Abstract
Background
Data on clinical, laboratory, and radiographic characteristics and risk factors for in‐hospital mortality of lung cancer patients with COVID‐19 are scarce. Here, we aimed to characterize the early clinical features of lung cancer patients with COVID‐19 and identify risk factors associated with in‐hospital mortality.
Methods
All consecutive lung cancer patients with laboratory‐confirmed COVID‐19 admitted to 12 hospitals in Hubei province, China, from 3 January to 6 May 2020 were included in the study. Patients without definite clinical outcomes during the period were excluded. Data on initial clinical, laboratory and radiographic findings were compared between survivors and nonsurvivors. Univariable and multivariable logistic regression analyses were used to explore the risk factors associated with in‐hospital mortality.
Results
Of the 45 lung cancer patients (median [interquartile range] age, 66 [58–74] years; 68.9% males) included, 34 (75.6%) discharged and 11 (24.4%) died. Fever (73.3%) and cough (53.3%) were the dominant initial symptoms, and respiratory symptoms were common. Lung cancer patients also presented atypical appearances of COVID‐19. In the multivariable analysis, prolonged prolongation prothrombin time (PT) (OR = 2.1, 95% CI: 1.00–4.41, P = 0.0497) and elevated high sensitivity cardiac troponin I (hs‐TNI) (OR = 7.65, 95% CI: 1.24–47.39, P = 0.0287) were associated with an increased risk of in‐hospital mortality.
Conclusions
Lung cancer patients with COVID‐19 have high in‐hospital mortality. Prolonged PT and elevated hs‐TNI are independent risk factors for in‐hospital mortality of lung cancer patients with COVID‐19.
Key points
Significant findings of the study
Lung cancer patients with COVID‐19 have atypical early symptoms and imaging features.
The prolonged prothrombin time and elevated high sensitivity cardiac troponin I are independent risk factors for in‐hospital mortality of lung cancer patients with COVID‐19.
What this study adds
This study characterizes the early clinical features of lung cancer patients with COVID‐19 in China, and identifies the risk factors associated with in‐hospital mortality of lung cancer patients with COVID‐19.
Keywords: Clinical features, COVID‐19, lung cancer, mortality, risk factors
Lung cancer patients with COVID‐19 have high in‐hospital mortality with unique early symptoms and imaging features. Prolonged PT and elevated hs‐TNI are independent risk factors for in‐hospital mortality of lung cancer patients with COVID‐19. They may be used to help clinicians predict prognosis at an early stage for lung cancer patients with COVID‐19.

Introduction
Coronavirus disease 2019 (COVID‐19) caused by severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) is now a global health, information and increasingly a wider socioeconomic crisis. 1 Patients with cancer have been shown to have a high risk of morbidity and mortality from COVID‐19. 2 , 3 Lung cancer represents a unique scenario of cumulative risk factors for COVID‐19 morbidity and mortality, including older age, multiple comorbidities, long smoking history, tumor‐related lung damage, as well as the unavoidable addition of treatment‐related immunosuppression. 4 , 5 , 6 Moreover, both mechanical tumor obstruction and previous lung resection surgery can cause defective pulmonary architecture, which may make lung cancer patients more predisposed to SARS‐CoV‐2 infection with a poor prognosis if COVID‐19 is suspected. However, data specifically focused on mortality, and the underlying risk factors for lung cancer patients with COVID‐19 are scarce. Rogado et al. first reported 17 lung cancer patients with COVID‐19 in Spain, when nine (52.9%) patients died. 6 However, the laboratory and radiographic findings of these patients were not described in the study. 6 Garassino et al. reported that only smoking history was associated with increased risk of death of patients with thoracic malignancies and COVID‐19. 5 However, this was a registry‐based study and clinical data, such as laboratory and radiographic characteristics of the patients, were not specified. 5
In this cohort study, we aimed to characterize the early clinical features of lung cancer patients with COVID‐19 in China, and identify the risk factors associated with in‐hospital mortality of lung cancer patients with COVID‐19.
Methods
Study design and participants
This retrospective study was conducted at 12 hospitals in Hubei province, China. All consecutive hospitalized patients diagnosed with lung cancer at any time and COVID‐19 on admission, or during hospitalization from 3 January to 6 May 2020, were identified. Patients with laboratory‐confirmed COVID‐19 and a definite clinical outcome (discharge or death) were included in the analysis. Laboratory‐confirmed COVID‐19 was defined when SARS‐CoV‐2 nucleic acid was positive as detected by reverse transcription‐polymerase chain reaction (RT‐PCR) assay, as described previously. 7 Patients who were transferred to other hospitals were excluded. The Research Ethics Commissions of Jinyintan Hospital approved this study and granted a waiver of informed consent from study participants (No.KY‐2020‐73.01).
Procedures of data collection and definitions
A trained team of physicians collected data from the electronic medical records using a standardized data collection form. Data included age, sex, smoking status, comorbidities, laboratory, radiographic, and electrocardiographic findings, pathology of lung cancer, active anticancer therapy (within four weeks of COVID‐19 diagnosis), cancer status (present vs. in remission), onset date, clinical manifestations and treatment of COVID‐19 and outcomes. Current lung cancer patients included all cases with newly diagnosed, partial remission, progressive, or recurrent cancer, while lung cancer patients in remission included those treated who went into complete remission. 8 The date of COVID‐19 onset was defined as the day when COVID‐19‐related symptoms such as fever, cough, chest tightness, shortness of breath, etc. were first noticed, and the severity of the disease was classified according to WHO guidelines. 9 Sepsis was defined as the Third International Consensus Definitions for Sepsis. 10 The diagnosis of acute respiratory distress syndrome (ARDS) was based on the Berlin Definition. 11 Patients were considered to have an acute cardiac injury if serum levels of high sensitivity cardiac troponin I (hs‐TNI) were above the 99th percentile upper reference limit, or if new abnormalities were revealed on electrocardiography (ECG) and echocardiography. 12
Chest computed tomography (CT) images were independently assessed for the presence and distribution of abnormalities by three chest radiologists. Ground‐glass opacity (GGO) was defined as hazy increased opacity in the lung, with the preservation of bronchial and vascular margins. 13 , 14 When there was a discrepancy, a final decision was reached by consensus.
Outcomes
Outcomes included in‐hospital mortality and discharge. The criteria for discharge were normal body temperature for at least three days, clinical remission of respiratory symptoms, substantial resolution of inflammation as shown by chest radiography, and two consecutive negative results for SARS‐CoV‐2 RNA obtained at least 24 hours apart. 7
Statistical analysis
Continuous variables are presented as median and interquartile ranges (IQR), and categorical variables are expressed as counts and percentages. Continuous variables to compare the differences between survivors and nonsurvivors were assessed using a Wilcoxon sum test, and categorical variables were analyzed using Chi‐square analysis or Fisher's exact test as appropriate; odds ratio (OR) and 95% confidence interval (CI) were calculated.
To explore the risk factors for in‐hospital mortality, univariate and multivariate logistic regression models were used. Multivariate logistic regression was performed, first by adjusting for the factors with a P‐value of <0.2 in univariate analysis, and then limiting final multivariate models to risk factors or confounders that were statistically significant in analyses. No imputation was made for missing data. If the RT‐PCR assay for SARS‐CoV‐2 was positive when the patient died, the date of death was used as the end date. All statistical tests were two‐sided, and a P‐value of <0.05 was considered statistically significant. For all analyses, SAS 9.3 (SAS Institute, Cary, NC) was used.
Results
Demographic, clinical, and tumor characteristics
From 3 January 2020, to 6 May 2020, 50 patients with lung cancer were identified from 11 660 patients clinically diagnosed with COVID‐19 and admitted to 12 hospitals in Hubei Province. Due to the newly constructed hospitals during the epidemic of COVID‐19, some patients, including lung cancer patients with COVID‐19, were transferred to the new hospitals for a reasonable allocation of medical resources. Five patients were then excluded, and thus 45 patients were included in the analysis. As of 6 May 2020, 34 patients were discharged, and 11 died, with an in‐hospital mortality rate of 24.4%.
Demographic, clinical, and tumor characteristics for patients are shown in Table 1. The median (IQR) age of the patients was 66 (58–74) years. There was a significant difference in age between survivors and nonsurvivors (61.5 [57.0–72.0] vs. 70.0 [67.0–80.0], P = 0.0207), but no difference in the sex ratio (P = 0.1318). Comorbidities were present in 30 (66.7%) patients, with hypertension being the most common comorbidity, followed by coronary artery disease and diabetes. The prevalence of hypertension was higher in nonsurvivors than in survivors (63.6% vs. 23.5%, P = 0.0256).
Table 1.
Patient demographic, clinical, and tumor characteristics
| Total N = 45 | Survivor N = 34 | Nonsurvivor N = 11 | P‑value | |
|---|---|---|---|---|
| Age, years | 66.0 (58.0–74.0) | 61.5 (57.0–72.0) | 70.0 (67.0–80.0) | 0.0207 |
| Sex | 0.1318 | |||
| Male | 31 (68.9) | 21 (61.8) | 10 (90.9) | |
| Female | 14 (31.1) | 13 (38.2) | 1 (9.1) | |
| History of smoking | 11 (24.4) | 8 (23.5) | 3 (27.3) | 1.0000 |
| Comorbidity | ||||
| Hypertension | 15 (33.3) | 8 (23.5) | 7 (63.6) | 0.0256 |
| Coronary artery disease | 7 (15.6) | 4 (11.8) | 3 (27.3) | 0.3368 |
| Diabetes | 6 (13.3) | 3 (8.8) | 3 (27.3) | 0.1459 |
| Other | 19 (42.2) | 13 (38.2) | 6 (54.5) | 0.4851 |
| Early symptoms of COVID‐19 | ||||
| Fever | 33 (73.3) | 25 (73.5) | 8 (72.7) | 1.0000 |
| Cough | 24 (53.3) | 18 (52.9) | 6 (54.5) | 1.0000 |
| Chest tightness | 8 (17.8) | 6 (17.6) | 2 (18.2) | 1.0000 |
| Shortness of breath | 7 (15.6) | 6 (17.6) | 1 (9.1) | 0.6628 |
| Fatigue | 6 (13.3) | 5 (14.7) | 1 (9.1) | 1.0000 |
| Sputum production | 5 (11.1) | 2 (5.9) | 3 (27.3) | 0.0853 |
| COVID‐19 disease severity | <0.0001 | |||
| Mild | 4 (8.9) | 4 (11.8) | 0 (0.0) | |
| Moderate | 18 (40.0) | 18 (52.9) | 0 (0.0) | |
| Severe | 12 (26.7) | 12 (35.3) | 0 (0.0) | |
| Critical | 11 (24.4) | 0 (0.0) | 11 (100.0) | |
| Time from COVID‐19 onset to hospital admission, days | 10.0 (6.5–14.5) | 10.0 (7.0–18.0) | 8.0 (2.0–12.0) | 0.0724 |
| Type of lung cancer | 0.0468 | |||
| Lung adenocarcinoma | 10 (22.2) | 6 (17.6) | 4 (36.4) | |
| Lung squamous cell cancer | 5 (11.1) | 4 (11.8) | 1 (9.1) | |
| Small cell lung cancer | 2 (4.4) | 0 (0) | 2 (18.2) | |
| No information | 28 (62.2) | 24 (70.6) | 4 (36.4) | |
| Prior thoracic surgery | 19(42.2) | 15(44.1) | 4(36.4) | 0.7363 |
| Cancer status | 0.4578 | |||
| Remission | 14 (31.1) | 12 (35.3) | 2 (18.2) | |
| Present | 31 (68.9) | 22 (64.7) | 9 (81.8) | |
| Time from lung cancer to COVID‐19, years † | 1.0 (0.0–3.0) | 1.0 (0.0–3.0) | 1.0 (1.0–1.0) | 0.9126 |
| Cancer treatment within four weeks of COVID‐19 diagnosis | ||||
| Lung resection surgery | 3 (6.7) | 2 (5.9) | 1 (9.1) | 1.000 |
| Chemotherapy | 4 (8.9) | 2 (5.9) | 2 (18.2) | 0.2470 |
| Treatment with immune checkpoint inhibitors | 4 (8.9) | 3 (8.8) | 1 (9.1) | 1.0000 |
Data are presented as the median (interquartile), or number (%), where appropriate.
Times from the diagnosis of lung cancer to the diagnosis of COVID‐19.
COVID‐19, coronavirus disease 2019.
The two most common early symptoms of COVID‐19 were fever (33 [73.3%]), and cough (24 [53.3%]). Other early symptoms of COVID‐19 included chest tightness (8 [17.8%]), shortness of breath (7 [15.6%]), fatigue (6 [13.3%]) and sputum production (5 [11.1%]), dyspnea (2 [4.4%]), loss of appetite (2 [4.4%]), myalgia (1 [2.2%]), nausea (1 [2.2%]), diarrhea (1 [2.2%]), seizure (1 [2.2%]), dysphagia (1 [2.2%]), headache (1 [2.2%]), and dizziness (1 [2.2%]). Among them, a 68‐year‐old man with a history of epilepsy and small cell lung cancer had a seizure on admission. He presented with respiratory symptoms (sputum production and dyspnea) during hospitalization and died 10 days following admission. There were no significant differences in the early symptoms of COVID‐19 between nonsurvivors and survivors (Table 1).
The most common lung cancer was lung adenocarcinoma (10 [22.2%]), followed by lung squamous cell cancer (5 [11.1%]) and small cell lung cancer (2 [4.4%]). The lung cancer types of 28 patients were not available in the electronic medical records. There were 31 (68.9%) current lung cancer patients and 14 (31.1%) patients in remission. There was no difference between the survivors and nonsurvivors in terms of cancer status and anticancer therapy (Table 1).
Laboratory, radiographic and electrocardiographic findings
Table 2 shows the laboratory, radiographic and ECG findings on admission. On admission, 33 (73.3%) patients had lymphocytopenia. In univariate analysis, there were significant differences in the lymphocyte count, platelet count, total bilirubin, urea, prothrombin time (PT), D‐dimer, hs‐TNI, and myoglobin between survivors and nonsurvivors.
Table 2.
Laboratory, radiographic and electrocardiographic findings of patients on admission
| Total N = 45 | Survivor N = 34 | Non‐survivor N = 11 | P value | |
|---|---|---|---|---|
| Laboratory findings | ||||
| White blood cell count ×109/L | 5.7 (4.0–7.5) | 5.2 (3.9–7.5) | 5.9 (4.4–8.4) | 0.4840 |
| Neutrophil count ×109/L | 3.8 (2.8–6.8) | 3.7 (2.8–6.0) | 4.4 (2.1–7.4) | 0.4129 |
| Lymphocyte count ×109/L | 0.8 (0.5–1.2) | 0.9 (0.6–1.4) | 0.6 (0.2–0.9) | 0.0223 |
| Monocyte count ×109/L | 0.5 (0.3–0.6) | 0.5 (0.4–0.6) | 0.3 (0.1–0.6) | 0.1424 |
| Red blood cell count ×1012/L | 4.0 (3.4–4.3) | 4.1 (3.7–4.4) | 3.5 (3.1–4.1) | 0.0725 |
| Hemoglobin, g/L | 120.0 (103.0–131.1) | 120.5 (106.0–133.0) | 120.0 (102.0–131.0) | 0.5347 |
| Platelet count ×109/L | 187.0 (133.0–245.0) | 203.0 (160.0–258.0) | 119.0 (66.0–199.0) | 0.0028 |
| <125 | 11 (24.4) | 5 (14.7) | 6 (54.5) | 0.0143 |
| ≥125 | 34 (75.6) | 29 (85.3) | 5 (45.5) | |
| ALT, U/L | 0.7049 | |||
| ≤40 | 34 (75.6) | 25 (73.5) | 9 (81.8) | |
| >40 | 11 (24.4) | 9 (26.5) | 2 (18.2) | |
| AST, U/L | 0.0707 | |||
| ≤40 | 31 (68.9) | 26 (76.5) | 5 (45.5) | |
| >40 | 14 (31.1) | 8 (23.5) | 6 (54.5) | |
| LDH, U/L | ||||
| ≤245 | 21 (53.8) | 18 (58.1) | 3 (37.5) | 0.4324 |
| >245 | 18 (46.2) | 13 (41.9) | 5 (62.5) | |
| Total bilirubin, μmol/L | 0.0125 | |||
| ≤21 | 41 (93.2) | 33 (100) | 8 (72.7) | |
| >21 | 3 (6.8) | 0 (0) | 3 (27.3) | |
| Urea, mmol/L | 5.5 (4.0–8.0) | 4.8 (3.7–6.8) | 8 (7.2–10.9) | 0.0013 |
| Creatinine, μmol/L | 0.1434 | |||
| ≤133 | 42 (93.3) | 33 (97.1) | 9 (81.8) | |
| >133 | 3 (6.7) | 1 (2.9) | 2 (18.2) | |
| PT, s | 11.8 (10.9–12.9) | 11.6 (10.8–12.3) | 13.0 (11.8–13.9) | 0.0168 |
| APTT, s | 29.4 (24.8–32.8) | 29.4 (25.0–32.2) | 30.0 (23.8–38.1) | 0.7311 |
| D‐dimer, mg/L | 2.0 (0.7–10.7) | 1.5 (0.7–3.8) | 19.1 (1.8–37.3) | 0.0096 |
| C‐reactive protein, mg/L | 0.4101 | |||
| <10 | 9 (22) | 8 (25.8) | 1 (10) | |
| ≥10 | 32 (78) | 23 (74.2) | 9 (90) | |
| Procalcitonin, ng/mL | 0.0635 | |||
| <0.5 | 35 (83.3) | 28 (90.3) | 7 (63.6) | |
| ≥0.5 | 7 (16.7) | 3 (9.7) | 4 (36.4) | |
| hs‐TNI | 0.0084 | |||
| Normal | 23 (62.2) | 20 (76.9) | 3 (27.3) | |
| Elevated | 14 (37.8) | 6 (23.1) | 8 (72.7) | |
| CK–MB | 0.6582 | |||
| Normal | 33 (80.5) | 25 (83.3) | 8 (72.7) | |
| Elevated | 8 (19.5) | 5 (16.7) | 3 (27.3) | |
| Myoglobin, ng/mL | 76.9 (35.2–144.6) | 52.8 (30.0–87.7) | 153.2 (100.1–218.3) | 0.0020 |
| NT‐proBNP, pg/mL | 254.6 (56.4–1061) | 197.4 (37.1–648.8) | 1061 (224.0–1383.0) | 0.0661 |
| Radiological findings | ||||
| GGO with reticular and/or interlobular septal thickening in unilateral lung | 4 (8.9) | 3 (8.8) | 1 (9.1) | 1.0000 |
| GGO with reticular and/or interlobular septal thickening in bilateral lungs | 40 (88.9) | 30 (88.2) | 10 (90.9) | 1.0000 |
| Pleural effusion | 4 (8.9) | 2 (5.9) | 2 (18.2) | 0.2470 |
| Nodule | 10 (22.2) | 9 (26.5) | 1 (9.1) | 0.4087 |
| Thoracic lymphadenopathy | 5 (11.1) | 5 (14.7) | 0 (0.0) | 0.3131 |
| Pleural thickening | 2 (4.4) | 2 (5.9) | 0 (0.0) | 1.0000 |
| Pericardial thickening | 3 (6.7) | 3 (8.8) | 0 (0.0) | 0.5651 |
| Electrocardiographic findings | 14 (31.1) | 8 (23.5) | 6 (54.6) | 0.0707 |
| Arrhythmia | 6 (13.3) | 3 (8.8) | 3 (27.3) | 0.1459 |
| ST segment abnormalities | 6 (13.3) | 3 (8.8) | 3 (27.3) | 0.1459 |
| Right bundle branch block | 3 (6.7) | 2 (5.9) | 1 (9.1) | 1.0000 |
| QT prolongation | 2 (4.4) | 1 (2.9) | 1 (9.1) | 0.4333 |
Data are presented as the median (interquartile), or number (%), where appropriate.
ALT, alanine aminotransferase; AST, Aspartate aminotransferase; LD, lactate dehydrogenase; PT, prothrombin time; APTT, activated partial thromboplastin time; hs‐TNI, high‐sensitivity troponin I; CK‐MB, creatinine kinase–myocardial band; NT‐proBNP, N‐terminal pro–brain natriuretic peptide; GGO, ground‐glass opacity.
The most common pattern on initial chest CT was GGO with reticular and/or interlobular septal thickening in bilateral lungs (40[88.9%]). Other findings included pleural effusion, nodule, thoracic lymphadenopathy, pleural thickening, and pericardial thickening (Table 2). The median duration from COVID‐19 onset to CT scan was 7.8 days. Radiographic abnormalities did not differ between survivors and nonsurvivors (Table 2).
A total of 14 (31.1%) patients presented with ECG abnormalities on admission. There was no significant difference in ECG abnormalities between survivors and nonsurvivors (Table 2).
Treatment of COVID‐19 patients and outcomes
Treatment of COVID‐19 patients and outcomes are shown in Table 3. Oxygen therapy use differed significantly between nonsurvivors and survivors (P < 0.0001); More patients needed noninvasive and invasive mechanical ventilation in nonsurvivors than survivors (45.5% vs. 8.8%, and 27.3% vs. 0%, respectively). More patients were admitted to the intensive care unit (ICU) in nonsurvivors than survivors (45.5% vs. 2.9%, OR = 27.5, 95% CI: 2.71–278.87).
Table 3.
Treatments of COVID‐19 and outcomes
| Total N = 45 | Survivor N = 34 | Nonsurvivor N = 11 | P‐value | |
|---|---|---|---|---|
| Treatments of COVID‐19 | ||||
| Oxygen therapy | <0.0001 | |||
| No oxygen therapy | 13 (28.9) | 13 (38.2) | 0 (0) | |
| High‐flow nasal cannula oxygen therapy | 21 (46.7) | 18 (52.9) | 3 (27.3) | |
| Noninvasive mechanical ventilation | 8 (17.8) | 3 (8.8) | 5 (45.5) | |
| Invasive mechanical ventilation | 3 (6.7) | 0 (0) | 3 (27.3) | |
| Antibiotics | 36 (80) | 26 (76.5) | 10 (90.9) | 0.4157 |
| Antiviral treatment | 40 (88.9) | 30 (88.2) | 10 (90.9) | 1.0000 |
| Systematic corticosteroids | 12 (26.7) | 7 (20.6) | 5 (45.5) | 0.1306 |
| Intravenous immunoglobulin | 5 (11.1) | 4 (11.8) | 1 (9.1) | 1.0000 |
| Renal replacement therapy | 1 (2.2) | 0 (0) | 1 (9.1) | 0.2444 |
| Outcomes | ||||
| Respiratory failure | 12 (26.7) | 2 (5.9) | 10 (90.9) | <0.0001 |
| Acute cardiac injury | 10 (22.2) | 4 (11.8) | 6 (54.5) | 0.0074 |
| ARDS | 9 (20.0) | 1 (2.9) | 8 (72.7) | <0.0001 |
| Heart failure | 6 (13.3) | 2 (5.9) | 4 (36.4) | 0.0247 |
| Acute kidney injury | 4 (8.9) | 0 (0) | 4 (36.4) | 0.0022 |
| Sepsis | 4 (8.9) | 0 (0) | 4 (36.4) | 0.0022 |
| Secondary infection | 11 (24.4) | 6 (17.6) | 5 (45.5) | 0.1037 |
| ICU admission | 6 (13.3) | 1 (2.9) | 5 (45.5) | 0.0020 |
| Time from illness onset to fever, days | 1.0 (1.0–1.0) | 1.0 (1.0–1.0) | 1.0 (1.0–1.0) | 0.4890 |
| Time from illness onset to sputum production, days | 1.0 (1.0–2.5) | 1.0 (1.0–3.0) | 1.0 (1.0–1.5) | 0.4175 |
| Time from illness onset to cough, days | 1.0 (1.0–2.0) | 1.0 (1.0–3.0) | 1.0 (1.0–1.0) | 0.1066 |
| Time from illness onset to respiratory failure, days | 11.0 (8.0–14.0) | 9.0 (5.0–13.0) | 11.0 (10.0–14.0) | 0.4765 |
| Time from illness onset to acute cardiac injury, days | 12.0 (7.0–15.0) | 12.0 (7.5–20.0) | 12.0 (7.0–14.0) | 0.5212 |
| Time from illness onset to ARDS, days | 12.0 (4.5–14.0) | 13.0 (13.0–13.0) | 11.0 (1.0–15.0) | 0.8252 |
| Time from illness onset to heart failure, days | 14.5 (12.0–18.0) | 14.0 (12.0–16.0) | 15.5 (8.0–32.0) | 0.8170 |
| Time from illness onset to ICU admission, days | 14.0 (2.0–15.0) | 13.0 (13.0–13.0) | 14.5 (7.5–16.5) | 0.7237 |
| Time from illness onset to corticosteroids treatment, days | 12.0 (9.0–23.0) | 20.0 (9.0–32.0) | 9.5 (8.0–15.0) | 0.1066 |
| Duration of treatment with corticosteroids, days | 7.0 (6.0–10.0) | 6.0 (6.0–10.0) | 8.5 (6.0–11.0) | 0.7022 |
| Duration of viral shedding after COVID‐19 onset, days | 12.0 (7.0–17.5) | 11.0 (7.0–17.0) | 14.5 (10.0–18.0) | 0.4406 |
| Time from illness onset to death or discharge, days | 25.5 (19–36.5) | 30.5 (22.0–38.0) | 18.0 (16.0–22.0) | 0.0367 |
Data are presented as the median (interquartile), or number (%), where appropriate.
ECMO, extracorporeal membrane oxygenation; ICU, intensive care unit; COVID‐19, coronavirus disease 2019; ARDS, acute respiratory distress syndrome; MODS, multiple organ dysfunction syndrome.
Respiratory failure was the most frequently observed complication (26.7%), followed by acute cardiac injury (22.2%), secondary infection (24.4%), ARDS (20.0%), heart failure (13.3%), acute kidney injury (8.9%), and sepsis (8.9%). The incidence of all comorbidities in nonsurvivors was significantly higher than survivors, except for secondary infection (Table 3).
Risk factors associated with in‐hospital mortality
Univariable analyses revealed that patients who were older (OR = 1.07, 95% CI: 1.01–1.14, P = 0.0342), with hypertension (OR = 5.69, 95% CI: 1.32–24.54, P = 0.0198), prolonged prothrombin time (PT) on admission (OR = 2.1, 95% CI: 1.14–3.88, P = 0.0176), and elevated high sensitivity cardiac troponin I (hs‐TNI) on admission (OR = 8.89, 95% CI, 1.78–44.47) were associated with an increased risk of in‐hospital mortality, whereas elevated platelet count (≥125 × 109/L) (OR = 0.14, 95% CI: 0.03–0.66, P = 0.0123) was associated with a decreased risk. In the multivariable analysis, only prolonged PT (OR = 2.1, 95% CI: 1.00–4.41, P = 0.0496) (Fig 1) and elevated hs‐TNI (OR = 7.65, 95% CI: 1.24–47.39, P = 0.0287) (Fig 2) on admission were associated with an increased risk of in‐hospital mortality.
Figure 1.
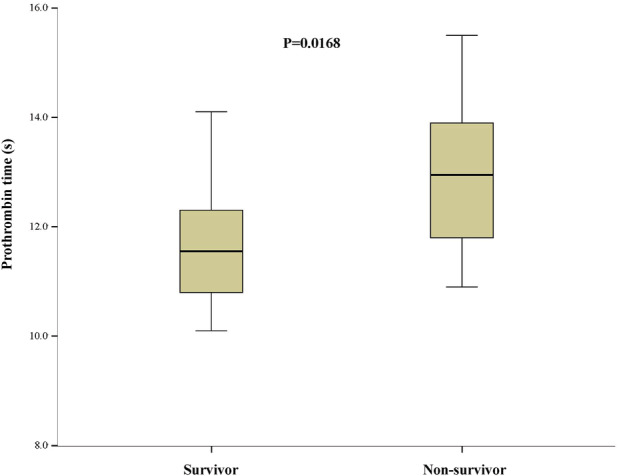
Prothrombin time on admission in lung cancer patients with COVID‐19.
Figure 2.
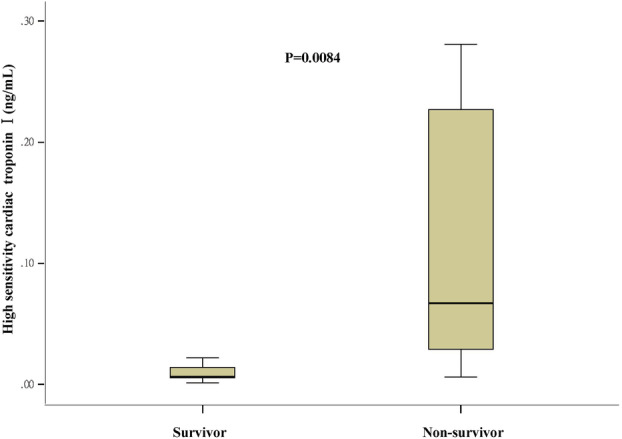
High sensitivity cardiac troponin I on admission in lung cancer patients with COVID‐19.
Discussion
Our study suggests that the early clinical manifestations, laboratory, and radiographic findings of COVID‐19 in lung cancer patients were similar to those previously reported in patients without cancer in Hubei, especially Wuhan city, China. 12 , 15 , 16 However, lung cancer patients also presented with atypical appearances of COVID‐19. The prolonged PT and elevated hs‐TNI on admission were identified as independent risk factors for the in‐hospital mortality of lung cancer patients with COVID‐19.
Our results show that the in‐hospital mortality rate in lung cancer patients with COVID‐19 in China was 24.4%, while previous studies reported that the fatality rate of noncancer patients in the same age groups with confirmed COVID‐19 in China ranged from 1.3% to 8.0%. 17 , 18 Our data indicate a significantly high risk of in‐hospital mortality posed to lung cancer patients with COVID‐19.
The lung cancer symptoms may overlap with symptoms of COVID‐19, since they both directly impair lung function. In our lung cancer cohort, fever and cough were the dominant initial symptoms, and respiratory symptoms were common. Lung cancer patients with COVID‐19 also presented with some nonspecific symptoms, which have been reported in older people or immunosuppressed patients. 9 , 19 Also, we observed a 68‐year‐old man with a history of epilepsy and small cell lung cancer who presented with seizures before the onset of respiratory symptoms. The seizures may have occurred due to occult brain metastasis or leptomeningeal carcinomatosis caused by the progression of small cell lung cancer. However, a recent retrospective study of 214 patients in Wuhan reported neurological symptoms in 36.4% of COVID‐19 patients, and the rate rose to 45.5% when the most severely affected patients were considered. 20 Autopsy of patients who died with COVID‐19 also showed that the brain tissue was hyperemic and edematous, with degeneration of neurons. 21 Therefore, there is a possibility that the invasion of the nervous system by SARS‐CoV‐2 may be one of the pathogenic mechanisms. 20 , 22 Our findings suggests that lung cancer patients may present with neurological manifestation without typical symptoms such as fever and cough at the early stage of COVID‐19. Clinicians should be aware of the possibility of COVID‐19 presenting nonspecifically, including seizures, in order that there will not be a delayed or misdiagnosis.
The main CT findings of lung cancer patients with COVID‐19 have been reported to be similar to radiological findings, commonly reported in patients with COVID‐19. 13 , 14 , 23 We also found some uncommonly reported imaging features. These imaging features may be atypical appearances of general COVID‐19 patients, 23 , 24 but they may represent unique appearances of patients with lung cancer.
More importantly, we identified that prolongation of PT on admission was associated with an over two‐fold increased risk of in‐hospital mortality in lung cancer patients with COVID‐19. This finding is in agreement with a previous study demonstrating that coagulation abnormalities may be associated with an increased risk of death in patients with COVID‐19. 25 The most typical findings reported in patients with COVID‐19 and coagulopathy are an increased D‐dimer concentration, a decrease in platelet count, and a prolongation of PT. 25 , 26 , 27 Although subclinical changes in the coagulation‐fibrinolytic system are often present in lung cancer, 28 , 29 coagulation parameters have not previously been studied in lung cancer patients with COVID‐19. In the present study, the nonsurvivors had a prolonged PT, increased D‐dimer concentration, and decreased platelet count, compared to survivors. Based on the findings of the present study and the data from published literature, we recommend closely monitoring coagulopathy in lung patients with COVID‐19 by measuring PT, platelet count, and D‐dimer concentrations.
Several studies have reported that cardiac injury is a common condition among hospitalized patients with COVID‐19, and is associated with a higher risk of in‐hospital mortality 11 , 30 The present study also demonstrates that an elevated hs‐TNI on admission was associated with an over seven‐fold increased risk of death in lung cancer patients with COVID‐19. The evidence that SARS‐CoV‐2 directly impairs cardiac function has been demonstrated in a study by Crackower et al. in which the targeted disruption of ACE2 in mice resulted in a severe heart contractility defect, increased angiotensin II levels, and upregulation of hypoxia‐induced genes in the heart. 31
This study has several limitations. First, the number of patients involved in this observational study is relatively small, and thus one should take this limitation into account when interpreting the results and conclusions. Second, from this dataset, we found that lung resection surgery, chemotherapy, and treatment with immune checkpoint inhibitors given within four weeks before the diagnosis of laboratory‐confirmed COVID‐19 were not associated with increased risk with in‐hospital mortality. Similar observations were reported for chemotherapy, immunotherapy, hormonal therapy, targeted therapy, and radiotherapy in cancer patients with COVID‐19 in a study by Lee et al. 3 but the number of lung cancer patients who received active anticancer therapy in their study was small. Further investigation with a greater number of patients will allow us to confirm or refute these findings. Since there has been no previously published study on a large series of lung cancer patients with COVID‐19, and data on clinical, laboratory, and radiographic characteristics lung cancer patients with COVID‐19 are scarce, we believe that this preliminary study is of value in guiding the management of lung cancer patients with COVID‐19.
In conclusion, lung cancer patients with COVID‐19 have high in‐hospital mortality. Prolonged PT and elevated hs‐TNI are independent risk factors for in‐hospital mortality of lung cancer patients with COVID‐19. They may be used to assist clinicians predict prognosis at an early stage for lung cancer patients with COVID‐19.
Disclosure
All authors have no conflict of interest to declare.
Acknowledgments
National Natural Science Foundation of China (Grant No. 82003480).
Science and Technology Planning Project of Hubei Province (Grant No. 2018CKB922).
References
- 1. Coronavirus disease (COVID‐19) Situation Report – 162. [cited 30 June 2020.] Available from URL: https://www.who.int/docs/default-source/coronaviruse/20200630-covid-19-sitrep-162.pdf?sfvrsn=e00a5466_2
- 2. Kuderer NM, Choueiri TK, Shah DP et al Clinical impact of COVID‐19 on patients with cancer (CCC19): A cohort study. Lancet 2020; 6736: 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lee LYW, Cazier JB, Starkey T et al COVID‐19 mortality in patients with cancer on chemotherapy or other anticancer treatments: A prospective cohort study. Lancet 2020; 395: 1919–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Russano M, Citarella F, Vincenzi B, Tonini G, Santini D. Coronavirus disease 2019 or lung cancer: What should we treat? J Thorac Oncol 2020; 15: e105–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Garassino MC, Whisenant JG, Huang L‐C et al COVID‐19 in patients with thoracic malignancies (TERAVOLT): First results of an international, registry‐based, cohort study. Lancet Oncol 2020; 21: 914–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Rogado J, Pangua C, Serrano‐montero G et al Covid‐19 and lung cancer: A greater fatality rate? Lung Cancer 2020; 146: 19–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Zhou F, Yu T, Du R et al Clinical course and risk factors for mortality of adult inpatients with COVID‐19 in Wuhan, China: A retrospective cohort study. Lancet 2020; 395: 1054–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. He W, Chen L, Chen L et al COVID‐19 in persons with haematological cancers. Leukemia 2020; 34: 1637–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. World Health Organization . Clinical management of COVID‐19: interim guidance. [Cited 27 May 2020.] Available from URL: https://www.who.int/publications/i/item/clinical-management-of-covid-19
- 10. Singer M, Deutschman CS, Seymour C et al The third international consensus definitions for sepsis and septic shock (sepsis‐3). JAMA 2016; 315: 801–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Guo T, Fan Y, Chen M et al Cardiovascular implications of fatal outcomes of patients with coronavirus disease 2019 (COVID‐19). JAMA Cardiol 2020; 5: 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Huang C, Wang Y, Li X et al Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020; 395: 497–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Song F, Shi N, Shan F et al Emerging 2019 novel coronavirus (2019‐NCoV) pneumonia. Radiology 2020; 295: 210–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Zhou Z, Guo D, Li C et al Coronavirus disease 2019: Initial chest CT findings. Eur Radiol 2020; 30: 4398–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Chen N, Zhou M, Dong X et al Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: A descriptive study. Lancet 2020; 395: 507–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wang D, Hu B, Hu C et al Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus‐infected pneumonia in Wuhan, China. JAMA 2020; 323: 1061–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Onder G, Rezza G, Brusaferro S. Case‐fatality rate and characteristics of patients dying in relation to COVID‐19 in Italy. JAMA 2020; 323: 1775–6. 10.1001/jama.2020.4683. [DOI] [PubMed] [Google Scholar]
- 18. Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID‐19) outbreak in China: Summary of a report of 72314 cases from the Chinese Center for Disease Control and Prevention. JAMA 2020; 323: 1239 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- 19. Dziewas R, Warnecke T, Zürcher P et al Dysphagia in COVID‐19‐multilevel damage to the swallowing network? Eur J Neurol 2020. 10.1111/ene.14367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Mao L, Jin H, Wang M et al Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan, China. JAMA Neurol 2020; 77: 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. National Health Commission of the People's Republic of China. Diagnosis and treatment of the novel coronavirus pneumonia (Trial version 7) [D]. Published 2020. [3 March 2020.] Available from URL: http://www.nhc.gov.cn/yzygj/s7653p/202003/46c9294a7dfe4cef80dc7f5912eb1989/files/ce3e6945832a438eaae415350a8ce964.pdf
- 22. Tassorelli C, Mojoli F, Baldanti F et al COVID‐19: What if the brain had a role in causing the deaths? Eur J Neurol 2020; 1–2. 10.1111/ene.14275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Simpson S, Kay FU, Abbara S et al Radiological Society of North America expert consensus statement on reporting chest CT findings related to COVID‐19. Endorsed by the Society of Thoracic Radiology, the American College of Radiology, and RSNA. J Thorac Imaging 2020; 35: 219–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Suppli MH, Riisgaard de Blanck S, Elgaard T, Josipovic M, Pøhl M. Early appearance of COVID‐19 associated pulmonary infiltrates during daily radiotherapy imaging for lung cancer. J Thorac Oncol 2020; 15: 1081–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Levi M, Thachil J, Iba T, Levy JH. Coagulation abnormalities and thrombosis in patients with COVID‐19. Lancet Haematol 2020; 7: e438–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Thachil J, Tang N, Gando S et al ISTH interim guidance on recognition and management of coagulopathy in COVID‐19. J Thromb Haemost 2020; 18: 1023–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Tang N, Li D, Wang X, Sun Z. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J Thromb Haemost 2020; 18: 844–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Tas F, Kilic L, Serilmez M, Keskin S, Sen F, Duranyildiz D. Clinical and prognostic significance of coagulation assays in lung cancer. Respir Med 2013; 107: 451–7. [DOI] [PubMed] [Google Scholar]
- 29. Ferrigno D, Buccheri G, Ricca I. Prognostic significance of blood coagulation tests in lung cancer. Eur Respir J 2001; 17: 667–73. [DOI] [PubMed] [Google Scholar]
- 30. Shi S, Qin M, Shen B et al Association of cardiac injury with mortality in hospitalized patients with COVID‐19 in Wuhan, China. JAMA Cardiol 2020; 395: 497–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Crackower MA, Sarao R, Oudit GY et al Angiotensin‐converting enzyme 2 is an essential regulator of heart function. Nature 2002; 417: 822–8. [DOI] [PubMed] [Google Scholar]


