Abstract
At the beginning of 2020, the outbreak of coronavirus disease 2019 (COVID-19) led to a worldwide pandemic and mass panic. The number of infected people has been increasing exponentially since, and the mortality rate has also been concomitantly increasing. At present, no study has summarized the mortality risk of COVID-19 in patients with chronic kidney disease (CKD). Therefore, the aim of the present study was to conduct a literature review and meta-analysis to understand the frequency of mortality among CKD patients infected with COVID-19. A comprehensive systematic search was conducted on the PubMed, Embase, and Cochrane databases to find articles published until May 15, 2020. Study quality was assessed using a modified version of the Newcastle–Ottawa Scale. After careful screening based on the inclusion and exclusion criteria, 3,867,367 patients from 12 studies were included. The mortality rate was significantly higher among CKD patients with COVID-19 infection than among CKD patients without COVID-19 infection, as indicated by a pooled OR of 5.81 (95% CI 3.78–8.94, P < 0.00001, I2 = 30%). The patients were then stratified into ≥ 70 and < 70 years, and subgroup analysis revealed that among CKD patients with COVID-19 infection, the mortality rate was higher in the < 70 years group (OR 8.69, 95% CI 7.56–9.97, P < 0.0001) than in the ≥ 70 years group (OR 2.44, 95% CI 0.75–6.63, P = 0.15). Thus, COVID-19 patients with CKD have a high mortality risk and require a comprehensive multidisciplinary management strategy.
Keywords: COVID-19, SARS-CoV-2, Chronic kidney disease, Mortality
Introduction
In December 2019, the first outbreak of coronavirus disease (COVID-19) caused by severe acute respiratory syndrome novel beta-coronavirus (SARS-CoV-2) was reported in Wuhan, China [1]. Since then, the novel coronavirus has dealt a severe blow to the global health care industry and led to mass panic all over the world [2]. As of July 1, 2020, a total of 10,596,241 confirmed cases and 514,242 deaths were reported across 212 countries (source: World Health Organization website), and the number of confirmed cases and deaths continues to rise. SARS-CoV-2 is mainly transmitted from person to person through respiratory droplets, which are usually released when an infected person coughs or sneezes. Its main clinical manifestations are fever, cough, myalgia, malaise, and diarrhea [3, 4]. The diagnosis of COVID-19 is usually based on PCR detection of SARS-CoV-2 in nasopharyngeal swabs or other specimens [5].
Patients with severe disease may present with dyspnea, a respiratory rate higher than 30 breaths per minute, blood oxygen saturation less than 93%, a PaO2:FiO2 ratio ≤ 300 mm Hg, and invasion of more than 50% of the lung fields within 24–48 h of symptom onset [6]. In patients with COVID-19, comorbidities (including cardiovascular disease, chronic kidney disease, diabetes, and other chronic diseases) are associated with a higher risk of severe complications and death [7]. In particular, chronic kidney disease (CKD) is associated with an increase in the hospitalization rate of patients with novel coronavirus-associated pneumonia, and the mortality rate seems to be 14–16 times higher than that of the general population [8]. Additionally, a meta-analysis suggested that CKD should be considered as an important risk factor for COVID-19 [9], and Cheng et al. showed that COVID-19 patients had a high prevalence of kidney disease at admission and a high in-hospital mortality rate [10]. Therefore, there is an urgent need to understand the risks in patient populations with such co-morbidities. This meta-analysis responds to this need by focusing on studies that report the risk of clinical death in patients with CKD complicated with COVID-19 and evaluating the reliability of the evidence. The purpose of this article is to explore the frequency of clinical deaths in patients with CKD complicated with COVID-19.
Methods
Search strategy
This meta-analysis was conducted and reported in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analysis and Meta-Analysis of Observational Studies in Epidemiology (PRISMA) guidelines [11]. To find the relevant studies, two authors (RY Cai and JS Zhang) conducted a comprehensive search of the PubMed, EMBASE, and Cochrane databases for studies published as of May 15, 2020. The search terms used were “coronavirus,” “COVID-19,” “SARS-CoV-2,” “mortality,” “outcomes,” “chronic kidney disease,” and “chronic renal disease.” The reference lists of the eligible articles were also reviewed to search for relevant articles. The supervising authors reviewed the included studies and data.
Inclusion and exclusion criteria
This review only includes studies on humans that were published in English and reported confirmed cases of COVID-19 in patients with CKD, with data for both survivors and non-survivors. Studies that were published in duplicate, case reports, reviews, and letters were excluded, as were studies that focused only on the mortality rate of CKD or only on COVID-19. Two authors (RY Cai and JS Zhang) independently screened the titles and abstracts of all potentially relevant studies and reviewed the full text of the articles that met the inclusion criteria.
Data extraction and quality check
The following features were extracted from the relevant articles: first author’s name, country, source of data, sample size, and age and gender of both survivors and non-survivors. The quality of the studies was assessed using the Newcastle–Ottawa scale (NOS) [12]. Quality assessment was independently conducted by two authors (RY Cai and JS Zhang), and any disagreement was resolved via discussion.
Statistical analysis
Our meta-analysis was performed with Review Manager 5.3. Heterogeneity was assessed by calculating the I2 index. The fixed- or random-effects model was utilized, as appropriate. Sensitivity analysis was used to evaluate the impact of each study by eliminating studies one at a time or alternating between random-effects and fixed-effects models. The findings from the meta-analysis are graphically represented by a forest plot. Publication bias was determined and presented using a funnel plot. For categorical outcomes, an odds ratio (OR) with 95% CI (for observational studies) was calculated for each study. The pooled odds ratios (ORs) of different studies and the corresponding 95% confidence intervals (CIs) were used to estimate the frequency of clinical deaths in patients with CKD complicated with COVID-19. All p-values in this study were two tailed, and statistical significance was set at ≤ 0.05.
Results
Characteristics of the included studies
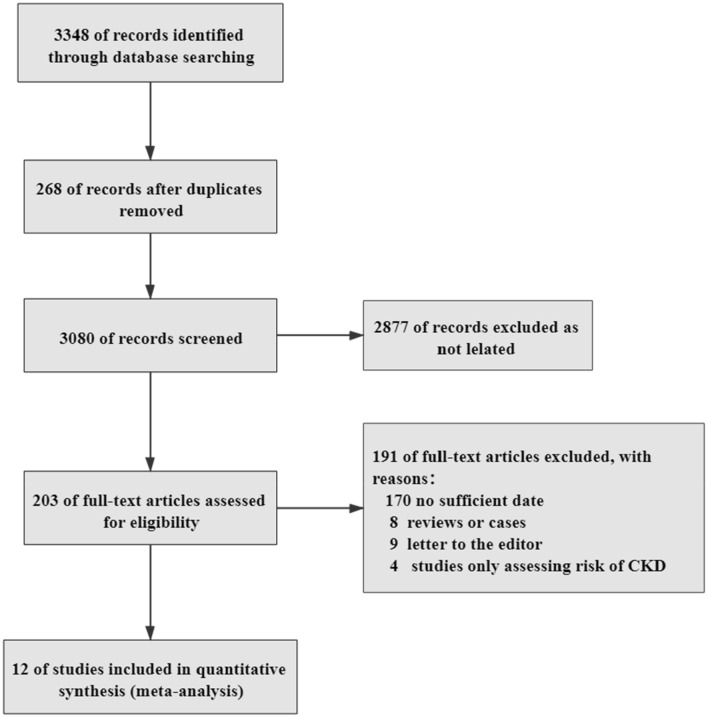
A total of 3348 articles were selected from the databases, but 268 were excluded because of duplication. The titles and abstracts of the articles were screened, and 2877 were excluded because they did not meet the inclusion criteria. After the full text of the articles was read, 191 articles with inconsistent data were deleted. Finally, 12 articles that met all the inclusion criteria were included [13–24]. The selection process is depicted in Fig. 1, and the common baseline characteristics of the patients are shown in Table 1. The NOS score of the included studies was 7–8, and the quality of the articles was evaluated as high (Table 2).
Fig. 1.
Flow chart depicting the search strategy to identify articles on the relationship between CKD and the mortality of patients with COVID-19
Table 1.
Baseline characteristics of studies included in the systematic review
| Author | Source of data | Country | Dead (n, %) | Mean/median age (years) | Female (%) |
|---|---|---|---|---|---|
| Age ≥ 70 years | |||||
| Chen et al. [22] | Zhongnan Hospital of Wuhan University | China | 19, 34.55% | 74 | 38.18 |
| Wang et al. [15] | Renmin Hospital of Wuhan University | China | 65, 19.17% | 71 | 51.03 |
| Age < 70 years | |||||
| Banerjee et al. [24] | Hospitals in UK | England | 38,847, 1.01% | 48 | 50.70 |
| Cao et al. [23] |
Wuhan University Zhongnan Hospital |
China | 17, 16.67% | 54 | 48.04 |
| Chen et al. [21] | Tongji Hospital | China | 113, 41.54% | 62 | 37.87 |
| Nikpouraghdam et al. [20] | Baqiyatallah Hospital | China | 239, 8.06% | 56 | 34.04 |
| Ruan et al. [19] | Tongji Hospital | China | 68, 45.33% | 58.5 | 32 |
| Shi et al. [17] | Renmin Hospital of Wuhan University | China | 62, 9.24% | 63 | 52.01 |
| Shi et al. [18] |
Renmin Hospital of Wuhan Zhongnan Hospital |
China | 47, 15.36% | 64.5 | 50.98 |
| Wang et al. [16] |
Zhongnan Hospital Xishui Hospital |
China | 19, 17.76% | 51 | 46.73 |
| Yan et al. [14] | Tongji Hospital | China | 108, 54.55% | 64 | 39.90 |
| Zhou et al. [13] |
Jinyintan Hospital Wuhan Pulmonary Hospital |
China | 54, 28.27% | 56 | 37.70 |
Table 2.
Newcastle–Ottawa score of the included studies
| Study ID | Exposed cohort representative | Non-exposed cohort selected from same source | Exposure ascertained |
Outcome of study was not present at start of the study | Comparability | Adequate assessment | Follow-up was long enough | Adequate follow-up | Quality score |
|---|---|---|---|---|---|---|---|---|---|
| Chen et al. [22] | Yes | Yes | Yes | Yes | 0 | Yes | Yes | Yes | 7 |
| Wang et al. [15] | Yes | Yes | Yes | Yes | 0 | Yes | Yes | Yes | 7 |
| Banerjee et al. [24] | Yes | Yes | Yes | Yes | 0 | Yes | Yes | Yes | 7 |
| Cao et al. [23] | Yes | Yes | Yes | Yes | 0 | Yes | Yes | Yes | 7 |
| Chen et al. [21] | Yes | Yes | Yes | Yes | 1 | Yes | Yes | Yes | 8 |
| Nikpouraghdam et al. [20] | Yes | Yes | Yes | Yes | 0 | Yes | Yes | Yes | 7 |
| Ruan et al. [19] | Yes | Yes | Yes | Yes | 0 | Yes | Yes | Yes | 7 |
| Shi et al. [17] | Yes | Yes | Yes | Yes | 0 | Yes | Yes | Yes | 7 |
| Shi et al. [18] | Yes | Yes | Yes | Yes | 0 | Yes | Yes | Yes | 7 |
| Wang et al. [16] | Yes | Yes | Yes | Yes | 0 | Yes | Yes | Yes | 7 |
| Yan et al. [14] | Yes | Yes | Yes | Yes | 0 | Yes | Yes | Yes | 7 |
| Zhou et al. 13 | Yes | Yes | Yes | Yes | 0 | Yes | Yes | Yes | 7 |
Pooled analysis
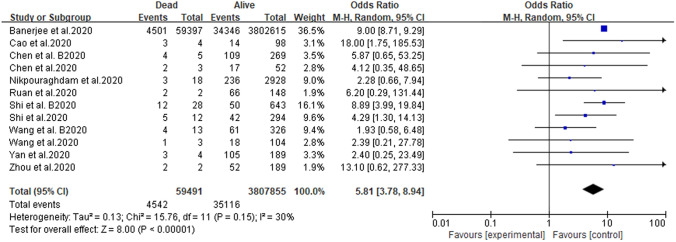
The forest plot of mortality in patients with CKD infected with COVID-19 is shown in Fig. 2. REM analysis showed that CKD patients with COVID-19 had a higher mortality rate (pooled OR 5.81, 95% CI 3.78–8.94, P < 0.00001, I2 = 30%). As shown in Fig. 2, although most of the samples were from the UK, this did not create a bias in the study results.
Fig. 2.
Forest plot depicting the relationship between CKD and the mortality of patients with COVID-19
Subgroup analysis and sensitivity analysis
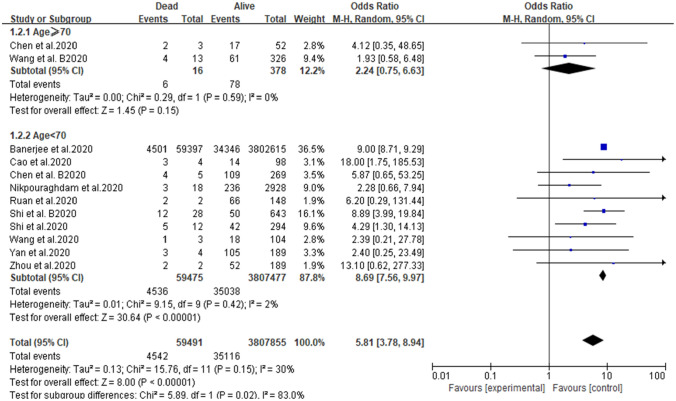
Patients were divided into two subgroups according to their age: ≥ 70 years and < 70 years. The results of the two groups were significantly different (≥ 70 years: OR 2.44, 95% CI 0.75–6.63, P = 0.15; < 70 years: OR 8.69, 95% CI 7.56–9.97, P < 0.0001) (Fig. 3). When all the studies were analyzed, low heterogeneity was observed (I2 = 30%), but after the studies of Chen and Wang were excluded, the heterogeneity decreased significantly (I2 = 2%). Therefore, Chen and Wang’s study may be responsible for the heterogeneity of this meta-analysis. There was no evidence of a publication bias, according to the findings of funnel plot analysis (Fig. 4).
Fig. 3.
Forest plot depicting the relationship between CKD and the mortality of COVID-19 patients after subgroup analysis according to age
Fig. 4.
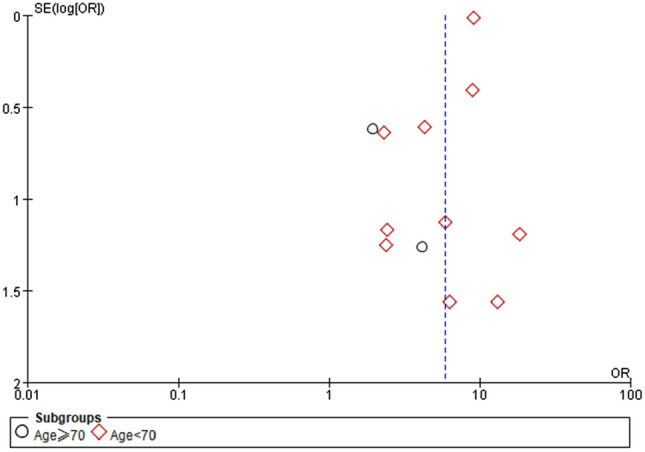
Funnel plot depicting publication bias
Discussion
This study presents a literature review and meta-analysis of the information reported so far on the mortality rate of CKD patients with COVID-19. According to the literature review in the current study, no meta-analysis has analyzed the mortality of CKD patients with COVID-19. Nonetheless, based on the data of the relevant studies extracted from the literature, our research found that COVID-19 infection was closely associated with mortality in CKD patients. Although cardiovascular disease is the leading cause of death in CKD, the death rate associated with pulmonary infection in CKD patients is also high [25, 26]. Our findings confirm this, as the CKD patients with COVID-19 infection had a higher mortality rate than those without COVID-19 infection (pooled OR 5.81). This is probably because in CKD patients, the levels of pro-inflammatory cytokines are increased, and this leads to an increase in oxidative stress that eventually produces an inflammatory immune response. The resulting immune system damage may increase susceptibility to bacterial and viral infections, and this might be the main reason for the increased risk of pulmonary inflammation [27]. When the patients in the current meta-analysis were stratified into those older than 70 years and those younger than 70 years (≥ 70 years: OR 2.44, 95% CI 0.75–6.63, P = 0.15; < 70 years: OR 8.69, 95% CI 7.56–9.97, P < 0.0001), we found that among CKD patients complicated with COVID-19, those younger than 70 years had a higher mortality rate than those who were 70 years or older. This is probably because elderly patients are more likely to have other complications (such as hypertension, diabetes, coronary heart disease, and COPD). These comorbidities will increase the risk of death associated with COVID-19 in the elderly, so the specific condition associated with the increased risk of death in CKD patients is not clear. Moreover, the high mortality rate in elderly patients is probably related to the higher incidence of these comorbidities, rather than CKD [28]. However, misdiagnosis or delayed diagnosis of COVID-19 is common in elderly patients with CKD, on account of low immune response, mild clinical symptoms, and atypical CT imaging findings of the chest [29]. This may explain why an increase in mortality is not found in elderly CKD patients with COVID-19 infection. As CKD can be prevented and treated to a large extent, it deserves more attention in health policy decision-making globally. Therefore, it is necessary to establish a comprehensive disease management strategy that includes lifestyle, nutrition, medications, and other methods to reduce the mortality rate of CKD patients.
Our study has several limitations. One of the main limitations is that there were no data about the disease stage of the CKD patients, the disease course, and the drugs that were administered. Second, the data were not adjusted for confounding factors, such as gender, race, and BMI, which may have affected the results. Third, CKD patients with COVID-19 may have other chronic diseases (such as hypertension, diabetes, COPD, and cardiovascular and cerebrovascular diseases) that may have affected the results, but the related data were not considered in the analysis. Fourth, the age stratification carried out was not comprehensive enough.
Conclusion
The results of the present meta-analysis on the limited published data indicate that regardless of age, CKD patients with COVID-19 infection have a high mortality risk. Thus, CKD patients infected with SARS-CoV-2 must be carefully monitored and managed to lower the risk of death.
Acknowledgments
We would like to thank Dr. Zhou, Dr. Yan, Dr. Wang, Dr. Wang, Dr. Shi, Dr. Shi, Dr. Ruan, Dr. Nikpouraghdam, Dr. Chen, Dr. Chen, and Dr. Cao for providing data for this study.
Funding
This work was supported by the General Project Funds from the Health Department of Zhejiang Province (Grant No. 2017KY213) and the Natural Science Foundation of Zhejiang Province (Grant No. LQ19H050003).
Data availability
All data needed to evaluate the conclusions in the paper are present in the paper.
Compliance with ethical standards
Conflict of interest
The authors declare that there is no conflict of interest regarding the publication of this paper.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Wang D, Hu B, Hu C, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China [J] JAMA. 2020;323(11):1061. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Phelan AL, Katz R, Gostin LO. The Novel Coronavirus Originating in Wuhan, China: Challenges for Global Health Governance [J] JAMA. 2020;323(8):709–710. doi: 10.1001/jama.2020.1097. [DOI] [PubMed] [Google Scholar]
- 3.Chen NS, Zhou M, Dong X, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study [J] Lancet. 2020;395(10223):507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Huang CL, Wang YM, Li XW, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China [J] Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gandhi RT, Lynch JB, Rio CD. Mild or Moderate Covid-19 [J] N Engl J Med. 2020;383(18):1757–1766. doi: 10.1056/NEJMcp2009249. [DOI] [PubMed] [Google Scholar]
- 6.Berlin DA, Solomon CG, Gulick RM et al (2020) Severe Covid-19 [J]. New England Journal of Medicine [DOI] [PubMed]
- 7.Singh AK, Gillies CL, Singh R, et al. Prevalence of comorbidities and their association with mortality in patients with COVID-19: A Systematic Review and Meta-analysis [J] Diabetes Obes Metab. 2020;22(10):1915–1924. doi: 10.1111/dom.14124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sarnak MJ, Jaber BL. Pulmonary infectious mortality among patients with end-stage renal disease [J] Chest. 2001;120:1883–1887. doi: 10.1378/chest.120.6.1883. [DOI] [PubMed] [Google Scholar]
- 9.Henry BM, Lippi G. Chronic kidney disease is associated with severe coronavirus disease 2019 (COVID-19) infection [J] Int Urol Nephrol. 2020;52(6):1193–1194. doi: 10.1007/s11255-020-02451-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cheng Y, Luo R, Wang K, et al. Kidney disease is associated with in-hospital death of patients with COVID-19 [J] Kidney Int. 2020;97(5):829–838. doi: 10.1016/j.kint.2020.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration [J] BMJ. 2009;339:b2700. doi: 10.1136/bmj.b2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wells GA, Shea B, O'Connell D et al (2014) The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses [J]. Ottawa Health Research Institute Web site
- 13.Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study [J] Lancet. 2020;395(10229):1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yan Y, Yang Y, Wang F, et al. Clinical characteristics and outcomes of patients with severe covid-19 with diabetes [J] BMJ Open Diabetes Res Care. 2020;8(1):e001343. doi: 10.1136/bmjdrc-2020-001343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang L, He W, Yu X, et al. Coronavirus disease 2019 in elderly patients: characteristics and prognostic factors based on 4-week follow-up [J] J Infect. 2020;80(6):639–645. doi: 10.1016/j.jinf.2020.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang D, Yin Y, Hu C, et al. Clinical course and outcome of 107 patients infected with the novel coronavirus, SARS-CoV-2, discharged from two hospitals in Wuhan, China [J] Crit Care. 2020;24(1):188. doi: 10.1186/s13054-020-02895-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shi S, Qin M, Cai Y, et al. Characteristics and clinical significance of myocardial injury in patients with severe coronavirus disease 2019 [J] Eur Heart J. 2020;41(22):2070–2079. doi: 10.1093/eurheartj/ehaa408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shi Q, Zhang X, Jiang F, et al. Clinical characteristics and risk factors for mortality of COVID-19 patients with diabetes in wuhan, China: a two-center, retrospective study [J] Diabetes Care. 2020;43(7):1382–1391. doi: 10.2337/dc20-0598. [DOI] [PubMed] [Google Scholar]
- 19.Ruan Q, Yang K, Wang M, et al. Correction to: clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China [J] Intensive Care Med. 2020;46(6):1294–1297. doi: 10.1007/s00134-020-06028-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nikpouraghdam M, Farahani AJ, Alishiri G, et al. Epidemiological characteristics of coronavirus disease 2019 (COVID-19) patients in IRAN: a single center study [J] J Clin Virol. 2020;127:104378. doi: 10.1016/j.jcv.2020.104378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen T, Wu D, Chen H, et al. Clinical characteristics of 113 deceased patients with coronavirus disease 2019: retrospective study [J] BMJ. 2020;368:m1091. doi: 10.1136/bmj.m1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen T, Dai Z, Mo P, et al. Clinical characteristics and outcomes of older patients with coronavirus disease 2019 (COVID-19) in Wuhan, China (2019): a single-centered, retrospective study [J] J Gerontol A Biol Sci Med Sci. 2020;75(9):1788–1795. doi: 10.1093/gerona/glaa089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cao J, Tu WJ, Cheng W, et al. Clinical features and short-term outcomes of 102 patients with corona virus disease 2019 in Wuhan, China [J] Clin Infect Dis. 2020;71(15):748–755. doi: 10.1093/cid/ciaa243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Banerjee A, Pasea L, Harris S, et al. Estimating excess 1-year mortality associated with the COVID-19 pandemic according to underlying conditions and age: a population-based cohort study [J] Lancet. 2020;395(10238):1715–1725. doi: 10.1016/S0140-6736(20)30854-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bikbov B, Purcell CA, Levey AS, et al. Global, regional, and national burden of chronic kidney disease, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017 [J] Lancet. 2020;395(10225):709–733. doi: 10.1016/S0140-6736(20)30045-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kessler M, Hoen B, Mayeux D, et al. Bacteremia in patients on chronic hemodialysis [J] Nephron. 1993;64(1):95–100. doi: 10.1159/000187285. [DOI] [PubMed] [Google Scholar]
- 27.Betjes MG. Immune cell dysfunction and inflammation in end-stage renal disease [J] Nat Rev Nephrol. 2013;9(5):255–265. doi: 10.1038/nrneph.2013.44. [DOI] [PubMed] [Google Scholar]
- 28.Lee JY, Kim HA, Huh K, et al. Risk factors for mortality and respiratory support in elderly patients hospitalized with COVID-19 in Korea [J] J Korean Med Sci. 2020;35(23):e223. doi: 10.3346/jkms.2020.35.e223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li R, Li SR, Du XB, et al. Clinical observations in the very elderly patients with COVID-19 in Wuhan [J] Geriatr Gerontol Int. 2020;20(7):709–714. doi: 10.1111/ggi.13974. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data needed to evaluate the conclusions in the paper are present in the paper.