Abstract
Background
Allocation of scarce resources during a pandemic extends to the allocation of vaccines when they eventually become available. We describe a framework for priority vaccine allocation that employed a cross-disciplinary approach, guided by ethical considerations and informed by local risk assessment.
Methods
Published and grey literature was reviewed, and augmented by consultation with key informants, to collate past experience, existing guidelines and emerging strategies for pandemic vaccine deployment. Identified ethical issues and decision-making processes were also included. Concurrently, simulation modelling studies estimated the likely impacts of alternative vaccine allocation approaches. Assembled evidence was presented to a workshop of national experts in pandemic preparedness, vaccine strategy, implementation and ethics. All of this evidence was then used to generate a proposed ethical framework for vaccine priorities best suited to the Australian context.
Findings
Published and emerging guidance for priority pandemic vaccine distribution differed widely with respect to strategic objectives, specification of target groups, and explicit discussion of ethical considerations and decision-making processes. Flexibility in response was universally emphasised, informed by real-time assessment of the pandemic impact level, and identification of disproportionately affected groups. Model outputs aided identification of vaccine approaches most likely to achieve overarching goals in pandemics of varying transmissibility and severity. Pandemic response aims deemed most relevant for an Australian framework were: creating and maintaining trust, promoting equity, and reducing harmful outcomes.
Interpretation
Defining clear and ethically-defendable objectives for pandemic response in context aids development of flexible and adaptive decision support frameworks and facilitates clear communication and engagement activities.
Keywords: Pandemic vaccine, Vaccine allocation, Vaccine ethics, Priority populations
1. Background
A key feature of any pandemic, apparent already for coronavirus disease 2019 (COVID-19), is the need to allocate scarce resources. By March 2020, there had already been shortages of RNA extraction kits required for SARS-CoV-2 testing [1], personal protective equipment, intensive care unit (ICU) beds, ventilators and clinical staff with the expertise to operate them [2], [3]. Pandemic mitigation measures aim to reduce pressure on health system resources by slowing the growth of an epidemic. For example, physical distancing limits transmission events thereby increasing the likelihood that hospital beds are available for those who need them [4]. Another important objective for non-pharmaceutical mitigation strategies is to delay local epidemics while developing vaccines.
Vaccines remain the most effective and practical means of preventing transmission in an epidemic and, if widely available, could circumvent the psychological and economic consequences of prolonged non-pharmaceutical interventions [5], [6]. They are thus an essential component of pandemic response plans. For influenza, vaccine could be expected within weeks using existing technology, while for novel pathogens, such as SARS-CoV-2, vaccine availability may be dependent on the development of new technology, which could take many months. Indeed, it has taken some 9 months since the COVID-19 pandemic was declared for vaccines to become available [7]. However, as the 2009 influenza A(H1N1)pdm09 pandemic highlighted [8], there may be additional delays and challenges in producing, distributing and administering strain-specific pandemic vaccines that limited their timely, equitable and effective use.
The WHO’s Pandemic Influenza Risk Management guidance recommends that member states establish goals and priorities for use of pandemic influenza vaccines, guided by ethical considerations, and informed by local risk assessment [9]. Similar goals and priorities will apply to COVID-19 vaccines. To address this recommendation, a cross-disciplinary team of researchers was contracted by the Australian Government Department of Health in 2018 to provide advice on best use of first-available vaccines in the early phases of pandemic response. This paper outlines the process by which a flexible tiered framework to guide priority vaccine allocation was developed for use in the Australian context and the outcomes of that process. While this body of work was completed for pandemic influenza, the insights gained are applicable to COVID-19.
2. Methods
This multi-faceted approach included reviews of existing pandemic plans, reviews of the lessons learnt from 2009 and relevant ethical considerations, local and international consultation, and simulation modelling. Assembled evidence was presented to a workshop of national experts and used to generate a proposed ethical framework for vaccine priorities.
2.1. Review of existing plans
2.1.1. Pandemic vaccine allocation strategies
Current pandemic influenza plans of the Organisation for Economic Co-operation and Development (OECD) countries were reviewed, with particular attention to countries with similar pandemic response capabilities. Several search strategies were used, including a review of World Health Organization (WHO) and European Centre for Disease Prevention and Control (ECDC) webpages on pandemic planning, and a Google search using ‘influenza pandemic plan’ and ‘country’. Only the most recently published English version of any national plan was included. Each was evaluated according to the following criteria [10] (see Table 1 ):
-
(1)
Does the country have a list of pandemic vaccine priority groups?
-
(2)
Are pandemic vaccine priority groups ranked and if so, how?
-
(3)
What is the rationale for the prioritisation concept?
-
(4)
Were ethical committees involved in establishing this concept?
-
(5)
Which types of institutions were involved in development of vaccine priority groups?
Table 1.
Summary of pandemic plans reviewed and updated since 2009.
| United States | Canada | New Zealand | United Kingdom | France | Switzerland | |
|---|---|---|---|---|---|---|
| List of pandemic vaccine priority groups | Yes, specified in figure. | Yes, but only as proposed priority groups. A framework guides development of prioritisation recommendations during the pandemic vaccine production process. | No, prioritisation will be published when a pandemic vaccination campaign is deemed to be necessary and in consideration of various factors. | Yes – presumed to be clinical risk groups and front-line health and social care workers, but dependent on the emerging profile of at-risk groups for a new pandemic virus. | Yes, but only suggested groups. Decision-making is deferred to when the pandemic occurs. | No, but examples of groups that might be prioritised are given. |
| Ranking of pandemic vaccine priority groups | Yes | Not applicable, but plan has flexibility to adopt group prioritisation rankings. | Not applicable | No | Not applicable | Not applicable |
| Rationale for pandemic vaccine prioritisation | Maintain infrastructure and functioning of the health care system, protecting children, maintaining homeland and national security. | Reduce morbidity and mortality, limit social disruption. | Not applicable | Reduce mortality and morbidity | Choice or combination of limiting dissemination in the population, and reducing serious morbidity and mortality. | Flexible to accommodate the scenario. |
| Evidence cited for pandemic vaccine prioritisation | Rigorous scientific assessments conducted, but references not listed. | Not applicable, but framework for pandemic vaccine prioritisation asks key questions with respect to scientific evidence. | Not applicable | Limited | No | Not applicable |
| Ethical committee involvement in prioritisation strategy | Yes | Yes | Not applicable, but pandemic plan has ethical framework. | No | Not indicated | Yes, plan has a strong ethical foundation. |
2.1.2. Lessons learned from the influenza A(H1N1) pdm09 pandemic
To identify gaps between pre-existing plans and implementation of vaccine strategies during the 2009 pandemic response, a literature search was undertaken in OVID Medline using the Medical Subject Headings (MeSH) terms ‘Pandemics’, ‘Influenza A Virus, H1N1 Subtype’, ‘Disaster Planning’, ‘Health Planning’, ‘Health Policy’, ‘Public Health Administration’, ‘Communicable Disease Control’, ‘Evaluation Studies’, ‘Program Evaluation’, ‘Immunization’ and ‘Immunization Programs’. A text word search was conducted on evaluation or reviews of influenza pandemic plans. Search results were de-duplicated, limited to ‘Humans’, English language and publication years 2009–2018. In addition, a grey literature search for reports on pandemic vaccine program evaluation commenced with Google Scholar keywords ‘pandemic’, ‘plan’, ‘influenza’, ‘lessons’, ‘review’, ‘response’, ‘evaluation’, and combinations of these terms with country names. Government health websites from Australia, the US, Canada, NZ and the UK were searched, along with key agencies such as the US Centers for Disease Control and Prevention (CDC) and Public Health England (PHE).
2.1.3. Ethical considerations relevant to pandemic vaccine allocation
Papers were initially selected via a Google Scholar search and from the authors’ reference collection. A snowball technique followed up citations in key publications. A systematic literature search was not conducted, as terms in ethics are not uniformly used or indexed in databases, leading to poor sensitivity and specificity [11].
2.1.4. Individual consultation with key informants
Semi-structured interviews were conducted with representatives of communicable disease and pandemic preparedness programs from each Australian state and territory, Canada, Japan, Singapore, NZ, the UK, the US, the WHO and ECDC (see Supplementary Material). Findings were transcribed and synthesised for recurrent themes and salient recommendations.
2.2. Modelling vaccine strategies
A previously developed mathematical model of pandemic influenza [12] was adapted, which simulates differing pandemic scenarios, here constrained to 3 scenarios: (1) low transmissibility/high severity, (2) moderate transmissibility/moderate severity and (3) high transmissibility/high severity. We assumed vaccine delivery six weeks into the local epidemic, with sensitivity analyses of zero and twelve weeks. To compare proposed vaccine allocation strategies additional population strata and vaccine administration were included in the model framework. Model outputs captured outcomes of public health importance for each group including clinical attack rate, hospitalisations, intensive care unit (ICU) admissions and deaths in hospital. A full description of model structure and assumptions is in the Supplementary Material .
Broadly, the model considered two dimensions of strategic vaccine use:
-
(1)
Comparison of a direct protection strategy focused on preventing severe outcomes by targeting ‘at risk’ groups, with an indirect protection strategy aimed at reducing transmission by vaccinating school children;
-
(2)
For scenarios where two vaccine doses are required for optimal protection, comparison of the impact of a two dose (complete) schedule, with that of a single dose administered to twice as many individuals.
Vaccine strategies were considered in isolation to assess their likely contribution to reducing infection and disease burden, without confounding due to the influence of other public health measures that may be differentially applied to population subgroups.
2.3. Expert consultation workshop
Assembled evidence was presented to a workshop of national experts in infectious diseases epidemiology, vaccinology, ethics, policy, Aboriginal health and program implementation, with representatives of the Communicable Diseases Network of Australia (CDNA), the Australian Health Protection Principal Committee (AHPPC), and OHP. Workshop aims were:
-
(1)
Develop a shared understanding of the evidence, and of the framework for prioritisation and distribution of future pandemic vaccines;
-
(2)
Identify ethical considerations for prioritisation and distribution;
-
(3)
Decide on the most relevant evidence supporting the framework for presentation to community groups that might subsequently be engaged in the process of decision making.
2.4. Constructing the ethics framework
All of the different elements of the project as outlined above were used in constructing an ethics framework, which is a practical heuristic to help decision makers with difficult and complex judgments. A good framework seeks to provide a ‘frame’ for such decisions built upon all relevant empirical and ethical considerations [13] in response to the particular circumstances of a pandemic. It requires judgment and flexibility. Unlike some regulatory documents it does not seek to provide a list of rules or all of the answers. The framework clearly articulates a set of steps in decision-making: defining clear aims, setting up processes for decision-making, distinguishing decisions and actions that can be made pre-pandemic and those that will have to wait until the characteristics of the pandemic and vaccine are known.
3. Findings
3.1. Review of existing plans
3.1.1. Pandemic vaccine allocation strategies
Twelve pandemic plans were retrieved, six published or updated since 2009. Only post-2009 plans incorporated flexible vaccine prioritisation strategies, proportionate to pandemic risk profile and impact. Eleven plans prioritised individuals at high risk of death and complications from influenza, including First Nations populations, pregnant women, age-defined risk groups, and individuals working in high exposure (i.e. clinical or institutional) settings. Health care workers and essential service providers were specifically named in nine, in different priority orders compared with at-risk individuals, and with varying granularity in description of services provided. Four plans included healthy adults and children within whole-population vaccine delivery, but generally in low priority order. Three plans recommended childhood immunisation to reduce transmission, while a fourth prioritised children to safeguard the country’s future (Section 3, Supplementary Material).
Pandemic plans of Canada [14], NZ [15], France [16] and Switzerland [17] did not detail vaccine priority groups, leaving determinations to be made based on epidemiological and vaccine characteristics, including timeliness of availability, during a pandemic. The US [18] had the most comprehensive prioritisation strategy, including priority tiers and severity scenarios, but retained flexibility to adapt to pandemic-specific factors. The UK prioritised allocation of pre-pandemic vaccines to frontline healthcare workers and clinical at-risk groups, with a more flexible strategy for distribution of pandemic vaccines to minimise morbidity and mortality [19]. Japan prioritised vaccine allocation by scenario, based on the age distribution of severe disease and whether the rationale for response was to reduce short term harms or protect the country’s future [20]. NZ, Switzerland and Canada were noteworthy in documenting ethical considerations in their priority allocation.
3.1.2. Lessons learned from the influenza A(H1N1) pdm09 pandemic
Experience of vaccine use in 2009 was reviewed at multinational level [21], [22], [23], [24], [25] (Supplementary Table S4). A key lesson was a requirement for flexible and adaptable pandemic vaccine prioritisation strategies. Changes in approach may be required depending on observed epidemiology, characteristics and availability of pandemic vaccines, underscoring the need for maintaining currency of the data used to inform the approach. However, flexibility may need to be weighed against perceptions of a lack of consistency in messaging. The timeliness and quantity of vaccines available will influence whether planned strategies can be implemented.
Communication of the rationale and objectives of vaccine prioritisation strategies was a critical determinant of uptake among target groups, with implications for coverage and impact. Health care workers were a group in whom priority vaccination helps maintain health care services, protect vulnerable patients and promote professional and public confidence in the vaccination program. Safety concerns need to be acknowledged, particularly where these concerns are associated with vaccine hesitancy among priority groups.
3.1.3. Ethical considerations relevant to pandemic vaccine allocation
Vaccine rationing involves deciding who to vaccinate first in a situation where many may seek vaccination. Relevant literature focused on the ethics of prioritising vaccine or included vaccines in a broader discussion of scarce resource allocation in a pandemic. The conditions of a well-resourced democracy, such as Australia, were assumed.
A large number of priority groups were described in the literature, reflecting an absence of consensus on ethical principles to guide early vaccine allocation [26]. Some groupings were social [27], some based on anticipated medical need or vaccine response [28], and others based on stage of life [29]. Listed priority groups depended on the aims of a vaccine program, which again differed widely, were often opaque, and were influenced by pandemic severity. Even where saving lives was the primary goal, opinions differed about which lives were most valuable. Documents that considered the normative value of potential target groups tended to favour children and the young [29], [30]. Additional motivations for priority included maintenance of services and supporting resilience in the recovery phase [31]. Ethical justifications for prioritising particular groups tended to be utilitarian, maximising ‘good’ by minimising illness and death. Others made justice-based arguments that supported prioritising those held to be ‘worst off.’
There was consistent emphasis on the need for procedural justice, a set of principles focused on transparency and accountability. Clear, respectful communication and information dissemination across all affected population groups was held to be important. Public health decision makers may face unanticipated challenges in implementation, and decision-making needs to be responsive. The place of vaccination must be considered within a suite of measures some of which may be more or less appropriate or accessible to some populations.
3.1.4. Individual consultation with key opinion leaders
Representatives of jurisdictions interviewed reflected differing currency of pandemic plans, with several in the process of reviewing vaccine allocation strategies. Clear goals were deemed essential to prioritisation, with recognition that these might include maintaining either or both of the healthcare system and the economy.
Healthcare workers, including primary care professionals, were uniformly prioritised for vaccination given their importance in implementation, increased exposure risk, and the need to maintain trust. The process by which other essential service workers were identified varied and would be modified by pandemic severity, with additional priority groups also defined in real-time. The value of pandemic special studies, modelling and stakeholder consultation was emphasised and would be expedited by having predefined data requirements and reliable denominators.
A salient theme was the need for flexibility, recognising that public perception and political considerations will further influence decision making. Moreover, as epidemiological information becomes available the priority framework may change. For example, in 2009 the UK plan de-prioritised children once it became clear from serosurveillance that there were already high rates of infection in this age group.
Pragmatic issues related to vaccine production, distribution and administration were highlighted, given potential for bottlenecks in implementation. As a pandemic evolves, perceptions of disease risk vary, with subsequent implications for demand. Concerns about vaccine wastage were raised, associated with oversupply or maldistribution, and use of multi-dose vials (associated with safety concerns).
The incorporation of ethical principles varied widely, with many jurisdictions relying on in-house expertise or formal advice from ethics advisory committees. The US and Canada were notably proactive in consulting with relevant communities. Vaccine safety concerns were viewed as especially problematic by some, particularly in children.
Consistency of communications about vaccine timeliness, prioritisation groups and safety was deemed essential, particularly where multiple jurisdictions are involved in implementing regional or subnational responses. Respondents emphasised the need to prime communities with clear messaging to increase acceptability, and avoid confusion and mistrust.
3.2. Modelling vaccine strategies
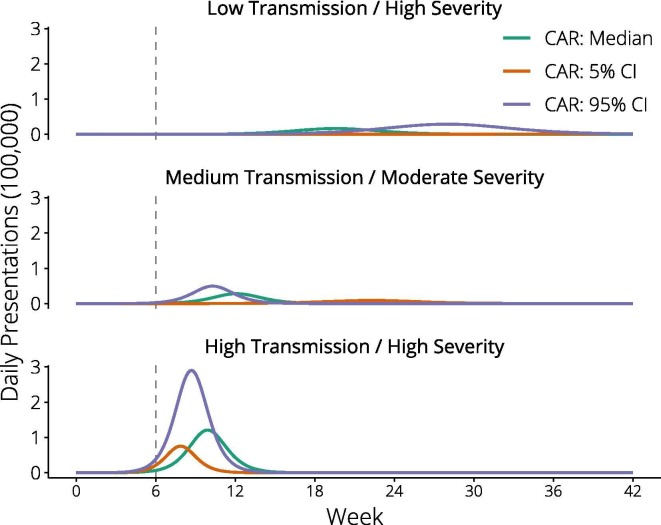
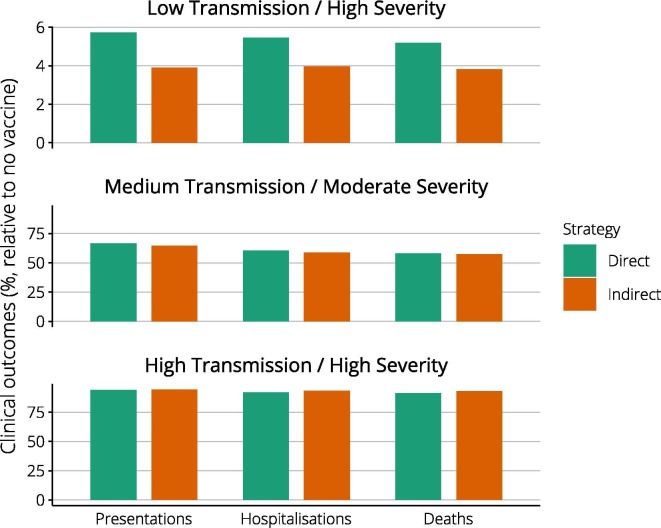
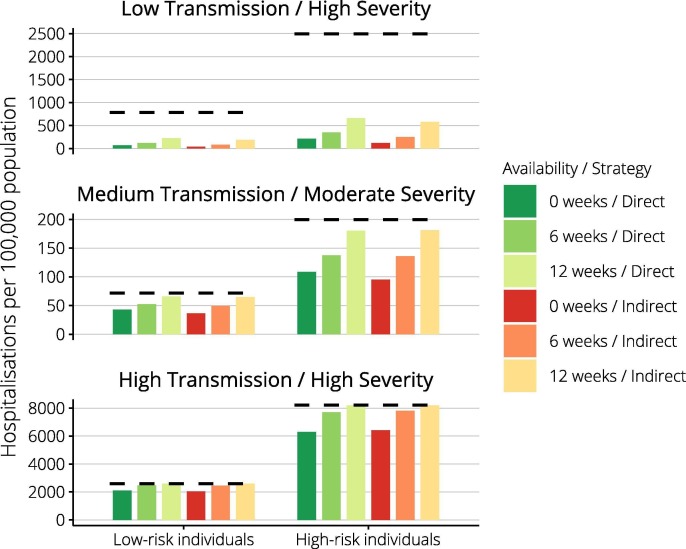
Of the three pandemic impact scenarios explored, only the low transmission/high severity event evolved sufficiently slowly for vaccines administered from twelve weeks to consistently precede the epidemic peak and curtail the epidemic (Fig. 1 ). For each pandemic scenario, we compared the impact of direct (high-risk) and indirect (school-based) vaccine approaches implemented from week six. Fig. 2 reports public health outcomes for each strategy as a percentage of those that would have been observed without vaccine. Vaccine impact was greatest if transmissibility was low, with presentations reduced to less than 10% of baseline, and minimal in the high transmissibility case. Comparing strategies within each scenario, the indirect approach was potentially more effective. Supplementary Fig. S1 reinforces the importance of vaccine timeliness, showing vaccine programs initiated at zero and twelve weeks. Vaccine was able to reduce inequities in health outcomes by underlying risk status in the low transmission/high severity case, whether vaccines were available immediately or at six weeks (Fig. 3 ). For other pandemic scenarios, inequities were not reduced.
Fig. 1.
Epidemic curves with respect to vaccine timeliness. Epidemic curves depict the three pandemic impact scenarios evaluated, of differing transmissibility and severity. The vertical dashed line at six weeks indicates our ‘base case’ assumption of early vaccine availability, based on the alignment of this timepoint with the initial epidemic phase.
Fig. 2.
Achievable harm reductions with direct and indirect vaccine strategies. For each of the pandemic scenarios evaluated, columns report the percentage of harmful outcomes (presentations, hospitalisations, deaths) achieved under direct (green) and indirect (brown) vaccine approaches, compared with those observed without vaccine. Note that axis values differ markedly, reflecting the substantial benefits achieved in the low transmission case, in stark contrast to the high transmission scenario. Note the different scales used for the Y-axes. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Fig. 3.
Vaccine benefits by underlying risk status. By pandemic scenario, achievable reductions in hospitalisation incidence are reported separately for low-risk and high-risk individuals. Dashed horizontal lines depict outcomes in the absence of a vaccine, columns show the achieved incidence for either of direct or indirect vaccine strategies. Note the different scales used for the Y-axes.
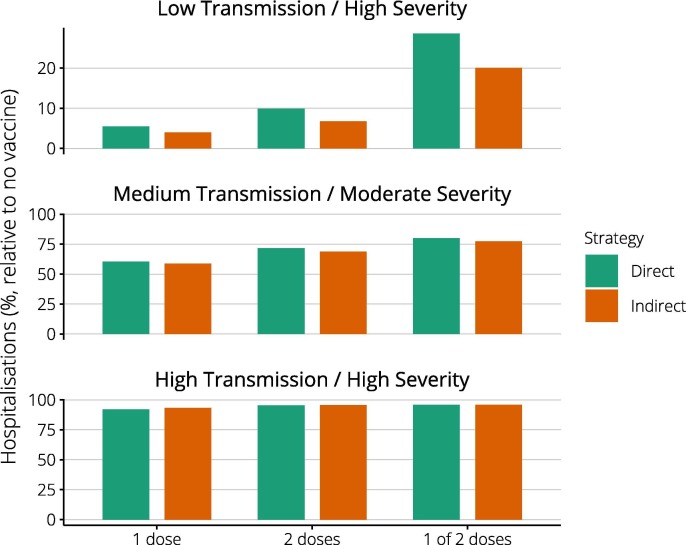
In 2009, a single vaccine dose was sufficient to protect older individuals, here taken as a base case. Recognising that two vaccine doses might be required for optimal protection, we compared the impact of a two dose (complete) schedule, with a single dose administered to twice as many individuals (Fig. 4 ). Across all scenarios, a single dose requirement resulted in greatest impact. If two doses were needed, coverage was lower, and the delay to maximum efficacy for each individual resulted in the prevention of fewer hospitalisations, particularly using the ‘high risk’ strategy. Providing only one of two required doses substantially reduced vaccine impact in the low transmission scenario, compared with complete vaccination of half the number of people. This difference was not observed in the faster growing medium and high transmission scenarios, because most individuals received only one of two scheduled doses before the epidemic peaked, limiting impact.
Fig. 4.
Impact of complete versus partial vaccine dosing. Columns report the percentage of hospitalisations observed for each vaccine strategy and pandemic scenario, compared with no vaccine. Best outcomes are observed where ‘1 dose’ only is needed for full protection. Where ‘2 doses’ are needed, provision of both results in better outcomes than administering only ‘1 of 2 doses’. Note the different scales used for the Y-axes.
3.3. Key goals for an ethics framework for priority vaccine allocation
More than 20 experts with wide ranging experience and perspectives participated in a full day workshop at which the above evidence was presented and discussed. All agreed that the allocation framework should be flexible and dynamic but should provisionally identify likely priority groups to enable pre-emptive community engagement and development of culturally appropriate communication and implementation strategies. Three overarching response goals were proposed. While the first is a constant background requirement, the other two will need to be interpreted and applied in the face of unfolding evidence in a health emergency.
-
(1)
Creating and maintaining trust
Trust is an essential part of successful pandemic management [32]. It must be developed and maintained between relevant parties including the public, government and non-government agencies, patients and healthcare providers. Trust is created and strengthened through clear and open procedures (e.g. transparency and accountability in decision-making), honest and responsive communications, and clear and appropriate goals and values.
-
(2)
Promoting equity
We should account for and, where feasible, address pre-existing disadvantage by promoting equity. This may mean prioritising populations who have relatively poor health outcomes, face barriers to accessing healthcare, or experience systematic discrimination. During a pandemic, it is highly likely that some groups will be more adversely affected, exacerbating existing disadvantage [33]. Where such evidence exists, we have a moral obligation to do what we can to ameliorate disparities.
-
(3)
Focusing on outcomes
We should aim to reduce the negative impacts of a pandemic during planning, response and recovery phases. Much work can be done in advance by focusing on identifying possible and perceived harms and seeking to prevent them, rather than just responding to actual harms. ‘Harm’ is a deliberately wide category encompassing a range of interests or concerns that negatively impact upon peoples’ welfare, such as anxiety, social disruption, and perceptions of unfairness and not simply morbidity and mortality. Should an especially severe pandemic threaten the future of society, relevant actions may include those most likely to preserve future generations.
4. Conclusions
Real-time prioritisation in a health emergency benefits from advanced articulation of pandemic response goals and how they relate to priority groups. Our process defined pandemic response objectives in the Australian context to be maintaining trust, promoting equity and reducing harms. We identified ‘Level 1’ priority groups to whom early vaccine access should be prioritised:
-
(a)
Healthcare workers most likely to be in contact with pandemic influenza-affected patients during the first wave: Protecting healthcare workers will promote trust in vaccines, maintain continuity of healthcare, and minimise infection spread. As they are placing themselves at risk, society is obliged to protect them.
-
(b)
People who self-identify as First Nations Australians: Health status in this population is lower than other groups, increasing the likelihood of severe morbidity and mortality. Historical evidence shows that previous pandemics have exacted a disproportionately heavy toll on First Nations communities, requiring development and incorporation of appropriate health services and values into all aspects of pandemic planning [34]. We also have ongoing justice obligations resulting from current inequities and past wrongs.
Groups suggested for ‘Level 2′ priority are to be defined and ordered depending on chosen outcomes, and iteratively informed by an evolving understanding of specific pathogen and vaccine characteristics in a pandemic.
-
(a)
Demographic groups selected on equity and outcomes grounds might include those who are socially or economically disadvantaged or have reduced health system access, such as recent migrants. Depending on pandemic severity, priority may be given to children and adults of working age, or alternatively to elder populations. Early vaccination of workers essential to social continuity, including military personnel and utility services, would be justified by a focus on outcomes, particularly in severe events.
-
(b)
Individuals with underlying health risks may be prioritised to promote equity and best outcomes, including those for whom seasonal influenza vaccines are recommended and funded, such as pregnant women, the elderly and people with chronic disease.
-
(c)
Social units like schools and residential institutions where close mixing occurs amplify influenza spread, making targeted immunisation strategies efficient to reduce harms. Underlying risk conditions may be prevalent in settings like aged care facilities and prisons, further justifying targeted interventions to achieve equity goals.
5. Relevance to COVID-19
While this process was undertaken for pandemic influenza vaccine, its relevance to COVID-19 is clear: anticipated shortages of vaccine in the face of global demand will necessitate rationing of any COVID-19 vaccines once developed. Many of the populations at greatest risk from COVID-19 correspond to those for pandemic influenza [35]. Having defined these priority groups, COVID-19-specific studies can inform updates to model parameters such as the latent and infectious periods, reproductive number, contact rates, risk of severe outcomes and the role of children in transmission [35], [36]. Clarity is needed around whether infection is immunising, especially given reports of reinfections [37], [38], and the utility of serosurveys to estimate susceptibility within priority groups [39]. Denominators need to be updated or determined. The specific vaccine schedule (number of doses and timing) remains unknown. The relevance of our model to the phase of any given country’s COVID-19 epidemic will depend on the level of constraint achieved with case-targeted and social distancing interventions [4], and we note that based on notifications to the WHO, most of the world’s countries are still in the early stages of their epidemics [40]. Early priority allocation remains essential even in countries that are heading into their second or third epidemics. A majority of countries remain in an early phase – the majority of populations are still susceptible. Even in some countries which have experienced large epidemics, like the US, a majority of the population remain susceptible.
Once COVID-19 vaccines are available, governments will need to communicate their allocation plans effectively and transparently, among all levels of government responsible for procurement and delivery, health professionals and the public. Suspicion of government decision-making is evident during this pandemic [41] and risks undermining careful planning. This is underscored by a recent global survey of acceptance of a COVID-19 vaccines which identified increased acceptance in nations where respondents had higher levels of trust [42].
Our decision-making process was undertaken over the course of 8 months, with many follow-up activities ongoing. Countries without updated pandemic plans may be able to use our template, with rapid adaptation to their own context, feeding into current vaccine allocation planning. It needs updated data, development of relevant frameworks to support ethical decision-making and transparent communication and engagement strategies to optimise trust, promote equity, and focus on outcomes.
6. Authors’ contributions
This multi-part project was led by JMcV, with input from all contributors at the study design phase. Thematic literature reviews were authored by JF (Pandemic vaccine allocation strategies), FB and KM (Lessons learned from the influenza A/(H1N1) pdm09 pandemic), JW and AD (Ethical considerations relevant to pandemic vaccine allocation). The individual consultation with key opinion leaders was conducted by SGS. Vaccine strategy modelling was undertaken by RM, JMcC and JMcV. The expert consultation workshop was led by PM, with KC and AD. AD, JW and GG developed the ethics framework. JMcV drafted the manuscript. SGS managed all revisions.
7. Role of the funding source
This project was directly funded by the Australian Government Department of Health Office of Health Protection Approach to Market: Health/17-18/73536 Investigate and Model Initial Pandemic Influenza Vaccination Target Groups, who have seen and approved the final version of the manuscript.
The Australian Partnership for Preparedness Research on Infectious Disease Emergencies is a Centre of Research Excellence funded by the Australian Government National Health and Medical Research Council (NHMRC) GNT1116530.
Jodie McVernon is supported by a NHMRC Principal Research Fellowship GNT1117140.
The WHO Collaborating Centre for Reference and Research on Influenza is supported by the Australian Government Department of Health.
8. Ethics committee approval
Not required.
Funding
Australian Government Department of Health, Office of Health Protection; Australian Government National Health and Medical Research Council
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.vaccine.2020.12.053.
Appendix A. Supplementary material
The following are the Supplementary data to this article:
References
- 1.Akst J. RNA Extraction Kits for COVID-19 Tests Are in Short Supply in US. The Scientist. 2020 March 11.
- 2.Emanuel E.J., Persad G., Upshur R., Thome B., Parker M., Glickman A., et al. Fair allocation of scarce medical resources in the time of covid-19. N Engl J Med. 2020 doi: 10.1056/NEJMsb2005114. [DOI] [PubMed] [Google Scholar]
- 3.Ranney M.L., Griffeth V., Jha A.K. Critical supply shortages — the need for ventilators and personal protective equipment during the covid-19 pandemic. N Engl J Med. 2020 doi: 10.1056/NEJMp2006141. [DOI] [PubMed] [Google Scholar]
- 4.Moss R, Wood J, Brown D, Shearer F, Black A, Cheng A, et al. Modelling the impact of COVID-19 in Australia to inform transmission reducing measures and health system preparedness; 2020. [DOI] [PMC free article] [PubMed]
- 5.Zhang SX, Wang Y, Rauch A, Wei F. Unprecedented disruptions of lives and work: Health, distress and life satisfaction of working adults in China one month into the COVID-19 outbreak. medRxiv. 2020:2020.03.13.20034496. [DOI] [PMC free article] [PubMed]
- 6.Fernandes N. Economic Effects of Coronavirus Outbreak (COVID-19) on the World Economy SSRN; 2020.
- 7.Mahase E. Covid-19: pfizer and BioNTech submit vaccine for US authorisation. BMJ. 2020;371 doi: 10.1136/bmj.m4552. [DOI] [PubMed] [Google Scholar]
- 8.Smith G.J., Vijaykrishna D., Bahl J., Lycett S.J., Worobey M., Pybus O.G., et al. Origins and evolutionary genomics of the 2009 swine-origin H1N1 influenza a epidemic. Nature. 2009;459(7250):1122–1125. doi: 10.1038/nature08182. [DOI] [PubMed] [Google Scholar]
- 9.Black A.J., Geard N., McCaw J.M., McVernon J., Ross J.V. Characterising pandemic severity and transmissibility from data collected during first few hundred studies. Epidemics. 2017;19:61–73. doi: 10.1016/j.epidem.2017.01.004. [DOI] [PubMed] [Google Scholar]
- 10.Straetemans M., Buchholz U., Reiter S., Haas W., Krause G. Prioritization strategies for pandemic influenza vaccine in 27 countries of the european union and the global health security action group: a review. BMC Public Health. 2007;7 doi: 10.1186/1471-2458-7-236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McDougall R. Reviewing literature in bioethics research: increasing rigour in non-systematic reviews. Bioethics. 2015;29(7):523–528. doi: 10.1111/bioe.12149. [DOI] [PubMed] [Google Scholar]
- 12.Moss R., McCaw J.M., Cheng A.C., Hurt A.C., McVernon J. Reducing disease burden in an influenza pandemic by targeted delivery of neuraminidase inhibitors: mathematical models in the Australian context. BMC Infect Dis. 2016;16(1):552. doi: 10.1186/s12879-016-1866-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Grill K., Dawson A. Ethical frameworks in public health decision-making: defending a value-based and pluralist approach. Health Care Anal. 2017;25(4):291–307. doi: 10.1007/s10728-015-0299-6. [DOI] [PubMed] [Google Scholar]
- 14.Government of Canada. Canadian Pandemic Influenza Preparedness: Planning Guidance for the Health Sector - Vaccine Annex 2017 [Available from: https://www.canada.ca/en/public-health/services/flu-influenza/canadian-pandemic-influenza-preparedness-planning-guidance-health-sector/table-of-contents.html#a4d.
- 15.Ministry of Health. New Zealand influenza pandemic plan: a framework for action 2017 [Available from: https://www.health.govt.nz/publication/new-zealand-influenza-pandemic-plan-framework-action.
- 16.Secrétariat général de la défense et de la sécurité nationale. National influenza pandemic prevention and response plan 2011 [Available from: http://solidarites-sante.gouv.fr/IMG/pdf/PlanPandemieGrippale-Version_Anglais.pdf.
- 17.Federal Office of Public Health. Swiss influenza pandemic plan 2018 [Available from: https://www.bag.admin.ch/bag/en/home/service/publikationen/broschueren/publikationen-uebertragbare-krankheiten/pandemieplan-2018.html.
- 18.U.S. Department of Health and Human Services. Guidance on allocating and targeting pandemic influenza vaccine 2007 [Available from: https://www.cdc.gov/flu/pandemic-resources/pdf/allocatingtargetingpandemicvaccine.pdf.
- 19.DH Pandemic Influenza Preparedness Team. UK Influenza Pandemic Preparedness Strategy 2011 [Available from: https://www.gov.uk/government/uploads/system/uploads/attachment_data/file/213717/dh_131040.pdf.
- 20.Pandemic Influenza Experts Advisory Committee. Vaccination guideline for pandemic influenza 2007 [Available from: http://www.mhlw.go.jp/bunya/kenkou/kekkaku-kansenshou04/pdf/09-e09.pdf.
- 21.Racine T. 2009 Influenza A/H1N1 Mass Vaccination Strategy: A Multinational Comparison National Collaborating Centre for Infectious Diseases; 2012 [Available from: https://nccid.ca/publications/2009-influenza-ah1n1-mass-vaccination-strategy-a-multinational-comparison/.
- 22.Stern E, Young S, Amlot R, Blake A. Assessment Report on EU-wide Pandemic Vaccine Strategies: European Commissions; 2010 [Available from: https://ec.europa.eu/health//sites/health/files/communicable_diseases/docs/assessment_vaccine_en.pdf.
- 23.Hashim A., Jean-Gilles L., Hegermann-Lindencrone M., Shaw I., Brown C., Nguyen-Van-Tam J. Did pandemic preparedness aid the response to pandemic (H1N1) 2009? a qualitative analysis in seven countries within the WHO European Region. J Infect Public Health. 2012;5(4):286–296. doi: 10.1016/j.jiph.2012.04.001. [DOI] [PubMed] [Google Scholar]
- 24.Hanquet G., Van Damme P., Brasseur D., De Cuyper X., Gregor S., Holmberg M., et al. Lessons learnt from pandemic A(H1N1) 2009 influenza vaccination. highlights of a European workshop in Brussels (22 March 2010) Vaccine. 2011;29(3):370–377. doi: 10.1016/j.vaccine.2010.10.079. [DOI] [PubMed] [Google Scholar]
- 25.Poland G.A. The 2009–2010 influenza pandemic: effects on pandemic and seasonal vaccine uptake and lessons learned for seasonal vaccination campaigns. Vaccine. 2010;7(28):024. doi: 10.1016/j.vaccine.2010.08.024. [DOI] [PubMed] [Google Scholar]
- 26.Williams J.H., Dawson A. Prioritising access to pandemic influenza vaccine: a review of the ethics literature. BMC Med Ethics. 2020;21(1):40. doi: 10.1186/s12910-020-00477-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Buccieri K., Gaetz S. Ethical vaccine distribution planning for pandemic influenza: prioritizing homeless and hard-to-reach populations. Public Health Ethics. 2013;6(2):185–196. [Google Scholar]
- 28.Gostin L. Medical countermeasures for pandemic influenza: ethics and the law. JAMA. 2006;5:554–556. doi: 10.1001/jama.295.5.554. [DOI] [PubMed] [Google Scholar]
- 29.Bambery B., Douglas T., Selgelid M., Maslen H., Giubilini A., Pollard A., et al. Influenza vaccination strategies should target children. Public Health Ethics. 2018;11(2):221–234. doi: 10.1093/phe/phx021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Emanuel E., Wertheimer A. Who should get influenza vaccine when not all can? Science. 2006;312(5775):854–855. doi: 10.1126/science.1125347. [DOI] [PubMed] [Google Scholar]
- 31.Kass N., Otto J., O’Brien D., Minson M. Ethics and severe pandemic influenza: maintaining essential functions through a fair and considered response. Biosecur Bioterror. 2008;6(3):227–236. doi: 10.1089/bsp.2008.0020. [DOI] [PubMed] [Google Scholar]
- 32.Upshur R., Faith K., Gibson J., Thompson A., Tracy C., Wilson K., et al. University of Toronto Joint Centre for Bioethics; 2005. Stand On Guard For Thee: Ethical considerations in pareparedness planning for pandemic influenza. [Google Scholar]
- 33.Flint S.M., Davis J.S., Su J.Y., Oliver-Landry E.P., Rogers B.A., Goldstein A., et al. Disproportionate impact of pandemic (H1N1) 2009 influenza on indigenous people in the top end of Australia's Northern Territory. Med J Aust. 2010;192(10):617–622. doi: 10.5694/j.1326-5377.2010.tb03654.x. [DOI] [PubMed] [Google Scholar]
- 34.Review of Australia's Health Sector Response to Pandemic (H1N1) 2009. Lessons identified. Australian Government Department of Health and Ageing. 2011.
- 35.World Health Organization. Q&A: Similarities and differences – COVID-19 and influenza Geneva: World Health Organization; 2020 [Available from: https://www.who.int/news-room/q-a-detail/q-a-similarities-and-differences-covid-19-and-influenza.
- 36.World Health Organization. Coronavirus disease (COVID-19) technical guidance: The Unity Studies: Early Investigations Protocols Geneva: World Health Organization; 2020 [Available from: https://www.who.int/emergencies/diseases/novel-coronavirus-2019/technical-guidance/early-investigations.
- 37.Tillett R.L., Sevinsky J.R., Hartley P.D., Kerwin H., Crawford N., Gorzalski A., et al. Genomic evidence for reinfection with SARS-CoV-2: a case study. Lancet Infect Dis. 2020 doi: 10.1016/S1473-3099(20)30764-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Van Elslande J., Vermeersch P., Vandervoort K., Wawina-Bokalanga T., Vanmechelen B., Wollants E., et al. Symptomatic SARS-CoV-2 reinfection by a phylogenetically distinct strain. Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa1330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Altmann D.M., Douek D.C., Boyton R.J. What policy makers need to know about COVID-19 protective immunity. Lancet. 2020 doi: 10.1016/S0140-6736(20)30985-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.World Health Organization. WHO COVID-19 Explorer Geneva: World Health Organization; 2020 [Available from: https://worldhealthorg.shinyapps.io/covid/.
- 41.Larson H.J. Blocking information on COVID-19 can fuel the spread of misinformation. Nature. 2020 doi: 10.1038/d41586-020-00920-w. [DOI] [PubMed] [Google Scholar]
- 42.Lazarus J.V., Ratzan S.C., Palayew A., Gostin L.O., Larson H.J., Rabin K., et al. A global survey of potential acceptance of a COVID-19 vaccine. Nat Med. 2020 doi: 10.1038/s41591-020-1124-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.