Abstract
Background
The use of autologous platelet-rich plasma as an ovarian treatment has not been standardized and remains controversial.
Case Presentation
A 41½-year old woman with diminished ovarian reserve (serum anti- Müllerian hormone = 0.163 mg/mL) and a history of 10 unsuccessful in vitro fertilization cycles presented for reproductive endocrinology consult. She and her partner declined donor oocyte in vitro fertilization. They were both in good general health and laboratory tests were unremarkable, except for mild thrombocytosis (platelets = 386K; normal range 150-379K) discovered in the female. The patient underwent intraovarian injection of fresh platelet-derived growth factor concentrate administered as an enriched cell-free substrate. Serum anti- Müllerian hormone increased by 115% within 6 wks of treatment. Spontaneous ovulation occurred the month after injection and subsequently the serum human chorionic gonadotropin was noted at 804 mIU/mL. Following an uneventful obstetrical course, a male infant was delivered at term without complication.
Conclusion
This is the first description of intraovarian injection of enriched platelet-derived growth factors followed by unassisted pregnancy and live birth. As a refinement of conventional ovarian platelet-rich plasma therapy, this procedure may be particularly valuable for refractory cases where prognosis for pregnancy appears especially bleak. A putative role for thrombocytosis is also viewed in parallel with mechanisms of action as advanced earlier. With continued experience in ovarian application of autologous platelet growth factors, additional research will evaluate laboratory protocol/sample preparation, injection technique, and patient selection.
Keywords: Ovarian rejuvenation, Platelet-rich plasma, Cytokines, Infertility, IVF.
1. Introduction
It is well-known that as women age, both the quality and the quantity of eggs decline; the low ovarian reserve observed among older infertile patients occurs as an expected physiological consequence of normal ovarian senescence. In such cases, even the use of high-dose gonadotropin protocols is generally futile, leaving oocyte donation/IVF as the only clinically effective treatment (1-3).
As an investigational alternative to egg donation, the surgical placement of autologous platelet-rich plasma (PRP) into ovarian tissue first began to attract attention in 2016 (4). This pioneering technique of ovarian “rejuvenation” was followed by two publications describing similar use of PRP for poor-prognosis patients as a precursor to IVF. Specifically, four patients with undetectable or very low ovarian reserve (mean age 42 years) who had planned for donor egg treatment instead underwent the PRP treatment; all four patients developed blastocysts from their oocytes (5), one of them has since undergone thaw, transfer, and had a healthy term delivery. Experts in Greece also described three poor-responder IVF patients (mean age 38 years) with similar “revolutionary” responses (6). At least one patient who produced only embryos with genetic errors was able to achieve “ploidy rescue” following intraovarian injection of platelet-derived growth factors (PDGFs) before IVF, culminating in successful term live birth (7). Ovarian PRP has been formally evaluated in a descriptive pilot study including 150 patients, where no significant change was observed in the serum AMH of most patients (8). However, the measured response rate after ovarian PRP (28%) approximates the overall IVF pregnancy rate in the USA. While ovarian treatment with growth factors has generally been framed as a precursor to IVF (5), almost no data exist on the reproductive outcome in the absence of IVF. Here, intraovarian injection of enriched, cell-free platelet growth factors followed by healthy term delivery - with no gonadotropins or IVF - is presented.
2. Case Presentation
A 41½-year old woman presented with her husband (aged 41) for reproductive endocrinology consultation. They were both in good general health and took no regular medication. Her past surgical history was significant for an uncomplicated laparoscopic myomectomy in 2018. The assessment of endometrial cavity contour after the procedure was normal. At age 38, the patient was provisionally diagnosed with primary ovarian insufficiency (POI) based on repeatedly elevated levels of follicle-stimulating hormone (FSH) and “undetectable” anti-Müllerian hormone (AMH). The remainder of the work-up in this case was essentially unremarkable, including a normal endocrine profile and negative pregnancy test, although a mild thrombocytosis (platelets = 386K; normal range 150-379K) was discovered in the female. Moreover, two pregnancies were established without medical assistance 5 yrs ago, but both were electively terminated without complication. The couple had initiated at least 10 IVF cycles elsewhere before consultation, however, none were successful; chart review attributed these failures to poor follicular response, “empty follicles,” fertilization failure, or culture arrest. Additional IVF attempts were discouraged, and for personal reasons the couple declined donor egg IVF.
Here, the patient was counseled and a written informed consent was obtained for the injection of enriched autologous PDGFs into both ovaries. This treatment was offered as an extension of a previous IRB-approved prospective clinical trial (8). PDGFs were isolated first via obtaining autologous PRP activated by calcium gluconate as previously described (8), followed by additional enrichment by centrifugation at 250 g 15 min with phosphate-buffered saline (Thermo Fisher Scientific, Carlsbad Calif USA) irrigation of the platelet pellet 3. Thereafter, the resuspended platelet pellet was next processed through a centrifugation sequence (300 g for 10 min, 2,000 g for 10 min) to subtract debris, based on prior protocols (9-11). The resulting cell-free supernatant was maintained fresh at room temperature for ovarian insertion. After processing, a volume of approximately 1.5 mL was injected into ovarian stroma (subcapsular) under direct transvaginal ultrasound guidance with instrumentation as for conventional PRP dosing (5). The procedure was well tolerated with no complications, completed in 10 min, and required no anesthesia or sedation. Using a uniform assay (12), the serum AMH was checked again the following month, and the level had increased from 0.163 ng/mL pretreatment to 0.352 ng/mL six weeks later (Figure 1). Ovulation occurred without medical assistance and serum human chorionic gonadotropin was subsequently noted at 804 mIU/mL. Her prenatal course was uneventful and she delivered a healthy 3,740 gr male infant without complication by elective cesarean at 39 weeks gestation. Both the mother and the baby continue to do well.
Figure 1.
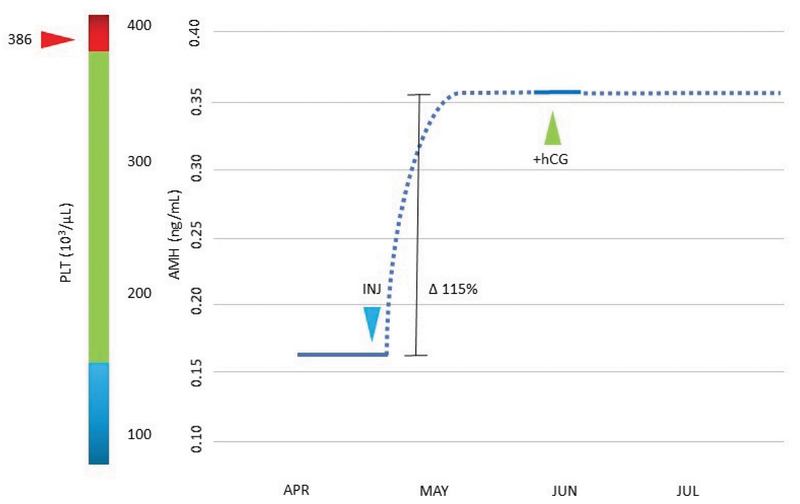
Serum AMH levels measured before versus after bilateral intraovarian injection of platelet-derived growth factors. Approximately six weeks after the treatment (blue arrow), an unassisted pregnancy was confirmed (green arrow). Baseline thrombocytosis is depicted at left (red arrow) to show nominally elevated pretreatment platelet concentration relative to the expected reference range (vertical bar). AMH: Anti-mullerian hormone; INJ: Intraovarian injection of enriched platelet growth factors; hCG: Human chorionic gonadotropin; PLT: Platelet concentration; Dashed/solid line: Estimated/verified data.
Ethical consideration
Written informed consent was obtained from the patient, who read and approved the manuscript before publication.
3. Discussion
For both patients and providers, the problem of recurrent IVF failure is difficult and usually leads to discussion about donor oocytes. Although this approach is an established component of advanced fertility treatment since first introduced in the 1980s (13, 14), it is still sometimes unacceptable to some patients. For this reason, any advances to open safe and effective opportunities for patients would be welcomed. While successful pregnancy has been reported even when POI has been validated (15), the numerous IVF failures experienced by our patient focused renewed attention on intraovarian injection of autologous platelet-derived cytokines. Although no consensus exists regarding any preferred PRP sample preparation or injection technique, there is even less agreement regarding an optimal methodology for the newer approach for incubation and/or isolation of platelet growth factors.
As summarized in Figure 2, selected platelet releasate components are shown with depleted platelets (DEP). These include TGF-β, a transcription activator modulating genes for differentiation, chemotaxis, and proliferation and activation of immune system cells; vascular endothelial growth factor, a signal protein stimulating blood vessel formation; insulin-like growth factors (1 and 2), proteins required for cell stimulation; PDGF, critical to blood vessel growth from adjacent capillaries, mitogenesis, and proliferation of mesenchymal cells including fibroblasts, osteoblasts, tenocytes, vascular SMCs, and mesenchymal stem cells; Interleukin-1β (IL-1β), an inflammatory marker involved in cell growth, differentiation, and programmed death; Interleukin-8 which initiates angiogenesis, perfusion, and movement to injury/infection sites; epidermal growth factor, a key messenger in cell proliferation, differentiation, and survival; as well as basic fibroblast growth factor, a mediator with mitogenic and cell survival activities including embryonic development, cell growth, morphogenesis, and tissue repair.
Placing such growth factors within ovarian tissue may potentiate higher AMH output and improve IVF response in several ways (16). One possibility is that any follicle emerging after intraovarian injection of these growth factors was merely latent, not completely absent. Perhaps more controversially, PDGFs could engage with uncommitted ovarian stem cells (17) and work along multiple signaling pathways to evoke differentiation to de novo oocytes. Indeed, PRP has been shown to induce proliferation of some cell populations to improve stemness and to enhance in vitro expression of receptivity markers (18, 19). Since these factors also have angiogenic properties, it is plausible that improving capillary flow and thus tissue oxygen delivery might induce beneficial ovarian effects after injection (20).
While it is tempting to ascribe any effects observed here to platelet cytokines, it is not possible to separate this component from the injection process itself where ovarian micro-puncture alone might be therapeutic (21, 22). However, we believe the link between the treatment and subsequent pregnancy here is supported by the brief interval between treatment and pregnancy, the absence of any other therapy, the serum AMH pattern after injection, and thrombocytosis. This latter issue draws notice to the role of platelet dynamics when ovarian PRP and related treatments are critically assessed; ambient platelet count has been identified as a modulator of AMH response independent of patient age, infertility duration, or pre-injection AMH level (5, 8).
We agree that limitations exist whenever case data are considered. For example, the marked uptick in serum AMH and the favorable reproductive outcome attained here after treatment are associative and not necessarily causative. Additional study should help clarify signaling pathways involved in follicular development, thereby providing potential techniques to make pregnancy possible even for older patients with low or absent ovarian reserve.
Figure 2.
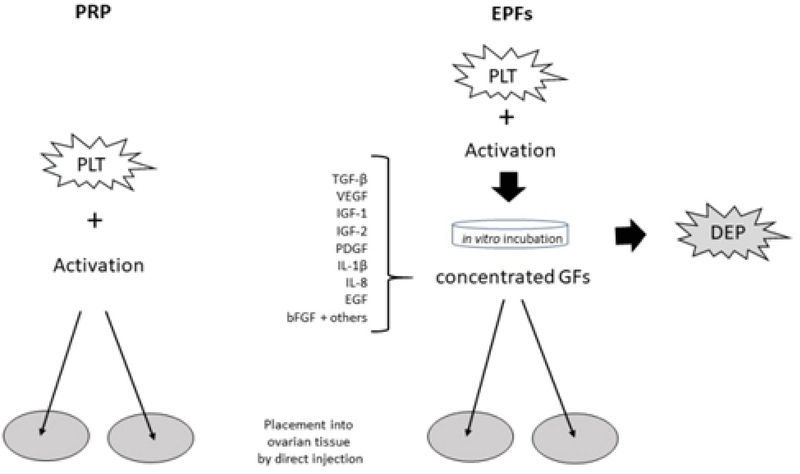
Outline comparing standard platelet-rich plasma (PRP) versus enriched platelet factors (EPF) ovarian treatment.
Conflict of Interest
ESS and SHW have received a provisional U.S. patent for the process & treatment of ovarian disorders using platelet cytokine derivatives. NSR has no conflicts to disclose.
References
- 1.Lefebvre J, Antaki R, Kadoch IJ, Dean NL, Sylvestre C, Bissonnette F, et al. 450 IU versus 600 IU gonadotropin for controlled ovarian stimulation in poor responders: A randomized controlled trial. Fertil Steril 2015; 104: 1419–1425. [DOI] [PubMed]
- 2.Ghahremani-Nasab M, Ghanbari E, Jahanbani Y, Mehdizadeh A, Yousefi M. Premature ovarian failure and tissue engineering. J Cell Physiol 2020; 235: 4217–4226. [DOI] [PubMed]
- 3.Sills ES, Brady AC, Omar AB, Walsh DJ, Salma U, Walsh APH. IVF for premature ovarian failure: first reported births using oocytes donated from a twin sister. Reprod Biol Endocrinol 2010; 8: 31–33. [DOI] [PMC free article] [PubMed]
- 4.Pantos K, Nitsos N, Kokkali G, Vaxevanoglou T, Markomichali C, Pantou A, et al. Ovarian rejuvenation and folliculogenesis reactivation in peri-menopausal women after autologous platelet-rich plasma treatment. Hum Reprod 2016 (Suppl.): P–401.
- 5.Sills ES, Rickers NS, Li X, Palermo GD. First data on in vitro fertilization and blastocyst formation after intraovarian injection of calcium gluconate-activated autologous platelet rich plasma. Gynecol Endocrinol 2018; 34: 756–760. [DOI] [PubMed]
- 6.Sfakianoudis K, Simopoulou M, Nitsos N, Rapani A, Pantou A, Vaxevanoglou T, et al. A case series on platelet-rich plasma revolutionary management of poor responder patients. Gynecol Obstet Invest 2019; 84: 99–106. [DOI] [PubMed]
- 7.Sills ES, Rickers NS, Svid Ch, Rickers JM, Wood SH. Normalized ploidy following 20 consecutive blastocysts with chromosomal error: Healthy 46,XY pregnancy with IVF after intraovarian injection of autologous enriched platelet-derived growth factors. Int J Mol Cell Med 2019; 8: 84–90. [DOI] [PMC free article] [PubMed]
- 8.Sills ES, Rickers NS, Petersen JL, Li X, Wood SH. Regenerative effect of intraovarian injection of autologous platelet rich plasma: Serum anti-Mullerian hormone levels measured among poor-prognosis in vitro fertilization patients. Int J Regen Med 2020; 3: 1–5.
- 9.Steller D, Herbst N, Pries R, Juhl D, Hakim SG. Impact of incubation method on the release of growth factors in non-Ca2+-activated PRP, Ca2+-activated PRP, PRF and A-PRF. J Craniomaxillofac Surg 2019; 47: 365–372. [DOI] [PubMed]
- 10.Théry C, Amigorena S, Raposo G, Clayton A. Isolation and characterization of exosomes from cell culture supernatants and biological fluids. Curr Protoc Cell Biol 2006; 30: 302201–3022029. [DOI] [PubMed]
- 11.Guo ShCh, Tao ShC, Yin WJ, Qi X, Yuan T, Zhang ChQ. Exosomes derived from platelet-rich plasma promote the re-epithelization of chronic cutaneous wounds via activation of YAP in a diabetic rat model. Theranostics 2017; 7: 81–96. [DOI] [PMC free article] [PubMed]
- 12.Marron KD, Sills ES, Cummins PL, Harrity C, Walsh DJ, Walsh AP. Impact of pre-mixing AMH serum samples with standard assay buffer: Ovarian reserve estimations and implications for clinical IVF providers. J Reprod Endocrinol Infertil 2016; 2: 10.
- 13.Rosenwaks Z, Navot D, Veeck L, Liu HC, Steingold K, Kreiner D, et al. Oocyte donation. The norfolk program. Ann N Y Acad Sci 1988; 541: 728–741. [DOI] [PubMed]
- 14.Sauer MV, Paulson RJ, Lobo RA. Reversing the natural decline in human fertility: An extended clinical trial of oocyte donation to women of advanced reproductive age. JAMA 1992; 268: 1275–1279. [DOI] [PubMed]
- 15.Gu Y, Xu Y. Successful spontaneous pregnancy and live birth in a woman with premature ovarian insufficiency and 10 years of amenorrhea: A case report. Front Med 2020; 7: 18. [DOI] [PMC free article] [PubMed]
- 16.Sills ES, Wood SH. Autologous activated platelet rich plasma injection into adult human ovary tissue: Molecular mechanism, analysis, and discussion of reproductive response. Biosci Rep 2019; 39: pii: BSR20190805. 1–15. [DOI] [PMC free article] [PubMed]
- 17.Johnson J, Canning J, Kaneko T, Pru JK, Tilly JL. Germline stem cells and follicular renewal in the postnatal mammalian ovary. Nature 2004; 428: 145–150. [DOI] [PubMed]
- 18.Zhang S, Li P, Yuan Zh, Tan J. Effects of platelet-rich plasma on activity of human menstrual blood-derived stromal cells in vitro. Stem Cell Res Ther 2018; 48: 1–11. [DOI] [PMC free article] [PubMed]
- 19.Zhang S, Li P, Yuan Zh, Tan J. Platelet-rich plasma improves therapeutic effects of menstrual blood-derived stromal cells in rat model of intrauterine adhesion. Stem Cell Res Ther 2019; 61: 1–12. [DOI] [PMC free article] [PubMed]
- 20.Wood SH, Sills ES. Intraovarian vascular enhancement by stromal injection of platelet-derived growth factors: Exploring subsequent oocyte chromosomal status and IVF outcomes. Clin Exp Reprod Med 2020; 47: 94–100. [DOI] [PMC free article] [PubMed]
- 21.Marschalek J, Ott J, Aitzetmueller M, Mayrhofer D, Weghofer A, Nouri K, et al. The impact of repetitive oocyte retrieval on the ovarian reserve: a retrospective cohort study. Arch Gynecol Obstet 2019; 299: 1495–1500. [DOI] [PMC free article] [PubMed]
- 22.Hatrnaz Ş, Tan SL, Hatrnaz E, Çelik Ö, Kanat-Pektaş M, Dahan MH. Vaginal ultrasound-guided ovarian needle puncture compared to laparoscopic ovarian drilling in women with polycystic ovary syndrome. Arch Gynecol Obstet 2019; 299: 1475–1480. [DOI] [PubMed]


