Abstract
Background:
Prediabetes awareness in adults has been associated with improved weight management. Whether youth with prediabetes diagnosis experience similar improvements is unknown.
Objective:
To investigate the association between prediabetes identification and body mass index (BMI) trajectory in overweight and obese adolescents.
Subjects:
Youth who were followed longitudinally in a large academic-affiliated primary care network and who were overweight/obese while 10 to 18 years old.
Methods:
Retrospective cohort study. Subjects were categorized as “screened” if at least 1 hemoglobin A1c (HbA1c) result was available. Time series analysis was used to determine the difference in difference (DID) in BMI Z-score (BMI-Z) slope before and after HbA1c between: (a) screened youth found to have prediabetes-range HbA1c (5.7%-6.4%, 39-46 mmol/mol) versus normal HbA1c and (b) screened versus age-matched unscreened obese youth.
Results:
A total of 4184 (55.6% female) screened subjects (median follow-up 9.7 years) were included. In which, 637 (15.2%) had prediabetes-range HbA1c. Prediabetes was associated with a greater decrease in BMI-Z slope than normal HbA1c (DID: −0.023/year [95% CI: −0.042 to −0.004]). When compared to age-matched unscreened subjects (n = 2087), screened subjects (n = 2815) experienced a greater decrease in BMI-Z slope after HbA1c than unscreened subjects at a matched age (DID: −0.031/y [95% CI −0.042 to −0.021]).
Conclusions:
BMI-Z trajectory improved more among youth with prediabetes-range HbA1c but also stabilized in screened youth overall. Prospective studies are needed to identify provider- and patient-level drivers of this observation.
Keywords: adolescent, body mass index, glycated hemoglobin a, obesity, prediabetic state
1 ∣. INTRODUCTION
Debate exists about the utility of screening for type 2 diabetes (T2D) in overweight or obese adolescents due to the low prevalence of undiagnosed T2D.1,2 However, prediabetes, an intermediate state of glucose metabolism, occurs in approximately one-quarter of obese youth.3 Although prediabetes in youth can be transient and related to insulin resistance of puberty,4 it may also present an opportunity for T2D risk reduction. In adults, awareness of prediabetes status is associated with improvements in weight and glycemic status.5,6 Therefore, we investigated whether prediabetes diagnosis by hemoglobin A1c (HbA1c) screening is associated with improved body mass index (BMI) trajectory in youth.
2 ∣. METHODS
This retrospective cohort study of data from the Children’s Hospital of Philadelphia (CHOP) Primary Care Network (2000—2018) was approved by the CHOP Institutional Review Board. The cohort included non-diabetic (by International Classification of Disease-9/10 codes, 249.x, 250.x; E08.x-E.11x., E13.x) youth who were overweight or obese (BMI Z-score [BMI-Z] ≥1.04) when 10 to 18 years old. “Screened” youth were those who underwent T2D screening via HbA1c between ages 10 to 18 years; if ≥1 HbA1c was available, only the first was considered. “Unscreened” youth were those without available HbA1c result while 10 to 18 years old. All screened subjects had ≥1 BMI before and after HbA1c. Unscreened obese youth were matched by age at start of follow up with screened youth and had ≥1 BMI before and after the matched age of the corresponding screened subject.
To minimize confounding of the relationship between BMI and hyperglycemia, subjects were excluded if they were prescribed anti-psychotics, frequent systemic glucocorticoids (>4 courses), or growth hormone, or had a transplant of any type or cystic fibrosis. Follow-up was from first to last BMI measurement or age 19 years. Among screened subjects, two groups were defined by HbA1c: prediabetes (5.7%-6.4%, 39-46 mmol/mol) and normal (<5.7%, <39 mmol/mol). Subjects with HbA1c ≥6.5% (≥48 mmol/mol) were excluded to reduce confounding due to hyperglycemia-related weight loss.
Prior to analysis, anthropometric measurements identified as likely errors were excluded using an automated method.7-9 The primary outcome was change in age- and sex-adjustedBMI-Z10 trajectory after HbA1c. To account for skewness, BMI-Z ≥1.96 were replaced with a modified SD score.11
Time series analysis was used to determine the impact of: (a) HbA1c result (normal vs prediabetes among the screened group) and (b) the act of screening (screened vs unscreened) on BMI-Z trajectory. Plots of unadjusted mean BMI-Z per year were generated to depict trends by group. To account for intra-subject correlation, generalized estimating equations with autoregressive correlation structure and identity link were used, with BMI-Z as the dependent variable. Independent variables included time before or after HbA1c, indicator variable for pre/post-A1c, and HbA1c group (prediabetes vs normal) or screened versus unscreened. The outcome of interest, difference in difference (DID) in BMI-Z slope between groups before and after screening or matched age, was determined using an interaction term between time, pre/post-screening, and group.12 The model adjusted for BMI-Z at HbA1c, age at HbA1c, sex, race (Caucasian, African American, Asian, multiple races, other), ethnicity (Hispanic, Non-Hispanic), and insurance type (private, public).
Two sensitivity analyses were conducted. First, BMI, BMI-Z without adjustment for extreme values, weight, and height (falsification test: height trajectory would not be modifiable) were used as dependent variables in separate models. Second, we repeated analyses using “normal” and “high” HbA1c of < or ≥6.0% (42 mmol/mol), based on clinical recommendations at our institution to consider referral to Endocrinology at that threshold, as well as the higher likelihood of persistent or progressive dysglycemia above that threshold in adolescents.4
Analyses were conducted using Stata 14 (Stata Corporation, TX). Descriptive analyses included means and standard deviations of continuous variables and distributions of categorical variables. Differences of means were assessed using Student’s t-test. Group differences in categorical variables were assessed using Pearson’s chi-squared test. Two-sided hypothesis tests were used; P < 0.05 was considered statistically significant.
3 ∣. RESULTS
After exclusion of 18 subjects with diabetes-range HbA1c, the screened cohort included 4184 (n = 2327, 55.6% female) adolescents followed for a median of 9.7 years (IQR 8.0-10.7), contributing 38 694 BMIs, with a median follow-up of 2.3 (IQR 1.4-3.0) years after HbA1c measurement. 637 (15.2%) had prediabetes-range HbA1c (mean [SD] 5.9% (0.1%), 41 (1) mmol/mol). Mean age at testing was slightly higher among those with normal HbA1c (13.2 [95% CI 13.1-13.3] vs 12.9 [95% CI 12.8-13.1] years). Weight, BMI, and BMI Z-score were higher among the prediabetes group at testing (all P < 0.005). Gender distribution was not different (P = 0.2). The prediabetes group had more African American youth (64% vs 42%, P < 0.0001).
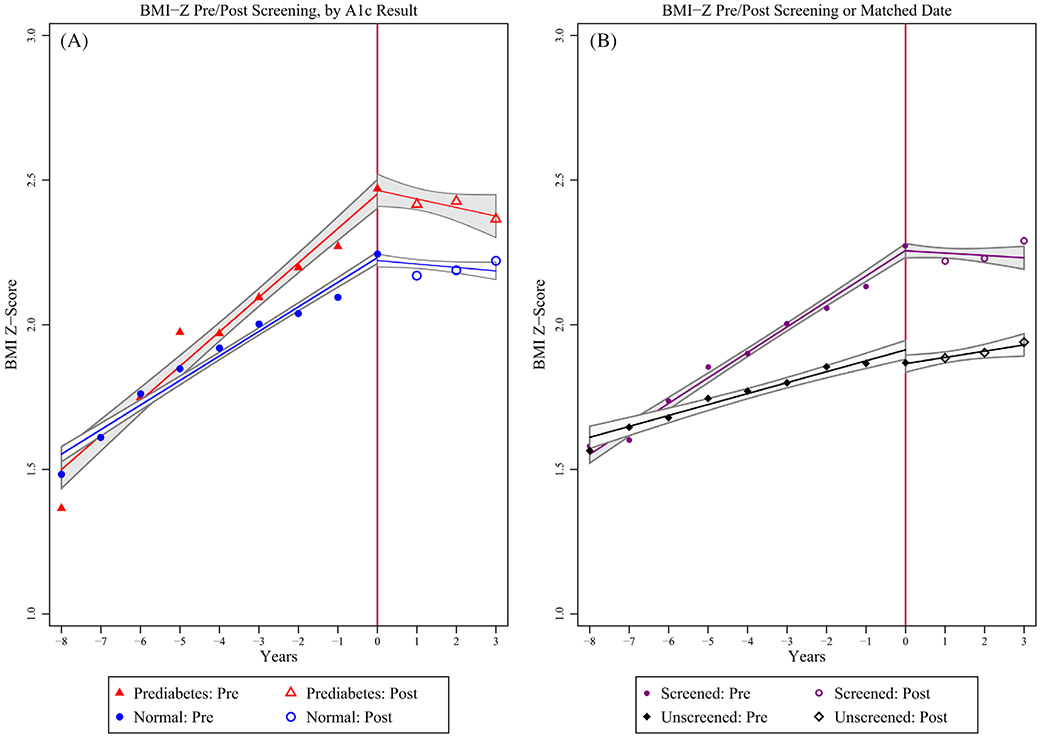
BMI-Z was similar at start of follow-up (P = 0.5), gradually diverged, then plateaued (normal HbA1c) or decreased (prediabetes) after HbA1c (Figure 1A). In time series analysis, BMI-Z increase was significantly greater among the prediabetes group than the normal HbA1c group prior to screening (0.061/y [95% CI 0.049-0.073] vs 0.036/y [95% CI 0.030-0.042]). Post-HbA1c, both had significantly decreased slopes (prediabetes: 0.011/y [95% CI −0.006-0.029] vs normal: 0.009/y [95% CI 0.002-0.017]), but the improvement was greater in the prediabetes group: pre/post difference of −0.050/y (prediabetes) versus −0.027 (normal), DID = −0.023/y (95% CI: −0.042 to −0.004).
FIGURE 1.
Mean BMI Z-score by year before and after initial HbA1c. Vertical line at “0” indicates screening HbA1c. Line and gray bands represent linear regression line fit and associated 95% confidence intervals. A, Full screened cohort and B, age-matched screened and unscreened cohort
To assess the impact of screening regardless of result, 2815 screened subjects were age-matched with 2087 unscreened obese subjects. Screened subjects were more often female (55.3% vs 41.7%, P < 0.001) and African American (46.3% vs 30.6%, P < 0.001). Screened subjects had a plateau in BMI-Z after screening, while unscreened did not after matched age (Figure 1B). Adjusting for BMI-Z at HbA1c (or matched age), sex, age, race, ethnicity, and insurance type, BMI-Z slope decreased more in the screened than the unscreened group (DID: −0.031/y [95% CI −0.042 to −0.021]).
3.1 ∣. Sensitivity analyses
Among the screened cohort, a greater improvement in slope in the prediabetes group was again found for BMI and unmodified BMI-Z, but not for weight or height (BMI DID: −0.13/y [95% CI −0.23 to −0.03]; unmodified BMI-Z DID: −0.013/y [95% CI −0.025 to −0.001]). The differential reduction in BMI-Z slope was even greater when a higher HbA1c threshold to define “abnormal” was used: DID = −0.049/y (95% CI −0.082 to −0.015).
4 ∣. DISCUSSION
To our knowledge, this is the first study to report an improved BMI trajectory after prediabetes identification in overweight and obese youth. In addition, our demonstration of greater slowing of BMI-Z gain after screening versus no screening suggests that the act of screening may have value even if results are normal. Our study has several strengths, including the large, diverse cohort of adolescents screened by HbA1c, a median follow-up of nearly a decade, and use of a robust algorithm to identify implausible growth data.
One possible explanation for the greater improvement in BMI trajectory after prediabetes diagnosis is intrinsic motivation due to perceived risk.13 Alternatively, differences in counseling by pediatricians may be operative. If elucidated, the factors responsible for this differential improvement could be harnessed to increase the utility of identifying prediabetes in adolescents. The differential improvement among screened versus unscreened patients may reflect actions taken by pediatrician at the time of screening. This should be further explored to determine whether the act of ordering screening, beyond any associated counseling, is necessary to achieve the observed improvement in BMI trajectory.
We acknowledge limitations, including the inability to determine how prediabetes diagnosis was conveyed or patients counseled. We were unable to assess (a) patient-level factors influencing completion of ordered screening tests or (b) provider-level factors related to screening some obese adolescents but not others, but we suspect additional risk factors, such as higher-risk race/ethnicity, informed some decision-making.14 In addition, our findings are limited to prediabetes defined by HbA1c, which is 0.3% to 0.4% higher in African American compared to White non-diabetic adults even after adjustment for age and adiposity and does not have 100% concordance with fasting glucose and oral glucose tolerance tests.15,16 Nonetheless, HbA1c is widely used for diabetes screening in pediatric practice.17 Finally, the possible concurrent identification of other obesity-or family-related health risks was not investigated in this study. However, a previous study using data from the same primary care network found that routine lipid screening was not associated with altered BMI trajectory in overweight and obese youth.18
Whether results from T2D screening in overweight and obese adolescents can be leveraged to promote BMI improvement is unknown, but our findings suggest that HbA1c-based screening may be useful beyond its intended goal of T2D identification.
ACKNOWLEDGMENTS
Dr. Vajravelu was supported by grant 5K12DK094723-08. Dr. Lee is supported by grants NIH R01HD074559-01-A1 and NIH UH3HD087979, the Juvenile Diabetes Research Foundation United States of America, and the M-Diabetes Center of Excellence at the University of Michigan. Dr. Amaral was supported by grants R01DK110749, R01DK120886, and R01HD091185. Dr. Kelly was supported by grant R01DK115648.
Funding information
Eunice Kennedy Shriver National Institute of Child Health and Human Development, Grant/Award Numbers: R01HD074559-01-A1, R01HD091185, UH3HD087979; Juvenile Diabetes Research Foundation United States of America; M-Diabetes Center of Excellence at the University of Michigan; National Institute of Diabetes and Digestive and Kidney Diseases, Grant/Award Numbers: 5K12DK094723-08, R01DK110749, R01DK115648, R01DK120886
Footnotes
CONFLICT OF INTEREST
Dr. Lee serves as a consultant to T1D Exchange and receives grant funding from Lenovo. All other authors have no relevant conflicts of interest to disclose.
ETHICS STATEMENT
This study was approved by the Children’s Hospital of Philadelphia Institutional Review Board.
REFERENCES
- 1.Wu EL, Kazzi NG, Lee JM. Cost-effectiveness of screening strategies for identifying pediatric diabetes mellitus and dysglycemia. JAMA Pediatr. 2013;167(1):32–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zeitler P, Arslanian S, Fu J, et al. ISPAD clinical practice consensus guidelines 2018: type 2 diabetes mellitus in youth. Pediatr Diabetes. 2018;19(Suppl 27):28–46. [DOI] [PubMed] [Google Scholar]
- 3.Andes LJ, Cheng YJ, Rolka DB, Gregg EW, Imperatore G. Prevalence of prediabetes among adolescents and young adults in the United States, 2005–2016. JAMA Pediatr. 2019:e194498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Love-Osborne KA, Sheeder JL, Nadeau KJ, Zeitler P. Longitudinal follow up of dysglycemia in overweight and obese pediatric patients. Pediatr Diabetes. 2018;19(2):199–204. [DOI] [PubMed] [Google Scholar]
- 5.Gopalan A, Lorincz IS, Wirtalla C, Marcus SC, Long JA. Awareness of prediabetes and engagement in diabetes risk-reducing behaviors. Am J Prev Med. 2015;49(4):512–519. [DOI] [PubMed] [Google Scholar]
- 6.Owei I, Umekwe N, Ceesay F, Dagogo-Jack S. Awareness of prediabetes status and subsequent health behavior, body weight, and blood glucose levels. J Am Board Fam Med. 2019;32(1):20–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gerber JS, Bryan M, Ross RK, et al. Antibiotic exposure during the first 6 months of life and weight gain during childhood. Jama. 2016; 315(12):1258–1265. [DOI] [PubMed] [Google Scholar]
- 8.Daymont C, Neal A, Prosnitz A, Cohen MS. Growth in children with congenital heart disease. Pediatrics. 2012;131(1):e236–e242. [DOI] [PubMed] [Google Scholar]
- 9.Daymont C, Ross ME, Russell Localio A, Fiks AG, Wasserman RC, Grundmeier RW. Automated identification of implausible values in growth data from pediatric electronic health records. J Am Med Inform Assoc. 2017;24(6):1080–1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kuczmarski RJ, Ogden CL, Guo SS, et al. CDC growth charts for the United States: methods and development. Vital Health Stat 11. 2000; 2002(246):1–190. [PubMed] [Google Scholar]
- 11.Prevention. NCfCDPaHP-CfDCa. Cut-Offs to Define Outliers in the 2000 CDC Growth Charts 2014; http://www.cdc.gov/nccdphp/dnpa/growthcharts/resources/BIV-cutoffs.pdf. Accessed March 30, 2019.
- 12.Kontopantelis E, Doran T, Springate DA, Buchan I, Reeves D. Regression based quasi-experimental approach when randomisation is not an option: interrupted time series analysis. BMJ. 2015;350:h2750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rosenstock I, Stretcher V, Becker M. Social learning theory and the health belief model. Health Educ Q. 1988;15(2):175–183. [DOI] [PubMed] [Google Scholar]
- 14.American Diabetes A. 2. Classification and diagnosis of diabetes: standards of medical Care in Diabetes-2019. Diabetes Care. 2019;42 (Suppl 1):S13–S28. [DOI] [PubMed] [Google Scholar]
- 15.Vajravelu ME, Lee JM. Identifying prediabetes and type 2 diabetes in asymptomatic youth: should HbA1c be used as a diagnostic approach? Curr Diab Rep. 2018;18(7):43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Herman WH, Dungan KM, Wolffenbuttel BH, et al. Racial and ethnic differences in mean plasma glucose, hemoglobin A1c, and 1,5-anhydroglucitol in over 2000 patients with type 2 diabetes. J Clin Endocrinol Metab. 2009;94(5):1689–1694. [DOI] [PubMed] [Google Scholar]
- 17.Lee JM, Eason A, Nelson C, Kazzi NG, Cowan AE, Tarini BA. Screening practices for identifying type 2 diabetes in adolescents. J Adolesc Health. 2014;54(2):139–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gregory EF, Miller JM, Wasserman RC, Seshadri R, Rubin DM, Fiks AG. Routine cholesterol tests and subsequent change in BMI among overweight and obese children. Acad Pediatr. 2019;19(7): 773–779. [DOI] [PubMed] [Google Scholar]