Abstract
Background
Continuous remote monitoring of vital signs on the hospital ward gained popularity during the Severe Acute Respiratory Syndrome coronavirus 2 pandemic due to its ability to support early detection of respiratory failure, and the possibility to do so without physical contact between patient and clinician. The effect of continuous monitoring on patient room visits has not been established yet.
Objectives
To assess the impact of continuous monitoring on the number of patient room visits for patients suspected of Corona Virus Disease 2019 (COVID-19) and the use of personal protection equipment.
Design and methods
We performed a before-after study at a ward with private rooms for patients suspected of COVID-19 at a tertiary hospital in Nijmegen, The Netherlands. Non-participant observers observed hospital staff during day, evening and night shifts to record patient room visits and personal protection equipment usage. After eleven days, wearable continuous vital sign monitoring was introduced. An interrupted time series analysis was applied to evaluate the effect of continuous monitoring on the number of patient room visits, visits for obtaining vital signs (Modified Early Warning Score visits) and the amount of personal protection equipment used.
Results
During the 45 day study period, 86 shifts were observed. During each shift, approximately six rooms were included. A total of 2347 patient room visits were recorded. The slope coefficient for the number of patient room visits did not change after introducing continuous vital sign monitoring (B -0.003, 95% confidence interval -0.022/0.016). The slope coefficients of the number of Modified Early Warning Score visits and the amount of personal protection equipment used did not change either (B -0.002, 95% confidence interval -0.021/0.017 and B 0.046, 95% confidence interval -0.008/0.099). The number of Modified Early Warning Score visits did show a decline over the entire study period, however this decline was not influenced by the intervention. Evening and night shifts were associated with fewer patient room visits compared to day shifts.
Conclusion
Introduction of continuous vital sign monitoring at a general ward for patients with suspected COVID-19 did not reduce the number of patient room visits or the usage of personal protection equipment by hospital staff. The number of Modified Early Warning Score visits declined over time, but this was not related to the introduction of continuous monitoring. Detailed analysis of the influence of continuous monitoring on the workflow of hospital staff reveals key points to increase efficacy of this intervention.
Keywords: Nurse-patient relations, Patient isolation, Patient monitoring, Vital signs, Workflow
What is already known about the topic?
-
•
Continuous remote monitoring of vital signs has the potential to support early detection of deterioration of patients on the general ward.
-
•
Continuous remote monitoring has been installed with urgency in many hospitals during the SARS-CoV-2 pandemic since it supports close monitoring of patients in isolation for infection control.
-
•
The effect of continuous remote monitoring on the number of contact moments and corresponding use of personal protection equipment has not been established yet.
What this paper adds
-
•
Introduction of a continuous remote vital sign monitoring system at a general ward with patients suspected of a novel infectious disease, isolated in private rooms, does not necessarily reduce the number of room visits and corresponding usage of personal protective equipment.
-
•
The number of visits primarily for obtaining vital signs decreased during the first period of the SARS-CoV-2 pandemic, but this could not be explained by the introduction of the continuous monitoring system.
-
•
By studying the changes in health care professionals’ workflow related to introduction of a continuous vital sign monitoring system, the efficacy of this system in the management of isolated patients can be better understood and improved.
1. Introduction
The 2020 Severe Acute Respiratory Syndrome coronavirus 2 (SARS-CoV-2) pandemic has posed major challenges for health care centers worldwide. This virus causes a systemic disease known as Corona Virus Disease 2019 (COVID-19) with predominant signs and symptoms of upper and lower airway infection (Lechien et al., 2019). Approximately 80% of patients have only mild disease, however 20% require hospital admission for supportive care and approximately 5% require intensive care unit admission (Cevik et al., 2020). With currently over 21 million cases reported globally, this pandemic puts a significant strain on hospital organizations, medical professionals and health care supplies (WHO 2020).
SARS-CoV-2 is a novel coronavirus related to Severe Acute Respiratory Syndrome coronavirus (SARS) (Zhu et al., 2020) and is believed to have a similar mechanism of transmission including contact, droplet and possibly airborne transmission (Wax and Christian, 2020). To limit transmission, patients with COVID-19 are isolated and being cared for using personal protection equipment. The type of personal protection equipment used differs according to the expected size and number of droplets or airborne particles emitted during a contact moment (Lockhart et al., 2020). However, even with the appropriate personal protection equipment, it is recommended to reduce the time spent in close proximity of a patient with confirmed or suspected infection as much as possible to reduce the total amount of viral exposure and thus the risk of transmission (Lockhart et al., 2020).
Health care professionals, particularly nurses, face a dilemma when caring for patients with COVID-19 on general wards. They want to limit the amount of time spent in close proximity to the patient reducing the risk of disease transmission, but simultaneously want to stay vigilant of sudden hypoxic respiratory failure which is common in COVID-19 (Grasselli et al., 2020, Wang et al., 2020) and might occur without symptoms of dyspnea or tachypnea (Gattinoni et al., 2020; Jouffroy et al., 2020). Contact precautions in general are associated with higher adverse event rates and more delays in care (Morgan et al., 2009), although this association was not confirmed by a recent systematic review on clinical deterioration that focused specifically on patients isolated for infection control (Berry et al., 2020). A second dilemma for health care professionals is the high use of personal protection equipment compared with the imminent shortage of several personal protection equipment components, particularly Filtering Facepiece Particle (FFP) 2 masks. This results in the unique challenge of delivering the best possible care with the least possible number of visits.
A possible solution for close monitoring of patients with COVID-19 with limited patient contact is continuous remote vital sign monitoring. In recent years, continuous vital sign monitoring using wearable devices has gained interest to improve the detection of patient deterioration on the hospital ward (Downey et al., 2018; Cardona-Morrell et al., 2016; Leenen et al., 2020). Because of the possibility of wireless transfer and remote access to real time patient vital sign data, continuous monitoring was considered as a potentially valuable addition to conventional monitoring in the care for patients with COVID-19, both at home and in health care facilities (Michard et al., 2020). The promise of remote monitoring even prompted the Food and Drug Admistration to expand the use of certain wearable devices in order to ease the burden on health care providers (Coronavirus (COVID-19) Update 2021). However, the ability of continuous monitoring systems to support appropriate care with limited patient visits and reduction of personal protection equipment has not yet been established. The aim of this study was to assess the effect of continuous vital sign monitoring during the SARS-CoV-2 pandemic on the number of room visits of patients suspected of COVID-19, the number of visits primarily for obtaining vital signs and the amount of personal protection equipment used. We hypothesize that continuous monitoring will decrease the number of patient room visits, both primarily for obtaining vital signs and in total, and that it will consequently decrease the amount of personal protection equipment used.
2. Methods
2.1. Study design and setting
We performed an observational before-and-after study of the introduction of continuous remote vital sign monitoring at the Radboud University Medical centre, Nijmegen, The Netherlands, a tertiary hospital with a capacity of 1065 beds, lasting March 28 through May 10, 2020 (44 days). The study was conducted at a general ward with private rooms that was designated for patients suspected of COVID-19. The ward consisted of eleven single occupancy rooms (ward floor plan can be found in supplementary materials). Admission of patients was based on clinical presentation and/or CT-scan suspected for COVID-19. After confirmation of the SARS-CoV-2 infection by reverse transcription polymerase chain reaction test, the patient was transferred to a different COVID-19 cohort ward. Patients who tested negative twice and had no specific radiologic findings were transferred to a regular hospital ward and excluded from further evaluation. Between April 7 and April 10, the study ward was gradually equipped with a continuous remote monitoring system. Most staff on the ward had no experience with the continuous monitoring system. Data were prospectively collected before, during and after introduction of the system. Research evaluating continuous monitoring on the general ward was approved when introduced in 2018 in the hospital (Committee on Research Involving Human Subjects 2018–4330). Additional ethical approval for this study was waived by the institutional review board, under Dutch law. Since no personal data or patient data were collected for this study, informed consent was not required under hospital policy and the Dutch law.
2.2. Conventional vital sign measurements
Before introduction of the continuous monitoring system nurses intermittently registered respiratory rate, peripheral oxygen saturation, heart rate, systolic and diastolic blood pressure and core temperature in the electronic medical record. Measurements were obtained using an automated blood pressure measuring device and pulse oximeter (Dinamap, GE Healthcare, Germany), and a tympanic thermometer (Genius 3, Medtronic, United States of America). In addition, supplemental oxygen delivery (L/min) and consciousness using a modified AVPU score (Alert, Delirious, Voice, Pain or Unresponsive) were noted and the Modified Early Warning Score was calculated by the computer, according to the hospital protocol (see Table 1 ) (Eddahchouri et al., 2020). This protocol stipulates a vital sign measurement every 8 h in all patients and every 4 h in patients with mid-range Modified Early Warning Score (3–5) or when caregivers are worried. A high Modified Early Warning Score (6 or higher), requires hourly measurements and consultation of the ward physician or consideration of rapid response team involvement.
Table 1.
Weighted contribution of vital parameters of the Modified Early Warning Score.
| Score points | 3 | 2 | 1 | 0 | 1 | 2 | 3 |
|---|---|---|---|---|---|---|---|
| Oxygen delivery, L/min | None | < 5 | ≥ 5 | ||||
| Oxygen saturation,% | ≤ 91 | 92–93 | 94–95 | ≥ 96 | |||
| Respiratory rate, /min | ≤ 8 | 9–11 | 12–20 | 21–24 | ≥ 25 | ||
| Heart rate, /min | ≤ 40 | 41–50 | 51–90 | 91–110 | 111–130 | ≥ 131 | |
| Systolic blood pressure, mmHg | ≤ 90 | 91–100 | 101–110 | 111–219 | ≥ 220 | ||
| Consciousness | A | D | V/P/U | ||||
| Core temperature, Celsius | ≤ 35.0 | 35.1–36.0 | 36.1–38.0 | 38.1–39.0 | ≥ 39.1 |
A: alert, D: delirious, V: verbal, P: pain, U: unresponsive.
2.3. Continuous vital sign monitoring system
Continuous vital sign monitoring was performed using VisiMobileⓇ (Sotera Wireless, San Diego, California, USA). This wrist-worn device continuously measures respiratory rate, peripheral oxygen saturation, heart rate, blood pressure and skin temperature. Once every minute all vital signs, except skin temperature, were sent automatically to the electronic medical record and available to nurses for periodic validation and storage. A manually obtained temperature, the amount of supplemental oxygen delivery and the score for consciousness were added to allow the computer to calculate a Modified Early Warning Score. Real-time measurements were visible on monitoring screens at the nurse stations with the possibility to view vital sign data and trends of the preceding 96 h. If a vital parameter exceeded preset thresholds, the system provided a single channel alarm on the monitor. All nurses at the study ward followed a learning module before the system was implemented. The first part consisted of a custom made e-learning covering the technical and functional aspects of the system. The second part was a two hour practical skills training given by a continuous monitoring ‘nurse super user’. This training included connecting the wrist device, cables and patches, blood pressure calibration, checking the appropriate displaying of vital signs on the device and monitor, and authorization of vital signs in the electronic health record. Finally, nurses practiced the entire procedure with a patient while observed and debriefed by experienced colleagues, until they were able to perform the procedure sufficiently. A day and night medical and IT help and service desk was available during the study.
2.4. Data collection
Four medical students prospectively collected the data about the number of room visits and the use of personal protection equipment by observing hospital staff during their shift at the study ward. Only hospital staff was observed, including included physicians, nurses and supportive personnel (e.g. cleaning staff and transportation). Visits by family were not recorded. All students have had basic training in conducting clinical research and were familiar with care processes at a general ward from several clinical clerkships. They were briefed about the study protocol and the case record form by the researchers before the start of the study. The students could use the same help desk as the nurses regarding questions and problems during the study. Eight hour day, night or evening shifts were covered, based on the availability of the students. Every shift, one student would function as a non-participating observer. This student randomly selected three to four nurses for observation. Together, these nurses took care of approximately six patients who were admitted to patients rooms located at one hallway (see supplement 1, Floor plan of the study ward). Students approached the nurses at the beginning of their shift asking to inform them each time they planned entering a room. Because of their strategic position in the middle of the hallway they were able to control for any unannounced visit by any personnel. Data were collected per observed patient room, defined as ‘an individual patient room that is observed during one shift’. When using a complete set of one mask, one apron and one pair of gloves this was registered as one patient room visit. Before each visit, the medical student asked the staff member the question: “Would you still have entered the patient room if this was not for the Modified Early Warning Score?” If the answer was yes, the visit was counted in the total number of visits. If the answer was no, the visit was counted in the total number of visits and as separate ‘Visit due to Modified Early Warning Score’ (MEWS visit). The total amount of used personal protection equipment by all hospital staff members was noted per patient room and during each shift. No personal data of the patients or hospital staff was recorded. Data were collected on a paper-based case report form and entered into the database by the medical student at the end of each shift. The form included an instruction part and was piloted by the four students and researcher (YE) after which minor text were changed. The database was checked for inaccurate entries before analysis (HMRvG).
2.5. Study endpoints
The primary endpoint of this study was the total number of room visits. Secondary endpoints were the total amount of personal protection equipment used and the number of room visits for obtaining vital signs (MEWS visits).
2.6. Statistical analysis
Variables were checked for normality using the Shapiro-Wilk test. In descriptive statistics, the nonparametric variable ‘Visits per room per shift’ is expressed as median with interquartile ranges (IQR). Discrete variables (e.g. number of individual patients, number of shifts) are expressed as numbers with percentages. Multiple imputation was used to correct for missing values in the variable ‘patients per nurse’. An interrupted time series analysis with a negative binomial regression model was performed to analyze the association between continuous monitoring and the number of patient room visits over time (Kontopantelis et al., 2015). A slope change was chosen based on the expected effect of the intervention; a gradual decline in patient room visits (Bernal et al., 2017). The slope after was derived from the slope before and the slope change, in accordance with the primary model. More information on the statistical model formula of interrupted time series analyses can be found in the article by Bernal et al. (2017). A generalized estimating equations analysis with exchangeable correlation structure was used to correct for repeated measurements within the same patient. The before period was defined as all observed patient rooms before introduction of the continuous monitoring system. The intervention period was defined as all observed patient rooms after April 7th, the day the intervention was introduced. The analysis was also performed using a model that did not include the transitional period; this did not improve the fit of the model. Due to the number of time points (97), the study had enough power to detect an estimated effect size of a minimum of 34% reduction (Hawley et al., 2019). The outcome is expressed as the degree of change per time point (beta coefficient, B), with a confidence interval (CI) and p-value. Residual plots were used to check the fit of the model. Data packages Microsoft Excel 2016 and SPSS version 25.0.0.2 were used for analysis. A two-sided alpha of 0.05 was considered statistically significant.
3. Results
Table 2 offers an overview of the observed shifts. A total of 519 patient rooms were observed during 86 shifts. Eleven of the shifts were studied during the transition period of four days and therefore included both intermittent and continuous monitored patients. Three patients switched from intermittent to continuous monitoring during the introduction of the monitoring system. Two patients received continuous monitoring but were switched to intermittent monitoring; the reasons for switching were not recorded. After implementation of the continuous monitoring system, the nurse-to-patient ratio was lower, with 70.6% of the nurses caring for one or two patients compared to 55.2% during the intermittent monitoring period. The distribution of shift types before and after implementation was comparable.
Table 2.
Description of observed shifts.
| Total | Intermittent monitoring | Continuous monitoring | |
|---|---|---|---|
| Number of individual patients* | 209 | 93 | 121 |
| Number of shifts | 86 | 32 | 65 |
| -Day | 34 (39.5%) | 12 (37.5%) | 26 (40.0%) |
| -Evening | 36 (41.9%) | 14 (43.8%) | 27 (41.5%) |
| -Night | 16 (18.6%) | 6 (18.8%) | 12 (18.5%) |
| Number of observed patient rooms | 519 | 172 | 347 |
| Nurse-to-patient ratio total | |||
| −1:1 | 167 (32.2%) | 27 (15.7%) | 140 (40.3%) |
| −1:2 | 173 (33.3%) | 68 (39.5%) | 105 (30.3%) |
| −1:3 | 30 (5.8%) | 23 (13.4%) | 7 (2.0%) |
| −1:4 | 4 (0.8%) | 4 (2.3%) | 0 (0.0%) |
| -unknown | 145 (27.9%) | 50 (29.1%) | 95 (27.4%) |
Five patients were counted in the intermittent and continuous monitoring period at different time point.
As shown in the descriptive results in Table 3 , a total of 2347 patient room visits were registered during the study period, with a median of 4 (IQR 2–5.5) visits per room per shift during the intermittent monitoring period compared to a median of 5 (IQR 3–6) visits during the continuous monitoring period. This rise in room visits after introduction of the monitoring system was most evident during the day shifts. Of the 2347 room visits, 213 (9.1%) were visits for obtaining vital signs to calculate a Modified Early Warning Score (MEWS visits). This percentage decreased from 14.1% before to 6.9% after introduction of continuous monitoring. The decrease in MEWS visits was evident during all shifts. The median number of used personal protection equipment per room per shift increased from 15 (IQR 8.5–22) to 19 (IQR 12–25).
Table 3.
Descriptive results of number of room visits, number of MEWS visits and amount of PPE used.
| Total | Intermittent monitoring | Continuous monitoring | |
|---|---|---|---|
| Room visits | |||
| Total | |||
| • Total | 2347 | 711 | 1636 |
| • Per patient room* | 4 (2–6) | 4 (2–5.5) | 5 (3–6) |
| • MEWS visits | 213 (9.1%) | 100 (14.1%) | 113 (6.9%) |
| Day visits | |||
| • Total | 1188 | 328 | 860 |
| • Per patient room* | 6 (5–8) | 6 (4–8) | 7 (5–8) |
| • MEWS visits | 96 (8.1%) | 39 (11.9%) | 57 (6.6%) |
| Evening visits | |||
| • Total | 802 | 258 | 544 |
| • Per patient room* | 3 (2–5) | 3 (2–4) | 3 (2–5) |
| • MEWS visits | 80 (10.0%) | 42 (16.3%) | 38 (7.0%) |
| Night visits | |||
| • Total | 357 | 125 | 232 |
| • Per patient room* | 2.5 (2–4) | 3 (2–4) | 2 (2–4) |
| • MEWS visits | 37 (10.4%) | 19 (15.2%) | 18 (7.8%) |
| Used personal protection equipment | |||
| • Total | 9441 | 2870 | 6571 |
| • Per patient room* | 16 (10–24) | 15 (8.5–22) | 19 (12–25) |
| • MEWS visits | 647 (6.9%) | 306 (10.7%) | 341 (5.2%) |
Median (interquartile range).
PPE: personal protection equipment. MEWS visit: a visit primarily for obtaining vital signs to be able to calculate the Modified Early Warning Score.
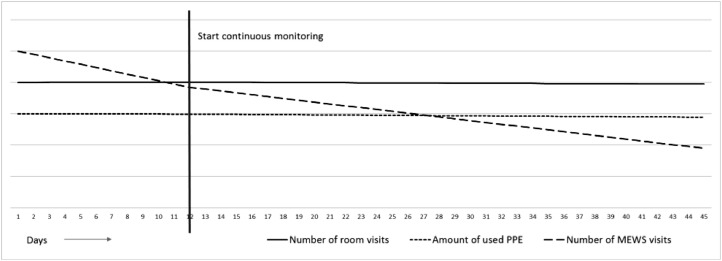
Although a difference in number of room visits before and after intervention was observed in the descriptive results, the interrupted time series analysis (Table 4 ) did not show an association between continuous vital sign monitoring and the total number of room visits, as demonstrated by an insignificant slope change of −0.003 (95% CI −0.022–0.016, p-value 0.761). Correspondingly, the number of used personal protection equipment was not affected (B −0.002 (95% CI −0.021–0.017, p-value 0.835)). Both outcomes, however, were influenced by the type of shift; evening and night shifts were associated with a reduction in the number of room visits and personal protection equipment usage, in contrast to day shifts. For the number of MEWS visits, a significant decline over time was found, both before and after the intervention. However, continuous monitoring did not impact this decline (B 0.046 (95% CI −0.008–0.099, p-value 0.097)) and even slightly increased the number of MEWS visits (B 0.614 (95% CI 0.000–1.228, p-value 0.050)). The evening was the only shift type that had a significant association with the number of MEWS visits (B-0.427 (95% CI −0.760- −0.093, p-value 0.012)). A graphic depicture of the slope changes over time is shown in Fig. 1 . Residual plots showed a proper fit of the model.
Table 4.
Interrupted time series analysis.
| Total number of room visits |
Total used PPE |
Total number of MEWS visits |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| B | 95% CI | p-value | B | 95% CI | p-value | B | 95%CI | p-value | |
| Day shift* | Reference | ||||||||
| Evening shift | −0.712 | −0.879 / −0.545 | 0.000 | −0.538 | −0.645 / −0.431 | 0.000 | −0.427 | −0.760 / −0.093 | 0.012 |
| Night shift | −0.536 | −0.640 / −0.432 | 0.000 | −0.722 | −0.892 / −0.552 | 0.000 | −0.385 | −0.944 / 0.174 | 0.177 |
| Nurse-to-patient ratio | −0.048 | −0.136 / 0.040 | 0.260 | −0.053 | −0.145 / 0.039 | 0.235 | −0.104 | −0.330 / 0.122 | 0.366 |
| Intermittent monitoring* | Reference | ||||||||
| Continuous monitoring | 0.105 | −0.065 / 0.275 | 0.226 | 0.112 | −0.059 / 0.282 | 0.200 | 0.614 | 0.000 / 1.228 | 0.050 |
| Slope before | 0.001 | −0.17 / 0.018 | 0.949 | −0.001 | −0.018 / 0.016 | 0.915 | −0.105 | −0.154 / −0.057 | 0.000 |
| Slope change | −0.003 | −0.022 / 0.016 | 0.761 | −0.002 | −0.021 / 0.017 | 0.835 | 0.046 | −0.008 / 0.099 | 0.097 |
| Slope after | −0.002 | −0.010 / 0.005 | 0.521 | −0.003 | −0.010 / 0.004 | 0.435 | −0.059 | −0.088 / −0.031 | 0.000 |
Day shift and Intermittent monitoring were used as reference values in the model.
PPE personal protection equipment; MEWS Modified Early Warning Score; CI confidence interval, B beta-coefficient (the degree of change per time point).
Fig. 1.
Graphic depicture of the interrupted time series analysis.
PPE personal protection equipment; MEWS Modified Early Warning Score.
4. Discussion
Although remote monitoring systems are claimed to reduce patient room visits by nurses with 30–50% (Kuraitis, 2007), no scientific evidence is supporting these claims so far. In contrast with our hypothesis, this study demonstrates no impact of continuous monitoring on the number of patient room visits or personal protection equipment usage when corrected for shift type, which does influence patient room visits. Even though the number of MEWS visits decreased over time, this was not the result of continuous vital sign monitoring.
Studies on the number of patient room visits are rare, however in a study by Cohen et al. in 2012, conducted on several wards of academic hospitals, the number of patient room visits was a tenfold higher compared to our study (Cohen et al., 2012). Isolated patients received a median of 5 visits per hour in their study (Cohen et al., 2012) but only a median of 4 visits per eight hours in our study. Differences in defining room visits and case-mix might explain the higher numbers. We did not include visits by relatives and other personal visitors, which was about one-quarter of the total number of visits in the study by Cohen et al. (2012). Notably, hospital policy restricted visits by relatives to one per day at our study ward. Only the day shifts were studied by Cohen et al., which was the shift with the highest number of room visits in our study. Although they did look at differences between intensive care units and general wards, and isolated patients and non-isolated patients, Cohen et al. did not report on numbers on isolated patients on the general ward specifically, the kind of patients that were included in our study.
According to previous research, assessment of patients and obtaining vital signs takes up only seven percent of nursing practice time (Hendrich, 2008), but is the reason for 49% of nurse-patient interactions (Cardona-Morrell et al., 2015). We expected a high number of MEWS visits for patients with COVID-19 signs and symptoms due to the severity and erratic course of the disease, and given that all patients were in private rooms; multi-patient rooms make it possible to combine taking vital signs of several patients in one visit. Moreover, we expected a decrease in both MEWS visits and room visits after introduction of continuous vital sign monitoring. Although the percentage of MEWS visits was halved, this had no effect on the total number of patient room visits; this number even increased slightly. Most likely, vital sign measurements were not only taken during MEWS visits, but also during visits with a different primary purpose, such as providing medication or assisting in personal hygiene. These vital sign measurements were not captured in this study. The ability of nurses to combine multiple tasks in one visit, thus effectively limiting the number of patient room visits themselves, has probably affected the impact of continuous monitoring. Conversely, rooms may have been entered due to deviant vital signs on the nurse monitors that needed to be checked at the bedside, which would lead to an increase rather than a decrease in MEWS visits. An increase in MEWS visits as a results of increased alertness by nurses, triggered by rapidly changing vital signs of these patients seen on the remote monitors, could be considered a benefit of remote continuous monitoring.
The continuous decline of the number of MEWS visits might be explained by two learning curves: nurses learning to use the continuous monitoring system, and all clinicians learning to manage a new infectious disease, COVID-19. Deploying a continuous monitoring system on the ward requires skills training and frequent practice until proficiency. Previous research shows that nurses perceived a higher value of continuous monitoring in their decision making if they are trained and confident in its use (Langhorne, 2010, Jeskey et al., 2011). Also, technical issues and false alarms are likely to decline and are solved during a planned room visits when nurses become more acquainted with the monitoring system. As the pandemic progressed, more information became available about the disease manifestations, and clinicians gained more experience with both patients with COVID-19 and continuous vital sign data. This resulted in more confidence interpreting changes in vital signs and decisions whether or not to (de)escalate care, supported by continuous data. Altogether this may have affected both MEWS visits and total number of patient room visits in either direction.
Although a decrease in patient room visits is desirable for limiting disease transmission and personal protection equipment usage, it can amplify patients’ feelings of social isolation. The number of patient room visits is reportedly lower in isolated non-COVID-19 patients (Morgan et al., 2009). High rates of depression and anxiety are found associated with private room isolation (Morgan et al., 2009; Abad et al., 2010). Remote vital sign monitoring system may further deprive social contact, an important concern raised by patients and clinicians previously (Prgomet et al., 2016; Weenk et al., 2019). Obviously, feelings of social deprivation are dependent not only on nurse-patient contacts but also contact with relatives and other patients, which were very limited for most patients suspected of COVID-19.
4.1. Limitations
The most prominent limitation of this study is the before-after design in a highly dynamic hospital environment with an unknown disease in the beginning of this pandemic. Although the hospital ward population was uniform, all patients suspected of COVID-19, the workflow and protocols on the ward were affected by the increasing knowledge on COVID-19 and the rapidly changing (inter)national guidelines. The used statistical strategy partly compensated for this, however we could only compensate for known confounders. Designs without this limitation, such as randomized controlled trials, were deemed inappropriate and have their own ethical and methodological drawbacks. Even though the hospital ward population was uniform based on disease type, the individual patients might have differed in various ways based on factors such as comorbidities and disease severity. Factors of hospital staff, such as experience, might have been of influence too. These factors were not taken into account in our study. Unexplained observations, such as the difference in nurse-to-patients before and after implementation, might also be explained by these factors. In observational studies of behavior there is always a risk of the Hawthorne effect. Hospital staff might have behaved differently because they know they are being observed. However, we do not think this will have influenced the outcomes of our study since this effect will have been similar in both the before and after period. Besides, hospital staff was already very aware they had to limit the number of patient room visits since supplies were limited. Because of the strict isolation measures, the observers were not able to follow hospital staff into the patient rooms and register their exact activities. Although the number of visits was not altered by the continuous monitoring, the timing and purpose of the visit might have been more tailored to the patient's need. This study did not have enough data to analyze day, evening and night shifts separately. During night shifts, nurses are low in staff (De Cordova et al., 2014) and are less inclined to disturb patients for measuring vital signs (Weenk et al., 2019). Continuous monitoring might have a greater effect on the number of room visits during the night, however this assumption need to be confirmed in future research. Another limitation is the type of monitoring system used. The VisiMobileⓇ is a fairly complete monitor; however, for measuring the core temperature and completing the Modified Early Warning Score the nurse still had to enter the patient's room. No wearable so far is able to measure all components of the Modified Early Warning Score (Leenen et al., 2020, Joshi et al., 2019), as core temperature and consciousness are technically challenging to monitor continuously by one wearable device, and more importantly as nurses’ worry cannot be measured without nurse-patient interaction (Douw et al., 2015).
4.2. Future research
During the study, the course and management of COVID-19 was roughly unknown for nurses and physicians. Repeating the study in a more stable situation, after formally training all hospital staff, would be of interest during the current and following outbreaks of COVID-19 and could also improve the generalizability of the study to other patients in isolation. In addition, the number of outcomes could be extended. Except for alarm fatigue (Hravnak et al., 2018) few aspects of the way workflow is influenced by continuous monitoring have been studied so far. A more advanced approach that may optimize the effects of continuous vital sign monitoring for patients in isolation is integrating this technology with an audio-video system, to enable nurses to acquire situational awareness. This way the urgency to enter the room can better be determined and patient can be remotely advised and instructed. Such technology is currently less common at a hospital ward compared to an intensive care unit or a nursing home, but bears the opportunity to optimize continuous monitoring by pervasive sensing and deep learning (Davoudi et al., 2019). Lastly, including the influence of multiple patient factors, such as comorbidities or functional status, and clinician related factors, such as experience or age, might improve our understanding of this complex intervention even more.
4.3. Conclusion
We conclude that introduction of a continuous remote vital sign monitoring system using a wearable at a ward for patients with suspected COVID-19 during the SARS-CoV-2 pandemic did not reduce the number of patient room visits or the usage of personal protection equipment by hospital staff. The number of visits for obtaining vital signs did decrease, probably due to altered nurses’ workflow, more frequent observation of vital signs derangements and increased experience with disease management. Further quantitative and qualitative analyses of the influence of continuous monitoring on the workflow of hospital staff will improve our understanding of this novel intervention, and could reveal key points to increase its efficacy.
CRediT authorship contribution statement
H.M.R. van Goor: Conceptualization, Methodology, Data curation, Validation, Formal analysis, Writing - original draft, Visualization. Y. Eddahchouri: Conceptualization, Methodology, Investigation, Data curation, Writing - original draft. K. van Loon: Conceptualization, Writing - review & editing. S.J.H. Bredie: Resources, Supervision. L. Schoonhoven: Writing - review & editing. H.A.H. Kaasjager: Conceptualization, Supervision, Writing - review & editing. H. van Goor: Conceptualization, Methodology, Supervision, Writing - review & editing.
Acknowledgments
Acknowledgments
We thank all ward nurses and medical students that were involved for their time. Our gratitude goes also to Dr. Henri van Werkhoven and Dr. Sonja van Roeden, both associated with the University Medical Center Utrecht, for their assistance in statistical analysis.
Funding
No external funding.
Declaration of Competing Interest
None.
Data statement
The datasets generated and/or analyzed during the present study are not publicly available, but they are available from the corresponding author on reasonable request.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.ijnurstu.2020.103868.
Appendix. Supplementary materials
References
- Abad C., Fearday A., Safdar N. Adverse effects of isolation in hospitalised patients: a systematic review. J. Hosp. Infect. 2010 doi: 10.1016/j.jhin.2010.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernal J.L., Cummins S., Gasparrini A. Interrupted time series regression for the evaluation of public health interventions: a tutorial. Int. J. Epidemiol. 2017 doi: 10.1093/ije/dyw098. Published Online First. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry D., Wakefield E., Street M., et al. Clinical deterioration and hospital-acquired complications in adult patients with isolation precautions for infection control: a systematic review. J. Adv. Nurs. 2020 doi: 10.1111/jan.14435. [DOI] [PubMed] [Google Scholar]
- Cardona-Morrell M., Prgomet M., Lake R., et al. Vital signs monitoring and nurse–patient interaction: a qualitative observational study of hospital practice. Int. J. Nurs. Stud. 2015 doi: 10.1016/j.ijnurstu.2015.12.007. Published Online First. [DOI] [PubMed] [Google Scholar]
- Cardona-Morrell M., Prgomet M., Turner R.M., et al. Effectiveness of continuous or intermittent vital signs monitoring in preventing adverse events on general wards: a systematic review and meta-analysis. Int. J. Clin. Pract. 2016;70:806–824. doi: 10.1111/ijcp.12846. [DOI] [PubMed] [Google Scholar]
- Cevik M., Bamford C.G.G., Ho A. COVID-19 pandemic—a focused review for clinicians. Clin. Microbiol. Infect. 2020 doi: 10.1016/j.cmi.2020.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen B., Hyman S., Rosenberg L., et al. Frequency of patient contact with health care personnel and visitors: implications for infection prevention. Jt. Comm. J. Qual. Patient Saf. 2012 doi: 10.1016/S1553-7250(12)38073-2. Published Online First. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coronavirus (COVID-19) Update: FDA Allows Expanded Use of Devices to Monitor patients’ Vital Signs Remotely | FDA. https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-allows-expanded-use-devices-monitor-patients-vital-signs-remotely.
- Davoudi N., Deán-Ben X.L., Razansky D. Deep learning optoacoustic tomography with sparse data. Nat. Mach. Intell. 2019 doi: 10.1038/s42256-019-0095-3. Published Online First. [DOI] [Google Scholar]
- De Cordova P.B., Phibbs C.S., Schmitt S.K., et al. Night and day in the VA: associations between night shift staffing, nurse workforce characteristics, and length of stay. Res. Nurs. Health. 2014 doi: 10.1002/nur.21582. Published Online First. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douw G., Schoonhoven L., Holwerda T., et al. Nurses’ worry or concern and early recognition of deteriorating patients on general wards in acute care hospitals: a systematic review. Crit. Care. 2015 doi: 10.1186/s13054-015-0950-5. Published Online First. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downey C.L., Chapman S., Randell R., et al. The impact of continuous versus intermittent vital signs monitoring in hospitals: a systematic review and narrative synthesis. Int. J. Nurs. Stud. 2018;84:19–27. doi: 10.1016/j.ijnurstu.2018.04.013. [DOI] [PubMed] [Google Scholar]
- Eddahchouri Y., Koeneman M., Plokker M., Brouwer E., van de Belt T.H., van Goor H.B.S. Low compliance to a vital sign safety protocol on general hospital wards: a retrospective cohort study. Int. J. Nurs. Stud. 2020 doi: 10.1016/j.ijnurstu.2020.103849. Published Online First. [DOI] [PubMed] [Google Scholar]
- Gattinoni L., Chiumello D., Caironi P., et al. COVID-19 pneumonia: different respiratory treatments for different phenotypes? Intensive Care Med. 2020 doi: 10.1007/s00134-020-06033-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grasselli G., Pesenti A., Grasselli C.M. Baseline Characteristics and Outcomes of 1591 Patients Infected With SARS-CoV-2 Admitted to ICUs of the Lombardy Region, Italy. JAMA J. Am. Med. Assoc. 2020 doi: 10.1001/jama.2020.4031. March. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawley S., Ali M.S., Berencsi K., et al. Sample size and power considerations for ordinary least squares interrupted time series analysis: a simulation study. Clin. Epidemiol. 2019 doi: 10.2147/CLEP.S176723. Published Online First. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendrich A. A 36-hospital time and motion study: how do medical-surgical nurses spend their time? Perm J. 2008 doi: 10.7812/tpp/08-021. Published Online First. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hravnak M., Pellathy T., Chen L., et al. A call to alarms: current state and future directions in the battle against alarm fatigue. J. Electrocardiol. 2018 doi: 10.1016/j.jelectrocard.2018.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeskey M., Card E., Nelson D., et al. Nurse adoption of continuous patient monitoring on acute post-surgical units: managing technology implementation. J. Nurs. Manag. 2011 doi: 10.1111/j.1365-2834.2011.01295.x. Published Online First. [DOI] [PubMed] [Google Scholar]
- Joshi M., Ashrafian H., Aufegger L., et al. Wearable sensors to improve detection of patient deterioration. Expert Rev. Med. Devices. 2019;16:145–154. doi: 10.1080/17434440.2019.1563480. [DOI] [PubMed] [Google Scholar]
- Jouffroy R., Jost D., Prunet B. Prehospital pulse oximetry: a red flag for early detection of silent hypoxemia in COVID-19 patients. Crit. Care. 2020 doi: 10.1186/s13054-020-03036-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kontopantelis E., Doran T., Springate D.A., et al. Regression based quasi-experimental approach when randomisation is not an option: interrupted time series analysis. BMJ. 2015 doi: 10.1136/bmj.h2750. Published Online First. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuraitis V. 2007. Five Lingering Questions Holding Back Remote Patient Monitoring (RPM) Adoption.http://e-caremanagement.com/five-lingering-questions-holding-back-remote-patient-monitoring-rpm-adoption/ [Google Scholar]
- Langhorne P. Continuous automated physiological monitoring in acute stroke: a meta-analysis of randomised controlled trials. Cerebrovasc. Dis. 2010 [Google Scholar]
- Lechien J.R., Chiesa-Estomba C.M., Place S., et al. Clinical and epidemiological characteristics of 1420 European patients with mild-to-moderate coronavirus disease. J. Intern. Med. 2019:2020. doi: 10.1111/joim.13089. Published Online First. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leenen J.P.L., Leerentveld C., van Dijk J.D., et al. Current evidence for continuous vital signs monitoring by wearable wireless devices in hospitalized adults: a systematic review (Preprint) J. Med. Internet Res. 2020 doi: 10.2196/18636. Published Online First. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lockhart S.L., Duggan L.V., Wax R.S., et al. Personal protective equipment (PPE) for both anesthesiologists and other airway managers: principles and practice during the COVID-19 pandemic. Can. J. Anesth. Can d'Anesth. 2020 doi: 10.1007/s12630-020-01673-w. Published Online First. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michard F., Saugel B., Vallet B. Rethinking the post-COVID-19 pandemic hospital: more ICU beds or smart monitoring on the wards? Intensive Care Med. 2020:1–2. doi: 10.1007/s00134-020-06163-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan D.J., Diekema D.J., Sepkowitz K., et al. Adverse outcomes associated with contact precautions: a review of the literature. Am. J. Infect. Control. 2009 doi: 10.1016/j.ajic.2008.04.257. Published Online First. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prgomet M., Cardona-Morrell M., Nicholson M., et al. Vital signs monitoring on general wards: clinical staff perceptions of current practices and the planned introduction of continuous monitoring technology. Int. J. Qual. Health Care. 2016 doi: 10.1093/intqhc/mzw062. Published Online First. [DOI] [PubMed] [Google Scholar]
- Wang D., Hu B., Hu C., et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA J. Am. Med. Assoc. 2020 doi: 10.1001/jama.2020.1585. Published Online First. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wax R.S., Christian M.D. Practical recommendations for critical care and anesthesiology teams caring for novel coronavirus (2019-nCoV) patients. Can. J. Anesth. 2020 doi: 10.1007/s12630-020-01591-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weenk M., Bredie S.J., Koeneman M., et al. Continuous monitoring of vital signs at the general ward using wearable devices: a qualitative analysis (Preprint) J. Med. Internet Res. 2019;22:1–11. doi: 10.2196/15471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO . 2020. WHO Situational Report - Weekly Epidemiological Update 1.https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200817-weekly-epi-update-1.pdf?sfvrsn=b6d49a76_4 accessed 18 Aug. [Google Scholar]
- Zhu N., Zhang D., Wang W., et al. A novel coronavirus from patients with pneumonia in China, 2019. N. Engl. J. Med. 2020 doi: 10.1056/NEJMoa2001017. Published Online First. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.