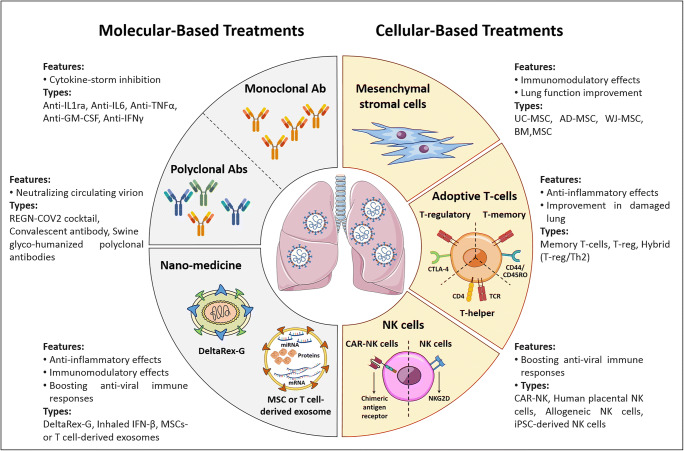
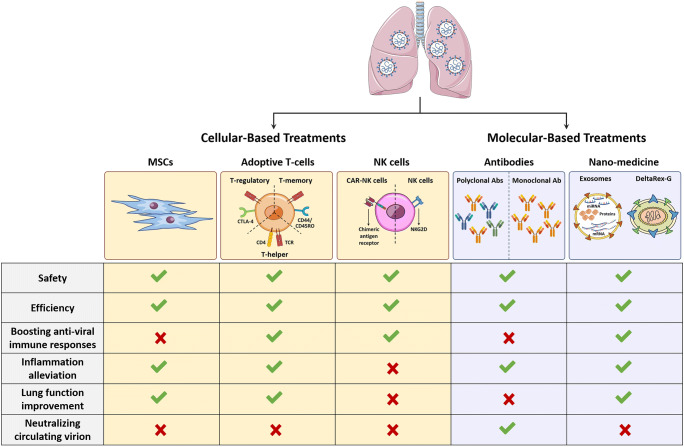
Although the exact mechanism of pathogenesis in COVID-19 is not fully understood, cytokine storm following viral infection plays an important role in the initiation and progression of disease. SARS-CoV-2 infection induces over-activation of the immune system and massive production of inflammatory cytokines. Therefore, it is necessary to develop new strategies to modulate inflammatory responses [1]. Despite many efforts to improve therapeutic protocols for COVID-19, there is no specific approved treatment or preventable vaccine for this disease [2, 3]. However, intensive research has been conducted to both prevent and treat COVID-19. This commentary is an update for our recent paper in “Journal of Molecular Medicine, June 2020” and highlights the recent achievements in terms of preventive and therapeutic approaches in COVID-19 [4].
Development of SARS-CoV-2 preventive vaccines
mRNA-1273 (Moderna TX, Inc.) is an mRNA vaccine that is composed of synthetic mRNA expressing the prefusion-stabilized SARS-CoV-2 spike trimer (mRNA-1273) [5, 6]. The efficacy and immunogenicity of Moderna vaccine investigated in a phase III clinical trial (NCT04470427). Moderna has announced its primary efficacy analysis (95%) and recently applied to the FDA (USA) for emergency use authorization.
ChAdOx1 nCOV-19 is another vaccine under evaluation in phase II/III clinical trials. This vaccine has been developed by Oxford University and produced due to the technology in which an adeno-viral vector encodes SARS-CoV-2 S protein (NCT04400838) [7]. The pre-clinical investigations showed that ChAdOx1 nCOV-19 was immunogenic in vaccinated mice and rhesus macaques and triggered robust humoral and cell-mediated responses [8]. Its safety and immunogenicity were evaluated in a phase II/III trial in a prime-boost regimen in young and old adults. In 14 days after receiving the boost dose, > 99% of participants had neutralizing antibodies [9].
BNT162b2 is a COVID-19 RNA vaccine candidate that has been announced by BioNTech/Pfizer. This vaccine encodes the receptor-binding domain (RBD) of the SARS-CoV-2 spike protein. Data from a phase III clinical trial showed vaccine efficiency over 95% [12, 13].
CoronaVac is inactivated SARS-CoV-2 manufactured by Sinovac Life Sciences (Beijing, China). Its safety, tolerability, and immunogenicity have been approved in healthy adults aged 18–59 years in a phase I/II clinical trial [14]; and now it is under investigation in a phase III clinical trial (NCT04582344).
Gam-COVID-Vac (Sputnik V) is a combined vector vaccine that consists of recombinant adenovirus type 26 (rAd26) and type 5 (rAd5) vectors. They carry the spike glycoprotein gene. Gam-COVID-Vac has been developed by Gamaleya National Research Center for Epidemiology and Microbiology (Moscow, Russia) [15]. Its safety and immunogenicity was approved in two formulations in a phase I/II clinical trial [15]. And now, the safety and efficiency of this vaccine is under assessment in a phase III clinical trial (NCT04530396).
Using Ad5 vector to carry the spike glycoprotein gene, CanSino Biologics Inc. (China) has developed a recombinant novel coronavirus vaccine which safety and efficiency has been being evaluated in a phase III clinical trial (NCT04526990).
The progress in vaccine development is critically discussed in the following recently published reviews in detail [10, 11].
SARS-CoV-2 therapeutic approaches
In our recently published paper entitled “Novel therapeutic approaches for treatment of COVID-19,” we grouped novel therapies into passive immunotherapy, cell-based therapies (including immune cell and non-immune cell therapies), monoclonal antibodies, and anti-viral drugs.
Searching terms “COVID-19” and “treatment” using https://clinicaltrials.gov/ resulted in more than 2200 clinical trials (October 29, 2020). Among these clinical trials, over 200 studies were related to cell-based therapies. They included mesenchymal stromal cell (MSC) therapies and adoptive T cell and natural killer (NK) cell therapies. Other studies applied monoclonal antibodies and nano-medicine to treat COVID-19 patients (Table 1) (Figs. 1 and 2).
Table 1.
SARS-CoV-2 therapeutic approaches
| Therapeutic approach | Number of studies | CT number | Status | Phase | The product used |
|---|---|---|---|---|---|
| MSC | 65 |
NCT04366063 NCT04333368 NCT04461925 NCT04486001 NCT04348435 NCT04473170 NCT04445454 NCT04349631 NCT04525378 NCT04392778 NCT04573270 NCT04447833 NCT04437823 NCT04288102 NCT04252118 NCT04273646 NCT04331613 NCT04537351 NCT04313322 NCT04299152 NCT04400032 NCT04382547 NCT04345601 NCT04565665 NCT04361942 NCT04527224 NCT04366271 NCT04339660 NCT04456361 NCT04390152 NCT04535856 NCT04457609 NCT04346368 NCT04371601 NCT04362189 NCT04467047 NCT04348461 NCT04416139 NCT04336254 NCT04452097 NCT04428801 NCT04390139 NCT04366323 NCT04355728 NCT04399889 NCT04429763 NCT04494386 NCT04269525 NCT04490486 NCT04371393 NCT04377334 NCT04397796 NCT04352803 NCT04389450 NCT04302519 NCT04466098 NCT04522986 NCT04315987 NCT04398303 NCT04524962 NCT03042143 NCT04367077 NCT04338347 NCT04451291 NCT04445220 |
Recruiting Recruiting Recruiting Not yet recruiting Enrolling by invitation Completed Recruiting Enrolling by invitation Recruiting Recruiting Completed Recruiting Recruiting Completed Recruiting Not yet recruiting Recruiting Recruiting Recruiting Recruiting Enrolling by invitation Not yet recruiting Recruiting Recruiting Not yet recruiting Recruiting Recruiting Active, not recruiting Not yet recruiting Not yet recruiting Recruiting Not yet recruiting Active, not recruiting Active, not recruiting Not yet recruiting Not yet recruiting Recruiting Recruiting Not yet recruiting Not yet recruiting Recruiting Recruiting Active, not recruiting Recruiting Not yet recruiting Recruiting Recruiting Not yet recruiting Recruiting Not yet recruiting Recruiting Not yet recruiting Recruiting Not yet recruiting Recruiting Not yet recruiting Not yet recruiting Not yet recruiting Not yet recruiting Recruiting Recruiting Available |
II/III I/II I/II I II II II II I I/II I I II II I - I/II I/II I II I I/II I I II II II I/II I I/II I I I/II I II I II II I/II I II I/II I/II I/II I/II II I/II II I III II I I II I II I II I/II I/II |
MSC, MSC + MSC-EVs UC-MSC Placenta-derived MSC/UC-MSC Allogenic AD-MSC AD-MSC Peripheral blood stem cells BM-MSC Autologous AD-MSC MSC MSC UC-MSC Allogenic BM-MSC UC-MSC UC-MSC MSC UC-MSC CAStem; regulatory cells from (hESCs) CYP-001(MSC from iPS) Wj-MSC BM-MSC Olfactory mucosa-derived MSCs Cord-blood MSC Cord-blood MSC MSC AD-MSC UC-MSC UC-MSC WJ-MSC WJ-MSC MSC UC-MSC BM-MSC UC-MSC AD-MSC MSC AD-MSC MSC DP-MSC UC-MSC AD-MSC WJ-MSC AD-MSC hCT-MSC UC-MSC UC-LSC UC-MSC UC-MSC Remestemcel-L BM-MSC BM-MSC Autologous AD-MSC placental mesenchymal-like adherent stromal cells DP-MSC MSC MSC NestaCell® UC-MSC MSCs or MSCs RNA-engineered UC-MSC MultiStem; BM-MSC |
| T cell | 7 |
NCT04351659 NCT04457726 NCT04482699 NCT04389385 NCT04406064 NCT04401410 NCT04468971 |
Recruiting Recruiting Not yet recruiting Active, not recruiting Not yet recruiting Not yet recruiting Recruiting |
I I/II I/II I II I I |
Convalescent donor Convalescent donors RAPA-501-ALLO (allogeneic hybrid TREG/Th2 Cells) T cell-derived exosomes Viral-specific T cells Specific T cell cord blood-derived T regulatory cells |
| NK cell | 5 |
NCT04324996 NCT04365101 NCT04280224 NCT04344548 NCT04363346 |
Recruiting Recruiting Recruiting Not yet recruiting Recruiting |
I/II I/II I I/II I |
NKG2D-ACE2 CAR-NK CYNK-001(human placental) NK Allogeneic NK cell transfer NK cell derived from an iPSC |
| CD34+ cells | 1 | NCT04522817 | Not yet recruiting | I | Peripheral blood-derived autologous CD34+ cells |
| Acellular product | 1 | NCT04384445 | Recruiting | I/II | Zofin; human amniotic fluid (HAF) |
| Monoclonal antibody | 80 |
NCT04413838 NCT04268537 NCT04464395 NCT04334044 NCT04390464 NCT04331665 NCT04439006 NCT04346277 NCT04441918 NCT04354766 NCT04425629 NCT04426695 NCT04483375 NCT04409509 NCT04391309 NCT04351152 NCT04341116 NCT04519437 NCT04432298 NCT04545060 NCT04452318 NCT04429529 NCT04324021 NCT04561076 NCT04351243 NCT04343651 NCT04386239 NCT04357808 NCT04305106 NCT04570397 NCT04435184 NCT04377750 NCT04516564 NCT04519424 NCT04447469 NCT04397497 NCT04454398 NCT04476979 NCT04347239 NCT04324073 NCT04365153 NCT04322773 NCT04331808 NCT04355494 NCT04369469 NCT04445272 NCT04479358 NCT04317092 NCT04345445 NCT04412772 NCT04331795 NCT04377659 NCT04412291 NCT04359667 NCT04335071 NCT04372186 NCT04356937 NCT04320615 NCT04377503 NCT04363736 NCT04363853 NCT04361032 NCT04409262 NCT04424056 NCT04332913 NCT04335305 NCT04560205 NCT04306705 NCT04310228 NCT04315480 NCT04339712 NCT04519385 NCT04423042 NCT04492501 NCT04380519 NCT04330638 NCT04486521 |
Not yet recruiting Not yet recruiting Recruiting Recruiting Recruiting Not yet recruiting Recruiting Available Recruiting Recruiting Recruiting Recruiting Recruiting Recruiting Not yet recruiting Recruiting Recruiting Recruiting Recruiting Recruiting Recruiting Active, not recruiting Recruiting Not yet recruiting Recruiting Active, not recruiting Not yet recruiting Recruiting Recruiting Not yet recruiting Recruiting Recruiting Recruiting Not yet recruiting Recruiting Not yet recruiting Recruiting Recruiting Recruiting Active, not recruiting Active, not recruiting Recruiting Active, not recruiting Available Recruiting Recruiting Recruiting Recruiting Not yet recruiting Recruiting Recruiting Recruiting Recruiting Not yet recruiting Recruiting Active, not recruiting Active, not recruiting Completed Not yet recruiting Completed Recruiting Not yet recruiting Recruiting Not yet recruiting Recruiting Recruiting Recruiting Recruiting Recruiting Active, not recruiting Recruiting Completed Not yet recruiting Completed Completed Recruiting Recruiting |
II II I I/II IV - II - I - I/II I/II I II II III I/II I II II/III III I II/III I II II I II - III II IV I II II/III II I II II II/III II II II - III II II II III III II II II II II III III III II II II III III III - II I - - II II - III - II/III III - |
Nivolumab PD-1 blocking antibody monoclonal antibody targeting the CD73 Ruxolitinib is an inhibitor of JAK1/2 Ravulizumab/Baricitinib Ruxolitinib Ibrutinib IC14, against human CD14 Anti-SARS-CoV-2 Anti-SARS-CoV-2 Anti-Spike (S) Anti-Spike (S) Anti-SARS-CoV-2 Garadacimab; anti-factor XIIa Antibody to CD14 Lenzilumab; anti GM-CSF Anti GM-CSF Anti-Spike (S) Pamrevlumab; anti-Connective tissue growth factor Anti-SARS-CoV-2 Anti-Spike (S) Anti-SARS-CoV-2 Emapalumab/anakinra Anti-Spike (S) Gimsilumab; Anti GM-CSF Leronlimab; Anti-CCR5 Sarilumab; Anti-IL-6 Sarilumab; Anti-IL-6 Bevacizumab; Anti-VEGF Ravulizumab; Anti- Complement component 5 Crizanlizumab; anti-P-selectin Tocilizumab; anti-IL-6R AK119; anti-CD73 CSL324; anti-GCSF Mavrilimumab; anti-GM-CSF-Rα Mavrilimumab; anti-GM-CSF-Rα Anti-Spike (S) Tocilizumab; anti-IL-6R Leronlimab; anti-complement component 5 Sarilumab; anti-IL-6 Canakinumab; anti-IL-1-β Tocilizumab; anti-IL-6R Tocilizumab; anti-IL-6R Eculizumab; anti-complement component 5 Ravulizumab; anti-complement component 5 Tocilizumab Tocilizumab Tocilizumab Tocilizumab Tocilizumab Tocilizumab Tocilizumab Tocilizumab/anakinra Tocilizumab Tocilizumab Tocilizumab Tocilizumab Tocilizumab Tocilizumab Tocilizumab Tocilizumab Tocilizumab Tocilizumab Anakinra, Tocilizumab, Ruxolitinib Tocilizumab Tocilizumab, Pembrolizumab Tocilizumab Tocilizumab Tocilizumab Tocilizumab Anakinra, Tocilizumab Tocilizumab Tocilizumab Tocilizumab Olokizumab Anakinra, Tocilizumab, Siltuximab Tocilizumab |
| Nanoparticle | 6 |
NCT04378244 NCT04517162 NCT04385095 NCT04276987 NCT04491240 NCT04493242 |
Not yet recruiting Recruiting Recruiting Completed Enrolling by invitation Not yet recruiting |
I I II I I/II II |
DeltaRex-G; mimic RNA virus SARS-CoV-2 by binding to viral receptors in human cells and may serve as a decoy Polymerized-type I collagen Inhaled IFN-β MSCs-derived exosomes MSCs-derived exosomes BM-derived MSC |
| Polyclonal antibody | 1 | NCT04453384 | Recruiting | II | Swine glyco-humanized polyclonal antibody |
Fig. 1.
Overview of molecular- and cellular-based treatments
Fig. 2.
Comparative analysis of therapeutic approaches to treat COVID-19
Mesenchymal stromal cells in COVID-19 treatment
Due to the immunomodulatory effects of MSCs [16, 17], clinical trials using MSCs from various sources including the umbilical cord, adipose tissue, and bone marrow have been registered for the treatment of acute respiratory distress syndrome (ARDS) caused by COVID-19 (NCT04341610, NCT04366063). Primary results showed that this strategy was safe and effective. The MSC therapy improved lung function, downregulated inflammatory cytokines, increased anti-inflammatory ones, and decreased mortality rate [18–20]. MSCs exert their anti-inflammatory properties through direct cell-cell contact, paracrine effects, and their extracellular vesicles such as exosomes [21, 22]. It seems that application of MSCs and their exosomes could be a promising approach for the management of respiratory complications in COVID-19.
Adoptive T cells in COVID-19 treatment
Some studies reported lymphopenia and functional exhaustion due to the over-activation of the immune system during infection [23]. COVID-19 specific T and TCD8+ cells play an important role in the virus clearance by producing inflammatory cytokines and their cytotoxicity effects [24]. Moreover, virus-specific memory T cells were isolated from the serum of the recovered patients [25–27]. Based on this evidence, recent clinical trials designed and used the adoptive T cells in severe COVID-19 patients. Using this treatment protocol, HLA-matched T cells from fully recovered patients were transfused into newly infected individuals. This approach may help patients who are at the risk of requiring mechanical ventilation (NCT04457726, NCT04401410, and NCT04406064).
Exosomes derived from adoptive T cells in COVID-19 treatment
In addition, another clinical trial used COVID-19-specific T cell-derived exosomes (CSTC-Exo) for the treatment of early infected patients in order to boost the IFN-γ production. Compared to the cells, CSTC-Exo does not need HLA-matching, and their administration route is an aerosol inhalation (NCT04389385). If it meets the endpoints, it could be a suitable alternative as an off-the-shelf product.
Since regulatory T cells (Treg) are known as major anti-inflammatory T cell subsets, Treg cell therapy may be a novel regenerative and anti-inflammatory treatment strategy for COVID-19. Infusion of cord blood-derived Treg cells (CK0802) may improve the ARDS symptoms in these patients (NCT04468971). RAPA-501-ALLO is a hybrid Treg/Th2 off-the-shelf reprogrammed Treg cell product produced by the healthy donors. RAPA-501-ALLO could have a dual advantage by modulating Th1 and Th17 subpopulations and inhibiting the massive production of inflammatory cytokines, as well as regenerating the damaged alveolar tissues [28]. This product may be a useful therapeutic option for the treatment of severe COVID-19 (NCT04482699).
NK cells in COVID-19 treatment
NK cells are an essential part of the innate immune system and play an important role in mediating virus-induced immune responses. So, interventional therapies using NK cells have been developed for the COVID-19 treatment. Recently, the adoptive transfer of allogenic NK cells has been developed to boost the antiviral immune responses and clearance of the infected cells in COVID-19 patients (NCT04344548, NCT04280224). NKG2D-ACE2 CAR-NK is an off-the-shelf product that has been investigated in a phase I/II clinical trial (NCT04324996). These cells simultaneously target ACE2 (the main receptor for SARS-CoV-2) [29] and NKG2D on the infected cells and removed them. Therefore, they could inhibit the SARS-CoV-2 infection through ACE2 blockade.
Monoclonal antibodies in COVID-19 treatment
It has been shown that monoclonal antibodies could be a promising treatment approach for COVID-19. Monoclonal antibodies against inflammatory cytokines such as anti-IL-1 receptor, IL-6 antagonist, anti-TNF-α, anti-GM-CSF, anti-IFN-γ, and C5a inhibitor have been studied in different clinical trials. Over 60 clinical trials have been registered to evaluate the treatment efficiency of Tocilizumab and Olokizumab (anti-IL-6 mAbs) [30–33]. The published studies showed that Tocilizumab (anti-IL-6 mAb) could improve the outcomes in COVID-19 patients and inhibit a cytokine storm [34]. Anakinra (IL-1ra) [35, 36] also showed beneficial effects for the treatment of COVID-19 patients and could decrease the mechanical ventilation need. Moreover, REGN-COV2 has been developed and consists of two neutralizing antibodies (REGN10987 + REGN10933) targeting SARS-CoV-2 spike protein [37, 38].
Nano-medicine in COVID-19 treatment
Using nano-medicine including aerosol inhalations of therapeutic agents attracts lots of attention. Recent studies have investigated the efficiency and safety of the MSC-derived exosome (NCT04491240, NCT04276987) and interferon beta inhalation (NCT04385095).
Now, most of the mentioned studies are ongoing. The growing number of clinical trials in this field could provide more validated designs and higher quality data. In this context, the increase in international collaborations to provide larger number of patients will be helpful to obtain more definite results [39]. Identifying the exact mechanisms of the COVID-19 immunopathogenesis will ensure the development of more effective therapies.
Funding
This work was supported by Royan Institute and the Russian Foundation for Basic Research (N. 20-04-60063).
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Peter Timashev, Email: timashev.peter@gmail.com.
Massoud Vosough, Email: masvos@Royaninstitute.org.
References
- 1.Tay MZ, Poh CM, Rénia L, MacAry PA, Ng LFP. The trinity of COVID-19: Immunity, inflammation and intervention. Nat Rev Immunol. 2020;20(6):363–374. doi: 10.1038/s41577-020-0311-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Touma M. COVID-19: Molecular diagnostics overview. J Mol Med (Berl) 2020;98(7):947–954. doi: 10.1007/s00109-020-01931-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Khoshdel-Rad N, Zahmatkesh E, Shpichka A, Timashev P, Vosough M. Outbreak of chronic renal failure: Will this be a delayed heritage of COVID-19? J Nephrol. 2020;2:1–3. doi: 10.1007/s40620-020-00851-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hossein-Khannazer N, Shokoohian B, Shpichka A, Aghdaei HA, Timashev P, Vosough M. Novel therapeutic approaches for treatment of COVID-19. J Mol Med (Berl) 2020;98(6):789–803. doi: 10.1007/s00109-020-01927-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Corbett KS, Edwards DK, Leist SR, et al. SARS-CoV-2 mRNA vaccine design enabled by prototype pathogen preparedness. Nature. 2020;586:567–571. doi: 10.1038/s41586-020-2622-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Anderson EJ, et al. Safety and immunogenicity of SARS-CoV-2 mRNA-1273 vaccine in older adults. N Engl J Med. 2020;383:2427–2438. doi: 10.1056/NEJMoa2028436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sharpe HR, Gilbride C, Allen E, Belij-Rammerstorfer S, Bissett C, Ewer K, Lambe T. The early landscape of coronavirus disease 2019 vaccine development in the UK and rest of the world. Immunology. 2020;160(3):223–232. doi: 10.1111/imm.13222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.van Doremalen N, Lambe T, Spencer A, Belij-Rammerstorfer S, Purushotham JN, Port JR, Avanzato V, Bushmaker T, Flaxman A, Ulaszewska M et al (2020) ChAdOx1 nCoV-19 vaccination prevents SARS-CoV-2 pneumonia in rhesus macaques. bioRxiv [Preprint] 2020.05.13.093195. Update in: Nature. 2020 Jul 30. 10.1101/2020.05.13.093195 [DOI] [PMC free article] [PubMed]
- 9.Ramasamy MN, Minassian AM, Ewer KJ, Flaxman AL, Folegatti PM, Owens DR, Voysey M, Aley PK, Angus B, Babbage G, et al. Safety and immunogenicity of ChAdOx1 nCoV-19 vaccine administered in a prime-boost regimen in young and old adults (COV002): A single-blind, randomised, controlled, phase 2/3 trial. Lancet. 2021;396(10267):1979–1993. doi: 10.1016/S0140-6736(20)32466-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Poland GA, Ovsyannikova IG, Kennedy RB. SARS-CoV-2 immunity: Review and applications to phase 3 vaccine candidates. Lancet. 2020;396:1595–1606. doi: 10.1016/S0140-6736(20)32137-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Krammer F. SARS-CoV-2 vaccines in development. Nature. 2020;586(7830):516–527. doi: 10.1038/s41586-020-2798-3. [DOI] [PubMed] [Google Scholar]
- 12.Walsh EE, Frenck R, Falsey AR, Kitchin N, Absalon J, Gurtman A, Lockhart S, Neuzil K, Mulligan MJ, Bailey R et al (2020) RNA-based COVID-19 vaccine BNT162b2 selected for a pivotal efficacy study. medRxiv:2020.08.17.20176651. 10.1101/2020.08.17.20176651
- 13.Dong Y, et al. A systematic review of SARS-CoV-2 vaccine candidates. Signal Transduct Target Ther. 2020;5(1):1–14. doi: 10.1038/s41392-019-0089-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang Y et al (2020) Safety, tolerability, and immunogenicity of an inactivated SARS-CoV-2 vaccine in healthy adults aged 18–59 years: A randomised, double-blind, placebo-controlled, phase 1/2 clinical trial. Lancet Infect Dis. 10.1016/S1473-3099(20)30843-4 [DOI] [PMC free article] [PubMed]
- 15.Logunov DY, Dolzhikova IV, Zubkova OV, Tukhvatullin AI, Shcheblyakov DV, Dzharullaeva AS, Grousova DM, Erokhova AS, Kovyrshina AV, Botikov AG, et al. Safety and immunogenicity of an rAd26 and rAd5 vector-based heterologous prime-boost COVID-19 vaccine in two formulations: two open, non-randomised phase 1/2 studies from Russia. Lancet. 2020;396(10255):887–897. doi: 10.1016/S0140-6736(20)31866-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hossein-Khannazer N, et al. Study of the immunomodulatory effects of osteogenic differentiated human dental pulp stem cells. Life Sci. 2019;216:111–118. doi: 10.1016/j.lfs.2018.11.040. [DOI] [PubMed] [Google Scholar]
- 17.Hashemi SM, Hassan ZM, Hossein-Khannazer N, Pourfathollah AA, Soudi S. Investigating the route of administration and efficacy of adipose tissuederived mesenchymal stem cells and conditioned medium in type 1 diabetic mice. Inflammopharmacology. 2020;28(2):585–601. doi: 10.1007/s10787-019-00661-x. [DOI] [PubMed] [Google Scholar]
- 18.Leng Z, Zhu R, Hou W, Feng Y, Yang Y, Han Q, Shan G, Meng F, du D, Wang S, et al. Transplantation of ACE2-mesenchymal stem cells improves the outcome of patients with COVID-19 pneumonia. Aging Dis. 2020;11(2):216–228. doi: 10.14336/AD.2020.0228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen X, Shan Y, Wen Y, Sun J, Du H. Mesenchymal stem cell therapy in severe COVID-19: A retrospective study of short-term treatment efficacy and side effects. J Infect. 2020;81(4):647–679. doi: 10.1016/j.jinf.2020.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ramezankhani R, Solhi R, Memarnejadian A, Nami F, Hashemian SMR, Tricot T, Vosough M, Verfaillie C. Therapeutic modalities and novel approaches in regenerative medicine for COVID-19. Int J Antimicrob Agents. 2020;56:106208. doi: 10.1016/j.ijantimicag.2020.106208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xiao K et al (2020) Mesenchymal stem cells: Current clinical progress in ARDS and COVID-19. Stem Cell Res Ther 11(1):1–7 [DOI] [PMC free article] [PubMed]
- 22.Golchin A, Seyedjafari E, Ardeshirylajimi A. Mesenchymal stem cell therapy for COVID-19: Present or future. Stem Cell Rev Rep. 2020;16(3):427–433. doi: 10.1007/s12015-020-09973-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mathew D, Giles JR, Baxter AE, Oldridge DA, Greenplate AR, Wu JE, Alanio C, Kuri-Cervantes L, Pampena MB, D'Andrea K, et al. Deep immune profiling of COVID-19 patients reveals distinct immunotypes with therapeutic implications. Science. 2020;369(6508):eabc8511. doi: 10.1126/science.abc8511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Olwenyi OA, Dyavar SR, Acharya A, Podany AT, Fletcher CV, Ng CL, Reid SP, Byrareddy SN. Immuno-epidemiology and pathophysiology of coronavirus disease 2019 (COVID-19) J Mol Med (Berl) 2020;98(10):1369–1383. doi: 10.1007/s00109-020-01961-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen Z, John Wherry E. T cell responses in patients with COVID-19. Nat Rev Immunol. 2020;20(9):529–536. doi: 10.1038/s41577-020-0402-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.De Biasi S et al (2020) Marked T cell activation, senescence, exhaustion and skewing towards TH17 in patients with COVID-19 pneumonia. Nat Commun 11(1):1–17 [DOI] [PMC free article] [PubMed]
- 27.Le Bert N, et al. SARS-CoV-2-specific T cell immunity in cases of COVID-19 and SARS, and uninfected controls. Nature. 2020;584(7821):457–462. doi: 10.1038/s41586-020-2550-z. [DOI] [PubMed] [Google Scholar]
- 28.Ghosh A, Menon A, Hussain A, Dubey M, Kumar R (2020) A review of mesenchymal stem cell therapy for severe SARS-CoV-2 infection. PUMRJ [Internet]. [cited 21Dec.2020];3(SPECIAL). Available from: http://www.praxisug.com/index.php/Praxis/article/view/104
- 29.Bellone M, Calvisi SL. ACE polymorphisms and COVID-19-related mortality in Europe. J Mol Med. 2020;98(11):1505–1509. doi: 10.1007/s00109-020-01981-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Luo P, Liu Y, Qiu L, Liu X, Liu D, Li J. Tocilizumab treatment in COVID-19: A single center experience. J Med Virol. 2020;92(7):814–818. doi: 10.1002/jmv.25801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang C, et al. The cytokine release syndrome (CRS) of severe COVID-19 and Interleukin-6 receptor (IL-6R) antagonist Tocilizumab may be the key to reduce the mortality. Int J Antimicrob Agents. 2020;55:105954. doi: 10.1016/j.ijantimicag.2020.105954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Xu X, Han M, Li T, Sun W, Wang D, Fu B, Zhou Y, Zheng X, Yang Y, Li X, et al. Effective treatment of severe COVID-19 patients with tocilizumab. Proc Natl Acad Sci. 2020;117(20):10970–10975. doi: 10.1073/pnas.2005615117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Russell B, Moss C, George G, Santaolalla A, Cope A, Papa S, Van Hemelrijck M. Associations between immune-suppressive and stimulating drugs and novel COVID-19-a systematic review of current evidence. Ecancermedicalscience. 2020;14:1022. doi: 10.3332/ecancer.2020.1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Comentale G, Manzo R, Pilato E. Sars-Cov-2 interference in HEME production: Is it the time for an early predictive biomarker? J Mol Med. 2020;98(8):1053–1054. doi: 10.1007/s00109-020-01945-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Navarro-Millán I, Sattui SE, Lakhanpal A, Zisa D, Siegel CH, Crow MK. Use of anakinra to prevent mechanical ventilation in severe COVID-19: A case series. Arthritis Rheum. 2020;72(12):1990–1997. doi: 10.1002/art.41422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Iglesias-Julián E, López-Veloso M, de-la-Torre-Ferrera N, Barraza-Vengoechea JC, Delgado-López PD, Colazo-Burlato M, Ubeira-Iglesias M, Montero-Baladía M, Lorenzo-Martín A, Minguito-de-la-Iglesia J, et al. High dose subcutaneous Anakinra to treat acute respiratory distress syndrome secondary to cytokine storm syndrome among severely ill COVID-19 patients. J Autoimmun. 2020;115:102537. doi: 10.1016/j.jaut.2020.102537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Baum A, Ajithdoss D, Copin R, Zhou A, Lanza K, Negron N, Ni M, Wei Y, Mohammadi K, Musser B, et al. REGN-COV2 antibodies prevent and treat SARSCoV-2 infection in rhesus macaques and hamsters. Science. 2020;370(6520):1110–1115. doi: 10.1126/science.abe2402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Matthews DB. A cocktail of antibodies for COVID-19 therapy. Nat Rev Immunol. 2020;20(10):591. doi: 10.1038/s41577-020-00431-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lythgoe MP, Middleton P. Ongoing clinical trials for the management of the COVID-19 pandemic. Trends Pharmacol Sci. 2020;41:363–382. doi: 10.1016/j.tips.2020.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]