Abstract
RNA endowed with both protein-coding and noncoding functions is referred to as ‘dual-function RNA’, ‘binary functional RNA (bifunctional RNA)’ or ‘cncRNA (coding and noncoding RNA)’. Recently, an increasing number of cncRNAs have been identified, including both translated ncRNAs (ncRNAs with coding functions) and untranslated mRNAs (mRNAs with noncoding functions). However, an appropriate database for storing and organizing cncRNAs is still lacking. Here, we developed cncRNAdb, a manually curated database of experimentally supported cncRNAs, which aims to provide a resource for efficient manipulation, browsing and analysis of cncRNAs. The current version of cncRNAdb documents about 2600 manually curated entries of cncRNA functions with experimental evidence, involving more than 2,000 RNAs (including over 1300 translated ncRNAs and over 600 untranslated mRNAs) across over 20 species. In summary, we believe that cncRNAdb will help elucidate the functions and mechanisms of cncRNAs and develop new prediction methods. The database is available at http://www.rna-society.org/cncrnadb/.
INTRODUCTION
Over the past decades, the ‘central dogma’ of molecular biology has been used to describe messenger RNA (mRNA) as the information-carrying intermediate in protein synthesis (1). Other RNA types, such as transfer RNA (tRNA) and ribosomal RNA (rRNA), are part of the protein-synthesizing machinery as well. However, the rapid development of high-throughput sequencing technologies has revealed the pervasive transcription of eukaryotic genomes (2). Subsequently, various kinds of noncoding RNAs (ncRNAs), such as microRNAs (miRNAs), long noncoding RNAs (lncRNAs) and circular RNAs (circRNAs) were discovered, with functions ranging from epigenetic regulation to transcriptional regulation and signal transduction (3–7). Consequently, the current narrative of RNA involved arbitrarily divided into mRNA and ncRNA according to their protein-coding capacity (8).
However, recent studies have shown that many previously annotated ncRNAs have protein-coding functions (translated ncRNAs) (9,10). For instance, pri-miR171b of Medicago truncatula and pri-miR165a of Arabidopsis thaliana can produce peptides that enhance the accumulation of their corresponding mature miRNAs (11). The lncRNA LINC00961 harbours a peptide named SPAR that can interact with the lysosomal v-ATPase to negatively regulate mTORC1 activation (12). The circRNA circβ-catenin can activate the Wnt pathway through a 370 aa peptide that it encodes (13). The rapid development of ribosome profiling and mass spectrometry has revealed pervasive translation outside of annotated protein-coding genes (14–18), and some databases have recorded many small open reading frames (smORFs) and micropeptides located in noncoding regions (19–23). All these findings indicate that there is a hidden world of small peptides/proteins produced by ncRNAs waiting to be explored (24). On the other hand, some mRNAs have also been proven to harbour noncoding functions independent of the proteins they encode (25). For example, the mRNA of P53 can interact with MDM2, blocking E3 ligase activity and stimulating translation of the P53 mRNA (26,27). The pre-mRNA of H2B can function as a scaffold in the formation of major nuclear bodies (28). Several mRNAs (CNOT6L, SERINC1 and VAPA) have been shown to regulate PTEN by competing for common miRNAs (29). These clues suggest that some mRNAs in certain situations may be able to fulfil regulatory functions similar to those of ncRNAs (30).
Given the above observations, the class of RNAs endowed with both protein-coding and noncoding functions has been referred to as ‘dual-function RNA’ (31–33), ‘binary functional RNA (bifunctional RNA)’ (8,34) or ‘cncRNA (this term was proposed as RNA with both coding and noncoding function in 2015)’ (35,36). In a sense, the discovery of cncRNA blurred the boundary between ‘coding’ and ‘noncoding’ RNAs and led researchers to reconsider the function, evolution and understanding of RNAs (8,25,37,38). Recently, an increasing number of cncRNAs have been identified, including translated ncRNAs and untranslated mRNAs (18,39–41), and some studies began to identify cncRNAs by combining different high-throughput experimental technologies, such as mass spectrometry, ribosome profiling and CRISPR-based screening (18,24,42,43). Here, we developed cncRNAdb (http://www.rna-society.org/cncrnadb/), a manually curated database of experimentally supported cncRNAs, which aims to provide a resource for efficient manipulation, browsing and analysis of cncRNAs. The current version of cncRNAdb documents about 2600 manually curated entries of cncRNA functions with experimental evidence, involving >2000 RNAs (including over 1,300 translated ncRNAs and over 600 untranslated mRNAs) across over 20 species. In summary, cncRNAdb provides a user-friendly interface to query and browse detailed information about these cncRNAs and will be of help in integrating, analysing and predicting cncRNAs, enabling faster structural and functional research of RNA.
DATA COLLECTION AND ORGANIZATION
The cncRNAs in cncRNAdb were curated manually from peer-reviewed literature (before July 2020). First, we retrieved the literature from the PubMed database using the following keywords: ‘cncRNA’, ‘bifunctional RNA’, ‘dual function RNA’, ‘translated ncRNA’, ‘untranslated mRNA’, ‘regulatory mRNA’, ‘scaffold mRNA’, ‘sponge mRNA’, ‘competitive endogenous mRNA’ and ‘ncRNA encode peptide’. Then, all retrieved literature was preliminarily reviewed by expert curators to remove irrelevant articles. Meanwhile, to avoid omitting any new cncRNAs, we further checked all the articles that cited at least one of the remaining relevant articles. Only the experimentally supported data were collected in the cncRNAdb. Besides, cncRNAdb collected 504 translated lncRNAs and 53 translated circRNAs from the SmProt (19) and circRNADb (44) databases, respectively.
In the most up to date version of cncRNAdb, all the cncRNAs were divided into two categories: translated ncRNAs, representing ncRNAs that harbour protein-coding functions, and untranslated mRNAs, representing mRNAs that possess noncoding functions that are independent of the proteins they encode (see Figure 1). Then, the translated ncRNAs was further divided into 6 subcategories: lncRNAs, pri-miRNAs, circRNAs, sRNAs, rRNAs and untranslated regions (UTRs). Meanwhile, after careful consideration common perspectives from multiple review articles (25,30,36,45) and the characteristics of the data, the untranslated mRNAs were further divided into three subcategories: regulatory mRNAs, scaffold mRNAs and sponge mRNAs. Regulatory mRNAs refer to mRNAs that play regulatory roles in various biological processes, such as protein binding, action, inhibition and chromatin remodelling (30,36). Scaffold mRNAs refer to the mRNAs that can serve as a scaffold to help form ribonucleoprotein complexes (30,36,45). Sponge mRNA refers to the mRNAs that can act as a competitive endogenous RNAs by competing with other RNAs (30,36,45). In addition, many studies have found some ncRNAs play new regulatory roles independent of their primary function (such as tRNA) (46). It should be noted that these RNAs were not included in cncRNAdb because of lacking evidence for coding capacity.
Figure 1.
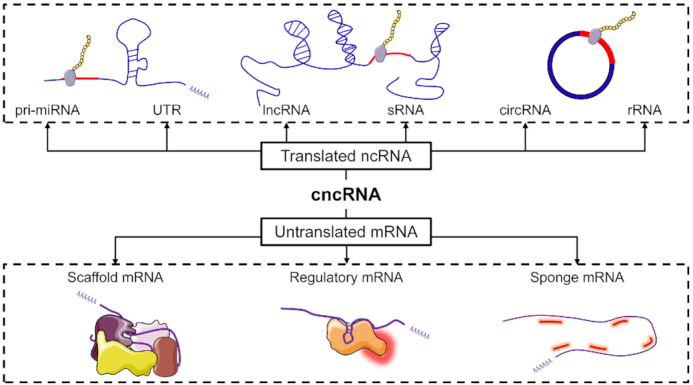
The classification of cncRNAs in cncRNAdb.
To unify the cncRNAs into authoritative reference databases, the translated ncRNAs were mapped to the Ensemble database (Ensemble Gene ID) (47), NCBI gene database (Entrez ID), RNAcentral database (RNAcentral ID) (48) and miRbase (miRbase Accession) (49). The untranslated mRNAs were mapped to the NCBI gene database (Entrez ID). The orthologous genes of cncRNA were obtained from Ensemble database (Ensemble Gene ID) (47).
DATABASE CONTENT
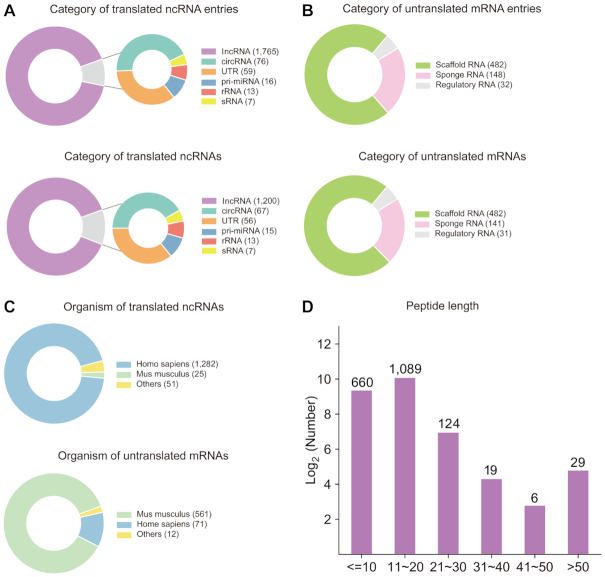
This current version of cncRNAdb documents 2598 manually curated cncRNA-associated function entries with experimental evidence (including 1,936 translated ncRNA entries and 662 untranslated mRNA entries) involving 2002 cncRNAs (including 1358 translated ncRNAs and 644 untranslated mRNAs) across 21 species. The distribution of the translated ncRNA entries and ncRNAs in categories is shown in Figure 2A, over 85% of translated ncRNAs are lncRNAs (1200/1358). In addition, we found 144 previously annotated translated ncRNAs that have been reannotated as protein-coding genes in the NCBI and/or Ensemble databases. The distribution of the untranslated mRNA entries and ncRNAs are shown in Figure 2B, there are 32 regulatory mRNA entries (31 mRNAs), 482 scaffold mRNA entries (482 mRNAs) and 148 sponge mRNA entries (141 mRNAs). The organismal distribution of translated ncRNAs and untranslated mRNAs is shown in Figure 2C, and over 95% of cncRNAs are from humans and mice. Moreover, the length distribution of the ncRNA-coding peptides is shown in Figure 2D, over 90% of the ncRNA-coding peptides contain fewer than 20 aa (1749/1927).
Figure 2.
Data statistics of cncRNAdb. (A) Subcategory distribution of translated ncRNA entries (upper) and ncRNAs (down). (B) Subcategory distribution of untranslated mRNA entries (upper) and mRNAs (down). (C) Organismal distribution of translated ncRNAs (upper) and untranslated mRNAs (down). (D) Sequence length distribution of peptide encoded by the translated ncRNAs.
DATA QUERYING, SEARCHING AND BROWSING
To make it convenient for users to query and browse data, cncRNAdb provided three different search methods on the ‘Search’ page (see Figure 3), including ‘By Keyword search’ (search by inputting keywords of RNA name or disease name, fuzzy search is supported), ‘By Locus search’ (search by inputting a locus of RNA with the associated option of organism) and ‘By Blast search’ (search by inputting an RNA or peptide sequence).
Figure 3.
The ‘Search’ page and ‘Detail’ page of cncRNAdb. cncRNAdb provides three different search methods on the ‘Search’ page, including ‘By Keyword search’, ‘By Locus search’ and ‘By Blast search’. The ‘Detail’ page presents more associated information on the cncRNAs, such as basic cncRNA information, peptide information (only for translated ncRNA), supporting experimental evidence, orthologues of the cncRNA, PubMed ID and description of the reference. Moreover, the disease, subcellular localization and interaction information (from OMIM database (50), MNDR v3.0 (51), RNALocate (52) and RNAInter (53)) of the cncRNAs were also provided.
A brief summary of the search results is presented in a table. Detailed descriptions, such as peptide sequence and description of the reference, are shown in ‘Detail’ page by clicking ‘more’. The ‘Detail’ page presents more information associated with the cncRNAs (see Figure 3), such as basic information for the cncRNA, peptide sequence and length (only for translated ncRNA), supporting experimental evidence, orthologous genes (Human, Chimpanzee, Mouse, Drosophila melanogaster and Zebrafish) of the cncRNA, PubMed ID and description of the reference. Moreover, the disease, subcellular localization and interaction information (from OMIM database (50), MNDR v3.0 (51), RNALocate (52) and RNAInter (53)) of the cncRNAs were also provided.
Furthermore, cncRNAdb provides the ‘Browse’ page to help quickly browse data. Users can browse translated ncRNA and untranslated mRNA in three different ways, ‘Browse by RNA type’, ‘Browse by Organism’ and ‘Browse by Method’. The related cncRNA information is presented by clicking each entry. Moreover, users can also quickly browse cncRNAs by clicking the schematic plots presented on the ‘Home’ page.
DISCUSSION AND FUTURE PROSPECTS
At present, many bifunctional RNAs have been identified by multiple experiments (in vitro and in vivo assay) (12,40). However, there is still a large part of bifunctional RNAs screened by high-through methods need further experimental validation (24). Systematically collecting, organizing, and grading these RNAs by diverse experimental evidence would be valuable for study on bifunctional RNA. Therefore, we developed cncRNAdb, a manually curated database of experimentally supported cncRNAs containing both translated ncRNAs and untranslated mRNAs. The current version of cncRNAdb documents about 2,600 manually curated entries on cncRNA function and provides stratified and detailed experimental evidence. On the one hand, the various unconventional functions of RNAs stored in cncRNAdb might provide new clues for the exploration and functional characterization of RNA. On the other hand, the continued identification and collection of cncRNAs will definitely extend our former understanding of the role of RNAs in cellular processes and organismal evolution, leading us to reconsider the function, evolution and understanding of RNAs (8,25,37,38). In addition, given the growing evidence that ncRNA translation products play important roles in various biological processes related to diseases (34), cncRNAdb might provide a useful resource for the identification and exploration of new disease biomarkers and/or drug targets. In summary, cncRNAdb provides a user-friendly interface for the query and browsing of detailed information about cncRNAs, and will help elucidate their functions and mechanisms of action for the development of new prediction methods. In the future, we will continue to collect cncRNAs from both translated ncRNAs and untranslated mRNAs and update cncRNAdb.
Contributor Information
Yan Huang, Shunde Hospital, Southern Medical University (The First People's Hospital of Shunde Foshan), Foshan 528308, China.
Jing Wang, Department of Bioinformatics, School of Basic Medical Sciences, Southern Medical University, Guangzhou 510515, China.
Yue Zhao, School of Basic Medical Sciences & Forensic Medicine, Hangzhou Medical College, Hangzhou 310053, China.
Huafeng Wang, Shunde Hospital, Southern Medical University (The First People's Hospital of Shunde Foshan), Foshan 528308, China.
Tianyuan Liu, Department of Bioinformatics, School of Basic Medical Sciences, Southern Medical University, Guangzhou 510515, China.
Yuhe Li, Department of Bioinformatics, School of Basic Medical Sciences, Southern Medical University, Guangzhou 510515, China.
Tianyu Cui, Department of Bioinformatics, School of Basic Medical Sciences, Southern Medical University, Guangzhou 510515, China.
Weiyi Li, Department of Bioinformatics, School of Basic Medical Sciences, Southern Medical University, Guangzhou 510515, China.
Yige Feng, Department of Bioinformatics, School of Basic Medical Sciences, Southern Medical University, Guangzhou 510515, China.
Jiaxin Luo, Department of Bioinformatics, School of Basic Medical Sciences, Southern Medical University, Guangzhou 510515, China.
Jiaqi Gong, Department of Bioinformatics, School of Basic Medical Sciences, Southern Medical University, Guangzhou 510515, China.
Lin Ning, Dermatology Hospital, Southern Medical University, Guangzhou 510091, China.
Yong Zhang, Shunde Hospital, Southern Medical University (The First People's Hospital of Shunde Foshan), Foshan 528308, China.
Dong Wang, Shunde Hospital, Southern Medical University (The First People's Hospital of Shunde Foshan), Foshan 528308, China; Department of Bioinformatics, School of Basic Medical Sciences, Southern Medical University, Guangzhou 510515, China; Dermatology Hospital, Southern Medical University, Guangzhou 510091, China.
Yang Zhang, Shunde Hospital, Southern Medical University (The First People's Hospital of Shunde Foshan), Foshan 528308, China.
FUNDING
National Key Research and Development Project of China [2019YFA0801800]; National Natural Science Foundation of China [82070109, 81770104, 62002153]; Basic and Applied Basic Research Fund of Guangdong Province [2019A1515010784, 2019A1515110701]. Funding for open access charge: National Key Research and Development Project of China [2019YFA0801800]; National Natural Science Foundation of China [82070109, 81770104, 62002153]; Basic and Applied Basic Research Fund of Guangdong Province [2019A1515010784, 2019A1515110701].
Conflict of interest statement. None declared.
REFERENCES
- 1. Crick F. Central dogma of molecular biology. Nature. 1970; 227:561–563. [DOI] [PubMed] [Google Scholar]
- 2. Carninci P., Kasukawa T., Katayama S., Gough J., Frith M.C., Maeda N., Oyama R., Ravasi T., Lenhard B., Wells C. et al.. The transcriptional landscape of the mammalian genome. Science. 2005; 309:1559–1563. [DOI] [PubMed] [Google Scholar]
- 3. Dunham I., Kundaje A., Aldred S.F., Collins P.J., Davis C.A., Doyle F., Epstein C.B., Frietze S., Harrow J., Kaul R. et al.. An integrated encyclopedia of DNA elements in the human genome. Nature. 2012; 489:57–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hon C.-C., Ramilowski J.A., Harshbarger J., Bertin N., Rackham O.J.L., Gough J., Denisenko E., Schmeier S., Poulsen T.M., Severin J. et al.. An atlas of human long non-coding RNAs with accurate 5′ ends. Nature. 2017; 543:199–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Zhang Y., Liu T., Chen L., Yang J., Yin J., Zhang Y., Yun Z., Xu H., Ning L., Guo F. et al.. RIscoper: a tool for RNA-RNA interaction extraction from the literature. Bioinformatics. 2019; 35:3199–3202. [DOI] [PubMed] [Google Scholar]
- 6. Li Y., Wang C., Miao Z., Bi X., Wu D., Jin N., Wang L., Wu H., Qian K., Li C. et al.. ViRBase: a resource for virus-host ncRNA-associated interactions. Nucleic Acids Res. 2015; 43:D578–D582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Liu T.Y., Zhang Y.C., Lin Y.Q., Hu Y.F., Zhang Y., Wang D., Wang Y., Ning L.. Exploration of invasive mechanisms via global ncRNA-associated virus-host crosstalk. Genomics. 2020; 112:1643–1650. [DOI] [PubMed] [Google Scholar]
- 8. Hubé F., Francastel C.. Coding and non-coding RNAs, the frontier has never been so blurred. Front. Genet. 2018; 9:140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Andrews S.J., Rothnagel J.A.. Emerging evidence for functional peptides encoded by short open reading frames. Nat. Rev. Genet. 2014; 15:193–204. [DOI] [PubMed] [Google Scholar]
- 10. Choi S.W., Kim H.W., Nam J.W.. The small peptide world in long noncoding RNAs. Brief Bioinform. 2019; 20:1853–1864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lauressergues D., Couzigou J.M., Clemente H.S., Martinez Y., Dunand C., Becard G., Combier J.P.. Primary transcripts of microRNAs encode regulatory peptides. Nature. 2015; 520:90–93. [DOI] [PubMed] [Google Scholar]
- 12. Matsumoto A., Pasut A., Matsumoto M., Yamashita R., Fung J., Monteleone E., Saghatelian A., Nakayama K.I., Clohessy J.G., Pandolfi P.P.. mTORC1 and muscle regeneration are regulated by the LINC00961-encoded SPAR polypeptide. Nature. 2017; 541:228–232. [DOI] [PubMed] [Google Scholar]
- 13. Liang W.C., Wong C.W., Liang P.P., Shi M., Cao Y., Rao S.T., Tsui S.K., Waye M.M., Zhang Q., Fu W.M. et al.. Translation of the circular RNA circbeta-catenin promotes liver cancer cell growth through activation of the Wnt pathway. Genome Biol. 2019; 20:84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ingolia N.T., Brar G.A., Stern-Ginossar N., Harris M.S., Talhouarne G.J., Jackson S.E., Wills M.R., Weissman J.S.. Ribosome profiling reveals pervasive translation outside of annotated protein-coding genes. Cell Rep. 2014; 8:1365–1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. van Heesch S., Witte F., Schneider-Lunitz V., Schulz J.F., Adami E., Faber A.B., Kirchner M., Maatz H., Blachut S., Sandmann C.L. et al.. The translational landscape of the human heart. Cell. 2019; 178:242–260. [DOI] [PubMed] [Google Scholar]
- 16. Wilhelm M., Schlegl J., Hahne H., Gholami A.M., Lieberenz M., Savitski M.M., Ziegler E., Butzmann L., Gessulat S., Marx H. et al.. Mass-spectrometry-based draft of the human proteome. Nature. 2014; 509:582–587. [DOI] [PubMed] [Google Scholar]
- 17. Friedman R.C., Kalkhof S., Doppelt-Azeroual O., Mueller S.A., Chovancova M., von Bergen M., Schwikowski B.. Common and phylogenetically widespread coding for peptides by bacterial small RNAs. BMC Genomics. 2017; 18:553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Chen J., Brunner A.D., Cogan J.Z., Nunez J.K., Fields A.P., Adamson B., Itzhak D.N., Li J.Y., Mann M., Leonetti M.D. et al.. Pervasive functional translation of noncanonical human open reading frames. Science. 2020; 367:1140–1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hao Y., Zhang L., Niu Y., Cai T., Luo J., He S., Zhang B., Zhang D., Qin Y., Yang F. et al.. SmProt: a database of small proteins encoded by annotated coding and non-coding RNA loci. Brief Bioinform. 2018; 19:636–643. [DOI] [PubMed] [Google Scholar]
- 20. Liu W., Xiang L., Zheng T., Jin J., Zhang G.. TranslatomeDB: a comprehensive database and cloud-based analysis platform for translatome sequencing data. Nucleic Acids Res. 2018; 46:D206–D212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Wang H., Yang L., Wang Y., Chen L., Li H., Xie Z.. RPFdb v2.0: an updated database for genome-wide information of translated mRNA generated from ribosome profiling. Nucleic Acids Res. 2019; 47:D230–D234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Olexiouk V., Van Criekinge W., Menschaert G.. An update on sORFs.org: a repository of small ORFs identified by ribosome profiling. Nucleic Acids Res. 2017; 46:D497–D502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Liu H., Zhou X., Yuan M., Zhou S., Huang Y.E., Hou F., Song X., Wang L., Jiang W.. ncEP: a manually curated database for experimentally validated ncRNA-encoded proteins or peptides. J. Mol. Biol. 2020; 432:3364–3368. [DOI] [PubMed] [Google Scholar]
- 24. Lu S., Zhang J., Lian X., Sun L., Meng K., Chen Y., Sun Z., Yin X., Li Y., Zhao J. et al.. A hidden human proteome encoded by 'non-coding' genes. Nucleic Acids Res. 2019; 47:8111–8125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Li J., Liu C.. Coding or noncoding, the converging concepts of RNAs. Front. Genet. 2019; 10:496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Candeias M.M., Malbert-Colas L., Powell D.J., Daskalogianni C., Maslon M.M., Naski N., Bourougaa K., Calvo F., Fåhraeus R.. P53 mRNA controls p53 activity by managing Mdm2 functions. Nat. Cell Biol. 2008; 10:1098–1105. [DOI] [PubMed] [Google Scholar]
- 27. Naski N., Gajjar M., Bourougaa K., Malbert-Colas L., Fåhraeus R., Candeias M.M.. The p53 mRNA-Mdm2 interaction. Cell Cycle. 2009; 8:31–34. [DOI] [PubMed] [Google Scholar]
- 28. Shevtsov S.P., Dundr M.. Nucleation of nuclear bodies by RNA. Nat. Cell Biol. 2011; 13:167–173. [DOI] [PubMed] [Google Scholar]
- 29. Tay Y., Kats L., Salmena L., Weiss D., Tan S.M., Ala U., Karreth F., Poliseno L., Provero P., Di Cunto F. et al.. Coding-independent regulation of the tumor suppressor PTEN by competing endogenous mRNAs. Cell. 2011; 147:344–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kloc M., Foreman V., Reddy S.A.. Binary function of mRNA. Biochimie. 2011; 93:1955–1961. [DOI] [PubMed] [Google Scholar]
- 31. Ulveling D., Francastel C., Hubé F.. When one is better than two: RNA with dual functions. Biochimie. 2011; 93:633–644. [DOI] [PubMed] [Google Scholar]
- 32. Raina M., King A., Bianco C., Vanderpool C.K.. Dual-function RNAs. Microbiol. Spectrum. 2018; 6:RWR-0032-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Gimpel M., Brantl S.. Dual-function small regulatory RNAs in bacteria. Mol. Microbiol. 2017; 103:387–397. [DOI] [PubMed] [Google Scholar]
- 34. Zhu S., Wang J., He Y., Meng N., Yan G.R.. Peptides/proteins encoded by non-coding RNA: a novel resource bank for drug targets and biomarkers. Front. Pharmacol. 2018; 9:1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kumari P., Sampath K.. cncRNAs: bi-functional RNAs with protein coding and non-coding functions. Semin. Cell Dev. Biol. 2015; 47-48:40–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Sampath K., Ephrussi A.. CncRNAs: RNAs with both coding and non-coding roles in development. Development. 2016; 143:1234–1241. [DOI] [PubMed] [Google Scholar]
- 37. Dinger M.E., Gascoigne D.K., Mattick J.S.. The evolution of RNAs with multiple functions. Biochimie. 2011; 93:2013–2018. [DOI] [PubMed] [Google Scholar]
- 38. Scheidler C.M., Kick L.M., Schneider S.. Ribosomal peptides and small proteins on the rise. Chembiochem. 2019; 20:1479–1486. [DOI] [PubMed] [Google Scholar]
- 39. Crerar H., Scott-Solomon E., Bodkin-Clarke C., Andreassi C., Hazbon M., Logie E., Cano-Jaimez M., Gaspari M., Kuruvilla R., Riccio A.. Regulation of NGF signaling by an axonal untranslated mRNA. Neuron. 2019; 102:553–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Chugunova A., Loseva E., Mazin P., Mitina A., Navalayeu T., Bilan D., Vishnyakova P., Marey M., Golovina A., Serebryakova M. et al.. LINC00116 codes for a mitochondrial peptide linking respiration and lipid metabolism. PNAS. 2019; 116:4940–4945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Pamudurti N.R., Bartok O., Jens M., Ashwal-Fluss R., Stottmeister C., Ruhe L., Hanan M., Wyler E., Perez-Hernandez D., Ramberger E. et al.. Translation of CircRNAs. Mol. Cell. 2017; 66:9–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Cao X., Khitun A., Na Z., Dumitrescu D.G., Kubica M., Olatunji E., Slavoff S.A.. Comparative proteomic profiling of unannotated microproteins and alternative proteins in human cell lines. J. Proteome Res. 2020; 19:3418–3426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Saghatelian A., Couso J.P.. Discovery and characterization of smORF-encoded bioactive polypeptides. Nat. Chem. Biol. 2015; 11:909–916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Chen X., Han P., Zhou T., Guo X., Song X., Li Y.. circRNADb: a comprehensive database for human circular RNAs with protein-coding annotations. Sci. Rep. 2016; 6:34985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Karapetyan A.R., Buiting C., Kuiper R.A., Coolen M.W.. Regulatory roles for long ncRNA and mRNA. Cancers. 2013; 5:462–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Geslain R., Pan T.. tRNA: vast reservoir of RNA molecules with unexpected regulatory function. Proc. Natl. Acad. Sci. U.S.A. 2011; 108:16489–16490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Yates A.D., Achuthan P., Akanni W., Allen J., Allen J., Alvarez-Jarreta J., Amode M.R., Armean I.M., Azov A.G., Bennett R. et al.. Ensembl 2020. Nucleic Acids Res. 2020; 48:D682–D688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. RNAcentral: a hub of information for non-coding RNA sequences. Nucleic Acids Res. 2019; 47:D1250–D1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Kozomara A., Birgaoanu M., Griffiths-Jones S.. miRBase: from microRNA sequences to function. Nucleic Acids Res. 2018; 47:D155–D162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Amberger J.S., Bocchini C.A., Scott A.F., Hamosh A.. OMIM.org: leveraging knowledge across phenotype-gene relationships. Nucleic Acids Res. 2019; 47:D1038–D1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Ning L., Cui T., Zheng B., Wang N., Luo J., Yang B., Du M., Cheng J., Dou Y., Wang D.. MNDR v3.0: mammal ncRNA–disease repository with increased coverage and annotation. Nucleic Acids Res. 2020; doi:10.1093/nar/gkaa707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Zhang T., Tan P., Wang L., Jin N., Li Y., Zhang L., Yang H., Hu Z., Zhang L., Hu C. et al.. RNALocate: a resource for RNA subcellular localizations. Nucleic Acids Res. 2017; 45:D135–D138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Lin Y., Liu T., Cui T., Wang Z., Zhang Y., Tan P., Huang Y., Yu J., Wang D.. RNAInter in 2020: RNA interactome repository with increased coverage and annotation. Nucleic Acids Res. 2020; 48:D189–D197. [DOI] [PMC free article] [PubMed] [Google Scholar]