Successful establishment of a seedling leads to an optimum capacity of growth and productivity of the plant. The present study revealed that bacterial strains (PS2 and PS10) in combination with nanozeolite, helped the maize plant to grow under field conditions and enhanced seed germination. Application of Bacillus sp. and nanozeolite individually also supported seed germination. The positive effect of nanocompounds on seed germination can be explained on the basis of the role of NPs in regulating aquaporins, the water channels, which regulate the permeability of water in the seeds and enhance the rate of seed germination and plant growth (Heinen et al. 2009; Khodakovskaya et al. 2009; Mahakham et al. 2017).
Stimulation of plant growth by using a combination of PGPR and nanocompounds (nanozeolite and nanochitosan) has also been reported by Kumari et al. (2020). They observed enhanced percent seed germination, plant height, total chlorophyll, protein content and soil enzyme activities over control where Fenugreek plants were treated with nanocompounds (50 mg/L) and PGPR. Ramesh et al. (2014) observed the beneficial effect of Zinc oxide nanoparticles (ZnONPs) on seed germination, chlorophyll and protein content in wheat when used at the rate of 250 mg/L.
In the present study, application of PGPR in the presence of nanozeolite may improve beneficial bacteria around the root zone, which enhanced nutrient uptake, released different growth hormones and may be supported shoot/ root growth and biomass in maize. Zeolites chelate nutrients, capture water and release them slowly in root zones which make the resources available for a longer duration and thus help the sustainable growth of Bacillus spp. (Khati et al. 2019a). Timmusk et al. (2018) have also reported similar observations in wheat when PGPR were inoculated with titania nanoparticles (50 ug/ml) under drought and salt stress. An increase in plant height may be related to increased level of gibberellic acid which is responsible for shoot elongation (Stepanova et al. 2007). Kukreti et al. (2020) reported that application of nanosilicon dioxide (20 mg/L) improved the maize and soil health.
Increased height of tomato plants in the presence of Zinc oxide NPs applied at the rate of 250 mg/kg was reported by Raliya et al. (2015). Application of silver nanoparticles at the concentrations of 20 and 40 mg/g increased shoot length, fresh and dry weight of shoot, chlorophyll, total carbohydrate and protein content in wheat plants (Hanan 2017). Increased fresh biomass in radish plants was observed when soil was treated with 50 mg/kg cerium oxide nanoparticles (CeO2 NPs). Enhanced level of chlorophyll content by 12.5, 12.9, and 12.2% as compared to control was observed in radish plants when treated with 10, 20 and 100 mg/kg CeO2 NPs (Gui et al. 2017). Wang et al. (2019) reported the negative impact of TiO2 and iron oxide nanoparticles (50 and 500 mg/L) on wheat plant growth. Similarly, Rui et al. (2018) also reported a negative aspect of copper nanoparticles (500 mg/kg) on peanut.
Photosynthesis is the basic and most essential physiological function of the plants to determine productivity. We also report a significant (P < 0.05) increase in chlorophyll and carotenoid content in treated maize plants as compared to control. This may be due to an increase in photosynthates as reported by Urbonaviciute et al. (2006). Venkatachalam (2017) showed enhanced growth and higher total biomass by 130.6% and 131% respectively over control in cotton plants when treated with Zinc oxide nanocompounds. He also observed an increased level of chlorophyll a (141.6%), b (134.7%), carotenoids (138.6%) and total soluble protein contents (179.4%) in treated plants as compared to control. Embedment of single-walled carbon nanotubes (SWCNTs) in chloroplasts increases the photosynthetic rate in Arabidopsis thaliana by three times in comparison to the control (Giraldo et al. 2014).
Total sugar, protein and phenol content were also enhanced in all treated plants this may be due to the positive and protected mechanisms shown by nanozeolite and PGPR. Carbon-based graphene nanoparticles, at lower doses (0.02 mg/L) have been demonstrated to trigger physiological processes in coriander (Coriandrum sativam) and garlic (Allium sativum). These nanocompounds act as a growth regulator in agricultural food crops (Chakravarty et al. 2015). Foliar application of ZnO NPs on cluster bean (Cyamopsis tetragonoloba L.) and tomato (Solanum lycopersicum) has also shown a positive response of the treatment in terms of biomass, chlorophyll and total soluble leaf protein over control (Raliya and Tarafdar 2013, 2015). Treatment of chitosan oligosaccharides induced resistance in Arabidopsis against tobacco mosaic virus (TMV) by activating Salicylic acid signalling pathway and resistance was associated with increased expression of PR-1 gene (Jia et al. 2016).
In the present study, combined treatment of PGPR with nanozeolite in maize plants showed the highest activity of CAT, POD and SOD till the end of the experiment. Application of nanozeolite and PGPR might have developed a significant increase in innate immune response by inducing catalase, peroxidase and SOD activities in maize plants. An increased level of enzyme activities might have protected maize plants from stress. Our findings can be correlated with the findings of Siddaiah et al. (2018) who observed 1.10, 1.96 and 3.09 folds higher SOD activity in pearl millet seedlings, treated with CNP in comparison to their bulk counterparts and control plants. Two-fold increase in CAT activity was recorded in CNP-treated seedlings compared to untreated control. Enhanced SOD and POX enzyme activities (264.2 and 182.8%) respectively over control were reported in cotton (Gossypisum hirsutum L.) plants when treated with zinc oxide NPs at the rate of 25–200 mg/L (Venkatachalam 2017).
Results of GC–MS analysis of treated maize plant extract also correlated with our findings. Treated plants had an enhanced level of secondary metabolites like ketones, phenols, acid esters and sugar as compared to control which provides protection to plants. An enhanced level of secondary metabolites in the treated maize plants might have protected plants from stress. Similar results were reported by Suriyaprabha et al. (2012) and Khati et al. (2017a, 2018).
Cob length, cob weight, grain yield and 100 grain weight per plot were also high in nanozeolite and PGPR treated plants and could be related to increased levels of plant health parameters which helped in the enhancement of productivity. Similar findings were observed by Siddaiah et al. (2018) who reported that treatment with Cu-chitosan on maize plants showed enhanced values of cob length, cob weight, grain yield and 100 grain weight.
In general, the performance of combined treatment of PGPR and nanozeolite was significantly (P < 0.05) better with respect to agronomical parameters, chlorophyll content, carotenoid, sugar, protein content and phenolic content, antioxidant enzymes and maize yield as compared to their respective counterparts and control.
Conclusion
Present findings strongly suggest the application of combined treatment of bio-inoculants and nanozeolite on different crops under field conditions. Interaction of nanocompounds and bio-inoculant with soil microorganisms can also significantly influence plant growth as well as the biochemical and defense response of the plants by inducing various antioxidants and phenolics. Low cost and eco-friendly technologies are needed in agriculture practices which do not affect the microbial diversity of soil. Hence, it is important to improve soil health by increasing the application of beneficial microbes as bio-inoculants. Bioformulation offers an environmentally sustainable approach to increase crop production by using microbial bio-inoculants and agriusable nanozeolite. However, more field trials and metagenomic studies are needed to assess and expand the results.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
Authors gratefully acknowledge the financial support provided by UCB, Haldi, Uttarakhand in a form of a project. Facilities provided by Department of Agronomy and Plant Breeding and Genetics are duly acknowledged.
Abbreviations
- CNPs
Chitosan nanoparticles
- TiO2
Titanium oxide
- NPs
Nanoparticles
- DMSO
Dimethyl sulfoxide
- SOD
Superoxide dismutase
- BSA
Bovine serum albumin
Author contributions
PC: experimental design and research work participated in all experiments and wrote the manuscript. PK: isolated and characterized the bacterial culture used in the experiment. AC: helped in data interpretation. SG: participated in analysis of agronomical parameters of maize crop. RK: provided the field facilities and nanozeolite used in the experiment. AS: provided the laboratory facilities and critical checking of the manuscript.
Compliance with ethical standards
Conflict of interest
Authors declare that they have no conflicts of interests.
Contributor Information
Parul Chaudhary, Email: parulchaudhary1423@gmail.com.
Priyanka Khati, Email: priyankakhati712@gmail.com.
Anuj Chaudhary, Email: anujkalihar1078@gmail.com.
Saurabh Gangola, Email: saindsaurabh@gmail.com.
Rajeew Kumar, Email: shuklarajeew@gmail.com.
Anita Sharma, Email: anitasharma14@yahoo.co.in.
References
- Ainsworth EA, Gillespie KM. Estimation of total phenolic content and other oxidation substrates in plant tissues using Folin-Ciocalteu reagent. Nat Protoc. 2007;2:875–877. doi: 10.1038/nprot.2007.102. [DOI] [PubMed] [Google Scholar]
- Aminiyan MM, Hosseini H, Heydariyan A. Microbial communities and their characteristics in a soil amended by nanozeolite and some plant residues: short time in-situ incubation. Eurasian J Soil Sci. 2018;7:9–19. [Google Scholar]
- Arora S, Sharma P, Kumar S, Nayan R, Khanna PK, Zaidi MGH. Gold-nanoparticle induced enhancement in growth and seed yield of Brassica juncea. Plant Growth Regul. 2012;66:303–310. [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Chakravarty D, Erande MB, Late DJ. Graphene quantum dots as enhanced plant growth regulators: effects on coriander and garlic plants. J Sci Food Agric. 2015;95:2772–2778. doi: 10.1002/jsfa.7106. [DOI] [PubMed] [Google Scholar]
- Chandlee JM, Scandalios JG. Analysis of variants affecting the catalase developmental program in maize scutellum. Theor Appl Genet. 1984;69:71–77. doi: 10.1007/BF00262543. [DOI] [PubMed] [Google Scholar]
- Chaudhary P, Sharma A. Response of nanogypsum on the performance of plant growth promotory bacteria recovered from nanocompound infested agriculture field. Environ Ecol. 2019;37:363–372. [Google Scholar]
- Chavan S, Sarangdhar V, Nadanathangam V. Toxicological effects of TiO2 nanoparticles on plant growth promoting soil bacteria. Emerg Contam. 2020;6:87–92. [Google Scholar]
- DuBois M, Gilles KA, Hamilton JK, Rebers PA, Smith F. Calorimetric method for determination of sugars and related substances. Anal Chem. 1956;28:350–356. [Google Scholar]
- Ge Y, Schimel JP, Holden PA. Evidence for negative effects of TiO2 and ZnO nanoparticles on soil bacterial communities. Environ Sci Technol. 2012;45:659–1664. doi: 10.1021/es103040t. [DOI] [PubMed] [Google Scholar]
- Ghervariya KK, Desai PB. Rhizobacteria of sugarcane: in vitro screening for their plant growth -promoting potentials. Res J Recent Sci. 2014;3:52–58. [Google Scholar]
- Giannopolitis CN, Ries SK. Superoxide dismutases I. Occurrence in higher plants. Plant Physio. 1977;59:309–314. doi: 10.1104/pp.59.2.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giraldo JP, Landry MP, Faltermeier SM, McNicholas TP, Iverson NM, Boghossian AA, Reuel NF, Hilmer AJ, Sen F, Brew JA, Strano MS. Plant nanobionics approach to augment photosynthesis and biochemical sensing. Nature Mat. 2014;13:109–117. doi: 10.1038/nmat3890. [DOI] [PubMed] [Google Scholar]
- Glick BR. Plant growth-promoting bacteria: mechanisms and applications. London: Hindawi Publishing Corporation, Scientifica; 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gui X, Rui M, Song Y, Ma Y, Rui Y, Zhang P, He X, Li Y, Zhang Z, Liu L. Phytotoxicity of CeO2 nanoparticles on radish plant (Raphanus sativus) Environ Sci Pollut Res. 2017;24:13775–13781. doi: 10.1007/s11356-017-8880-1. [DOI] [PubMed] [Google Scholar]
- Jia X, Meng Q, Zeng H, Wang W, Yin H. Chitosan oligosaccharide induces resistance to Tobacco mosaic virus in Arabidopsis via the salicylic acid–mediated signalling pathway. Sci Rep. 2016;6:26144. doi: 10.1038/srep26144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katiyar D, Hemantaranjan A, Singh B. Chitosan as a promising natural compound to enhance potential physiological responses in plant: a review Indian. J Plant Physiol. 2015;20:1–9. [Google Scholar]
- Khati P, Sharma A, Gangola S, Kumar R, Bhatt P, Kumar G. Impact of some agriusable nanocompounds on soil microbial activity: an indicator of soil health. Clean-Soil Air Water. 2017;45:1600458. [Google Scholar]
- Khati P, Chaudhary P, Gangola S, Bhatt P, Sharma A. Nanochitosan supports growth of Zea mays and also maintains soil health following growth. 3 Biotech. 2017;7:81. doi: 10.1007/s13205-017-0668-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khati P, Parul BP, Nisha KR, Sharma A. Effect of nanozeolite and plant growth -promoting rhizobacteria on maize. 3 Biotech. 2018;8:141. doi: 10.1007/s13205-018-1142-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khati P, Chaudhary P, Gangola S, Sharma A. Influence of nanozeolite on plant growth promotory bacterial isolates recovered from nanocompound infested agriculture field. Environ Ecol. 2019;37:521–527. [Google Scholar]
- Khati P, Sharma A, Chaudhary P, Singh AK, Gangola S, Kumar R. High- throughput sequencing approach to access the impact of nanozeolite treatment on species richness and evens of soil metagenome. Biocatal Agric Biotechnol. 2019;20:101249. [Google Scholar]
- Kirk JOT, Allen RL. Dependence of chloroplast pigment synthesis on protein synthesis: effect of actidione. Biochem Biophys Res Commun. 1965;21:523–530. doi: 10.1016/0006-291x(65)90516-4. [DOI] [PubMed] [Google Scholar]
- Kukreti B, Sharma A, Chaudhary P, Agri U, Maithani D. Influence of nanosilicon dioxide along with bioinoculants on Zea mays and its rhizospheric soil. 3 Biotech. 2020;10:345. doi: 10.1007/s13205-020-02329-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumari S, Sharma A, Chaudhary P, Khati P. Management of plant vigor and soil health using two agriusable nanocompounds and plant growth promotory rhizobacteria in Fenugreek. 3 Biotech. 2020;10:461. doi: 10.1007/s13205-020-02448-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latif HH, Ghareib M, Tahon MA. Phytosynthesis of silver nanoparticles using leaf extracts from Ocimum basilicumand Mangifira indicaand their effect on some biochemical attributes of Triticum aestivum. Gesunde Pflanzen. 2017;69:39–46. [Google Scholar]
- Mali PC, Vyas SP, Satish LL. Biochemical components of clusterbean genotypes in relation to bacterial blight. Indian Phytopathol. 1989;42:559–561. [Google Scholar]
- Mousavi-Kouhi SM, Lahouti M, Ganjeali A, Entezari MH. Comparative effects of ZnO nanoparticles, ZnO bulk particles, and Zn2þ on Brassica napus after long-term exposure: changes in growth, biochemical compounds, antioxidant enzyme activities, and Zn bioaccumulation. Water Air Soil Poll. 2015;226:364. [Google Scholar]
- Mukherjee A, Sun Y, Morelius E, Tamez C, Bandyopadhyay S, Niu G, White JC, Peralta-Videa JR, Gardea-Torresdey JL. Differential toxicity of bare and hybrid ZnO nanoparticles in green pea (Pisum sativum L.): a life cycle study. Front Plant Sci. 2016;6:1242. doi: 10.3389/fpls.2015.01242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nair P, Varghese SH, Nair BG, Maekawa T, Yoshida Y, Kumar DS. Nanoparticulate material delivery to plants. Plant Sci. 2010;9:154–163. [Google Scholar]
- Ok CH, Anderson SH, Ervin EH. Amendments and construction systems for improving the performance of sand-based putting greens. Agron J. 2003;95:1583–1590. [Google Scholar]
- Olczyk T (2005) Vegetarian news letter. Horticultural sciences department: a vegetable crops extension publication. Vegetarian
- Ozyigit II, Filiz E, Vatansever R, Kurtoglu KY, Koc I, Öztürk MX, Anjum NA. Identification and comparative analysis of H2O2-scavenging enzymes (ascorbate peroxidase and glutathione peroxidase) in selected plants employing bioinformatics approaches. Front Plant Sci. 2016;7:301. doi: 10.3389/fpls.2016.00301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raliya R, Tarafdar JC. ZnO nanoparticle biosynthesis and its effect on phosphorous-mobilizing enzyme secretion and gum contents in Clusterbean (Cyamopsis tetragonoloba L.) Agric Res. 2013;2:48–57. [Google Scholar]
- Raliya R, Nair R, Chavalmane S, Wang WN, Biswas P. Mechanistic evaluation of translocation and physiological impact of titanium dioxide and zinc oxide nanoparticles on the tomato (Solanum lycopersicum L.) plant. Metallomics. 2015;7:1584–1594. doi: 10.1039/c5mt00168d. [DOI] [PubMed] [Google Scholar]
- Ramesh K, Biswas AK, Somasundaram J, Subba Rao A. Nanoporous zeolites in farming: current status and issues ahead. Curr Sci. 2010;99:760–765. [Google Scholar]
- Ramesh M, Palanisamy K, Babu K, Sharma NK. Effects of bulk & nano-titanium dioxide and zinc oxide on physio-morphological changes in Triticum aestivum Linn. J Glob Biosci. 2014;3:415–422. [Google Scholar]
- Rui M, Ma C, White JC, Hao Y, Wang Y, Tang X, Yang J, Jiang F, Ali A, Rui Y, Cao W, Chen G, Xing B. Metal oxide nanoparticles alter peanut (Arachis hypogaea L.) physiological response and reduce nutritional quality: a life cycle study. Environ Sci Nano. 2018;5:2088–2102. [Google Scholar]
- Soil Survey Staff . Keys to soil taxonomy. 11. Washington: USDA-Natural Resources Conservation Service; 2010. [Google Scholar]
- Stepanova AN, Yun J, Likhacheva AV, Alonso JM. Multilevel interactions between ethylene and auxin in Arabidopsis roots. Plant Cell. 2007;19:2169–2185. doi: 10.1105/tpc.107.052068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suriyaprabha R, Karunakaran G, Yuvakkumar R, Rajendran V, Kannan N. Silica nanoparticles for increased silica availability in maize (Zea mays L.) seeds under hydroponic conditions. Curr Nanosci. 2012;8:902–908. [Google Scholar]
- Timmusk S, Seisenbaeva G, Behers L. Titania (TiO2) nanoparticles enhance the performance of growth-promoting rhizobacteria. Sci Rep. 2018;8:617. doi: 10.1038/s41598-017-18939-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tripathi DK, Shweta S, Singh S, Pandey R, Singh VP, Sharma NC, Prasad SM, Dubey NK, Chauhan DK. An overview on manufactured nanoparticles in plants: uptake, translocation, accumulation and phytotoxicity. Plant Physiol Biochem. 2016;7:1. doi: 10.1016/j.plaphy.2016.07.030. [DOI] [PubMed] [Google Scholar]
- Tyagi J, Varma A, Pudake RN. Evaluation of comparative effects of arbuscular Mycorrhiza (Rhizophagus intraradices) and endophyte (Piriformospora indica) association with finger millet (Eleusine coracana) under drought stress. Eur J Soil Biol. 2017;81:1–10. [Google Scholar]
- Urbonaviciute A, Samuoliene G, Sakalauskaite J, Duchovskis P, Brazaityte A, Siksnianiene JB, Ulinskaite R, Sabajeviene G, Baranauskis K. The effect of elevated CO2 concentrations on leaf carbohydrate, chlorophyll contents and photosynthesis in radish. Pol J Environ Stud. 2006;15:921–925. [Google Scholar]
- Venkatachalam P, Priyanka N, Manikandan K, Ganeshbabu I, Indiraarulselvi P, Geetha N, Muralikrishna K, Bhattacharya RC, Tiwari M, Sharma N, Sahi SV. Enhanced plant growth-promoting role of phycomolecules coated zinc oxide nanoparticles with P supplementation in cotton (Gossypium hirsutum L.) Plant Physiol Biochem. 2017;110:118–127. doi: 10.1016/j.plaphy.2016.09.004. [DOI] [PubMed] [Google Scholar]
- Wang Y, Jiang F, Ma C, Rui Y, Tsang DCW, Xing B. Effect of metal oxide nanoparticles on amino acids in wheat grains (Triticum aestivum) in a life cycle study. J Environ Manage. 2019;241:319–327. doi: 10.1016/j.jenvman.2019.04.041. [DOI] [PubMed] [Google Scholar]
- Yadav V. Nanotechnology, big things from a tiny world: a review. Adv Electr Electron Eng. 2013;3:771–778. [Google Scholar]
- Zakry FAA, Shamsuddin ZH, Khairuddin AR, Zakaria ZZ, Anuar AR. Inoculation of Bacillus sphaericus UPMB10 to young oil palm and measurement of its uptake of fixed nitrogen using the 15N isotope dilution technique. Microbes Environ. 2012;27:257–262. doi: 10.1264/jsme2.ME11309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao L. Stress response and tolerance of Zea maysto CeO2 nanoparticles: cross talk among H2O2, heat shock protein, and lipid peroxidation. ACS Nano. 2012;6:9615–9622. doi: 10.1021/nn302975u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao LJ, Peralta-Videa JR, Rico CM, Hernandez-Viezcas JA, Sun YP, Niu GH. CeO2 and ZnO nanoparticles change the nutritional qualities of cucumber (Cucumis sativus) J Agric Food Chem. 2014;62:2752–2759. doi: 10.1021/jf405476u. [DOI] [PubMed] [Google Scholar]
- Boczkowski J, Hoet P. What’s new in nanotoxicology? Implications for public health from a brief review of the 2008 literature. Nanotoxicology. 2009;4:1–14. doi: 10.3109/17435390903428844. [DOI] [PubMed] [Google Scholar]
- Siddaiah NC, Prasanth HVK, Satyanarayana RN, Mudili V, Gupta KV, Kalagatur KN, Satyavati T, Dai FX, Chen YJ, Mocan A, Singh PB, Srivastava RK. Chitosan nanoparticles having higher degree of acetylation induce resistance against pearl millet downy mildew through nitric oxide generation. Sci Rep. 2018;8:2485. doi: 10.1038/s41598-017-19016-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiscox JD, Israelstam GF. A method for the extraction of chlorophyll from leaf and tissue without maceration. Can J Bot. 1979;57:1332–1334. [Google Scholar]
- Heinen RB, Ye Q, Chaumont F. Role of aquaporins in leaf physiology. J Exp Bot. 2009;11:2971–2985. doi: 10.1093/jxb/erp171. [DOI] [PubMed] [Google Scholar]
- Khodakovskaya M, Dervishi E, Mahmood M, Xu Y, Li Z, Watanabe F. Carbon nanotubes are able to penetrate plant seed coat and dramatically affect seed germination and plant growth. ACS Nano. 2009;3:3221–3227. doi: 10.1021/nn900887m. [DOI] [PubMed] [Google Scholar]
- Mahakham W, Ajit K, Maensiri S, Theerakulpisut P. Nanopriming technology for enhancing germination and starch metabolism of aged rice seeds using phytosynthesized silver nanoparticles. Sci Rep. 2017;7:8263. doi: 10.1038/s41598-017-08669-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.