Abstract
Eukaryotic genomes encode thousands of small and large non-coding RNAs (ncRNAs). However, the expression, functions and evolution of these ncRNAs are still largely unknown. In this study, we have updated deepBase to version 3.0 (deepBase v3.0, http://rna.sysu.edu.cn/deepbase3/index.html), an increasingly popular and openly licensed resource that facilitates integrative and interactive display and analysis of the expression, evolution, and functions of various ncRNAs by deeply mining thousands of high-throughput sequencing data from tissue, tumor and exosome samples. We updated deepBase v3.0 to provide the most comprehensive expression atlas of small RNAs and lncRNAs by integrating ∼67 620 data from 80 normal tissues and ∼50 cancer tissues. The extracellular patterns of various ncRNAs were profiled to explore their applications for discovery of noninvasive biomarkers. Moreover, we constructed survival maps of tRNA-derived RNA Fragments (tRFs), miRNAs, snoRNAs and lncRNAs by analyzing >45 000 cancer sample data and corresponding clinical information. We also developed interactive webs to analyze the differential expression and biological functions of various ncRNAs in ∼50 types of cancers. This update is expected to provide a variety of new modules and graphic visualizations to facilitate analyses and explorations of the functions and mechanisms of various types of ncRNAs.
INTRODUCTION
The vast majority of human genome and other mammalian genomes are transcribed to encode thousands of short (sRNAs) and long non-coding RNAs (lncRNAs), which have been implicated in diverse physiological and pathological processes, such as tumorigenesis, development, imprinting, apoptosis and cell differentiation (1–5). Although thousands of ncRNA studies have been published in recent years, only a small fraction of ncRNAs have been well functionally studied.
Given the thousands of ncRNAs being discovered in various species, many databases have been developed to help researchers understand their diversity and functions in recent years. Notable examples include miRBase (6), a reference database of published miRNA sequences and GENCODE (7), a reference database for lncRNAs. In addition, a series of databases have been developed to explore ncRNA expression patterns, regulatory networks and biological functions, such as RNAcentral (8), LNCipedia (9), LncRNAdb (10), ChIPBase (11), NONCODE (12), LncRNADisease (13), starBase (14) and circBase (15). However, these databases focus on either specific ncRNA families or specific features of ncRNAs.
Tremendous amounts of deep-sequencing data have been generated by multiple consortium projects, such as the ENCODE (16), TCGA (17), ICGC (18), GTEx (19) and ERCC (20) projects, providing new opportunities to understand the functions of ncRNAs. A few databases have integrated TCGA RNA-seq data to explore the expression profiles of miRNAs and lncRNAs in cancers. Notable examples include starBase (14), which enables the pan-cancer analysis on miRNA-target and RBP-RNA interactions in ∼10 000 clinical samples of 32 types of cancers, and TANRIC (21) which is an interactive open platform for exploration of the functions of lncRNAs in cancer. However, these databases use only one consortium project (e.g. TCGA) to explore two types of ncRNAs (e.g. miRNAs and lncRNAs). There is a great need to integrate all deep-sequencing data produced by all large consortium projects to explore the dynamic expression, clinical implications and functions of various ncRNAs in physiological and pathological processes.
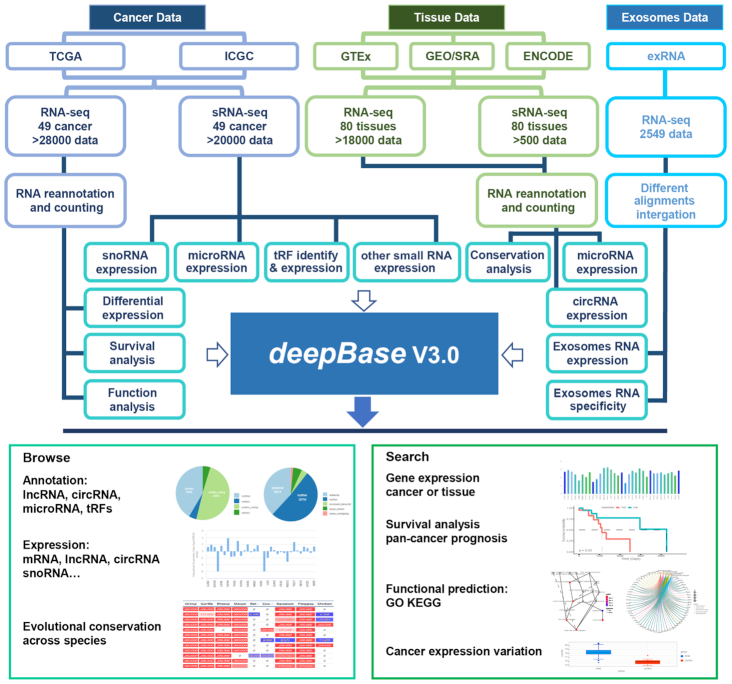
To overcome the abovementioned issues, we have updated deepBase (22) to version 3.0 (deepBase v3.0, Figure 1, Table 1). deepBase v3.0, for the first time, constructs the expression profiles of tRFs and snoRNAs by mining small RNA deep-sequencing data from TCGA. deepBase v3.0 also provides the most comprehensive expression profiles available for lncRNAs and other ncRNAs in normal and cancer tissues by integrating sequencing data from large consortium projects, including the ENCODE, TCGA, ICGC and GTEx projects. By analyzing sequencing data from the ERCC, we decoded the extracellular patterns of miRNAs, lncRNAs and circRNAs. In addition, deepBase v3.0 provides a variety of new web modules and graphic visualizations to facilitate analyses and explorations of the complex expression, functions and evolution of various types of ncRNAs.
Figure 1.
System-level overview of the deepBase v3.0 framework. We performed a large-scale integration and analysis of ∼46 000 RNA-seq datasets and 20 000 sRNA-seq datasets downloaded from large consortium projects and various databases. All small and large ncRNAs were identified and annotated. We constructed the most comprehensive database of expression, evolution, prognosis and extracellular patterns and functional predictions for various types of ncRNAs. All results are stored in MySQL relational databases and displayed in interactive webpages.
Table 1.
Major improvements of deepBase v3.0. This table describes the major improvements of data features and web-based functionalities in the updated deepBase v3.0 compared to previous versions
| Data features/functionalities | deepBase v2.0 | deepBase v3.0 |
|---|---|---|
| Total RNA-seq data | 558 | 46 384 |
| Total sRNA-seq data | 478 | 21 235 |
| Tissue RNA samples | 489 | 18 685 |
| LncRNA information entries | 367 539 | 436 436 |
| Cancer RNA profiling | none | 49 cancer types |
| Cancer RNA-seq data | none | 25 639 |
| Cancer sRNA-seq data | none | 20 707 |
| Patients clinical samples | none | 14 810 |
| Cancer mRNA expression | none | 19 814 genes |
| Cancer lncRNA expression | none | 14 855 genes |
| Cancer sRNA expression | none | 7495 genes |
| Cancer miRNA expression | none | 1881 genes |
| Cancer snoRNA expression | none | 1988 genes |
| tRFs annotation | none | >1100 |
| tRFs expression and variation | none | 31 cancer types |
| RNA differential expression | none | 24 cancer types |
| Clinical prognosis | none | 12 310 genes |
| Exosome RNA profiling | none | 9 biofluids types |
| Exosome RNA-seq data | none | 2549 |
| Exosome mRNA expression | none | 81 692 entries |
| Exosome lncRNA expression | none | 81 229 entries |
| Exosome miRNA expression | none | 3396 entries |
| Exosome circRNA expression | none | 918 entries |
MATERIALS AND METHODS
Integration of small RNA-seq and RNA-seq data
More than 28 000 RNA-seq and small RNA-seq (sRNA-seq) data from 14 species were collected from the GEO (23), GTEx (19), ENCODE (16), exRNA (20), TCGA (17) and ICGC (18). Datasets of normal tissue were retrieved from GTEx and ENCODE, and cancer-related datasets and the corresponding clinical data were downloaded from TCGA and ICGC. The human (Homo sapiens) reference genome was updated to GRCh38(NCBI GRCh38) (24). The genome sequences of other species, including the mouse (UCSC mm10), chicken (Gallus gallus, v4), chimp (Pan troglodytes, panTro4), gorilla (Gorilla gorilla gorilla, gorGor3), rhesus monkey (Macaca mulatta, rheMac3), rat (Rattus norvegicus, rn6), cow (Bos taurus, bosTau7), opossum (Monodelphis domestica, monDom5), platypus (Ornithorhynchus anatinus, ornAna1), X. tropicalis (Xenopus tropicalis, xenTro3), zebrafish (Danio rerio, danRer7) and C. elegans (Caenorhabditis elegans, ce10), were downloaded from the UCSC Genome Browser. Gene annotation files were downloaded from the UCSC Genome Browser corresponding to the genome versions (Table 2). For exosomes RNA, data for 2549 healthy samples were retrieved from exRNA database (20). Once collected, the data were classified into different species, tissue, cancer or exosome types according to the metafile descriptions or related literature.
Table 2.
The genome and annotation sources of 13 species. This table describes the annotation version of genome used in the integration of sRNA-seq and RNA-seq data
| Species | Assembly | Annotation Source |
|---|---|---|
| Homo sapiens | GRCh38(hg38) | UCSC Genome Browser |
| Mus musculus | GRCm38(mm10) | UCSC Genome Browser |
| Gallus gallus | galGal4 | UCSC Genome Browser |
| Pan troglodytes | panTro4 | UCSC Genome Browser |
| Gorilla gorilla gorilla | gorGor3 | UCSC Genome Browser |
| Macaca mulatta | rheMac3 | UCSC Genome Browser |
| Rattus norvegicus | RGSC6.0(rn6) | UCSC Genome Browser |
| Bos taurus | bosTau7 | UCSC Genome Browser |
| Monodelphis domestica | monDom5 | UCSC Genome Browser |
| Ornithorhynchus anatinus | ornAna1 | UCSC Genome Browser |
| Xenopus tropicalis | xenTro3 | UCSC Genome Browser |
| Danio rerio | danRer7 | UCSC Genome Browser |
| Caenorhabditis elegans | ce10 | UCSC Genome Browser |
Identification of tRFs from sRNA-seq datasets
tRNA-derived RNA fragments(tRFs) are 14–32 nt single-stranded RNAs (25,26). They are produced from pre-tRNAs (3′U tRFs) or mature tRNAs (3′ CCA tRFs, 5′ tRFs, tRF-i) (25). mirDeep2 (27) was first used to annotate miRNA sequences from TCGA raw sequencing data of small RNAs and the unaligned short sequences were kept for further analysis. tRFfinder (28) was then applied to identify tRFs. We next classified and annotated the identified tRFs to tRF-5 (5′ tRFs), tRF-3(3′ CCA tRFs), tRF-1 (3′U tRFs) and tRF-novel (tRF-i). The annotated tRFs were deposited in deepBase v3.0
Expression analysis of various kinds of RNAs
For RNA-seq data, after downloading and classification, the raw data were recomputed as the FPKM (Fragments Per Kilobase of transcript per Million mapped reads) values to calculate the expression of genes. The expression in different tissues or life stages were normalized by z-score or mean and deposited in deepBase v3.0.
For sRNA-seq data, miRNA data in RPM form were collected to determine the expression amounts. snoRNA annotations were downloaded from snoDB. We counted the snoRNAs with featureCounts (parameters: -M -s1 -fraction).
For exosome data, based on a series of alignment results and a description file downloaded from ERCC, the expression levels of mRNAs and lncRNAs were integrated in RPKM format, and those of miRNAs and circRNAs were collected in RPM format.
Differential expression analysis
The gene expression profiles identified from RNA-seq data were reannotated to GRCh38 and were then separated into different subclasses of RNAs. EdgeR (29) was used to perform differential expression analysis between cancer and normal samples. The differential expression of lncRNAs, mRNAs, small RNAs, tRNAs and snoRNAs was analyzed separately and then a comprehensive and detailed expression variation network of cancer RNAs was constructed. The differential expression changes and FDR values were deposited in deepBase and displayed in the web page.
Predicting functions of ncRNAs from RNA–RNA coexpression networks
With a considerable amount of data, we developed a pipeline to predict functions of ncRNAs from RNA–RNA coexpression networks. Given the distribution of RNA expression values cannot be treated as normal, spearman correlation coefficient has better performance than pearson correlation coefficient (30). So, the expression correlations between ncRNAs and protein-coding genes were estimated using spearman correlation analysis in the R stats package and the P-values were adjusted with the FDR (False Discovery Rate) (31). Protein-coding genes with correlation values higher than 0.5 and P-values ≤0.05 were considered as coexpressed genes. GO and KEGG analyses were completed by the R clusterProfiler package (32).
Prognostic analysis of differentially expressed ncRNAs
LncRNAs with apparent changes (|log FC| ≥ 1, P-value < 0.05) were collected from the differential expression data and clinical data from TCGA to complete a survival analysis using univariate Cox regression. Once an RNA was identified as apparently differentially expressed in one kind of cancer, it was brought into the analysis. deepBase set the log-rank P-value ≤0.05 to determine whether an RNA had an influence on patient survival. A KM survival plot was drawn for the qualified lncRNAs (33).
DATABASE CONTENT AND WEB INTERFACE
Web-based exploration of sRNAs, lncRNAs, circRNAs and tRFs
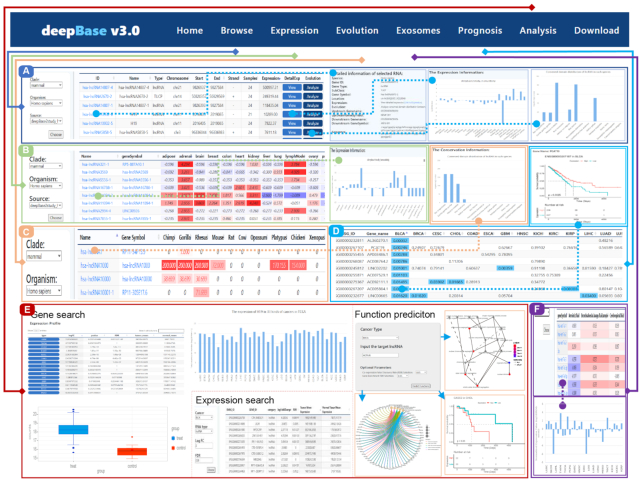
deepBase provides genome-wide identification of multiple types of RNAs, from lncRNAs to different types of small RNAs. In the Browse section, there are four web pages for user to browse different kinds of RNAs with annotations and expression profiles (Figure 2).
Figure 2.
Introduction and usage of deepBase v3.0. (A) Browse page for ncRNAs with its detailed information, expression, and evolutionary conservation pages. (B) Expression page for ncRNAs with a detailed expression profile. (C) Evolutionary conservation of lncRNAs across 14 species. (D) Prognoses associated with cancer-related lncRNAs and detailed pages containing KM-plots of corresponding ncRNAs. (E) Multiple analysis interfaces for gene, expression, and function predictions. (F) Exosome expression page and detailed information for exosome expression profiles.
The Browse pages for lncRNAs, small RNAs and circRNAs display identified and reannotated RNAs from different experiments with their detailed information including the genomic locus, strand, length, type, total expression and expressed sample numbers. Users can sort the data table by the ‘Sample’ column to determine the universality of RNA expression. Some RNAs are widely expressed in all samples, while some are expressed in only a few samples. By sorting the data table by the ‘Expression’ column, users can obtain the expression conditions of RNAs. On the lncRNAs and smallRNAs page, we provide an outbound link to a new page showing the detailed expression in different samples or tissues. User can simply click the gene name to obtain detailed information.
deepBase provides an overview of tRFs identified from TCGA deep-sequencing data and shows the type, expressed sample number and expression details in multiple cancers. The naming rules are similar to those we used to name the lncRNAs in deepBase v2.0. Users can also click the tRF name to see information about its tRNA location, sequence and structure. An outbound link to RNAfold for the 2D structure is also provided.
Expression profiles of various ncRNAs
Quantification of ncRNAs expression is one of the most important features of studies on RNA, and specific expression in certain stages, tissues or cells can imply the functions of ncRNAs in biological processes (34).
The expression sections are divided into two parts: normal tissues and cancers. In the normal tissue part, deepBase provides the expression profiles of all mRNAs, lncRNAs, miRNAs, circRNAs and small ncRNAs. deepBase v3.0 shows the normalized expression values (normalized by z-score or mean value) of RNAs in the form of a heatmap to give users a clear view of the relative expression in different tissues or samples. Users can click the gene name and jump to a detailed expression page. In the cancer part, the same normalization is applied to samples from different cancer types to show the expression differences between cancers. TCGA and ICGC data are displayed separately. The cancer small RNA page data are RNA-seq data, while the miRNA page data are sRNA-seq data, and the two types of data change in parallel. On the basis of the re-analysis of sRNA-seq data from TCGA, deepBase v3.0 also displayed the expression profiles of snoRNAs in various cancer types.
Expression profiles of exosomes
Extracellular RNAs (exRNAs) are a type of RNA molecule that is present in various biological fluids. ExRNAs from heterogeneous populations including small RNAs, circRNAs, lncRNAs and mRNAs. They exist in free form or associate with proteins to form complexes, participating in a variety of cell-to-cell communications and play significant roles in cancer and other diseases. More than 2500 sample data downloaded from the ERCC database were analyzed to construct an expression map for different exosomes and to provide an overview of human exosomes RNA expression. Users can obtain direct views of exRNA expression in different biofluids and of the expression specificity of exRNAs.
Prognostic analysis
With RNA-seq and sRNA-seq sequencing data, we also collected clinical data from TCGA and ICGC for deepBase v3.0. Combining expression data and clinical data, we applied univariate Cox regression to all differentially expressed genes (|log FC| ≥ 1, P-value < 0.05). deepBase shows all survival log-rank P-values in a data table. Genes that are not expressed in a specific cancer type or show no relationship to survival are not displayed. KM survival curve plots are provided for survival-related RNAs that pass the log-rank P-value threshold of 0.05.
Interactive analysis for different kinds of ncRNAs
deepBase provides several analysis interfaces for users to take in-depth looks at different kinds of RNA in different features.
The gene search page shows the detailed expression of a single RNA in different types of cancer. This page consists of four parts. The left search bar includes the input box and guide. In the right section, a data table shows the expression and related information, and a boxplot shows the specific expression in one cancer type. Users can click on the cancer name in the data table to change it. A bar plot shows the cancer-wide expression. This page was designed to give users a direct and quick access to specific RNA information.
The expression page displays an expression matrix of different types of RNA in a single cancer type, and users can set the P-value and FDR cutoff to obtain a custom data table for further inspection. The search and expression pages provide user with quick and easy ways to obtain primary information from cancer-related RNA studies.
A web-based tool to predict ncRNA functions in cancer was developed based on coding and non-coding coexpression networks. There are four parameters to customize: the cancer type, ncRNA ID or gene name, co-expression FDR and enrichment analysis FDR. After submitting data, a user jumps to a new result page that displays the GO enrichment results, the KEGG enrichment results and a Kaplan–Meier plot. While only the biological process (BP) GO terms are shown on the webpage, a text file containing all three kinds of GO terms can be downloaded for further study. The user can download the specific plot or the zipped data of all plots and data tables.
CONCLUSIONS
We introduce deepBase v3.0, which has significantly better web modules and functionalities than deepBase v2.0 (22). Previous versions of deepBase (22) have focused mainly on the expression patterns of miRNAs, lncRNAs and circRNAs in normal tissues or cell lines. In comparison to the previous release, deepBase v3.0 has several advances and improvements in data features and functionality (Table 1): (i) deepBase v3.0 provides the most comprehensive expression analysis of sRNAs and lncRNAs by mining 67 619 datasets for 14 species from large consortium projects and public deep-sequencing data. This will generate numerous differentially expressed ncRNAs for functional studies by bench biologists. (ii) To the best of our knowledge, this is the first attempt to construct the expression patterns of tRFs and snoRNAs from thousands of cancer and normal samples. It may help biologists select disease-related tRFs and snoRNAs for further functional validation. (iii) Gene expression data for miRNAs, lncRNAs and circRNAs from 2549 exRNA sequencing datasets have been newly added to deepBase. These data will help biologists discover noninvasive ncRNA biomarkers. (iv) The novel ‘Prognosis’ module has been developed to illustrate the correlations between ncRNAs and patient survival by linking a large number of expression profiles of ncRNAs with clinical data. (v) A new ‘Analysis’ module allows researchers to deeply investigate the functions of lncRNAs and other ncRNAs in tumor tumorigenesis by performing differential expression analysis and functional prediction based on protein–lncRNA coexpression networks across 42 types of cancers.
FUTURE DIRECTIONS
Various high-throughput sequencing methods, such as CLIP-seq, ChIP-seq and ribo-seq, have been developed to explore the biological function, regulatory networks and translational potential of ncRNAs. We are considering adding these kinds of data in the next version of deepBase to facilitate analyses and explorations of the complex regulation, functions and mechanisms of various types of ncRNAs. Moreover, more annotation data and additional species will be integrated to further expand this database. We will continue to improve the database to accept and analyze new data uploaded by users.
DATA AVAILABILITY
deepBase v3.0 is freely available at http://rna.sysu.edu.cn/deepbase3/index.html. The deepBase data files can be downloaded and used in accordance with the GNU Public License and the licenses of primary data sources.
Contributor Information
Fangzhou Xie, MOE Key Laboratory of Gene Function and Regulation, State Key Laboratory for Biocontrol, The Fifth Affiliated Hospital, Sun Yat-sen University, Guangzhou 510275, China.
Shurong Liu, MOE Key Laboratory of Gene Function and Regulation, State Key Laboratory for Biocontrol, The Fifth Affiliated Hospital, Sun Yat-sen University, Guangzhou 510275, China.
Junhao Wang, MOE Key Laboratory of Gene Function and Regulation, State Key Laboratory for Biocontrol, The Fifth Affiliated Hospital, Sun Yat-sen University, Guangzhou 510275, China.
Jiajia Xuan, MOE Key Laboratory of Gene Function and Regulation, State Key Laboratory for Biocontrol, The Fifth Affiliated Hospital, Sun Yat-sen University, Guangzhou 510275, China.
Xiaoqin Zhang, School of Medicine, South China University of Technology, Guangzhou 510275, China.
Lianghu Qu, MOE Key Laboratory of Gene Function and Regulation, State Key Laboratory for Biocontrol, The Fifth Affiliated Hospital, Sun Yat-sen University, Guangzhou 510275, China.
Lingling Zheng, MOE Key Laboratory of Gene Function and Regulation, State Key Laboratory for Biocontrol, The Fifth Affiliated Hospital, Sun Yat-sen University, Guangzhou 510275, China.
Jianhua Yang, MOE Key Laboratory of Gene Function and Regulation, State Key Laboratory for Biocontrol, The Fifth Affiliated Hospital, Sun Yat-sen University, Guangzhou 510275, China; Department of Interventional Medicine, The Fifth Affiliated Hospital, Sun Yat-sen University, Zhuhai 519000, China.
FUNDING
National Key R&D Program of China [2019YFA0802202, 2017YFA0504400]; National Natural Science Foundation of China [91940304, 31971228, 31770879, 31771459, 31370791, 31471223, 91440110, 31770879, 31900903, 81702945]; Youth science and technology innovation talent of guangdong TeZhi plan [2019TQ05Y181]; Guangdong Province [2017A030313106, 2017A030313483]; Guangzhou city [202002030351, 201904020041]; Fundamental Research Funds for the Central Universities [14lgjc18, 20lgpy112]; Science and Technology New Star in ZhuJiang Guangzhou city [201806010151]; Guangdong Province Key Laboratory of Computational Science [13lgjc05] (in part); Guangdong Province Computational Science Innovative Research Team [14lgjc18]. Funding for open access charge: National Key R&D Program of China [2019YFA0802202, 2017YFA0504400].
Conflict of interest statement. None declared.
REFERENCES
- 1. Cech T.R., Steitz J.A.. The noncoding RNA revolution-trashing old rules to forge new ones. Cell. 2014; 157:77–94. [DOI] [PubMed] [Google Scholar]
- 2. Barquist L., Vogel J.. Accelerating discovery and functional analysis of small RNAs with new technologies. Annu. Rev. Genet. 2015; 49:367–394. [DOI] [PubMed] [Google Scholar]
- 3. Liu S., Li B., Liang Q., Liu A., Qu L., Yang J.. Classification and function of RNA-protein interactions. Wiley Interdisciplinary Reviews RNA. 2020; e1601. [DOI] [PubMed] [Google Scholar]
- 4. Matera A.G., Terns R.M., Terns M.P.. Non-coding RNAs: lessons from the small nuclear and small nucleolar RNAs. Nat. Rev. Mol. Cell Biol. 2007; 8:209–220. [DOI] [PubMed] [Google Scholar]
- 5. Ulitsky I., Bartel D.P.. lincRNAs: genomics, evolution, and mechanisms. Cell. 2013; 154:26–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kozomara A., Birgaoanu M., Griffiths-Jones S.. miRBase: from microRNA sequences to function. Nucleic Acids Res. 2019; 47:D155–D62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Harrow J., Frankish A., Gonzalez J.M., Tapanari E., Diekhans M., Kokocinski F., Aken B.L., Barrell D., Zadissa A., Searle S. et al.. GENCODE: the reference human genome annotation for The ENCODE Project. Genome Res. 2012; 22:1760–1774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. The RC RNAcentral: a hub of information for non-coding RNA sequences. Nucleic Acids Res. 2019; 47:D1250–D1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Volders P.-.J., Anckaert J., Verheggen K., Nuytens J., Martens L., Mestdagh P., Vandesompele J.. LNCipedia 5: towards a reference set of human long non-coding RNAs. Nucleic Acids Res. 2019; 47:D135–D139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Quek X.C., Thomson D.W., Maag J.L., Bartonicek N., Signal B., Clark M.B., Gloss B.S., Dinger M.E.. lncRNAdb v2. 0: expanding the reference database for functional long noncoding RNAs. Nucleic Acids Res. 2015; 43:D168–D173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Zhou K.-.R., Liu S., Sun W.-.J., Zheng L.-.L., Zhou H., Yang J.-.H., Qu L.H.. ChIPBase v2. 0: decoding transcriptional regulatory networks of non-coding RNAs and protein-coding genes from ChIP-seq data. Nucleic Acids Res. 2017; 45:D43–D50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Zhao Y., Li H., Fang S., Kang Y., Wu W., Hao Y., Li Z., Bu D., Sun N., Zhang M.Q. et al.. NONCODE 2016: an informative and valuable data source of long non-coding RNAs. Nucleic Acids Res. 2016; 44:D203–D208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bao Z., Yang Z., Huang Z., Zhou Y., Cui Q., Dong D.. LncRNADisease 2.0: an updated database of long non-coding RNA-associated diseases. Nucleic Acids Res. 2019; 47:D1034–D1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Li JH., Liu S., Zhou H., Qu LH., Yang JH.. starBase v2.0: decoding miRNA-ceRNA, miRNA-ncRNA and protein-RNA interaction networks from large-scale CLIP-Seq data. Nucleic Acids Res. 2014; 42:D92–D97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Glažar P., Papavasileiou P., Rajewsky N.. circBase: a database for circular RNAs. RNA. 2014; 20:1666–1670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Moore J.E., Purcaro M.J., Pratt H.E., Epstein C.B., Shoresh N., Adrian J., Kawli T., Davis C.A., Dobin A., Kaul R. et al.. Expanded encyclopaedias of DNA elements in the human and mouse genomes. Nature. 2020; 583:699–710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Weinstein J.N., Collisson E.A., Mills G.B., Shaw K.R.M., Ozenberger B.A., Ellrott K., Shmulevich I., Sander C., Stuart J.M.. The cancer genome atlas pan-cancer analysis project. Nat. Genet. 2013; 45:1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Consortium ICG International network of cancer genome projects. Nature. 2010; 464:993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lonsdale J., Thomas J., Salvatore M., Phillips R., Lo E., Shad S., Hasz R., Walters G., Garcia F., Young N. et al.. The genotype-tissue expression (GTEx) project. Nat. Genet. 2013; 45:580–585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Murillo O.D., Thistlethwaite W., Rozowsky J., Subramanian S.L., Lucero R., Shah N., Jackson A.R., Srinivasan S., Chung A., Laurent C.D. et al.. exRNA atlas analysis reveals distinct extracellular RNA cargo types and their carriers present across human biofluids. Cell. 2019; 177:463–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Li J., Han L., Roebuck P., Diao L., Liu L., Yuan Y., Weinstein J.N., Liang H.. TANRIC: an interactive open platform to explore the function of lncRNAs in cancer. Cancer Res. 2015; 75:3728–3737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Zheng L.-.L., Li J.-.H., Wu J., Sun W.-.J., Liu S., Wang Z.-.L., Zhou H., Yang J.H., Qu L.H.. deepBase v2. 0: identification, expression, evolution and function of small RNAs, LncRNAs and circular RNAs from deep-sequencing data. Nucleic Acids Res. 2016; 44:D196–D202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Barrett T., Troup D.B., Wilhite S.E., Ledoux P., Evangelista C., Kim I.F., Tomashevsky M., Marshall K.A., Phillippy K.H., Sherman P.M. et al.. NCBI GEO: archive for functional genomics data sets–10 years on. Nucleic Acids Res. 2011; 39:D1005–D1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Pruitt K.D., Tatusova T., Maglott D.R.. NCBI reference sequences (RefSeq): a curated non-redundant sequence database of genomes, transcripts and proteins. Nucleic Acids Res. 2007; 35:D61–D65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kumar P., Kuscu C., Dutta A.. Biogenesis and function of transfer RNA-related fragments (tRFs). Trends Biochem. Sci. 2016; 41:679–689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lee Y.S., Shibata Y., Malhotra A., Dutta A.. A novel class of small RNAs: tRNA-derived RNA fragments (tRFs). Genes Dev. 2009; 23:2639–2649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Friedländer M.R., Mackowiak S.D., Li N., Chen W., Rajewsky N.. miRDeep2 accurately identifies known and hundreds of novel microRNA genes in seven animal clades. Nucleic Acids Res. 2012; 40:37–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Zheng L.-.L., Xu W.-.L., Liu S., Sun W.-.J., Li J.-.H., Wu J., Yang J.H., Qu L.H.. tRF2Cancer: a web server to detect tRNA-derived small RNA fragments (tRFs) and their expression in multiple cancers. Nucleic Acids Res. 2016; 44:W185–W193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Robinson M.D., McCarthy D.J., Smyth G.K.. edgeR: a Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics. 2010; 26:139–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Wang J.F., Zhou H., Chen Y.Q., Luo Q.J., Qu L.H.. Identification of 20 microRNAs from Oryza sativa. Nucleic Acids Res. 2004; 32:1688–1695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Benjamini Y., Hochberg Y.. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J. R. Stat. Soc.: Ser. B (Methodological). 1995; 57:289–300. [Google Scholar]
- 32. Yu G., Wang L.-.G., Han Y., He Q.-.Y.. clusterProfiler: an R package for comparing biological themes among gene clusters. Omics. 2012; 16:284–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kassambara A., Kosinski M., Biecek P., Fabian S.. 2017; survminer: Drawing Survival Curves using’ggplot2′. R package version 03, 1.
- 34. Cabili M.N., Trapnell C., Goff L., Koziol M., Tazon-Vega B., Regev A., Rinn J.L.. Integrative annotation of human large intergenic noncoding RNAs reveals global properties and specific subclasses. Genes Dev. 2011; 25:1915–1927. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
deepBase v3.0 is freely available at http://rna.sysu.edu.cn/deepbase3/index.html. The deepBase data files can be downloaded and used in accordance with the GNU Public License and the licenses of primary data sources.