Abstract
ADP-ribosylation is a protein modification responsible for biological processes such as DNA repair, RNA regulation, cell cycle and biomolecular condensate formation. Dysregulation of ADP-ribosylation is implicated in cancer, neurodegeneration and viral infection. We developed ADPriboDB (adpribodb.leunglab.org) to facilitate studies in uncovering insights into the mechanisms and biological significance of ADP-ribosylation. ADPriboDB 2.0 serves as a one-stop repository comprising 48 346 entries and 9097 ADP-ribosylated proteins, of which 6708 were newly identified since the original database release. In this updated version, we provide information regarding the sites of ADP-ribosylation in 32 946 entries. The wealth of information allows us to interrogate existing databases or newly available data. For example, we found that ADP-ribosylated substrates are significantly associated with the recently identified human protein interaction networks associated with SARS-CoV-2, which encodes a conserved protein domain called macrodomain that binds and removes ADP-ribosylation. In addition, we create a new interactive tool to visualize the local context of ADP-ribosylation, such as structural and functional features as well as other post-translational modifications (e.g. phosphorylation, methylation and ubiquitination). This information provides opportunities to explore the biology of ADP-ribosylation and generate new hypotheses for experimental testing.
INTRODUCTION
ADP-ribosylation is a reversible post-translational modification defined by the addition of one [mono(ADP-ribosyl)ation] or multiple [poly(ADP-ribosyl)ation] ADP-ribose moieties onto amino acid side chains with nucleophilic oxygen, nitrogen or sulfur (1,2). ADP ribose groups can be transferred by enzymatically active members of the family of 17 ADP-ribosyltransferases (commonly known as PARPs) as well as other enzymes, such as bacterial toxins and sirtuins (3–5). ADP-ribosylation has been implicated in fundamental biological processes (e.g. DNA damage repair, gene regulation and cell signaling) (6–8) as well as disease-related processes (e.g. microbial pathogenesis, carcinogenesis and inflammation) (9–11). While ADP-ribosylation was discovered in 1963 (12), studies of ADP-ribosylation have recently begun to benefit from the rapid development in proteomics technologies that identify ADP-ribosylated sites and characterize the substrate specificity of PARPs and other enzymes associated with ADP-ribosylation (13). In light of these developments, we created ADPriboDB in 2015 (14) to provide a one-stop informatics portal about ADP-ribosylated substrates across the proteomes from different species, similar to databases like dbPTM (15,16) and PhosphoSite (17) that provide information about other post-translational modifications such as ubiquitination and phosphorylation. In addition, ADPriboDB provides data entries pertaining to PARP inhibitor effects on the ADP-ribosylated proteome, aiming to bridge basic scientific knowledge about ADP ribosylation with information that physicians can use to garner insights on clinical benefits and side effects. Since its creation, the database has received over 702 500 hits and 13 300 unique visitors.
ADPriboDB 2.0 includes an expanded library of data, new addition of site information, and an improved user interface (Table 1). ADPriboDB 2.0 also integrates other post-translational modification sites and features a web interface that allows users to visualize protein domains and other features at and proximal to the modification sites.
Table 1.
Data summary of ADPriboDB versions
| ADPriboDB | v1.0 | v2.0 |
|---|---|---|
| Entries | 12 428 | 48 346 |
| Unique Proteins | 2389 | 9097 |
| Species | 28 | 41 |
| Entries with Site Information | 385 | 32 946 |
| Other PTM Information | No | Yes |
| R Shiny-based Visualization Tool | No | Yes |
NEW FEATURES
Expanded Library of Data
As in ADPriboDB, published papers were curated from PubMed, accessed via the following search terms: ‘PARylation’ or ‘PARsylation’ or ‘poly(ADP-ribosyl)ation’ or ‘poly-adp-ribosylation’ or ‘poly(ADP-ribosylation)’ or ‘PARylated’ or ‘PARsylated’ or ‘poly(ADP)ribosylation’ or ‘MARylation’ or ‘MARsylation’ or ‘mono(ADP-ribosyl)ation’ or ‘mono-adp-ribosylation’ or ‘mono(ADP-ribosylation)’ or ‘MARylated’ or ‘MARsylated’ or ‘mono(ADP)ribosylated.’ Search results were restricted between the dates of July 2015 and May 2019, where the initial release covered between January 1975 and June 2015 (14). ADPriboDB 2.0 now also included the terms ‘ADPr,’ ‘ADP-ribose’ and ‘ADPribosylation’ for literature search between January 1975 and May 2019. Information regarding protein identifiers as well as experimental and modification details were collected and assessed for inclusion in ADPriboDB by two independent curators.
Since 2010, research regarding ADP-ribosylation has accelerated, and particularly the identification of ADP-ribosylated substrates has risen (Figure 1A). In light of these trends, the latest iteration of ADPriboDB comprises 48 346 entries, including 9097 unique proteins across 610 papers, more than tripling the size of the initial release of the database. The database now includes data from 41 species, up from 28 species. ADPriboDB 2.0 has more than doubled the proportion of its entries bearing information regarding the enzyme responsible for the ADP-ribosylation. Reflecting a greater availability of technology (e.g. analog-sensitive PARP approaches), 65% of database entries include the information of enzyme–substrate specificity (Table 2). To enhance the speed with which queried database pages are loaded, we have used the open-source PHP framework Xataface to compress and save all requested pages to a cache. These caches are generated for each individual user. This feature allows the display of the frequently visited pages, thereby speeding-up the process of information loading and display.
Figure 1.
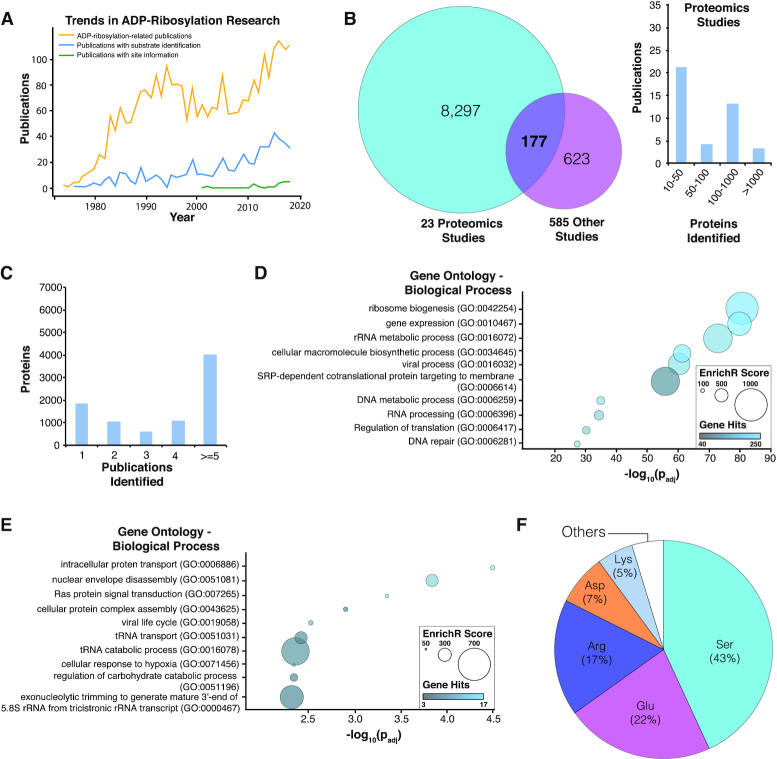
Analyses of ADPriboDB 2.0. (A) Trends in ADP-ribosylation research shows a progressive increase in the number of publications about ADP-ribosylation biology, substrates and sites. (B) Left: Venn diagram shows the overlap between proteins identified in proteomics-based studies (≥10 proteins) and those identified in other publications. Right: Histogram describes the number of proteins identified by these proteomics studies. (C) Distribution of the number of publications identifying a given ADP-ribosylated substrate. (D) Proteins identified in at least two publications were subjected to gene ontology analysis via EnrichR. Bubble chart shows the enrichment of selected pathways analyzed with REVIGO (26); the full enrichment analysis is available in Supplementary Data S1. (E) Enrichment analysis was also performed on ADP-ribosylated proteins within the SARS-CoV-2 interactome. Bubble chart shows the enrichment of selected pathways analyzed with REVIGO; the full enrichment analysis is available in Supplementary Data S2. (F) Distribution of ADP-ribosylated residues.
Table 2.
Number of unique proteins modified by each PARP
| Enzyme | Modified Proteins |
|---|---|
| PARP1 | 1524 |
| PARP10 | 1080 |
| PARP2 | 462 |
| PARP3 | 394 |
| PARP11 | 295 |
| PARP14 | 222 |
| Other PARPs | 58 |
In ADPriboDB 2.0, a growing number of entries were derived from proteomics studies, which account for ∼8000 unique proteins (Figure 1B). Approximately 75% of ADP-ribosylated proteins were identified in at least two publications (Figure 1C). Analysis of these independently verified ADP-ribosylated substrates via EnrichR (18) revealed a significant enrichment of gene sets associated with diverse biological processes, including DNA metabolism, RNA processing, protein targeting and viral-related processes (Figure 1D and Supplementary Data S1).
This expanded database therefore provides greater flexibility and depth to analyses of ADP-ribosylation, its function and its clinical implications. As an example, we used ADPriboDB to explore the biology of the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). SARS-CoV-2 encodes a protein domain (called macrodomain) that is conserved in all coronaviruses (19,20), and this macrodomain binds and hydrolyzes ADP-ribose from substrates (21,22), as in other viral macrodomains. Given the conserved nature of macrodomain and the critical importance of the enzymatic activity of macrodomain in the virulence of other coronaviruses (19,20), ADP-ribosylation is likely highly regulated in SARS-CoV-2 infection. Consistent with this hypothesis, human protein interactors of SARS-CoV-2 viral proteins (23) are statistically enriched with ADP-ribosylated substrates (P = 0.0096, Chi-square test with continuity correction) (Supplementary Data S2). No statistical significance was observed between SARS-CoV-2 protein interactors with the methylated proteomes (P = 0.1977, Chi-square test with continuity correction). Notably, this statistical enrichment with ADP-ribosylated substrates was present even though the SARS-CoV-2 protein interaction map did not include the association with nonstructural protein 3 (nsP3), which possesses the macrodomain (23). These ADP-ribosylated substrates associated with SARS-CoV-2 proteins were enriched with gene ontologies, including transfer RNA and ribosomal RNA regulation (Figure 1E and Supplementary Data S2).
Increased availability of ADP-ribosylation site information
For the last 6 years, evolving proteomics techniques have allowed the identification of sites of ADP-ribosylation for functional analyses (13,24). During curation of database entries, information regarding modified sites and/or peptide sequences identified by mass spectrometry were included when available. Sequence information was then aligned to protein sequences deposited in the UniProt database (25). Currently, ∼67% of ADPriboDB 2.0 entries includes information regarding ADP-ribosylation sites, with a total of 14 839 unique sites on a range of amino acids (Figure 1F). For each database entry, users can view the site and sequence information provided by the publication. Users can also find out whether the same sites were identified in other publications or whether there are other ADP-ribosylation sites from the same proteins.
Enhanced user interface features
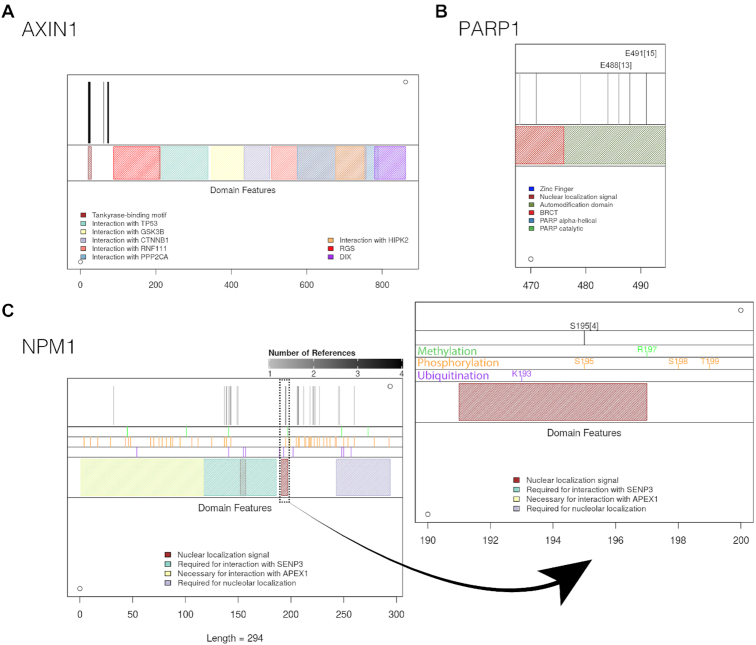
The new site information enables users to identify potential crosstalks between ADP-ribosylation and other post-translational modifications. ADPriboDB 2.0 currently features sites of phosphorylation, methylation and ubiquitination, sourced from dbPTM (15,16). To facilitate correlation analyses, ADPriboDB 2.0 includes a new Shiny-based tool to visualize the local structural and functional context of ADP-ribosylation sites, such as other post-translational modification sites [e.g. phosphorylation, as has been studied (26–28)], protein domains, as well as regions of protein–protein interaction. Vertical black lines are drawn to denote the location of ADP-ribosylation sites. For example, a high frequency of Axin1 ADP-ribosylation was found to localize around the motif that binds tankyrases—i.e. PARP5a and PARP5b (Figure 2A).
Figure 2.
New Graphical Interface. Representative images shown for (A) AXIN1 (all sites have one reference) (B) PARP1 and (C) NPM1.
To help identify ADP-ribosylation sites of high confidence, the number of publications that identified a given modified site is now provided. So far, ∼7% of sites (1018 unique sites) listed in the database have been identified by at least two publications (Supplementary Figure S1). The varying gray level of the vertical black lines corresponds to the number of publications identifying that particular site in a given protein. Users can then gauge the ‘confidence’ of a particular ADP-ribosylation site for mutagenesis studies. For example, E488 and E491 within the automodification domain of PARP1 were identified in >10 independent studies (Supplementary Data S3 and Figure 2B).
Besides, users can toggle zoom settings to visualize crowded regions at a single amino acid level (Figure 2C). For example, S195 in NPM1 within the nuclear localization signal was identified four times, and this site can also be phosphorylated (Figure 2C). Taken together, all these additional features enable users to prioritize which sites for further mechanistic analyses.
CONCLUSIONS AND FUTURE DEVELOPMENT
ADPriboDB 2.0 was updated in response to the growing volume of data regarding novel substrates and sites of ADP-ribosylation. We anticipate that the wealth of site information will stimulate new mechanistic studies, especially with the advent of new chemical approaches to generate model ADP-ribosylated peptides and methods to label ADP-ribose (29,30). Given that the database now includes a considerable amount of site and sequence information, we hope the database will encourage the development of novel algorithms for motif finding, site prediction and correlation analyses with various post-translational modifications (31,32).
With the development of chemical inhibitors and genetic ablation technologies (such as CRISPR) that can target specific PARPs, we anticipate future generations of ADPriboDB will include more information regarding substrate–enzyme specificity. As ADP-ribosylhydrolases also exhibit amino acid specificity (33), future entries may include information of enzymes that add and remove ADP-ribosylation from specific sites. Emergent studies indicate that cellular processes are regulated not only by the balance between the synthesis and removal of ADP-ribosylation, but also by maintaining the appropriate forms of ADP-ribosylation (34). Although mono(ADP-ribosyl)ation and poly(ADP-ribosyl)ation cannot be distinguished by the current proteomics methods (13), future entries will be focused on annotating this critical chain length information, especially when appropriate technologies are mature (30,35).
DATA AVAILABILITY
ADPriboDB is free and publicly available at http://adpribodb.leunglab.org/.
Supplementary Material
ACKNOWLEDGEMENTS
We thank the Leung lab members for critical comments on the manuscript and the database for improving its usability.
Contributor Information
Vinay Ayyappan, Department of Biomedical Engineering, The G.W.C. Whiting School of Engineering, Johns Hopkins University, Baltimore, MD 21218, USA.
Ricky Wat, Department of Biochemistry and Molecular Biology, Bloomberg School of Public Health, Johns Hopkins University, Baltimore, MD 21205, USA.
Calvin Barber, Department of Biophysics, Krieger School of Arts and Sciences, Johns Hopkins University, Baltimore, MD 21218, USA.
Christina A Vivelo, Department of Biochemistry and Molecular Biology, Bloomberg School of Public Health, Johns Hopkins University, Baltimore, MD 21205, USA.
Kathryn Gauch, Department of Biology, Krieger School of Arts and Sciences, Johns Hopkins University, Baltimore, MD 21218, USA.
Pat Visanpattanasin, Department of Biology, Krieger School of Arts and Sciences, Johns Hopkins University, Baltimore, MD 21218, USA.
Garth Cook, Department of Biology, Krieger School of Arts and Sciences, Johns Hopkins University, Baltimore, MD 21218, USA.
Christos Sazeides, Department of Biochemistry and Molecular Biology, Bloomberg School of Public Health, Johns Hopkins University, Baltimore, MD 21205, USA.
Anthony K L Leung, Department of Biochemistry and Molecular Biology, Bloomberg School of Public Health, Johns Hopkins University, Baltimore, MD 21205, USA; Department of Molecular Biology and Genetics, School of Medicine, Johns Hopkins University, Baltimore, MD 21205, USA; Department of Oncology, School of Medicine, Johns Hopkins University, Baltimore, MD 21205, USA.
SUPPLEMENTARY DATA
Supplementary Data are available at NAR Online.
FUNDING
American Cancer Society [129539-RSG-16–062-01-RMC]; National Institute of General Medical Sciences [R01-GM104135]; National Cancer Institute [T32CA009110 to C.A.V]. Funding for open access charge: American Cancer Society [129539-RSG-16-062-01-RMC].
Conflict of interest statement. None declared.
REFERENCES
- 1. Gupte R., Liu Z., Kraus W.L.. PARPs and ADP-ribosylation: recent advances linking molecular functions to biological outcomes. Genes Dev. 2017; 31:101–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Palazzo L., Mikoč A., Ahel I.. ADP-ribosylation: new facets of an ancient modification. FEBS J. 2017; 284:2932–2946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Houtkooper R.H., Pirinen E., Auwerx J.. Sirtuins as regulators of metabolism and healthspan. Nat. Rev. Mol. Cell Biol. 2012; 13:225–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hottiger M.O., Hassa P.O., Lüscher B., Schüler H., Koch-Nolte F.. Toward a unified nomenclature for mammalian ADP-ribosyltransferases. Trends Biochem. Sci. 2010; 35:208–219. [DOI] [PubMed] [Google Scholar]
- 5. Di Girolamo M., Dani N., Stilla A., Corda D.. Physiological relevance of the endogenous mono(ADP-ribosyl)ation of cellular proteins. FEBS J. 2005; 272:4565–4575. [DOI] [PubMed] [Google Scholar]
- 6. Hottiger M.O. Nuclear ADP-Ribosylation and Its Role in Chromatin Plasticity, Cell Differentiation, and Epigenetics. Annu. Rev. Biochem. 2015; 84:227–263. [DOI] [PubMed] [Google Scholar]
- 7. Ray Chaudhuri A., Nussenzweig A.. The multifaceted roles of PARP1 in DNA repair and chromatin remodelling. Nat. Rev. Mol. Cell Biol. 2017; 18:610–621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kim D.-S., Challa S., Jones A., Kraus W.L.. PARPs and ADP-ribosylation in RNA biology: from RNA expression and processing to protein translation and proteostasis. Genes Dev. 2020; 34:302–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Simon N.C., Aktories K., Barbieri J.T.. Novel bacterial ADP-ribosylating toxins: structure and function. Nat. Rev. Microbiol. 2014; 12:599–611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hou W.-H., Chen S.-H., Yu X.. Poly-ADP ribosylation in DNA damage response and cancer therapy. Mutat. Res. 2019; 780:82–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Fehr A.R., Singh S.A., Kerr C.M., Mukai S., Higashi H., Aikawa M.. The impact of PARPs and ADP-ribosylation on inflammation and host-pathogen interactions. Genes Dev. 2020; 34:341–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Chambon P., Weill J.D., Mandel P.. Nicotinamide mononucleotide activation of new DNA-dependent polyadenylic acid synthesizing nuclear enzyme. Biochem. Biophys. Res. Commun. 1963; 11:39–43. [DOI] [PubMed] [Google Scholar]
- 13. Daniels C.M., Ong S.-E., Leung A.K.L.. The promise of proteomics for the study of ADP-ribosylation. Mol. Cell. 2015; 58:911–924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Vivelo C.A., Wat R., Agrawal C., Tee H.Y., Leung A.K.L.. ADPriboDB: The database of ADP-ribosylated proteins. Nucleic Acids Res. 2016; 45:D204–D209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Huang K.-Y., Su M.-G., Kao H.-J., Hsieh Y.-C., Jhong J.-H., Cheng K.-H., Huang H.-D., Lee T.-Y.. dbPTM 2016: 10-year anniversary of a resource for post-translational modification of proteins. 2016; 44:D435–D446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Huang K.-Y., Lee T.-Y., Kao H.-J., Ma C.-T., Lee C.-C., Lin T.-H., Chang W.-C., Huang H.-D.. dbPTM in 2019: exploring disease association and cross-talk of post-translational modifications. Nucleic Acids Res. 2019; 47:D298–D308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hornbeck P.V., Zhang B., Murray B., Kornhauser J.M., Latham V., Skrzypek E.. PhosphoSitePlus, 2014: mutations. PTM Recalibr. 2015; 43:D512–D520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kuleshov M.V., Jones M.R., Rouillard A.D., Fernandez N.F., Duan Q., Wang Z., Koplev S., Jenkins S.L., Jagodnik K.M., Lachmann A. et al.. Enrichr: a comprehensive gene set enrichment analysis web server 2016 update. Nucleic Acids Res. 2016; 44:W90–W97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Leung A.K.L., McPherson R.L., Griffin D.E.. Macrodomain ADP-ribosylhydrolase and the pathogenesis of infectious diseases. PLoS Pathog. 2018; 14:e1006864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Alhammad Y.M.O., Fehr A.R.. The Viral Macrodomain Counters Host Antiviral ADP-Ribosylation. Viruses. 2020; 12:384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Frick D.N., Virdi R.S., Vuksanovic N., Dahal N., Silvaggi N.R.. Molecular Basis for ADP-Ribose Binding to the Mac1 Domain of SARS-CoV-2 nsp3. Biochemistry. 2020; 59:2608–2615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Alhammad Y.M.O., Kashipathy M.M., Roy A., Gagné J.-P., Nonfoux L., McDonald P., Gao P., Battaile K.P., Johnson D.K., Poirier G.G. et al.. The SARS-CoV-2 conserved macrodomain is a highly efficient ADP-ribosylhydrolase. bioRxiv doi:12 May 2020, preprint: not peer reviewed 10.1101/2020.05.11.089375. [DOI]
- 23. Gordon D.E., Jang G.M., Bouhaddou M., Xu J., Obernier K., White K.M., O’Meara M.J., Rezelj V.V., Guo J.Z., Swaney D.L. et al.. A SARS-CoV-2 protein interaction map reveals targets for drug repurposing. Nature. 2020; 583:459–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Zhen Y., Yu Y.. Proteomic Analysis of the Downstream Signaling Network of PARP1. Biochemistry. 2018; 57:429–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Consortium U.P. UniProt: a worldwide hub of protein knowledge. Nucleic Acids Res. 2019; 47:D506–D515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Bartlett E., Bonfiglio J.J., Prokhorova E., Colby T., Zobel F., Ahel I., Matic I.. Interplay of Histone Marks with Serine ADP-Ribosylation. Cell Rep. 2018; 24:3488–3502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Larsen S.C., Hendriks I.A., Lyon D., Jensen L.J., Nielsen M.L.. Systems-wide Analysis of Serine ADP-Ribosylation Reveals Widespread Occurrence and Site-Specific Overlap with Phosphorylation. Cell Rep. 2018; 24:2493–2505. [DOI] [PubMed] [Google Scholar]
- 28. Gibson B.A., Zhang Y., Jiang H., Hussey K.M., Shrimp J.H., Lin H., Schwede F., Yu Y., Kraus W.L.. Chemical genetic discovery of PARP targets reveals a role for PARP-1 in transcription elongation. Science. 2016; 353:45–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. van der Heden van Noort G.J. Chemical Tools to Study Protein ADP-Ribosylation. ACS Omega. 2020; 5:1743–1751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ando Y., Elkayam E., McPherson R.L., Dasovich M., Cheng S.-J., Voorneveld J., Filippov D.V., Ong S.-E., Joshua-Tor L., Leung A.K.L.. ELTA: Enzymatic Labeling of Terminal ADP-Ribose. Mol. Cell. 2019; 73:845–856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Li G.X.H., Vogel C., Choi H.. PTMscape: an open source tool to predict generic post-translational modifications and map modification crosstalk in protein domains and biological processes. Mol Omics. 2018; 14:197–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Chang C.-C., Tung C.-H., Chen C.-W., Tu C.-H., Chu Y.-W.. SUMOgo: Prediction of sumoylation sites on lysines by motif screening models and the effects of various post-translational modifications. Sci. Rep. 2018; 8:15512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Rack J.G.M., Palazzo L., Ahel I.. (ADP-ribosyl)hydrolases: structure, function, and biology. Genes Dev. 2020; 34:263–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Suskiewicz M.J., Zobel F., Ogden T.E.H., Fontana P., Ariza A., Yang J.-C., Zhu K., Bracken L., Hawthorne W.J., Ahel D. et al.. HPF1 completes the PARP active site for DNA damage-induced ADP-ribosylation. Nature. 2020; 579:598–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Martello R., Mangerich A., Sass S., Dedon P.C., Bürkle A.. Quantification of cellular poly(ADP-ribosyl)ation by stable isotope dilution mass spectrometry reveals tissue- and drug-dependent stress response dynamics. ACS Chem. Biol. 2013; 8:1567–1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
ADPriboDB is free and publicly available at http://adpribodb.leunglab.org/.