Abstract
Background
Non‐small cell lung cancer (NSCLC) is the first cause of cancer‐related death among men and the second among women worldwide. It also poses an economic threat to the sustainability of healthcare services. This study estimated the direct costs of care for patients with NSCLC by stage at diagnosis, and management phase of pathway recommended in local and international guidelines.
Methods
Based on the most up‐to‐date guidelines, we developed a very detailed “whole‐disease” model listing the probabilities of all potentially necessary diagnostic and therapeutic actions involved in the management of each stage of NSCLC. We assigned a cost to each procedure, and obtained an estimate of the total and average per‐patient costs of each stage of the disease and phase of its management.
Results
The mean expected cost of a patient with NSCLC is 21,328 € (95% C.I. −20 897−22 322). This cost is 16 291 € in stage I, 19530 € in stage II, 21938 € in stage III, 22175 € in stage IV, and 28 711 € for a Pancoast tumor. In the early stages of the disease, the main cost is incurred by surgery, whereas in the more advanced stages radiotherapy, medical therapy, treatment for progressions, and supportive care become variously more important.
Conclusions
An estimation of the direct costs of care for NSCLC is fundamental in order to predict the burden of new oncological therapies and treatments on healthcare services, and thus orient the decisions of policy‐makers regarding the allocation of resources.
Key points
Significant findings of the study
The high costs of surgery make the early stages of the disease no less expensive than the advanced stages.
What this study adds
An estimation of the direct costs of care is fundamental in order to orient the decisions of policy‐makers regarding the allocation of resources.
Keywords: Cost of illness, economic models, lung cancer burden, practice guidelines
The high costs of surgery make the early stages of the disease no less expensive than the advanced stages. An estimation of the direct costs of care is fundamental in order to orient the decisions of policy‐makers regarding the allocation of resources.
Introduction
Lung cancer is now the first cause of cancer‐related death in men and the second (after breast cancer) in women worldwide. 1 In this scenario, non‐small cell lung cancer (NSCLC), which accounts for approximately 87% of lung cancers, 2 clearly emerges as a “big killer” of our times. It has become a huge public health issue, not only in terms of the related morbidity and mortality, but also from an economic standpoint. Many new oncological drugs for NSCLC have arrived on the market in recent years, improving patients' progression‐free survival (PFS) and overall survival (OS)3, 4, 5, 6, 7, 8, 9, 10 however, they are extremely costly and are seriously threatening the sustainability of healthcare systems. 11
Standardized guidelines have been developed locally and internationally to help specialists make decisions regarding their patients' management, but also in an endeavor to ensure a more rational allocation of resources. 12 Furthermore clinical pathways derived by guidelines provide more details about organizational aspects and timing schedule of the care for the management of chronical pathology within an integrated network of hospital and primary care. 13 , 14
The cost components of lung cancer care are being analyzed more and more around the world nowadays to assess the effective economic impact of this disease on healthcare systems. Such studies have been conducted in Germany (Schwarzkopf et al. 15 ), in the Netherlands (Van der Linden et al. 16 ), in the Czech Republic (Šimrová et al. 17 ), and in Catalonia (Corral et al. 18 ). Comparisons were drawn between Western European countries by McGuire et al. 19 and between different parts of the U.S. by Sheehan et al. 20 and Cipriano et al. 21 A review on the situation in Latin America has been published by Raez et al. 22 However, all these studies were conducted before the approval for use of new costly therapies. The new therapies have been considered only referring to the USA, 23 which has a very different health care system, so it is not easy to apply these findings to Europe.
The purpose of the present study was therefore to develop a “whole‐disease model” 24 of the pathways of care for NSCLC, including all the steps of a patient's experience, from when the disease is suspected to the end of the first year of treatment, through the whole diagnostic and staging process, and all the possible interventions. Our aim was to predict with accuracy the direct costs of care for NSCLC by stage at diagnosis, and phase of its management, on the basis of the currently‐adopted clinical pathway.
Methods
We developed a model that estimated the costs, stratified by stage of diagnosis, of the clinical pathway of care for NSCLC patients over a one‐year time horizon, using a payer perspective, and only considering direct costs.
Context
Italy's health care system is a regionally‐managed national health service (NHS) that provides universal coverage at the point of delivery, and free of charge for patients who are exempt for reasons such as a cancer diagnosis. The system is grounded on fundamental values of universality, free access, freedom of choice, pluralism in provision, and equity. 25 Regional authorities plan and organize healthcare facilities and activities in accordance with a national health plan designed to assure an equitable provision of comprehensive care throughout the country.
Consistently with other international guidelines, 26 the AIOM (Italian Association of Medical Oncology) developed clinical guideline for the management of lung cancer, from diagnosis to all subsequent staging, treatment and follow‐up actions. 27 Veneto Region established a diagnostic and therapeutic patient care pathway according this national guideline to its regional context 28 to guarantee all of the region's residents an equitable and effective cancer care. In particular, the Veneto Oncological Institute drafted its “Place in Therapy” (PIT) document to help its specialists allocate stage IIIB and IV patients to the most suitable chemotherapy protocol currently available. 29
Based on the above‐mentioned clinical pathway for NSCLC, we developed our model.
Probabilities
The probabilities were divided into two categories, described as clinical and process probabilities.
Clinical probabilities were estimated mostly from the literature. In particular, the probability of a given stage distribution at diagnosis was assumed from a previous study by Benitez‐Majano et al. 30 Cases of superior sulcus tumor (also known as Pancoast tumors) were modeled as a separate group. 31 , 32 OS and PFS rates, by disease stage at diagnosis, were obtained from the literature (Table 1). Mortality rates due to natural causes were drawn from the data available from the Italian Statistics Institute (ISTAT), taking into account the average age of incidence for NSCLC cohort cases. 33 At all disease stages, patients could be classified as “surgical” or “nonsurgical” according literature probabilities: 81% of patients with stage I disease had surgery, 43 as did 63% of those with stage II, 44 11.6% of those with stage III, 45 4.5% of those with stage IV, 46 and 54% of those with a Pancoast tumor. 40
Process probabilities related to the diagnostic and therapeutic actions taken on patients. Most procedures were the same for all patients since their specific condition was deterministically associated with a specific clinical pathway (eg, all patients diagnosed with a stage IV NSCLC undergo a multidisciplinary clinical examination). However, clinical and diagnostic pathway leaves some decisions regarding certain procedures to the physician's discretion. Since the other national and international guidelines consulted 26 , 27 have the same discretionary elements, a number of NSCLC experts were asked to indicate the proportions of patients involved in such procedures, based on their professional experience, in order to estimate the probability of these procedures as accurately as possible. A survey was designed using the Delphi technique 47 to obtain a consensus among the experts consulted by means of a two‐round questionnaire that contained both aggregate information and any open suggestions made by the experts. The questionnaire was divided into three sections: oncological, surgical, and radiotherapy. The survey was conducted using a CAWI (Computer‐Assisted Web‐based Interviewing) method, and the questionnaire was sent by email to 21 experts (seven surgeons, six oncologists and eight radiotherapists). The process probabilities adopted in the model were estimated from the means of the proportions indicated by the experts. In all, 11 (response rate 52.4%) experts completed two successive questionnaires and contributed to our achievement of good results in terms of convergence. The respondents included three surgeons, five oncologists and three radiotherapists (and two oncologists and one radiotherapist were female). All the process probabilities estimated with the Delphi survey and their interquartile ranges are reported in the Supplementary Tables A, B and C .
Table 1.
Probabilities (and beta distribution parameters) of death and recurrence rate one year after diagnosis by stage at diagnosis
| Post‐surgery mortality rate (only for surgical patients) | Natural mortality rate | Tumor‐related mortality rate | Recurrence rate | Source | |
|---|---|---|---|---|---|
| I |
1.5% beta (1.5, 98.5) |
3.1% beta (3.1, 96.9) |
0.7% beta (0.7, 99.3) |
5.0% beta (5.0, 95.0) |
33, 34, 35, 36 |
| II |
1.4% beta (1.4, 98.6) |
3.1% beta (3.1, 96.9) |
6.1% beta (6.1, 93.9) |
10.0% beta (10.0, 90.0) |
33, 34, 35, 36 |
| III |
7.1% beta (7.1, 92.8) |
3.1% beta (3.1, 96.9) |
23.0% beta (23.0, 77.0) |
18.0% beta (18.0, 82.0) |
33, 34, 37 |
| IV |
1.9% beta (1.9, 98.1) |
3.1% beta (3.1, 96.9) |
53.2% beta (53.2, 46.8) |
‐ | 3, 4, 5, 6, 7, 8, 9, 33, 38 |
| Pancoast |
3.2% beta (3.2, 96.8) |
3.1% beta (3.1, 96.9) |
33.2% beta (33.2, 66.8) |
22.0% beta (22.0, 78.0) |
33, 39, 40, 41, 42 |
Costs
The study was conducted from the perspective of the Veneto Regional Healthcare Service, considering only the direct costs sustained by the public health authorities, and using cost data drawn from official reimbursement tariffs in effect in 2019. 48 , 49
We estimated the mean per‐patient cost at first after diagnosis, by disease stage.
The unit costs used in the model are given in Supplementary Tables D‐E‐F .
To assess the costs of adjuvant medical therapy, we estimated a monthly cost for each treatment package that included the cost of the drug, the cost of drug delivery, and the cost of routine laboratory tests. These costs were multiplied to complete the whole cycle of therapy (the standard duration of cycle of traditional chemotherapies was drawn from international protocols, whereas the duration of new targeted therapies treatment was assumed as the median progression‐free survival for each drug, derived from the registration trials) and up to a maximum of six months, in the first year, because the first six months of the first year after diagnosis was occupied by the diagnostic and staging phase, a surgery phase (if any), and neoadjuvant therapies (Fig 1 and Table 2). For patients interrupting their chemo‐ or radiotherapy in advance due to treatment toxicities, disease progression or death, the cost was assumed at 50% of the cost of the full treatment. Costs of treatment toxicities were considered, according to the literature, for traditional chemotherapy, 50 for radiotherapy 51 , 52 , 53 , 54 and for the modern target‐therapies. 55
Figure 1.
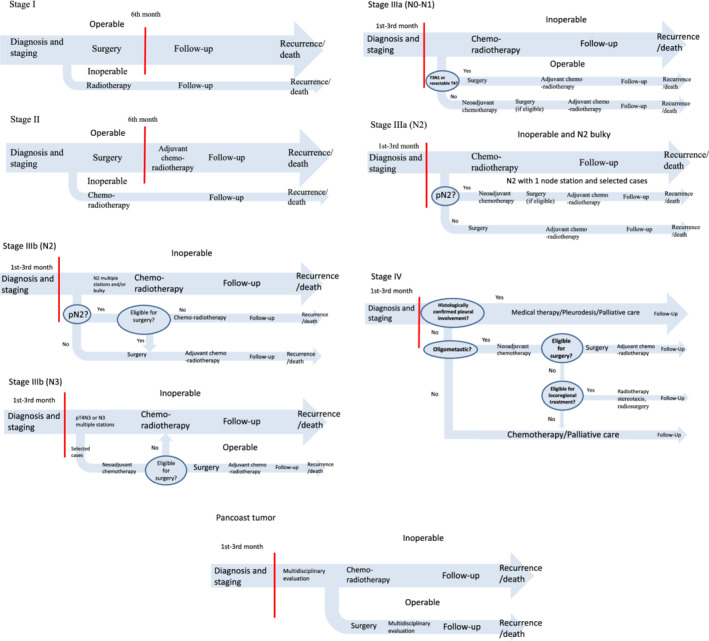
Essential diagnostic and therapeutic pathway for the different stages at diagnosis of NSCLC.
Table 2.
Average monthly costs of medical therapy † (€)
| Drug | Administration (i.v.) | Biochemical tests | Follow‐up visits | Total | |
|---|---|---|---|---|---|
| Gefitinib | 2.190 | 0 | 20 | 14 | 2.224 |
| Afatinib | 1.796 | 0 | 20 | 14 | 1.830 |
| Erlotinib | 1.864 | 0 | 20 | 14 | 1.898 |
| Crizotinib | 4.154 | 0 | 20 | 14 | 4.188 |
| Pemetrexed + cisplatin | 2.914 | 480 | 0 | 0 | 3.394 |
| Bevacizumab | 4604 | 480 | 0 | 0 | 5.007 |
| Pembrolizumab | 5.938 | 480 | 0 | 0 | 6.418 |
| Nivolumab | 4.215 | 755 | 0 | 0 | 4.970 |
| Nintedanib + docetaxel | 643 | 480 | 0 | 0 | 1.123 |
| Osimertinib | 5.437 | 0 | 20 | 14 | 5.471 |
| Alectinib (I line) | 5.208 | 0 | 20 | 14 | 5.240 |
| Alectinib (II line) | 3.958 | 0 | 20 | 14 | 3.990 |
| Atezolizumab | 4.395 | 480 | 0 | 0 | 4.875 |
| Ceritinib | 2.035 | 0 | 12 | 9 | 2.055 |
The table refers to the monthly cost of each drug, disregarding the duration of the therapy.
The costs of monitoring the courses of therapy were included in the follow‐up costs.
We also considered the costs associated in the case of NSCLC‐related deaths, we assumed that patients' medical therapy would be stopped in the last three months of life, and the costs of supportive care (derived from a previous study) were assumed for three months. 56
Sensitivity analysis
We simulated the probabilistic uncertainty analysis using Monte Carlo simulations and assuming a beta distribution for clinical outcomes' variability, as shown in Table 2. Repeated samples were then drawn from these distributions to establish an empirical distribution of the cost estimates. These analyses were done with the TreeAge Pro 2011 software.
Results
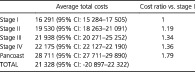
The mean per‐patient costs of care during the first year for NSCLC by disease stage and patient management phase are shown in Table 3: 21328 € (95% CI: –20 897−22 322) was the average cost for the first year after a patient was suspected of having lung cancer. The costs were 16 291 € (95% CI: 15 284‐17 505) for a patient with stage I disease (95% CI: 18 263–21 091) then dropped slightly for stages II (19 530 €), III (21 938 €) and IV (22 175 €).
Table 3.
Estimates of average (and confidence interval) per‐patient costs of care for NSCLC by disease stage (€) during the first year after diagnosis
| Average total costs | Cost ratio vs. stage I | |
|---|---|---|
| Stage I | 16 291 (95% CI: 15 284–17 505) | 1 |
| Stage II | 19 530 (95% CI: 18 263–21 091) | 1.19 |
| Stage III | 21 938 (95% CI: 20 271–25 252) | 1.34 |
| Stage IV | 22 175 (95% CI: 22 127–22 190) | 1.36 |
| Pancoast | 28 711 (95% CI: 27 711–29 890) | 1.79 |
| TOTAL | 21 328 (95% CI: ‐20 897–22 322) |
A Pancoast tumor is associated with higher costs 28 711 (95% CI: 27 711–29 890). The treatment of choice in this case is surgery preceded by neoadjuvant chemo‐radiotherapy, entailing high costs of staging and restaging procedures.
Discussion
This study enabled a prediction of the direct costs and outcomes for patients diagnosed with NSCLC on a one‐year timeline after disease is first clinically suspected.
Our results indicated higher costs than those estimated in previous studies, conducted before the introduction of the latest medical treatment options. For instance, the study published in 2015 by McGuire et al. 19 compared NSCLC treatment costs between Germany, France and England: the overall cost in the first year for a patient with metastatic versus nonmetastatic disease was estimated at 19227 € versus. 17 242 € in France, 10 144 € versus 8059 € in England, and 27 392 € versus 18 338 € in Germany. These last figures were essentially in line with another German study by Schwarzkopf et al. 15 published in the same year, which estimated overall spending for lung cancer at 20425 € per patient. A Spanish study 18 also reported per‐patient costs ranging from 13 218 € for stage III to 16 120 € for stage II disease. On the other hand, a Dutch study 16 that considered data for the year 2012 found an average cost for stage IV patients similar to ours: after 12.6 months of observation it was 26 109 € (i.e. 22 175 € a year). The high incidence and high costs of NSCLC confirm the huge effort required of our healthcare services, now and in the future, to cope with the distribution of new oncological drugs to all candidate patients. For the time being, new oncological drugs are only approved for patients with metastatic disease,. 6 , 7 , 8 , 9 , 10 , 11 , 12 However a new monoclonal antibody, durvalumab, is currently being released for stage III patients with positive PDL1 as a consolidation treatment after chemotherapy. 10 , 57 It is therefore reasonable to expect costs to increase for locally‐advanced disease in the near future, making some economic concerns even more urgent.
Overall, the high costs of surgery make the early stages of the disease no less expensive than the advanced stages. In fact the differences between the costs of the various stages are quite limited because, as the use of surgery begins to decline for more advanced disease, medical therapies, radiotherapy, treatment for progressions and supportive care take up an increasing share of the total costs (Pancoast tumors tend to carry the highest costs due to the associated diagnostic and staging/restaging techniques, neoadjuvant chemotherapy and surgery involved in the elective treatment of this particular subtype). Actually our cost data showed quite a low stage I to stage IV cost ratio (1/1.4), as seen in previous studies: in Spain it was also 1/1.1, for instance. The cost ratio between metastatic and nonmetastatic disease was 1/1.1 in France, 1/1.3 in England, and 1/1.5 in Germany. 15 These data support the conviction that prevention is still the best weapon for the purpose of controlling the rising costs of lung cancer. Screening for lung cancer has been proposed only recently, and there is no consensus as yet on its efficacy. 58 , 59 In short, stressing public health policies and campaigns against tobacco smoking is probably the main solution on which policy‐makers should focus to contain the costs of lung cancer. Primary prevention oriented towards containing environmental pollution should also be considered at national political level.
The strength of this study lies in the very detailed model developed by following the main guidelines currently available. This reinforces the reliability of the economic considerations drawn from the model, and made it possible to thoroughly analyze the single stages of NSCLC, and the different phases of the diagnosis, treatment and follow‐up of each stage of the disease.
Unfortunately, the present work also suffers from some pitfalls that should be mentioned here. First, the model was constructed referring to best practices, but real‐world everyday practice may sometimes differ. It is reasonable to assume that physicians do not always follow the guidelines, and that patients are not always wholly compliant. It would therefore be interesting to compare our findings on the expected costs with real‐world cost data.
Second, this study only considered the direct costs of NSCLC, disregarding the burden of indirect costs. The latter were investigated in another publication and are known to be considerable, both for the individual and for the health service. 60 In fact, although national social policies help NSCLC patients' families financially to sustain disease‐related costs, there is still a non‐negligible economic burden for patients and their informal caregivers (usually family members). These indirect costs also appear to be greater the higher the stage of disease at diagnosis.
Third, with regard to the cost of new cancer drugs, we did not consider that some patients may have been enrolled in clinical trials, and that the costs of their therapies would be covered by the pharmaceutical industries, not by the health services. Last but not least, trial data was used to estimate OS and PFS, although real‐world evidence has shown that patients receiving treatment may actually derive shorter survival compared to trial patients receiving the same treatment. 61 , 62
Disclosure
The authors declare that there are no conflicts of interest.
Supporting information
Table S1 Supporting information
Acknowledgments
Funding for this study was provided by ROV (Region Veneto Oncology Network): Grant Health Ministry Research NET‐2016‐02363853.
References
- 1. Torre LA, Siegel RL, Jemal A. Lung cancer statistics. Adv Exp Med Biol 2016; 893: 1–19. [DOI] [PubMed] [Google Scholar]
- 2. Morgensztern D, Ng SH, Gao F, Govindan R. Trends in stage distribution for patients with non‐small cell lung cancer: A National Cancer Database survey. J Thorac Oncol 2010; 5 (1): 29–33. [DOI] [PubMed] [Google Scholar]
- 3. Soria JC, Ohe Y, Vansteenkiste J et al Osimertinib in untreated EGFR‐mutated advanced non‐small‐cell lung cancer. N Engl J Med 2018; 378 (2): 113–25. [DOI] [PubMed] [Google Scholar]
- 4. Miller VA, Hirsh V, Cadranel J et al Afatinib versus placebo for patients with advanced, metastatic non‐small‐cell lung cancer after failure of erlotinib, gefitinib, or both, and one or two lines of chemotherapy (LUX‐Lung 1): a phase 2b/3 randomised trial. Lancet Oncol 2012; 13 (5): 528–38. [DOI] [PubMed] [Google Scholar]
- 5. Solomon BJ, Mok T, Kim DW et al First‐line crizotinib versus chemotherapy in ALK‐positive lung cancer. N Engl J Med 2014; 371 (23): 2167–77. [DOI] [PubMed] [Google Scholar]
- 6. Reck M, Rodríguez‐Abreu D, Robinson AG et al Pembrolizumab versus chemotherapy for PD‐L1‐positive non‐small‐cell lung cancer. N Engl J Med 2016; 375 (19): 1823–33. [DOI] [PubMed] [Google Scholar]
- 7. Peters S, Camidge DR, Shaw AT et al Alectinib versus crizotinib in untreated ALK‐positive non‐small‐cell lung cancer. N Engl J Med 2017; 377 (9): 829–38. [DOI] [PubMed] [Google Scholar]
- 8. Horn L, Spigel DR, Vokes EE et al Nivolumab versus docetaxel in previously treated patients with advanced non‐small‐cell lung cancer: Two‐year outcomes from two randomized, open‐label, phase III trials (CheckMate 017 and CheckMate 057). J Clin Oncol 2017; 35 (35): 3924–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Rittmeyer A, Barlesi F, Waterkamp D et al Atezolizumab versus docetaxel in patients with previously treated non‐small‐cell lung cancer (OAK): A phase 3, open‐label, multicentre randomised controlled trial. Lancet 2017; 389 (10066): 255–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Mezquita L, Planchard D. Durvalumab in non‐small‐cell lung cancer patients: Current developments. Future Oncol 2018; 14 (3): 205–22. [DOI] [PubMed] [Google Scholar]
- 11. Carrera PM, Olver I. The financial hazard of personalized medicine and supportive care. Support Care Cancer 2015; 23 (12): 3399–401. [DOI] [PubMed] [Google Scholar]
- 12. Rotter T, Kinsman L, James E et al Clinical pathways: Effects on professional practice, patient outcomes, length of stay and hospital costs. Cochrane Database Syst Rev. 2010; 17: CD006632. [DOI] [PubMed] [Google Scholar]
- 13. Piccinni C, Calabria S, Ronconi G et al Facts and figures of clinical pathways in Italy: Results from the PDTA net project. Recenti Prog Med 2019; 110 (4): 188–94. [DOI] [PubMed] [Google Scholar]
- 14. Martini N. I PDTA come indicatori del processo di cambiamento assistenziale in sanità. CARE 2017; 4: 29–31. [Google Scholar]
- 15. Schwarzkopf L, Wacker M, Holle R et al Cost‐components of lung cancer care within the first three years after initial diagnosis in context of different treatment regimens. Lung Cancer 2015; 90 (2): 274–80. [DOI] [PubMed] [Google Scholar]
- 16. Van der Linden N, Bongers ML, Coupé VM et al Costs of non‐small cell lung cancer in The Netherlands. Lung Cancer 2016; 91: 79–88. [DOI] [PubMed] [Google Scholar]
- 17. Šimrová J, Barták M, Vojtíšek R, Rogalewicz V. The costs and reimbursements for lung cancer treatment among selected health care providers in The Czech Republic. Ekon Manag 2014; 17: 74–85. [Google Scholar]
- 18. Corral J, Espinàs JA, Cots F et al Estimation of lung cancer diagnosis and treatment costs based on a patient‐level analysis in Catalonia (Spain). BMC Health Serv Res 2015; 15: 70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. McGuire A, Martin M, Lenz C, Sollano JA. Treatment cost of non‐small cell lung cancer in three European countries: Comparisons across France, Germany, and England using administrative databases. J Med Econ 2015; 18 (7): 525–32. [DOI] [PubMed] [Google Scholar]
- 20. Sheehan DF, Criss SD, Chen Y et al Lung cancer costs by treatment strategy and phase of care among patients enrolled in Medicare. Cancer Med 2019; 8 (1): 94–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Cipriano LE, Romanus D, Earle CC et al Lung cancer treatment costs, including patient responsibility, by disease stage and treatment modality, 1992 to 2003. Value Health 2011; 14 (1): 41–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Raez LE, Cardona AF, Santos ES et al The burden of lung cancer in Latin‐America and challenges in the access to genomic profiling, immunotherapy and targeted treatments. Lung Cancer 2018; 119: 7–13. [DOI] [PubMed] [Google Scholar]
- 23. Korytowsky B, Radtchenko J, Nwokeji ED, Tuell KW, Kish JK, Feinberg BA. Understanding total cost of care in advanced non‐small cell lung cancer pre‐ and postapproval of immuno‐oncology therapies. Am J Manag Care 2018; 24 (20 Suppl): S439–47. [PubMed] [Google Scholar]
- 24. Tappenden P, Chilcott J, Brennan A, Squires H, Stevenson M. Whole disease modelling to inform resource allocation decisions in cancer: A methodological framework. Value Health 2012; 15: 1127–36. [DOI] [PubMed] [Google Scholar]
- 25. World Health Organization . The Veneto Model – A Regional Approach to Tackling Global and European Health Challenges (2016). [Cited 3 Jul 2020.] Available from URL: http://www.euro.who.int/en/publications/abstracts/the-veneto-model-a-regional-approach-to-tackling-global-and-european-health-challenges-2016.
- 26. NCCN Non‐Small‐Lung‐Cancer Guidelines . [Cited 5 Dec 2019.] Available from URL: https://www.nccn.org/professionals/physician_gls/default.aspx.
- 27. Linee guida AIOM Neoplasie del Polmone . [Cited 5 Dec 2019.] Available from URL: https://www.aiom.it/linee-guida-aiom-2018-neoplasie-del-polmone/.
- 28.Rete Oncologica Veneta – PDTA per i pazienti affetti da tumore del polmone. [Cited 5 Dec 2019.] Available from URL: https://salute.regione.veneto.it/web/rov/polmone.
- 29. Regione Veneto, Decreto 68, 8 luglio 2019. Commissione tecnica regionale farmaci. [Cited 5 Dec 2019.] Available from URL: https://salute.regione.veneto.it/c/document_library/get_file?uuid=d9a05ad6-7310-45ab-81af-85f095b65fa6&groupId=534936
- 30. Benitez‐Majano S, Fowler H, Maringe C, di Girolamo C, Rachet B. Deriving stage at diagnosis from multiple population‐based sources: Colorectal and lung cancer in England. Br J Cancer 2016; 115 (3): 391–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Panagopoulos N, Leivaditis V, Koletsis E et al Pancoast tumors: Characteristics and preoperative assessment. J Thorac Dis 2014; 6 (Suppl 1): S108–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Parissis H, Young V. Treatment of Pancoast tumors from the surgeon's perspective: Re‐appraisal of the anterior‐manubrial sternal approach. J Cardiothorac Surg 2010; 5: 102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.ISTAT ‐ Mortality Tables of the Resident Population. [Cited 5 Dec 2019.] Available from URL: http://demo.istat.it/tvm2016/index.php?lingua=ita.
- 34. Chansky K, Detterbeck FC, Nicholson AG et al The IASLC lung cancer staging project: External validation of the revision of the TNM stage groupings in the eight edition of the TNM classification of lung cancer. J Thorac Oncol 2017; 12 (7): 1109–21. [DOI] [PubMed] [Google Scholar]
- 35. Valle LF, Jagsi R, Bobiak SN et al Variation in definitive therapy for localized NSCLC among national comprehensive cancer network institutions. Int J Radiat Oncol Biol Phys 2016; 94 (2): 360–7. [DOI] [PubMed] [Google Scholar]
- 36. Allen MS, Darling GE, Pechet TT et al Morbidity and mortality of major pulmonary resections in patients with early stage lung cancer: Initial results of the randomized, prospective ACOSOG Z0030 trial. Ann Thorac Surg 2006; 81 (3): 1013–9. [DOI] [PubMed] [Google Scholar]
- 37. Eberhardt WE, Pöttgen C, Gauler TC et al Phase III study of surgery versus definitive concurrent chemoradiotherapy boost in patients with resectable stage IIIA(N2) and selected IIIB non‐small‐cell lung cancer after induction chemotherapy and concurrent chemoradiotherapy (ESPATUE). J Clin Oncol 2015; 33 (35): 4194–201. [DOI] [PubMed] [Google Scholar]
- 38. Congedo MT, Cesario A, Lococo F et al Surgery for oligometastatic non‐small cell lung cancer: Long‐term results from a single center experience. J Thorac Cardiovasc Surg 2012; 144 (2): 444–52. [DOI] [PubMed] [Google Scholar]
- 39. Jeannin G, Merle P, Janicot H et al Combined treatment modalities in pancoast tumor: Results of a monocentric retrospective study. Chin Clin Oncol 2015; 4 (4): 39. [DOI] [PubMed] [Google Scholar]
- 40. Xue Z, Wu F, Pierson KE et al Survival in surgical and nonsurgical patients with superior sulcus tumors. Ann Thorac Surg 2017; 104 (3): 988–97. [DOI] [PubMed] [Google Scholar]
- 41. Solli P, Casiraghi M, Brambilla D et al Surgical treatment of superior sulcus tumors: A 15‐year single‐center experience. Semin Thorac Cardiovasc Surg 2017; 29 (1): 79–88. [DOI] [PubMed] [Google Scholar]
- 42. Waseda R, Klikovits T, Hoda MA et al Trimodality therapy for pancoast tumors: T4 is not a contraindication to radical surgery. J Surg Oncol 2017; 116 (2): 227–35. [DOI] [PubMed] [Google Scholar]
- 43. Kapadia NS, Valle LF, George JA et al Patterns of treatment and outcomes for definitive therapy of early stage non‐small cell lung cancer. Ann Thorac Surg 2017; 104 (6): 1881–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Driessen EJ, Aarts MJ, Bootsma GP, van Loon JG, Janssen‐Heijnen ML. Trends in treatment and relative survival among non‐small cell lung cancer patients in The Netherlands (1990‐2014): Disparities between younger and older patients. Lung Cancer 2017; 108: 198–204. [DOI] [PubMed] [Google Scholar]
- 45. Vinod SK, Wai E, Alexander C, Tyldesley S, Murray N. Stage III non‐small‐cell lung cancer: Population‐based patterns of treatment in British Columbia, Canada. J Thorac Oncol 2012; 7 (7): 1155–63. [DOI] [PubMed] [Google Scholar]
- 46. Little AG, Gay EG, Gaspar LE, Stewart AK. National survey of non‐small cell lung cancer in the United States: Epidemiology, pathology and patterns of care. Lung Cancer 2007; 57 (3): 253–60. [DOI] [PubMed] [Google Scholar]
- 47. Linstone HA, Turoff M. Delphi Method: Techniques and Applications. Journal of Marketing Research. 1975; 18 (3). [Google Scholar]
- 48.Tariffario DRG Veneto. [Cited 5 Dec 2019.] Available from URL: https://salute.regione.veneto.it/c/document_library/get_file?p_l_id=1026082&folderId=1026048&name=DLFE-32750.pdf.
- 49.Nomenclatore tariffario delle prestazioni di assistenza specialistica ambulatoriale. [Cited 5 Dec 2019.] Available from URL: https://www.regione.veneto.it/web/sanita/assistenza-ambulatoriale.
- 50. Ihbe‐Heffinger A, Paessens B, Berger K. The impact of chemotherapy‐induced side effects on medical care usage and cost in German hospital care—an observational analysis on non‐small‐cell lung cancer patients. Support Care Cancer 2013; 21 (6): 1665–75. [DOI] [PubMed] [Google Scholar]
- 51. Kong FM, Hayman JA, Griffith KA et al Final toxicity results of a radiation‐dose escalation study in patients with non‐small‐cell lung cancer (NSCLC): predictors for radiation pneumonitis and fibrosis. Int J Radiat Oncol Biol Phys 2006; 65 (4): 1075–86. [DOI] [PubMed] [Google Scholar]
- 52. Kong FM, Ten Haken R, Eisbruch A et al Non‐small cell lung cancer therapy‐related pulmonary toxicity: an update on radiation pneumonitis and fibrosis. Semin Oncol 2005; 32 (2 Suppl 3): S42–54. Review. [DOI] [PubMed] [Google Scholar]
- 53. Waddle MR, Chen RC, Arastu NH et al Unanticipated hospital admissions during or soon after radiation therapy: Incidence and predictive factors. Pract Radiat Oncol 2015; 5 (3): e245–53. [DOI] [PubMed] [Google Scholar]
- 54. Baker S, Fairchild A. Radiation‐induced esophagitis in lung cancer. Lung Cancer (Auckl) 2016; 7: 119–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Sgambato A, Casaluce F, Sacco PC et al Anti PD‐1 and PDL‐1 immunotherapy in the treatment of advanced non‐ small cell lung cancer (NSCLC): A review on toxicity profile and its management. Curr Drug Saf 2016; 11 (1): 62–8. [DOI] [PubMed] [Google Scholar]
- 56. Bremner KE, Krahn MD, Warren JL et al An international comparison of costs of end‐of‐life care for advanced lung cancer patients using health administrative data. Palliat Med 2015; 29 (10): 918–28. [DOI] [PubMed] [Google Scholar]
- 57. Antonia SJ, Villegas A, Daniel D et al Durvalumab after chemoradiotherapy in stage III non‐small‐cell lung cancer. N Engl J Med 2017; 377: 1919–29. [DOI] [PubMed] [Google Scholar]
- 58. Oudkerk M, Devaraj A, Vliegenthart R et al European position statement on lung cancer screening. Lancet Oncol 2017; 18 (12): e754–66. [DOI] [PubMed] [Google Scholar]
- 59. Usman Ali M, Miller J, Peirson L et al Screening for lung cancer: A systematic review and meta‐analysis. Prev Med 2016; 89: 301–14. [DOI] [PubMed] [Google Scholar]
- 60. Wood R, Taylor‐Stokes G. Cost burden associated with advanced non‐small cell lung cancer in Europe and influence of disease stage. BMC Cancer 2019; 19 (1): 214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Lamont EB, Schilsky RL, He Y et al Generalizability of trial results to elderly Medicare patients with advanced solid tumors (Alliance 70802). J Natl Cancer Inst 2014; 107 (1): 336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Khozin S, Carson KR, Zhi J et al Real‐world outcomes of patients with metastatic non‐small cell lung cancer treated with programmed cell death protein 1 inhibitors in the year following U.S. regulatory approval. Oncologist 2019; 24 (5): 648–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1 Supporting information


