Abstract
Cancer is not only the leading cause of mortality and morbidity but also poses a major economic burden. Until recently, onco-immunotherapy has dramatically changed the landscape of cancer treatment. For example, an inhibitor of programmed cell death (PD-1) plays a vital role by potentiating effective immune-mediated destruction of tumor cells. However, the spectrum of immune-related adverse events especially granulomatous sarcoid lesions has been recognized too. Such lesions involve the dermis and subcutaneous tissue (panniculitis) and lymph nodes. Herein we are presenting a case report of a patient, who developed a sarcoid-like reaction after treatment with pembrolizumab.
Keywords: sarcoid like reaction, pembrolizumab, lung cancer, case report, programmed cell death protein 1, tumour necrosis factor-α (tnf-α), heerfordet syndrome
Introduction
Pembrolizumab is a novel humanized monoclonal antibody that targets programmed cell death protein 1 (PD-1) cell signaling. PD-1 is a checkpoint protein on T cells where it normally acts as a type of “off switch” which prevent T cells from attacking other cells in the body. Some cancer cells produce a large amount of programmed death-ligand 1 (PD-L1) and use it as an escape mechanism from an immune attack. Pembrolizumab negates such an escape mechanism and thus restores anti-tumor-cell response against tumor cells. However, preventing the inhibition of T cell activation results in both immune and inflammatory reactions. Granulomatous/sarcoid-like lesions (clinical or radiological) are recognized as toxicity associated with pembrolizumab. Pembrolizumab-related lesions exhibit sarcoid granuloma on histopathological sections. Early recognition of such side effects is important as they often mimic disease recurrence and/or consequently may lead to cessation of therapy.
Case presentation
A 62-year-old female with a past medical history of hypertension and ex-smoker (> 50 pack-years) was diagnosed with T1C, N0, M0, adenocarcinoma of the right lung and underwent right video-assisted thoracoscopic surgery (VATS). Subsequent CT chest showed relapse of disease with progressive mediastinal, right hilar, and subcarinal lymphadenopathy. Furthermore, her biomarkers programmed death-ligand 1 (PD-L1) was positive (100%), epidermal growth factor receptor (EGFR), anaplastic lymphoma kinase (ALK) wild type (negative), and no ROS proto-oncogene 1 (ROS-1) translocation. Given the high level of PD-L1 positivity, she was placed on pembrolizumab 200 mg intravenously every three weeks.
After nine weeks of treatment, she developed right wrist pain and hyperpigmentation of her previous scars (surgical scars-episiotomy and accidental scars). Additionally, she also reported some new skin lesions on her face, especially involving nasolabial groove and noticed subcutaneous thickening on the right forearm. Ultrasound of forearm revealed an ill-defined subcutaneous abnormality for a length of about 9.5 cm extending from the ulnar aspect of the wrist to the level of mid-forearm. It showed extensive subcutaneous edema, ill-defined hyperechoic areas with marked vascularity.
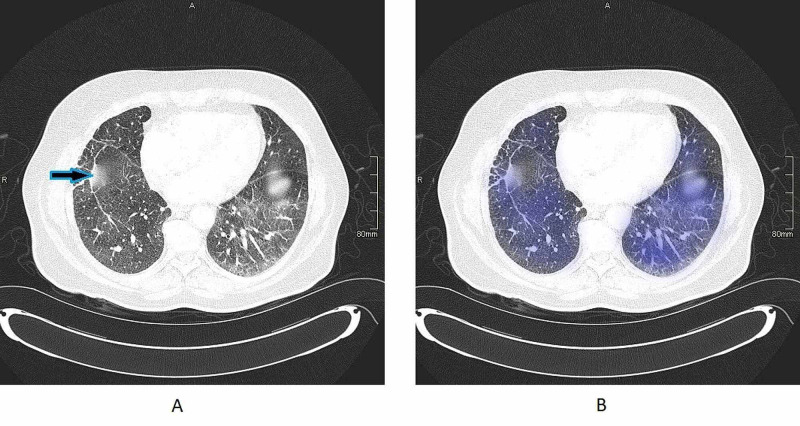
Physical examination revealed sclerotic scars on bilateral shins. Additionally, face examination revealed an erythematous palpable dermal change to a small scar on the right cheek, numerous erythematous papules to the left cheek which are not related to previous scars. Angiotensin-converting enzyme (ACE) was 116 (normal range 8-52 IU/L) and erythrocyte sedimentation rate (ESR) was 7 (normal range 1-14 mm/h). Polymerase chain reaction (PCR) for Mycobacterium tuberculosis and Ziehl-Neelsen (ZN) staining were negative. CT scan thorax (Figure 1A,B) revealed ground glass changes more marked on left lower lobe.
Figure 1. A shows bilateral ground glass appearance with arrowhead showing old surgical scar. B shows shaded areas which explain areas of ground glass changes.
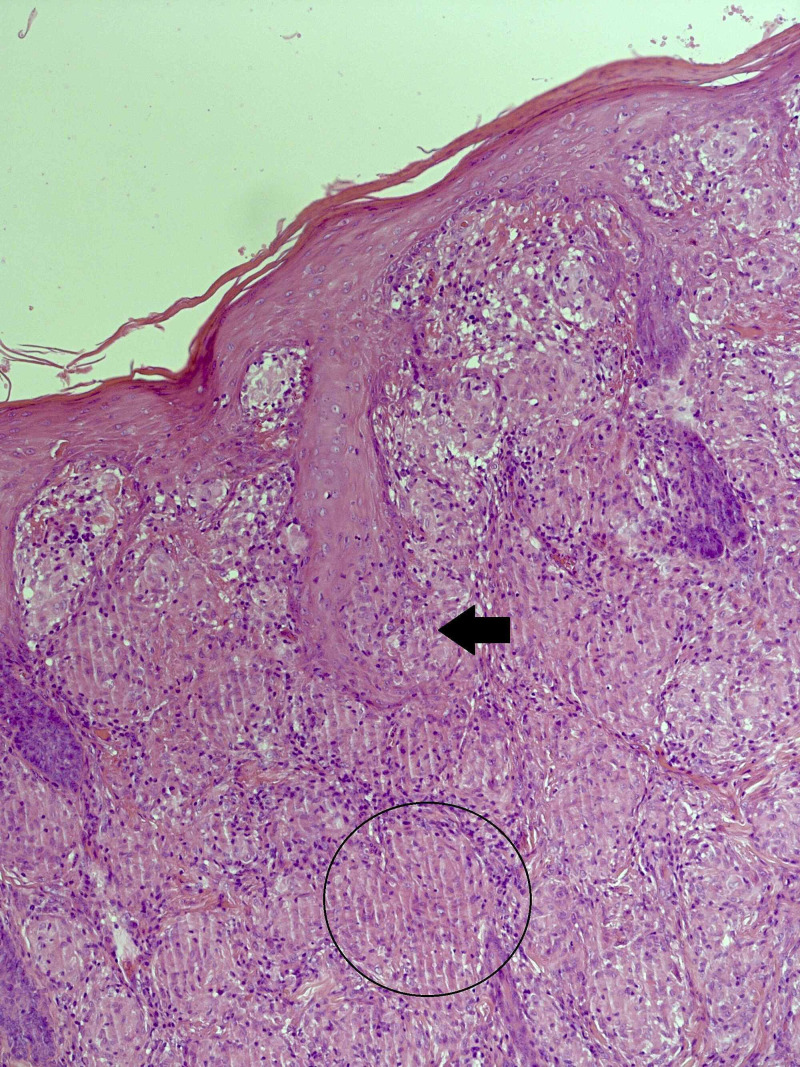
Skin punch biopsies (Figures 2-3) performed from cheek and shins showed plentiful non-necrotizing granulomas within the dermis, along with a small number of admixed lymphocytes and giant cells. There is no evidence of atypia or malignancy.
Figure 2. A section of skin stained with H&E. The dermis is packed with relatively well-defined non-necrotizing granulomata, some of which are surrounded by a loose cuff of lymphocytes (circled area). The skin adnexae are involved and disrupted by the granulomata (black arrow).
H&E, hematoxylin and eosin
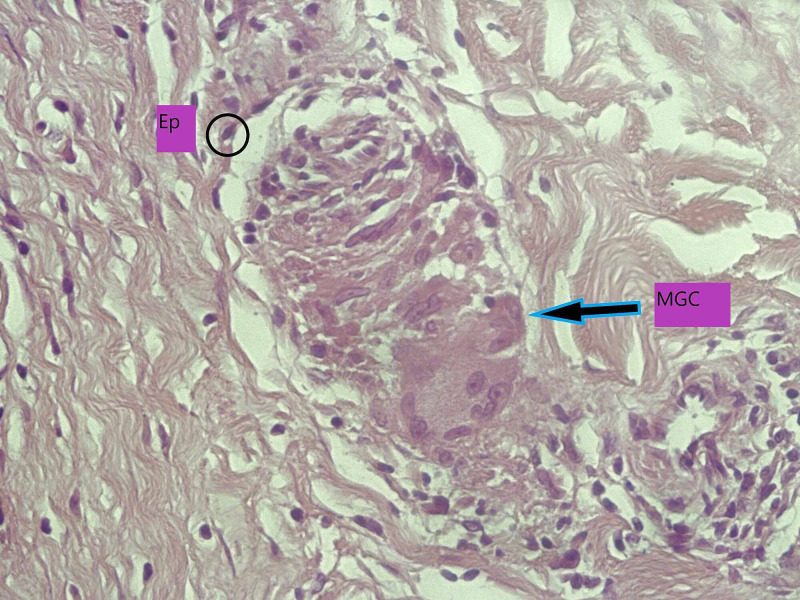
Figure 3. A high-power image of a non-necrotizing granuloma (H&E), showing epithelioid histiocytes (circle) and admixed multinucleated giant cell (arrow).
Ep, epitheiod histocyte; MGA, multinucleated giant cells; H&E, hematoxylin and eosin
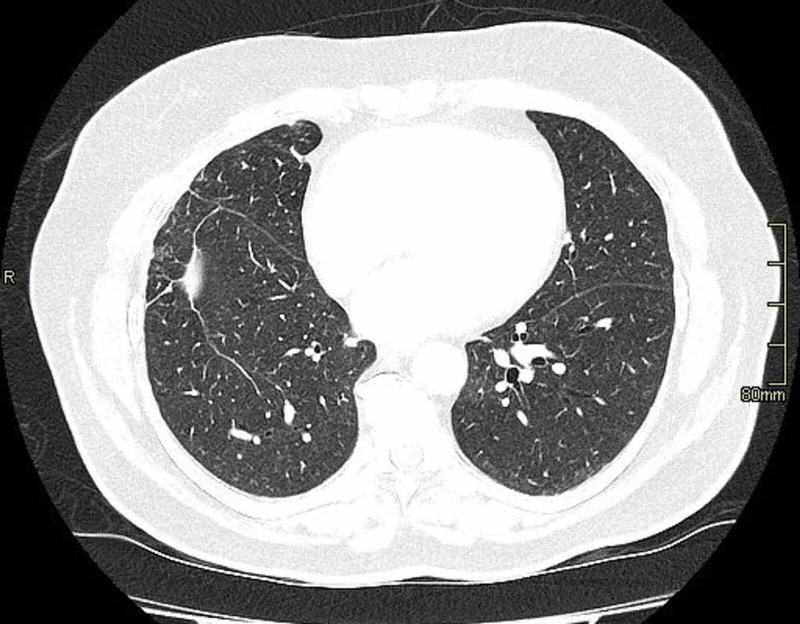
Given the consistent ground glass changes on radiological studies, noncaseating granuloma on histological findings and the correlation in timeline with treatment, the diagnosis of pembrolizumab-related sarcoidosis-like granulomatous reaction was made. Pembrolizumab was stopped after discussion with the patient as there was evidence of systemic involvement. She was commenced on prednisolone 30 mg once a day orally and tapered over six weeks. She had marked improvement in her skin lesions and, repeat CT scan after eight weeks (Figure 4) showed resolution ground glass appearance. There was no evidence of disease progression. After eight weeks, follow up examination and restaging studies demonstrated stable disease and no evidence of skin lesions.
Figure 4. Resolution of ground glass changes after cessation of pembrolizumab.
Discussion
According to NICE guidelines [1], in adults with metastatic non-small cell lung cancer (NSCLC) which express PD-L1(at least 50% of tumor proportion score) and negative for epidermal growth factor, pembrolizumab is a recommended option. Several published trials have demonstrated the superiority of pembrolizumab over chemotherapy in patients with advanced NSCLC [2]. However, these treatments also have a wide range of side effects but sarcoid-like reactions are uncommon.
The term “sarcoid-like reaction” is usually used to describe localized reactions as compared to the systemic process seen in sarcoidosis. Yet, systemic involvement affecting the lungs, skin, and kidneys can be seen. Noncaseating granulomas are the hallmark of sarcoid-like reactions [3]. It is prudent to note that sarcoid-like reaction can masquerade as worsening or recurrence of cancer. In such cases, clinical presentation, coupled with appropriate biopsy, radiographic evidence of bilateral hilar lymphadenopathy (with paratracheal lymphadenopathy), and elevated serum ACE levels aid in distinguishing sarcoid-like reaction from recurrent or metastatic disease. In our patients, the diagnosis of the sarcoid-like reaction was suspected because of the typical pattern of skin lesion and noncaseating granuloma on biopsy and raised ACE levels. Sarcoid-like reactions are not limited to pembrolizumab but also associated with other checkpoint inhibitors, e.g. ipilimumab and nivolumab [4]. As these drugs are becoming standard of care for several malignancies, it is crucial for both oncologist and radiologists to be vigilant about sarcoid-like reaction as it is important for the appropriate management and possible measurement of therapeutic response.
At the cellular level, the interaction of monocyte-derived histiocytes and CD4+ Th1 cells is the plausible mechanism behind the pathogenesis of sarcoid reactions. Tumor necrosis factor-α (TNF-α) plays a central role not only in the formation but also in the maintenance of the “sarcoid” granulomata [5]. For example, anti-TNF-alpha monoclonal antibodies (i.e., infliximab and adalimumab) have been successfully used to treat sarcoidosis [6-7]. Paradoxically, some patients with inflammatory bowel disease, psoriasis, arthritis, or even sarcoidosis who received anti-TNF-α therapy developed sarcoidosis-like reactions to therapy [8]. These findings led the researcher to suggest that there is cytokine imbalance and promotion of Th1 immune response, which resulted from the impaired regulatory role of anti-TNF-α on autoreactive cells [9]. Either hypoactive or hyperactive immune response is responsible for sarcoidosis [10]. For instance, sarcoidosis unrelated to pembrolizumab has demonstrated hypoactive immune response with decreased T-cell proliferation and PD-1 up-regulation [11]. On contrary, there is a hyperactive immune response with increased T-cell proliferation and inhibition of PD-1 signaling in sarcoidosis related to immune-modulating drugs [9].
Sarcoidosis is a multi-systemic disease of unknown etiology and mostly a diagnosis of exclusion. A presumptive diagnosis of sarcoidosis is based on a constellation of clinical symptoms and radiographic features [12]. The hallmark radiological feature is bilateral hilar lymphadenopathy. In most cases, patients are asymptomatic or may present with erythema nodosum-like panniculitis [13]. Alternatively, the patient may present with a Heerfordet syndrome, the triad of uveitis, parotiditis, and fever. There may be an abnormal gallium imaging with increased uptake in the parotid and lacrimal gland and mediastinal nodes, i.e. paratracheal and bilateral hilar uptake [12].
As per guidelines for managing immune-mediated adverse effects, treatment depends on the severity of the disease [14]. For instance, asymptomatic cases can be managed by discontinuing and close monitoring, while corticosteroids should be reserved for systemic involvement or progression, while immunosuppressants can be added in severe cases [15]. Fortunately, the sarcoid-like reactions are reversible as per published cases. The adverse effects of our patient, for example, resolved with discontinuing of drug and commencement of steroids.
Conclusions
Pembrolizumab-induced granulomatous sarcoid-like reaction is immune-mediated toxicity. It is prudent for both oncologists and radiologists to recognize such uncommon adverse effect of pembrolizumab as it can masquerade both clinically and radiologically as progression or recurrence of underlying cancer. Early diagnosis with the cessation of pembrolizumab can change the outcome of a clinical course for patients.
The content published in Cureus is the result of clinical experience and/or research by independent individuals or organizations. Cureus is not responsible for the scientific accuracy or reliability of data or conclusions published herein. All content published within Cureus is intended only for educational, research and reference purposes. Additionally, articles published within Cureus should not be deemed a suitable substitute for the advice of a qualified health care professional. Do not disregard or avoid professional medical advice due to content published within Cureus.
The authors have declared that no competing interests exist.
Human Ethics
Consent was obtained by all participants in this study
References
- 1.Pembrolizumab for treating PD-L1-positive non-small-cell lung cancer after chemotherapy. https://www.nice.org.uk/guidance/ta428
- 2.Pembrolizumab in lung cancer: current evidence and future perspectives. Palumbo G, Carillio G, Manzo A, Montanino A. Future Oncol. 2019;15:3327–3336. doi: 10.2217/fon-2019-0073. [DOI] [PubMed] [Google Scholar]
- 3.Immune-related sarcoidosis observed in combination ipilimumab and nivolumab therapy. Suozzi KC, Stahl M, Ko CJ, et al. JAAD Case Rep. 2016;2:264–268. doi: 10.1016/j.jdcr.2016.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pembrolizumab-induced sarcoid-like reactions during treatment of metastatic melanoma. Cheshire SC, Board RE, Lewis AR, Gudur LD, Dobson MJ. Radiology. 2018;289:564–567. doi: 10.1148/radiol.2018180572. [DOI] [PubMed] [Google Scholar]
- 5.Immunopathogenesis of granulomas in chronic autoinflammatory disease. Timmermans WM, van Laar JA, van Hagen PM, van Zelm MC. Clin Transl Immunol. 2016;5:118. doi: 10.1038/cti.2016.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Treatment of sarcoidosis with infliximab. Doty JD, Mazur JE, Judson MA. Chest. doi: 10.1378/chest.127.3.1064. [DOI] [PubMed] [Google Scholar]
- 7.Systemic sarcoidosis with bone marrow involvement responding to therapy with adalimumab: a case report. Patel SR. J Med Case Rep. 2009;3:8573. doi: 10.4076/1752-1947-3-8573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Development of sarcoidosis during adalimumab therapy for chronic plaque psoriasis. Marcella S, Welsh B, Foley P. Australas J Dermatol. 2011;52:8–11. doi: 10.1111/j.1440-0960.2010.00660.x. [DOI] [PubMed] [Google Scholar]
- 9.Sarcoidosis appearing during anti-tumour necrosis factor-alpha therapy: a new “class effect” paradoxical phenomenon. Two case reports and literature review. Massara A, Cavazzini L, La Corte R, Trotta F. Semin Arthritis Rheum. 2010;39:313–319. doi: 10.1016/j.semarthrit.2008.11.003. [DOI] [PubMed] [Google Scholar]
- 10.Nivolumab-related cutaneous sarcoidosis in a patient with lung adenocarcinoma. Birnbaum MR, Ma MW, Fleisig S, et al. JAAD Case Rep. 2017;3:208–211. doi: 10.1016/j.jdcr.2017.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Programmed death-1 inhibition of phosphatidylinositol 3-kinase/AKT/mechanistic target of rapamycin signalling impairs sarcoidosis CD4(+) T cell proliferation. Celada LJ, Rotsinger JE, Young A, et al. Am J Respir Cell Mol Biol. 2017;56:74–82. doi: 10.1165/rcmb.2016-0037OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.A concise review of pulmonary sarcoidosis. Baughman RP, Culver DA, Judson MA. Am J Respir Crit Care Med. 2011;183:573–581. doi: 10.1164/rccm.201006-0865CI. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sarcoidosis in melanoma patients: case report and literature review. Beutler BD, Cohen PR. Cancers (Basel) 2015;7:1005–1021. doi: 10.3390/cancers7020821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Monitoring and management of immune-related adverse events associated with programmed cell death protein-1 axis inhibitors in lung cancer. O'Kane GM, Labbé C, Doherty MK, Young K, Albaba H, Leighl NB. https://pubmed.ncbi.nlm.nih.gov/27534573/ Oncologist. 2017;22:70–80. doi: 10.1634/theoncologist.2016-0164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sarcoidosis-like granulomatous reaction associated with pembrolizumab immunotherapy. García-Mansoa IG, del Mar García Ródenasa M, Barroso Medela ME, Illán Gambínb FJ. https://www.archbronconeumol.org/en-sarcoidosis-like-granulomatous-reaction-associated-with-articulo-S1579212918303252-comprar. Arch Bronconeumol. 2018 doi: 10.1016/j.arbres.2018.04.010. [DOI] [PubMed] [Google Scholar]