Abstract
Patients with adenomatous polyposis, usually defined as patients with >10 adenomatous polyps in the colorectum, are at increased risk for colorectal cancer (CRC). Since surgical and endoscopic treatment do not completely eliminate the potential for future polyps or extraintestinal neoplasms, there is an unmet medical need to identify pharmacological agents to delay major surgical interventions. We present two cases of patients with adenomatous polyposis who developed chronic myelogenous leukaemia and were treated with imatinib as part of their chemotherapy. A sustained regression of the colonic polyps documented in both cases was observed after the initiation of the tyrosine kinase inhibitor. Despite the presence of potential confounders, we hypothesise the potential role of imatinib as a chemopreventive agent in patients with familial adenomatous polyposis.
Keywords: familial adenomatous polyposis, colorectal cancer, chemoprevention, cancer prevention
Introduction
Patients with adenomatous polyposis, usually defined as patients with >10 adenomatous polyps in the colorectum, are at increased risk for colorectal cancer (CRC). Familial adenomatous polyposis (FAP) is an autosomal dominant inherited disorder caused by mutations in the APC or MUTYH genes and characterised by the progressive development of multiple adenomas throughout the colon and rectum.1 2 In a meaningful proportion of patients, adenomatous polyposis is not associated to a germline mutation. In FAP, regular colonoscopy surveillance is recommended until colectomy is planned. However, there are many potential complications associated with surgery, and it does not prevent from lower gastrointestinal surveillance in patient with retained rectum of ileal pouch. Accordingly, in this group of patients, it is essential to develop strategies for the prevention of both adenoma and CRC development. Chemoprevention refers to a pharmacological intervention to stop or reverse the process of carcinogenesis. As chemoprevention is intended to be taken for long periods, it must meet certain requirements such as low or no toxicity, ease of administration and cost-effectiveness.3 Whereas multiple pharmacological agents (ie, sulindac, COX-2 inhibitors) have been tested for this purpose, chemoprevention is not yet currently the standard of care in FAP.4 5
Here, we describe two clinical cases of patients with adenomatous polyposis in whom imatinib, a tyrosine kinase inhibitor, was administered as part of their treatment for a haematological entity (chronic myelogenous leukaemia, CML). Despite the presence of potential confounders, we believe the present observations deserve more investigation about the potential role of imatinib as a chemopreventive agent.
Case 1
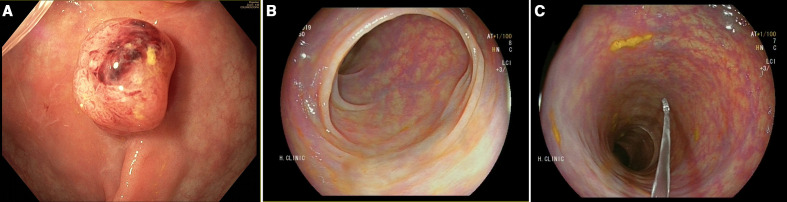
The first case is a 69-year-old man with a history of heart transplant in 2001 due to an ischaemic cardiomyopathy (current treatment cyclosporine 75 mg/day, azathioprine 50 mg/day and acetylsalicylic acid (ASA) 100 mg/day), radical cystectomy due to urothelial tumour managed with Bricker-type urethro-ileostomy in 2009, chronic kidney disease on haemodialysis since 2015 and diagnosis of Philadelphia-positive (BCR-ABL fusion transcript) CML in 2011. Patient was treated with imatinib 300 mg/day from 2011 to 2015, discontinued due to chronic kidney disease, starting dasatinib (a highly potent inhibitor of BCR-ABL) 50 mg/day in 2015. The patient participated in the CRC population screening programme in 2016, and presented a positive faecal immunochemical test. He underwent screening colonoscopy on July 2016 with poor preparation finding 50 polyps throughout the colon that were classified as Paris 0-Is and 0-IIa (sizes 6–12 mm) (figure 1A). The examination could reach only the hepatic flexure. Due to the incompleteness of the procedure and poor prep, only four polyps were resected in the rectum, with the pathology report showing tubular-villous adenomas with low-grade dysplasia. The patient had a family history of CRC (two maternal granduncles at age of 76 years, a granduncle and a maternal cousin over 60 years). A germline genetic analysis was performed which was negative for MUTYH and APC mutations. Due to comorbidities, in agreement with the patient, neither treatment nor surveillance was indicated. Regarding CML treatment, in 2017 dasatinib was suspended due to associated pulmonary complication (pleural effusion and pulmonary fibrosis), and imatinib was restarted in the beginning of 2018, initially at 400 mg/day with gradual reduction to 200 mg as a result of associated infectious complications. At the end of 2018, he presented abdominal pain and change in bowel habit with diarrhoea. Drug and infectious aetiology were ruled out and a colonoscopy study was repeated, this time reaching the cecum with adequate preparation. Diverticula were identified at sigmoid colon and a single 5 mm sessile polyp in the rectum was identified managed with cold snare polypectomy (figure 1B, C). The pathology report showed a tubular adenoma with low-grade dysplasia.
Figure 1.
Case 1. (A) Colonoscopy before the administration of imatinib, a 10 mm sessile polyp is shown. (B) and (C) Descending colon explored with LCI (linked colour imaging; FUJIFILM) after the administration of imatinib for 1 year.
Case 2
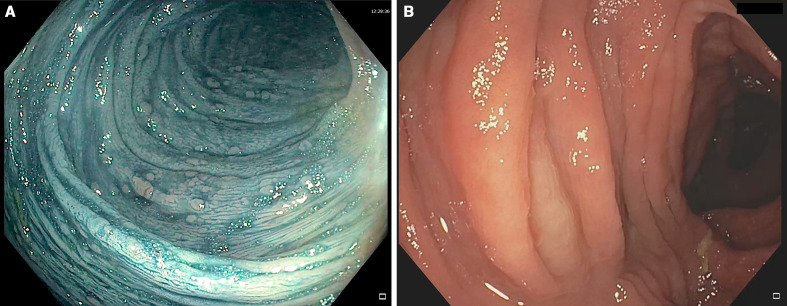
A 33-year-old man with an APC pathogenic germline variant started surveillance in our centre in 2014. Colonoscopy showed 100 adenomas <10 mm throughout that colon, with rectal sparing (figure 2A). The patient participated in the randomised clinical trial CPP-FAP-310 (NCT01483144) aimed at determining if the combination of eflornithine plus sulindac was superior to sulindac or eflornithine as single agents in delaying time to any FAP-related event.6 He initiated the trial medication (eflornithine alone) in October 2014. Surveillance colonoscopy in November 2015 evidenced 10–12 polyps of 1–3 mm in the ascending colon, with no polyps in the rest of the study. The trial was extended until January 2017, when eflornithine was suspended due to diagnosis of Philadelphia-positive (BCR-ABL) CML. The patient began treatment with imatinib at a dose of 400 mg/day, with adequate tolerance and clinical response to treatment. Surveillance colonoscopy in September 2017 showed no evidence of lesions in the colon. Later in September 2020, an endoscopic control was performed and only around 10 polyps <3 mm in the recto-sigmoid were observed (figure 2B).
Figure 2.
Case 2. (A) Colonoscopy with chromoendoscopy with indigo carmine showing several sessile polyps 1–3 mm in size in the descending colon. (B) Transverse colon after the administration of imatinib for 30 months without polyps.
Discussion
Imatinib is a derivative of 2-phenylaminopyrimidine, a potent inhibitor directed against protein tyrosine kinases, including BCR-ABL, c-kit and the platelet-derived growth factor receptor (PDGF-R).7 In CML, the drug appears to work primarily by occupying the ATP binding site of the BCR-ABL oncoprotein and, therefore, prevents phosphorylation of the substrate and interrupts contact with the effector protein, resulting in the nullification of the enzymatic function of the BCR-ABL oncoprotein.8 Although its mechanism of action is not directly involved in preventing the development of cancer precursor lesions in the colon, we describe two cases in which regression of the polyps is observed after administration.
The precise potential mechanism of imatinib in the colorectal carcinogenesis remains poorly understood. To date, it is known that EphB tyrosine kinase receptors regulate the positioning and promote the proliferation of intestinal stem and progenitor cells.9 10 In tumour growth, these receptors play dual roles, acting as tumour promoters during adenoma development but as tumour suppressors in the progression to invasive CRC.11 12 Experimental evidence suggests that inhibiton of EphB signalling pathway by imatinib in the cell could explain the suppression of development of intestinal tumours. The relationship of this pathway to the inhibition of transduction signals from PDGF-R or c-Kit remain unclear. In the case reported by Itsukuma et al,13 the authors described that the immunohistochemical analysis of adenomas did not show expression of c-Kit and the anti-PDGFR alpha (PDGFRA) antibodies did not provide conclusive information on the expression of PDGFRA. There are experimental studies10 14 in which EphB tyrosine kinase receptors were shown to promote the proliferation of intestinal stem and progenitor cells. They also determined the inhibitory effect of imatinib on this receptor in a model with Knockout mice with an APC mutation.10 Treatment with imatinib inhibited the tumour-promoting effects of EphB signalling in Knockout mice, without attenuating EphB-mediated tumour suppression, demonstrating a role for EphB signalling in the initiation of intestinal tumours. The imatinib treatment regimen extended the lifespan of ApcMin/+ mice and reduced cell proliferation in cultured sections of adenomas from patients with familial adenomatous polyposis.
We report two cases of adenomatous polyposis and CML both treated with imatinib. The coexistence of these two entities is very rare, being found only in case reports such as the one published in 2007 by Itsukuma et al,13 in which a 38-year-old man with an FAP complicated by CML was treated with imatinib achieving haematological and cytogenetic remission in 6 months. Numerous polyps, 2–3 mm in diameter, observed in the rectum prior to the administration of imatinib, regressed in size, but not in number, after 1 year of treatment with imatinib. In our cases, there was a total regression of the polyps concerning those visualised in the initial endoscopic studies with a similar therapeutic approach.
Although we cannot infer causality in neither of our cases, we believe our observation deserves further studies. Regarding case 1, although the patient began treatment with tyrosine kinase inhibitors in 2011, this medication had to be continuously adjusted, changed and restarted in 2018 due to its multiple comorbidities, among which chronic kidney disease that entered the final stage. Once the patient was on imatinib treatment, it was surprisingly noticed that the polyps almost disappeared compared with the previous examination.
In case 2, a patient with a germline mutation in the APC gene,1 there is a clear confounding factor which is having received eflornithine, an active chemopreventive agent for 27 months prior to the initiation of imatinib. Eflornithine (also known as DMFO) is an irreversible inhibitor of ornithine decarboxylase. This enzyme is normally negatively regulated by APC and overexpressed in tumour tissues. In our case, in the setting of the study, we already observed a clinical response 12 months after being on this medication. However, despite suspending eflornithine, he showed a sustained response with the disappearance of the polyps 3 years after, while he continued on imatinib.
In summary, we describe two cases that highlight the potential effect of imatinib as a chemopreventive agent in colorectal carcinogenesis. Imatinib is usually well-tolerated but long-term administration carries serious side effects, including cardiotoxicity.15 However, short-term or intermittent treatment would reduce the side effects substantially, and could potentially reduce polyp burden in severe duodenal or rectum/pouch cases. Although still speculative, if this hypothesis is confirmed, imatinib could be properly evaluated as a chemopreventive drug in the setting of FAP patients.
Acknowledgments
We thank the High-Risk Colorectal Cancer Clinic and the Endoscopy Unit of Hospital clinic for providing the facilities and for their hard work in this study.
Footnotes
Contributors: FB was responsible for the study concept and design. AT, TO and PO were responsible for the acquisition of data, analysis and interpretation. AT, PO and FB drafted the manuscript. MP, FB and AT contributed to final revision of the manuscript. FB was responsible for the study supervision.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement
All data relevant to the study are included in the article or uploaded as supplementary information and aditional data is available upon request.
Ethics statements
Patient consent for publication
Obtained.
References
- 1.Sabela C, Maria LA, Leticia M, et al. Hereditary colorectal cancer syndromes10.2217/CRC.13.80. 2014 future medicine LTD. Colorect. Cancer 2014;3:1–20. [Google Scholar]
- 2.Galiatsatos P, Foulkes WD. Familial adenomatous polyposis. Am J Gastroenterol 2006;101:385–98. 10.1111/j.1572-0241.2006.00375.x [DOI] [PubMed] [Google Scholar]
- 3.Umezawa S, Higurashi T, Komiya Y, et al. Chemoprevention of colorectal cancer: past, present, and future. Cancer Sci 2019;110:3018–26. 10.1111/cas.14149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mangas-Sanjuan C. Vigilancia tras resección de pólipos de colon Y de cáncer colorrectal. Actualización 2018. Gastroenterol Hepatol 2018. [DOI] [PubMed] [Google Scholar]
- 5.Cubiella J, et al. Guía de práctica clínica. Diagnóstico Y prevención del cáncer colorrectal Actualización 2018. Gastroenterología y Hepatología 2018;41:585–96. [DOI] [PubMed] [Google Scholar]
- 6.Burke CA, Dekker E, Lynch P, et al. Eflornithine plus sulindac for prevention of progression in familial adenomatous polyposis. N Engl J Med 2020;383:1028–39. 10.1056/NEJMoa1916063 [DOI] [PubMed] [Google Scholar]
- 7.Heinrich MC, Griffith DJ, Druker BJ, et al. Inhibition of c-kit receptor tyrosine kinase activity by STI 571, a selective tyrosine kinase inhibitor. Blood 2000;96:925–32. 10.1182/blood.V96.3.925 [DOI] [PubMed] [Google Scholar]
- 8.Mughal TI, Schrieber A. Principal long-term adverse effects of imatinib in patients with chronic myeloid leukemia in chronic phase. Biologics 2010;4:315–23. 10.2147/BTT.S5775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kundu P, Genander M, Straat K. An EphB-Abl signaling pathway is associated with intestinal tumor initiation and growth. Sci Transl Med 2015;281:ra44. [DOI] [PubMed] [Google Scholar]
- 10.Batlle E, Henderson JT, Beghtel H, et al. Beta-Catenin and TCF mediate cell positioning in the intestinal epithelium by controlling the expression of EphB/ephrinB. Cell 2002;111:251–63. 10.1016/S0092-8674(02)01015-2 [DOI] [PubMed] [Google Scholar]
- 11.Genander M, Halford MM, Xu N-J, et al. Dissociation of EphB2 signaling pathways mediating progenitor cell proliferation and tumor suppression. Cell 2009;139:679–92. 10.1016/j.cell.2009.08.048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Genander M, Frisén J. Eph receptors tangled up in two: independent control of cell positioning and proliferation. Cell Cycle 2010;9:1865–6. 10.4161/cc.9.10.11677 [DOI] [PubMed] [Google Scholar]
- 13.Itsukuma T, Ishikawa H, Misawa M, et al. Familial adenomatous polyposis complicated by chronic myelogenous leukemia: response to imatinib mesylate. J Gastroenterol 2007;42:402–5. 10.1007/s00535-007-2009-0 [DOI] [PubMed] [Google Scholar]
- 14.Batlle E, Bacani J, Begthel H, et al. EphB receptor activity suppresses colorectal cancer progression. Nature 2005;435:1126–30. 10.1038/nature03626 [DOI] [PubMed] [Google Scholar]
- 15.Kalmanti L, Saussele S, Lauseker M, et al. Safety and efficacy of imatinib in CML over a period of 10 years: data from the randomized CML-study IV. Leukemia 2015;29:1123–32. 10.1038/leu.2015.36 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data relevant to the study are included in the article or uploaded as supplementary information and aditional data is available upon request.