Abstract
Background
Coronavirus Disease 2019 (COVID-19) caused by severe acute respiratory syndrome coronavirus 2 appeared in December 2019 in Wuhan, China.
Objective
To investigate the clinical manifestations including signs and symptoms, laboratory results, and perinatal outcomes in pregnant women with COVID-19.
Materials and Methods
Scholarly databases such as PubMed via LitCovid hub, Embase, Scopus, Web of sciences, and Google scholar were searched on April 7, 2020. Meta-analysis was performed via comprehensive meta-analysis software using the Mantel-Haenszel method. The event rate with 95% CI was calculated for each variable.
Results
Ten studies were selected. The pooled prevalence for fever, post-partum fever, cough, myalgia, fatigue, dyspnea, sore throat, and diarrhea were 66.8%, 37.1%, 35%, 24.6 %, 14.9%, 14.6%, 11.5%, and 7.6%, respectively. Laboratory test results were 49.8% for lymphopenia, 47.7% for leukocytosis, 83.7% for elevated neutrophil ratio, 57% for elevated C-reactive protein, and 71.4% for decreased lymphocyte ratio. The rate of cesarean section for delivery in all cases was 84%. Of the newborns of the corona-positive mothers, only one had a positive test result. Also, there was only one death due to a decreased lymphocyte ratio.
Conclusion
Fever was the most common sign and symptom in pregnant women with COVID-19. Among the laboratory tests, the highest amount was related to elevated neutrophil ratio. It seems that due to the differences between pregnant women and the general population, special measures should be considered to treat these patients.
Keywords: COVID-19, Pregnancy, Diagnosis, Signs and symptoms, Meta-analysis.
1. Introduction
Coronavirus Disease 2019 (COVID-19) caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) appeared for the first time in December 2019 in Wuhan, China. Following that, the disease spread rapidly around the world to the point where it was confirmed a pandemic by the World Health Organization (WHO) (1, 2). COVID-19 is an infectious disease with respiratory symptoms almost similar to SARS (2003) and MERS (2012) epidemics (3, 4). In some cases, the disease can lead to a sensitive respiratory condition, many of which require specialized management in the intensive care unit (ICU) (5).
Moreover, respiratory droplets along with close contact transmission are the considerable routes of transmission. Aerosol transmission is also possible in a close environment when exposed to high concentrations of aerosol for a protracted period (6). On the other hand, touching surfaces or objects that are touched by an infected person can also transmit the disease (7). A study has also shown that older age and comorbidity play an important role in determining the severity and clinical consequences of the disease (8).
Because most studies have focused on patients infected with the new coronavirus in the general population, bounded details are available regarding pregnancy outcomes in women infected with COVID-19. It has caused particular concern among pregnant women, as both SARS-CoV and MERS-CoV viruses have been shown to cause severe side effects in pregnant women (9, 10). In 2004, Wong and colleagues conducted a study on pregnant women with SARS in Hong Kong and observed that the pregnant women showed higher rates of death and mortality (11). Similarly, a study by Mertz and colleague showed that women infected with influenza were at a higher risk than healthy pregnant women (12). Chen and co-authors also reported that pregnancy with pneumonia could be associated with the risk of cesarean delivery, preterm delivery, a decrease in the baby's Apgar score, weight loss at birth, etc. (13).
It is obvious that a parturient woman has a relatively depressed immunity or immune suppression, and in theory, they could be more at risk of contracting the virus. Also, confronting the SARS-CoV-2 during pregnancy is a serious threat to pregnant women and their fetuses (14, 15). Therefore, it is pertinent to prevent pregnant women from being infected during the epidemic/pandemic period such as that of COVID-19, a disease without an approved treatment.
Pregnant women are at a risk of infection to respiratory pathogens and severe pneumonia because they are in an immunosuppressive state and changes in physiological adaptation during pregnancy (e.g., increased diaphragm levels, increased oxygen consumption) can cause hypoxia intolerance in such patients. For instance, the outbreak of influenza in 1918 caused a total mortality of 2.6% in the population, however in pregnant women, it was about 37% (16). Additionally, pregnant women were also observed to have a higher risk of complications from the H1N1 epidemic influenza virus infection in 2009 and were hospitalized fourfold more than the other patients (relative risk 4.3 95% CI: 2.3-7.8) (17). Therefore, it is important to study the signs and symptoms of COVID-19 in pregnant women as understanding the disease and its effects on newborns are very important.
Thus, this study is aimed at investigating the clinical manifestation including the signs and symptoms, laboratory results, and prenatal outcomes in pregnant women with COVID-19.
2. Materials and Methods
This systematic review and meta-analysis was followed by the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) statement (18).
Eligibility criteria
All included studies were investigated COVID-19 in pregnant women or during pregnancy and were in the English language. Studies were excluded if the researchers didn't have access to the full-text of the article or the data about the outcomes were not sufficient. Also, studies that were not peer reviewed were excluded.
Information sources and search
Scholarly databases including PubMed via LitCovid hub, Embase, Scopus, Web of Sciences, and Google Scholar were searched using specific keywords (“2019 nCoV" OR 2019nCoV OR “2019 novel coronavirus" OR “COVID 19" OR COVID19 OR “new coronavirus" OR “novel coronavirus" OR “SARS CoV-2" OR (Wuhan AND coronavirus) OR “COVID 19" OR “SARS-CoV" OR “2019-nCoV" OR “SARS-CoV-2" AND pregnancy OR “pregnant women”) on April 7, 2020. Our search was not limited by the type of study or publication date but by the studies with full-text in the English language. We also searched the references of the included studies for capturing potential studies in the field. For incomplete data, the corresponding author of the article was contacted for more information.
Study selection
After importing the records to EndNote X7, the duplicate records were removed and then screened based on the title, abstract, and full-text considering the eligibility criteria. All stages were conducted using two independent reviewers and the potential disagreements were solved through consultation with a third reviewer.
Quality appraisal
Two independent reviewers assessed the included studies for quality issues. Because the final studies were case-series and case-control, the JBI checklists related to this type of study were used. These checklists include 10 questions for case-control and case-series studies. These questions investigate issue regarding domain such as inclusion criteria, reliability and validity of methods, sampling process, transparency in data and results, and statistical analysis. The detail about each question has been mentioned at the end of the questionnaire (Supplementary 1). We scored one for yes and zero for no in each question (19, 20).
Outcomes measures
The investigated outcomes were signs and symptoms (cough, diarrhea, dyspnea, fatigue, fever, myalgia, sore throat, and post-partum fever), laboratory test results (lymphopenia, leukocytosis, elevated neutrophil ratio, elevated C-reactive protein, and decreased lymphocyte ration), type of delivery (cesarean), and perinatal outcomes (COVID-19 positive, low birth weight, premature, complication, and death). For all outcome variables, we extracted the number of events and sample size.
Data analyses
Meta-analysis was performed for the signs and symptoms, laboratory tests, and type of delivery using the event rate (the proportion of the occurrence of an event in the subjects to the total subjects under study) with CMA (version 2) software using the Mantel-Haenszel method. In addition, narrative synthesis was used for reporting the results of the perinatal outcome. The Q-value was applied to discover between-study heterogeneity, and I values were calculated to assess statistical heterogeneity. Random-effect model was used based on the level of heterogeneity. Based on the Cochrane criteria, we used the random-effect model when the heterogeneity was over 50% (21). The event rate with 95% CI was calculated for each variable. Egger's test and visual inspection of the funnel plot were used for assessing publication bias. In addition, a meta-regression was conducted for an association between the mean age and each sign and symptoms, laboratory test, and type of delivery.
3. Results
Description of search
After searching all international databases, 4,721 articles were found; after removing the duplicate articles, 3,985 articles were examined in terms of title and abstract, of which 17 articles were passed to the next stage. Finally, after reviewing the full texts of the articles, 10 articles entered the systematic review (22-31). In the screening stages of studies, they were excluded for a variety of reasons, which included unrelated topics (two articles), unassociated population (four articles), and duplicate study (one article). The overall sample size of the included studies was 135 pregnant women diagnosed with COVID-19 (Figure 1).
Characteristics of included studies
Based on the geographical location, all included studies were performed in China. Table I shows the summary characteristics of the included studies.
Quality assessment
Based on the results of the quality assessment, seven studies were good quality and three were average (Table II).
Heterogeneity
Based on the data analysis, a high level of heterogeneity was not observed in the findings. In some cases with high heterogeneity, the random effect was used (Table III).
Synthesis of results
Signs and symptoms
Various signs and symptoms have been reported in studies. Of these, the highest was fever with 66.8% (95% CI; 48.3-81.2). Other reported signs and symptoms were: post-partum fever (37.1%, 95% CI; 18.5-60.6), cough (35.5.9%, 95% CI; 23.1-50.2), myalgia (24.6%, 95% CI; 12.1-43.5), fatigue (14.9%, 95% CI; 7-29.1), dyspnea (14.6%, 95% CI; 9.2-22.3), sore throat (11.5%, 95% CI; 4.8-25.1), and diarrhea (7.6%, 95% CI; 3.3-16.5) (Figure 2, Table III).
Laboratory tests
Based on data analysis, lymphopenia with 49.8% (95% CI; 30.1-69.6), leukocytosis 47.7% (95% CI; 31.6-64.2), elevated neutrophil ratio 83.7% (95% CI; 72.3-91.0), elevated C-reactive protein 57% (95% CI, 43.7-69.3), and decreased lymphocyte ratio 71.4% (95% CI; 16.4-96.9) were observed in the studies (Figure 3,Table III).
Type of delivery
According to the results, the rate of cesarean section for delivery in all cases was 84% (95% CI; 74-90.7) (Figure 4, Table III).
Perinatal outcomes
According to the results, of the newborns of the corona-positive mothers, only one had a positive test result. Also, there was only one death due to DIC (Table IV).
Results of meta-regression
According to the findings, the only factor that could be examined in this section was the mean age of pregnant women. Data analysis showed that older pregnant women have a significantly higher fever rate (Coefficient = 0.477, p = 0.033). For the type of delivery, the higher average age of pregnant women significantly associated with a higher rate in cesarean delivery (Coefficient = 0.433, p = 0.016) (Table V).
Publication bias
Visual inspection of funnel plot and Egger's tests did not indicate evidence of publication bias (p = 0.127).
Table 1.
Basic information about the included studies
|
| |||||||
| Author, year (Ref) | Country | Setting | Time period | Study design | Sample size | Mean age (Year) (Range) | Mean gestational age (Week) (Range) |
| Chen et al. , 2020-c (14) | China | Renmin hospital | From January 30-February 23, 2020 | Case series | 17 | 29.5 | 37 = 3 37 = 14 |
| Chen et al. , 2020-a (13) | China | Maternal and child hospital of Hubei province | From January 20-February 10, 2020 | Case series | 5 | 28.4 (25-31) | 38-40 |
| Chen et al. , 2020-b (22) | China | Zhongnan hospital | From January 20-January 31, 2020 | Case series | 9 | 32.5 (26-40) | 36-39 |
| Khan et al. , 2020 (25) | China | Renmin hospital | From January 28-March 1, 2020 | Case series | 3 | 29.3 (27-33) | (34-39) |
| Li et al. , 2020 (24) | China | Hubei provincial maternal and child health center | From January 24-February 29, 2020 | Case-control | 16 | 30.9 | 38 |
| Liu et al. , 2020-c (28) | China | Hospitals outside of Wuhan | From December 8, 2019-February 25, 2020 | Case series | 13 | 29.6 (22-36) | (25-38) |
| Liu et al. , 2020-b (26) | China | Multicenter | From January 27-February 14, 2020 | Case-control | 41 | 30.5 (22-42) | NR |
| Liu et al. , 2020-a (27) | China | Union hospital | From January 20, 2020-February 10, 2020 | Case series | 15 | 32 (23-40) | 32 (12-38) |
| Yu et al. , 2020 (30) | China | Tongji hospital | From January 1-February 8, 2020 | Case series | 7 | 32 (29-34 ) | 39 (37-41) |
| Zhu et al. , 2020 (31) | China | Multicenter (five hospitals) | From January 20-February 5, 2020 | Case series | 9 | 29.5 (25-35) | (31-42) |
| NR: Not reported | |||||||
Table 2.
JBI critical appraisal checklist applied to the included studies
|
| ||||||||||||
| Author name/year (Ref) | Q1 | Q2 | Q3 | Q4 | Q5 | Q6 | Q7 | Q8 | Q9 | Q10 | Overall quality | |
| Case series | ||||||||||||
| Liu et al. , 2020-a (27) | Yes | Yes | Yes | Yes | Yes | No | Yes | N/A | No | Yes | 7/10 | |
| Liu et al. , 2020-c (28) | Yes | Yes | Yes | Yes | N/A | Yes | Yes | Yes | N/A | Yes | 8/10 | |
| Zhu et al. , 2020 (31) | Yes | Yes | Yes | Yes | No | Yes | Yes | Yes | No | Yes | 8/10 | |
| Yu et al. , 2020 (30) | Yes | Yes | Yes | Yes | No | No | Yes | Yes | N/A | Yes | 7/10 | |
| Khan et al. , 2020 (25) | Yes | Yes | N/A | Yes | No | Yes | N/A | No | No | Yes | 5/10 | |
| Chen et al. , 2020-a (13) | Yes | Yes | N/A | Yes | No | Yes | N/A | Yes | No | Yes | 6/10 | |
| Chen et al. , 2020-b (22) | Yes | Yes | Yes | Yes | Yes | N/A | N/A | Yes | No | Yes | 7/10 | |
| Chen et al. , 2020-c (14) | Yes | Yes | Yes | Yes | No | No | Yes | Yes | No | Yes | 7/10 | |
| Case-control | ||||||||||||
| Liu et al. , 2020-b (26) | Yes | Yes | Yes | Yes | Yes | Yes | N/A | Yes | Yes | No | 8/10 | |
| Li et al. , 2020 (24) | Yes | Yes | Yes | Yes | No | Yes | N/A | N/A | Yes | No | 6/10 | |
| Case series design questions: Q1. Were there clear criteria for inclusion in the case series? Q2. Was the condition measured in a standard, reliable way for all participants included in the case series? Q3. Were valid methods used for identification of the condition for all participants included in the case series? Q4. Did the case series have consecutive inclusion of participants? Q5. Did the case series have complete inclusion of participants? Q6. Was there clear reporting of the demographics of the participants in the study? Q7. Was there clear reporting of the clinical information of the participants? Q8. Were the outcomes or follow-up results of cases reported? Q9. Was there clear reporting of the presenting site(s)/clinic(s) demographic information? Q10. Was the statistical analysis appropriate? Case-control design questions: Q1. Were the groups comparable other than the presence of disease in cases of the absence of disease in controls? Q2. Were cases and controls matched appropriately? Q3. Were the same criteria used for the identification of cases and controls? Q4. Was exposure measured in a standard, valid, and reliable way? Q5. Was exposure measured in the same way for cases and controls? Q6. Were confounding factors identified? Q7. Were strategies to deal with confounding factors stated? Q8. Were outcomes assessed in a standard, valid, and reliable way for cases and controls? Q9. Was the exposure period of interest long enough to be meaningful? Q10. Was an appropriate statistical analysis used? | ||||||||||||
Table 3.
Results of heterogeneity among included studies
|
| ||||||||
| Variable | Sub-groups | #No. of studies | Event rate (%) 95% CI | Q-value | Df (Q) | I | P-value | Selected model |
| Signs and symptoms | Cough | 9 | 35.5, (23.1-50.2) | 13.93 | 8 | 42.60 | 0.083 | Random |
| Diarrhea | 6 | 7.6 (3.3-16.5) | 2.65 | 5 | 0.0 | 0.754 | Random | |
| Dyspnea | 9 | 14.6 (9.2-22.3) | 2.55 | 8 | 0.0 | 0.895 | Random | |
| Fatigue | 3 | 14.9 (7-29.1) | 2.79 | 2 | 28.52 | 0.247 | Random | |
| Fever | 10 | 66.8 (48.3-81.2) | 26.63 | 9 | 66.2 | 0.002 | Random | |
| Myalgia | 3 | 24.6 (12.1-43.5) | 0.59 | 2 | 0.0 | 0.744 | Random | |
| Sore throat | 4 | 11.5(4.8-25.1) | 1.71 | 3 | 0.0 | 0.633 | Random | |
| Post-partum fever | 4 | 37.1 (18.5-60.6) | 6.01 | 3 | 60.1 | 0.016 | Random | |
| Laboratory tests | Lymphopenia | 7 | 49.8 (30.1-69.6) | 17.59 | 6 | 65.90 | 0.007 | Random |
| Leukocytosis | 4 | 47.7 (31.6-64.2) | 4.57 | 3 | 34.38 | 0.206 | Random | |
| Elevated neutrophil ratio | 3 | 83.7 (72.3-91.0) | 0.236 | 2 | 0.0 | 0.889 | Random | |
| Elevated C-reactive protein | 7 | 57 (43.7-69.3) | 8.89 | 6 | 32.53 | 0.180 | Random | |
| Decreased lymphocyte ratio | 3 | 71.4 (16.4-96.9) | 19.47 | 2 | 89.73 | 0.001 | Random | |
| Type of delivery | Cesarean | 10 | 84 (74.0-90.7) | 9.76 | 9 | 7.84 | 0.370 | Random |
| NR: Not reported; CI: Confidence interval; DF (Q): Degrees of freedom (Cochran's Q); I: I square | ||||||||
Table 4.
Perinatal outcomes of pregnant women with COVID-19
|
| ||||||
| Author, year (reference number) | Total sample size | Covid-19 Positive | Low birth weight | Premature | Complication | Outcome (Died) |
| Liu et al. , 2020-a (27) | 13 | 0 | NR | 6 | 0 | 0 |
| Liu et al. , 2020-b (26) | NR | NR | NR | NR | NR | NR |
| Liu et al. , 2020-c (28) | 2 | 0 | NR | 0 | 0 | 0 |
| Zhu et al. , 2020 (31) | 10 | 0 | 7 | 5 | Multiple organ failure and DIC (1) | 1 |
| Yu et al. , 2020 (30) | 7 | 1 | 0 | NR | 0 | 0 |
| Khan et al. , 2020 (25) | 3 | 0 | 0 | 1 | 0 | 0 |
| Li et al. , 2020 (24) | 17 | 0 | 3 | 4 | Intrauterine fetal distress (2) | 0 |
| Chen et al. , 2020-a (13) | 17 | 0 | 0 | 0 | 0 | 0 |
| Chen et al. , 2020-b (22) | 5 | 0 | 0 | 0 | 0 | 0 |
| Chen et al. , 2020-c (14) | 9 | 0 | 2 | 4 | 0 | 0 |
| NR: Not reported | ||||||
Table 5.
Result of meta-regression
|
| ||||
| Variable | Sub-groups | Mean age | ||
| Coefficient | SE | P-value | ||
| Signs and symptoms | Cough | 0.192 | 0.207 | 0.354 |
| Diarrhea | 0.103 | 0.392 | 0.792 | |
| Dyspnea | -0.110 | 0.236 | 0.640 | |
| Fatigue | 0.676 | 0.405 | 0.095 | |
| Fever | 0.477 | 0.223 | 0.033 | |
| Myalgia | 0.0 | 0.322 | 0.999 | |
| Sore throat | -0.117 | 0.520 | 0.821 | |
| Post-partum fever | -0.001 | 0.192 | 0.995 | |
| Laboratory tests | Lymphopenia | 0.309 | 0.252 | 0.220 |
| Leukocytosis | 0.092 | 0.324 | 0.775 | |
| Elevated neutrophil ratio | 0.141 | 0.443 | 0.749 | |
| Elevated C-reactive protein | 0.057 | 0.231 | 0.802 | |
| Decreased lymphocyte ratio | -0.649 | 0.431 | 0.131 | |
| Type of delivery | Cesarean | 0.433 | 0.180 | 0.016 |
| SE: Standard error | ||||
Figure 1.
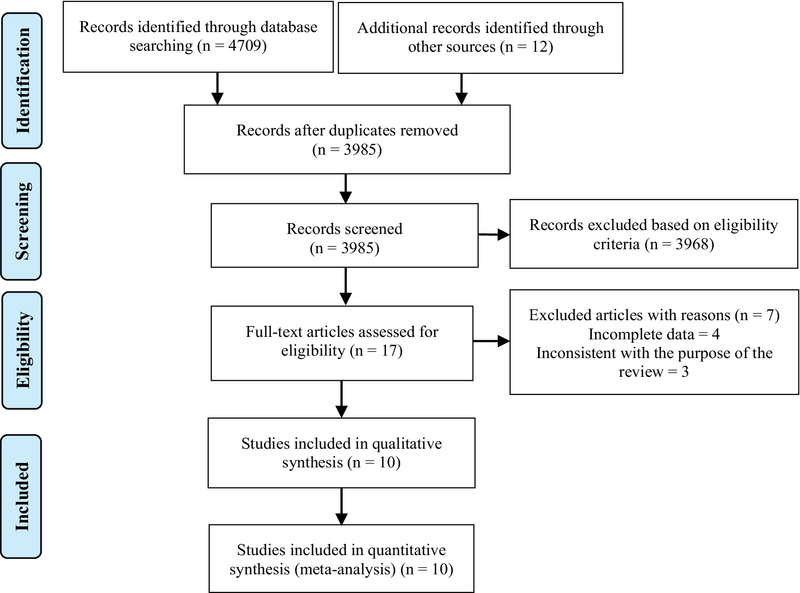
Study selection flow diagram.
Figure 2.
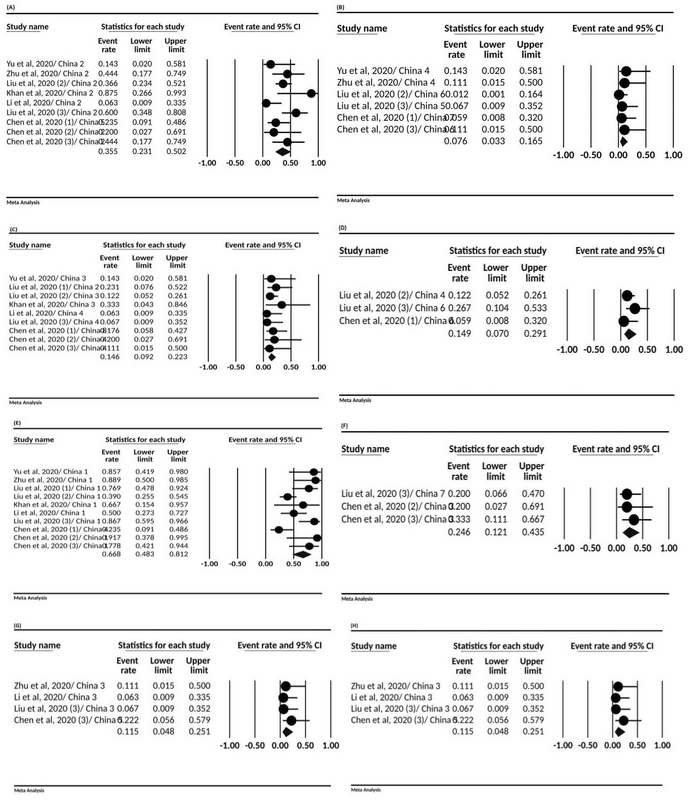
The forest plot presenting event rate and 95% CI for the signs and symptoms in pregnant women with COVID-19; (A) cough, (B) diarrhea, (C) dyspnea, (D) fatigue, (E) fever, (F) myalgia, (G) post-partum fever, and (H) sore throat.
Figure 3.
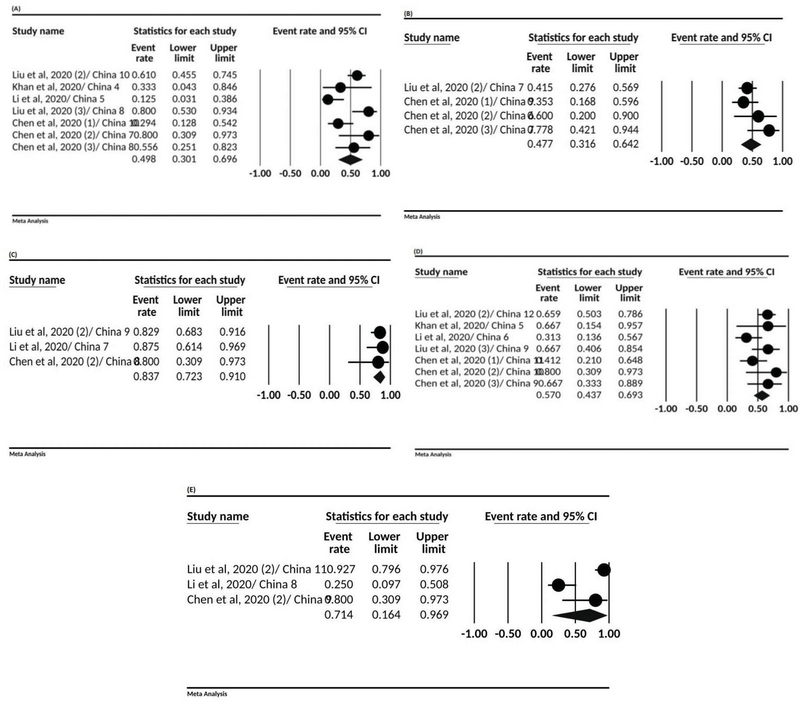
The forest plot presenting event rate and 95% CI for the laboratory tests in pregnant women with COVID-19; (A) lymphopenia, (B) leukocytosis, (C) elevated neutrophil ratio, (D) elevated C-reactive protein, and (E) and decreased lymphocyte ration.
Figure 4.
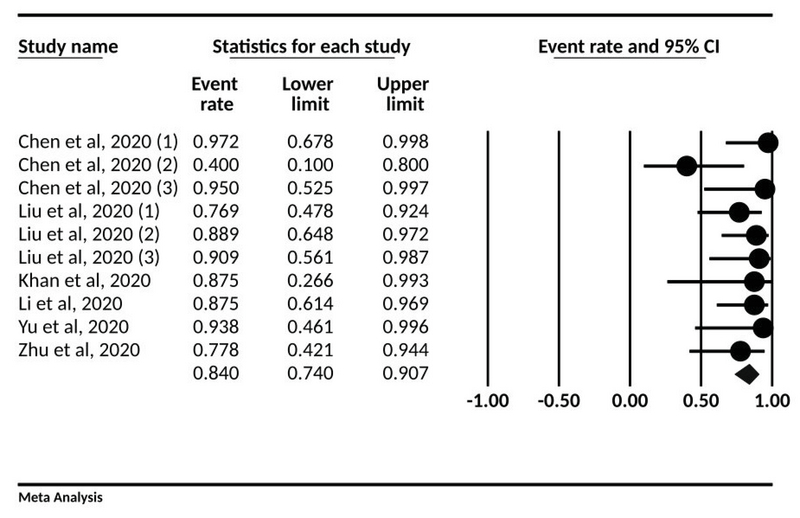
The forest plot presenting event rate and 95% CI for the type of delivery in pregnant women with COVID-19.
4. Discussion
A total of 10 articles were reviewed in this study, which analyzed 135 pregnant women, all of whom were in the third trimester of pregnancy (22-31). These summary findings help healthcare workers better manage pregnant women with COVID-19, which could potentially reduce the side effects for women as well as their newborns.
The common clinical manifestations of pregnant women with COVID-19 include fever and cough, and the less common symptoms are sore throat and diarrhea. Postpartum fever is also more common in women after childbirth. However, the rate of fever in our study was lower than that of Guan and colleagues', who studied the symptoms of non-pregnant coronary artery disease and reported an 87.9% rate of fever. However, similar to our study, in their study, diarrhea was the least common (32).
In terms of laboratory demonstrations, elevated neutrophil ratio and decreased lymphocyte ratio are common. On the other hand, the prevalence of CRP elevated in our study was 57%. However, in Zhang and co-authors' study, this prevalence in a group of people with non-severe and severe patients was 88.9% and 96.4%, respectively (33). This indicates a more pronounced inflammation in patients with more severe conditions and given that pregnant women in this study were not in severe disease conditions, a lower percentage of increased CRP prevalence is justified. On the other hand, in Rodriguez-Morales co-workers study, the increased CRP prevalence was 58.3%, which is similar to our study (8). These differences in numbers can be explained due to the severity of the disease, and on the other hand, a more comprehensive examination is needed.
Lymphopenia and leukocytosis were less common in our study. However, in the study of Zhang and colleagues and Wang and colleague, which was performed on patients with COVID-19 (normal population), lymphopenia was the most common laboratory symptom and was 75.4% and 70.3%, respectively (33, 34). However, it should be noted that these numbers are a decrease in absolute lymphocyte count.
In our study, the majority of pregnancies ended up with cesarean section, which is much higher than the WHO's recommendation for vaginal route delivery (35), which can be determined by a gynecologist to prevent maternal respiratory distress during pregnancy.
In the current study, which examined 135 pregnant women with COVID-19 pneumonia, none of the patients with severe or dead pneumonia were infected with COVID-19 infection. Although SARS-CoV-2 has a common sequence with SARS of up to 85%, we need to be aware of the possibility that the course of the disease and the prognosis of this disease can follow the same SARS process in pregnant women (36, 37).
The current study does have some limitations. First, all patients registered in the included articles were in the third trimester of pregnancy, and the effect of the virus infection on the fetus in the first or second trimester was unknown. Second, due to the short duration of the outbreak, the long-term consequences of the disease on infants have not been possible and more studies are needed. Third, the low number of samples of articles included is another limitation of the work. Fourth, all included studies were from China.
5. Conclusion
In conclusion, pregnant women with COVID-19 pneumonia had diverse symptoms; however, fever and cough were the main clinical symptoms in those women. Although one infant was born with COVID-19 in the included studies, there was little evidence that COVID-19 was transmitted from mother to infant in late pregnancy. Therefore, the study of long-term outcomes on mother and child, as well as the vertical transfer of mother to child in second-trimester pregnancies and the first months after delivery, requires further studies.
Conflict of Interest
The authors declare no conflict of interest.
Acknowledgements
None.
References
- 1.Xu XW, Wu XX, Jiang XG, Xu KJ, Ying LJ, Ma CL, et al. Clinical findings in a group of patients infected with the 2019 novel coronavirus (SARS-Cov-2) outside of Wuhan, China: retrospective case series. BMJ 2020; 368: 1–7. [DOI] [PMC free article] [PubMed]
- 2.Arab-Zozani M, Hassanipour S. Features and limitations of LitCovid hub for quick access to literature about COVID-19. Balkan Med J 2020; 37: 231–232. [DOI] [PMC free article] [PubMed]
- 3.Petrosillo N, Viceconte G, Ergonul O, Ippolito G, Petersen E. COVID-19, SARS and MERS: are they closely related? Clinical Microbiology and Infection 2020; 26: 729–734. [DOI] [PMC free article] [PubMed]
- 4.Meo SA, Alhowikan AM, Al-Khlaiwi T, Meo IM, Halepoto DM, Iqbal M, et al. Novel coronavirus 2019-nCoV: prevalence, biological and clinical characteristics comparison with SARS-CoV and MERS-CoV. Eur Rev Med Pharmacol Sci 2020; 24: 2012–2019. [DOI] [PubMed]
- 5.Fang Zh, Zhang Y, Hang Ch, Ai J, Li Sh, Zhang W. Comparisons of viral shedding time of SARS-CoV-2 of different samples in ICU and non-ICU patients. J Infect 2020; 81: 147–178. [DOI] [PMC free article] [PubMed]
- 6.Wax RS, Christian MD. Practical recommendations for critical care and anesthesiology teams caring for novel coronavirus (2019-nCoV) patients. Can J Anaesth 2020; 67: 568–576. [DOI] [PMC free article] [PubMed]
- 7.Chan JFW, Yuan Sh, Kok KH, To KKW, Chu H, Yang J, et al. A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person-to-person transmission: A study of a family cluster. Lancet 2020; 395: 514–523. [DOI] [PMC free article] [PubMed]
- 8.Rodriguez-Morales AJ, Cardona-Ospina JA, Gutiérrez-Ocampo E, Villamizar-Peña R, Holguin-Rivera Y, Escalera-Antezana JP, et al. Clinical, laboratory and imaging features of COVID-19: A systematic review and meta-analysis. Travel Med Infect Dis 2020; 34: 101623. [DOI] [PMC free article] [PubMed]
- 9.Schwartz DA, Graham AL. Potential maternal and infant outcomes from coronavirus 2019-nCoV (SARS-CoV-2) infecting pregnant women: Lessons from SARS, MERS, and other human coronavirus infections. Viruses 2020; 12: 194–209. [DOI] [PMC free article] [PubMed]
- 10.Favre G, Pomar L, Musso D, Baud D. 2019-nCoV epidemic: What about pregnancies? Lancet 2020; 395: e40. [DOI] [PMC free article] [PubMed]
- 11.Wong ShF, Chow KM, Leung TN, Ng WF, Ng TK, Shek ChC, et al. Pregnancy and perinatal outcomes of women with severe acute respiratory syndrome. Am J Obstet Gynecol 2004; 191: 292–297. [DOI] [PMC free article] [PubMed]
- 12.Mertz D, Geraci J, Winkup J, Gessner BD, Ortiz JR, Loeb M. Pregnancy as a risk factor for severe outcomes from influenza virus infection: A systematic review and meta-analysis of observational studies. Vaccine 2017; 35: 521–528. [DOI] [PMC free article] [PubMed]
- 13.Chen YH, Keller J, Wang IT, Lin ChCh, Lin HCh. Pneumonia and pregnancy outcomes: a nationwide population-based study. Am J Obstet Gynecol 2012; 207: 288. e1–e7. [DOI] [PMC free article] [PubMed]
- 14.Chen R, Chen J, Meng QT. Chest computed tomography images of early coronavirus disease (COVID-19). Can J Anaesth 2020; 67: 754–755. [DOI] [PMC free article] [PubMed]
- 15.Mor G, Cardenas I, Abrahams V, Guller S. Inflammation and pregnancy: the role of the immune system at the implantation site. Ann N Y Acad Sci 2011; 1221: 80–87. [DOI] [PMC free article] [PubMed]
- 16.Gottfredsson M. The spanish flu in iceland 1918. Lessons in medicine and history. Laeknabladid 2008; 94: 737–745. [PubMed]
- 17.Jamieson DJ, Honein MA, Rasmussen SA, Williams JL, Swerdlow DL, Biggerstaff MS, et al. H1N1 2009 influenza virus infection during pregnancy in the USA. Lancet 2009; 374: 451–458. [DOI] [PubMed]
- 18.Moher D, Liberati A, Tetzlaff J, Altman DG, The PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Int J Surg 2010; 8: 336–341.
- 19.Munn Z, Barker TH, Moola S, Tufanaru C, Stern C, McArthur A, et al. Methodological quality of case series studies: An introduction to the JBI critical appraisal tool. JBI Evidence Synthesis 2020; 18: 2127–2133. [DOI] [PubMed]
- 20.Joanna Briggs Institute. Critical appraisal checklist for case reports. Available at: https://joannabriggs.org/critical-appraisal-tools.
- 21.Arab-zozani M, Mostafazadeh N, Arab-zozani Z, Ghoddoosi-Nejad D, Hassanipour S, Soares JJF. The prevalence of elder abuse and neglect in Iran: A systematic review and meta-analysis. J Elder Abuse Negl 2018; 30: 408–423. [DOI] [PubMed]
- 22.Chen H, Guo J, Wang Ch, Luo F, Yu X, Zhang W, et al. Clinical characteristics and intrauterine vertical transmission potential of COVID-19 infection in nine pregnant women: A retrospective review of medical records. Lancet 2020; 395: 809–815. [DOI] [PMC free article] [PubMed]
- 23.Gidlöf S, Savchenko J, Brune T, Josefsson H. COVID-19 in pregnancy with comorbidities: More liberal testing strategy is needed. Acta Obstet Gynecol Scand 2020; 99: 948–949. [DOI] [PubMed]
- 24.Li N, Han L, Peng M, Lv Y, Ouyang Y, Liu K, et al. [Maternal and neonatal outcomes of pregnant women with COVID-19 pneumonia: A case-control study]. Clin Infect Dis 2020. (in Press) [DOI] [PMC free article] [PubMed]
- 25.Khan S, Peng L, Siddique R, Nabi Gh, Nawsherwan U, Xue M, et al. Impact of COVID-19 infection on pregnancy outcomes and the risk of maternal-to-neonatal intrapartum transmission of COVID-19 during natural birth. Infect Control Hosp Epidemiol 2020; 41: 748–750. [DOI] [PMC free article] [PubMed]
- 26.Liu H, Wang LL, Zhao SJ, Kwak-Kim J, Mor G, Liao AH. Why are pregnant women susceptible to COVID-19? An immunological viewpoint. J Reprod Immunol 2020; 139: 103122. [DOI] [PMC free article] [PubMed]
- 27.Liu D, Li L, Wu X, Zheng D, Wang J, Yang L, et al. Pregnancy and perinatal outcomes of women with coronavirus disease (COVID-19) pneumonia: A preliminary analysis. Am J Roentgenol 2020; 215: 127–132. [DOI] [PubMed]
- 28.Liu H, Liu F, Li J, Zhang T, Wang D, Lan W. Clinical and CT imaging features of the COVID-19 pneumonia: Focus on pregnant women and children. J Infect 2020; 80: e7–e13. [DOI] [PMC free article] [PubMed]
- 29.Zhang B, Liu Sh, Tan T, Huang W, Dong Y, Chen L, et al. Treatment with convalescent plasma for critically Ill patients with severe acute respiratory syndrome coronavirus 2 infection. Chest 2020; 158: e9–e13. [DOI] [PMC free article] [PubMed]
- 30.Yu N, Li W, Kang Q, Xiong Zh, Wang Sh, Lin X, et al. Clinical features and obstetric and neonatal outcomes of pregnant patients with COVID-19 in Wuhan, China: A retrospective, single-centre, descriptive study. Lancet Infect Dis 2020; 20: 559–564. [DOI] [PMC free article] [PubMed]
- 31.Zhu H, Wang L, Fang Ch, Peng S, Zhang L, Chang G, et al. Clinical analysis of 10 neonates born to mothers with 2019-nCoV pneumonia. Transl Pediatr 2020; 9: 51–60. [DOI] [PMC free article] [PubMed]
- 32.Guan WJ, Ni ZhY, Hu Y, Liang WH, Ou CHQ, He JX, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med 2020; 382: 1708–1720. [DOI] [PMC free article] [PubMed]
- 33.Zhang JJ, Dong X, Cao YY, Yuan YD, Yang YB, Yan YQ, et al. Clinical characteristics of 140 patients infected with SARS-CoV-2 in Wuhan, China. Allergy 2020; 75: 1730–1741. [DOI] [PubMed]
- 34.Wang D, Hu B, Hu Ch, Zhu F, Liu X, Zhang J, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA 2020; 323: 1061–1069. [DOI] [PMC free article] [PubMed]
- 35.De Brouwere V, Dubourg D, Richard F, Van Lerberghe W. Need for caesarean sections in west Africa. Lancet 2002; 359: 974–975. [DOI] [PubMed]
- 36.Schoeman D, Fielding BC. Coronavirus envelope protein: current knowledge. Virol J 2019; 16: 69–90. [DOI] [PMC free article] [PubMed]
- 37.Zhou P, Yang XL, Wang XG, Hu B, Zhang L, Zhang W, et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature 2020; 579: 270–273. [DOI] [PMC free article] [PubMed]


