Abstract
The occurrence of endotracheal/endobronchial metastasis (EEM) after complete resection of a primary lung cancer is rare. Here, we report the case of an 86‐year‐old woman in whom EEM occurred twice over a 20‐year period following complete resection of a primary adenocarcinoma localized to the left main bronchus and trachea. The presence of EEM was confirmed by establishing immunohistochemical homology of the metastases with the primary tumor. To the best of our knowledge, this is the first reported case of repetitive EEM of primary lung adenocarcinoma. Lymphatic invasion in the primary lesion suggested that a possible route for EEM was the peripheral lymphatic tract, explaining the slow recurrence rate. We conclude that observation of the trachea/bronchus over a long period post operation could be important in monitoring for EEM, particularly if lymphatic invasion is confirmed in the primary tumor.
Keywords: Adenocarcinoma, endotracheal/endobronchial metastasis, lung cancer, lymphatic metastasis, pathology
This is the first reported case of repetitive localized endotracheal/endobronchial metastasis of primary lung adenocarcinoma with mild lymphatic invasion that occurred twice over a 20‐year period after complete resection. Observation of the trachea/bronchus over a long period post operation could be important in monitoring for endotracheal/endobronchial metastasis, particularly if lymphatic invasion is confirmed in the primary tumor.
Introduction
There are few reported cases of endotracheal/endobronchial metastasis (EEM) of primary lung cancer following resection. 1 , 2 It is often difficult to distinguish between EEM and multiple primary lung cancer (MPLC), 3 although pathological findings and molecular background can help differentiate between these clinical courses. 4 , 5 In this study, we report the first case of repetitive reoccurrence of EEM over a 20‐year period following radical resection of a primary lung adenocarcinoma and confirm the presence of EEM using immunohistochemical techniques.
Case report
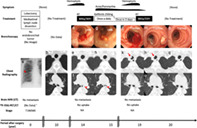
An 86‐year‐old woman with no history of smoking visited our hospital suffering with hemoptysis. She had been diagnosed with lung adenocarcinoma (T1N0M0; well‐differentiated papillary adenocarcinoma, bronchioloalveolar type) (Fig 1a) 19 years previously and had undergone a right middle lobectomy with lymphatic dissection. There had been no recurrence for 10 years after surgery and no treatment had been administered (Fig 1b, c). Five years ago, she experienced hemoptysis and was diagnosed with an endobronchial tumor localized in the left main bronchus, confirmed as EEM on the basis of histological homology (Fig 1d, f). Local irradiation was performed, resulting in complete tumor reduction (Fig 1g, i). Gefitinib treatment was initiated because of epidermal growth factor receptor (EGFR) mutation (exon 19 deletion positivity). There was no recurrence in the trachea during this period (Fig 1e, h).
Figure 1.
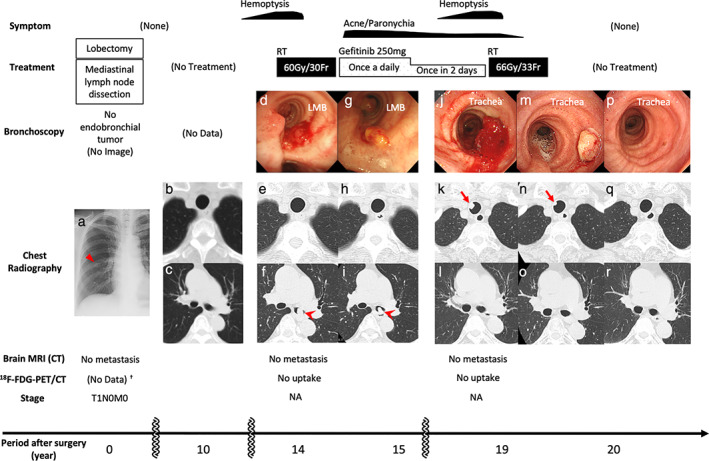
Clinical course. Initial chest X‐ray imaging (a, triangle) revealed a primary lung adenocarcinoma. Ten years after complete resection, there were no abnormal CT findings in the trachea (b) or LMB (c). Fourteen years after surgery, bronchoscopy (d) and CT (f, arrowhead) demonstrated the first EEM localized in the LMB, with no tumor in the trachea (e). Gefitinib was initiated after irradiation therapy (60 Gy/30 Fr). Because of these treatments, bronchoscopy (g) and CT showed no tumors in the trachea (h), and LMB lesions (i, arrowhead) were diminished and eventually disappeared (l, o, r). Nineteen years after surgery, the second EEM was confirmed by bronchoscopy (j) and CT (k, arrow) in the trachea, and RT was administered (66 Gy/33 Fr). One month later, bronchoscopy (m) and CT (n, arrow) revealed that the EEM had shrunk. One year after the last treatment, bronchoscopy (p) and CT (q) showed no endotracheal lesions. 18F‐FDG‐PET/CT and brain MRI confirmed no further metastasis during this period. †Instead of 18F‐FDG‐PET/CT, CT with contrast and bone scintigraphy was performed, and no metastasis was found. CT, computed tomography; EEM, endotracheal/endobronchial metastasis; FDG, fluorodeoxyglucose; Fr, fractions; LMB, left main bronchus; MRI, magnetic resonance imaging; PET, positron emission tomography; RT, radiation therapy; NA, not applicable.
When the patient visited our hospital, her general condition was good and vital signs were normal. There were no abnormal physical findings except facial acne and paronychia, probably caused by gefitinib treatment. Blood tests for tumor markers and chest X‐ray imaging revealed normal findings. Chest computed tomography showed a 10 mm protruding lesion in the upper trachea (Fig 1k), but the left main bronchus was intact (Fig 1l, o, r). Brain magnetic resonance imaging and 18F‐fluorodeoxyglucose positron emission tomography/computed tomography (18F‐FDG‐PET/CT) showed no further metastases. Bronchoscopy revealed a polypoid lesion with a clot at the periphery, six rings from the vocal cords (Fig 1j), and an endotracheal biopsy was performed. The lesion was diagnosed as a second EEM localized in the trachea. Gefitinib was discontinued, and radiation therapy was administered at the request of the patient. Hemoptysis ceased, and the endotracheal tumor disappeared after radiation therapy (Fig 1m, n). No further treatment was administered given the patient's age, and she maintained a good performance status with no recurrence one year after radiation therapy (Fig 1p, q).
To assess whether each of the EEM tumors originated in the primary lung adenocarcinoma, we performed hematoxylin and eosin (Fig 2a, e, i) staining and immunostaining for thyroid transcription factor‐1 (Fig 2b, f, j), Napsin A (Fig 2c, g, k), surfactant protein A, cytokeratin 5/6, caudal‐type homeobox protein‐2, thyroglobulin, and p40 (Fig 2d, h, l) in all samples. The results showed that the EEM had the characteristics of lung adenocarcinoma as revealed through morphological and immunohistochemical homology (Table 1) (Figure S1). EGFR exon 19 deletion was confirmed in the first and second EEM but was undetectable in the primary tumor because of poor gene amplification following gene degradation of the preserved sample. The first and second EEM were negative for exon 20 EGFR mutation T790M (Table 1).
Figure 2.
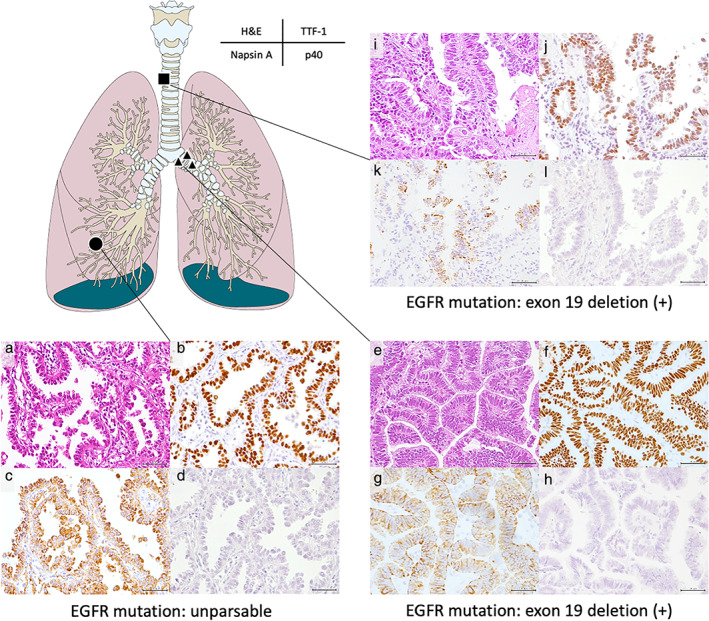
Pathological findings (magnification ×200). Primary invasive lung adenocarcinoma (a); the first EEM (e); and the second EEM (i) with papillary pattern (H&E stain). Immunohistochemically, TTF‐1 and Napsin A were positive in the primary tumor (b, c); the first EEM (f, g); and the second EEM (j, k). p40 was negative in the primary tumor (d); the first EEM (h), and the second EEM (l). H&E, hematoxylin and eosin; TTF‐1, thyroid transcription factor‐1; EGFR, epidermal growth factor receptor; EEM, endotracheal/endobronchial metastasis. ( ) initial, (
) initial, ( ) second, (
) second, ( ) third.
) third.
Table 1.
Immunohistochemical and molecular characteristics of each tumor
| EGFR mutation | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| TTF‐1 | Napsin A | SP‐A | CK5/6 | CDX‐2 | Thyroglobulin | p40 | Exon 19 deletion | Exon 20 T790M | |
| Primary | (+) | (+) | (+) | (−) | (−) | (−) | (−) | NA | NA |
| First EEM | (+) | (+) | (+) | (−) | (−) | (−) | (−) | (+) | (−) |
| Second EEM | (+) | (+) | (+−) | (+−) | (−) | (−) | (−) | (+) | (−) |
CDX‐2, caudal‐type homeobox protein‐2; CK5/6, cytokeratin 5/6; EEM, endotracheal/endobronchial metastasis; EGFR, epidermal growth factor receptor; NA, not applicable; SP‐A, surfactant protein A; TTF‐1, thyroid transcription factor‐1.
Discussion
This is the first reported case of repeated EEM of primary lung adenocarcinoma occurring 20 years after radical resection. EEM was first reported and defined in detail by Schoenbaum and Viamonte 6 and is considered a rare phenotype of metastasis. 7 The incidence rate of EEM ranges from 2% to 50% for pulmonary metastases from extrathoracic neoplasms. 7 , 8 There are few observed cases of localized EEM of primary lung adenocarcinoma. 1 , 2 , 9 Although it is often difficult to distinguish between EEM and MPLC, we succeeded in diagnosing EEM and propose a mechanism of metastasis via the lymphatic vessels, based on the pathological homology and characteristics of this case. The diagnosis of EEM is further supported by the presence of lymphatic invasion in the primary tumor. According to a retrospective study of 609 patients with resectable lung adenocarcinoma, 10 the time to recurrence was significantly longer in cases with negative lymph node metastasis than in positive cases. Patients with lymphatic invasion showed significantly higher recurrence rates than those without. Therefore, cases similar to this one, without lymph node metastasis but with lymphatic invasion, could be associated with the risk of slow but significant local metastasis and recurrence, and it is presumed that EEM gradually progressed via lymphatic vessels around the trachea/bronchus 6 in this case.
A limitation of our case study is that EEM was diagnosed only by pathological findings and EGFR gene analysis. For comprehensive analysis, genetic approaches such as next‐generation sequencing may be used to accurately discriminate between EEM and MPLC. It has been previously reported that surgery such as lymph node dissection could result in temporary alternation of the lymphatic flow. 11 , 12 We believe that the mechanism of lymphatic regurgitation after lobectomy and lymph node dissection may be related to late metastasis of two lesions in the contralateral main bronchus and trachea.
In conclusion, here we present the first case of repetitive EEM 20 years after resection of a primary lung adenocarcinoma. Even after a long postoperative period, careful observation of the trachea/bronchus is recommended to detect possible local metastases if lymphatic invasion is confirmed in the primary tumor.
Disclosure
None of the authors have any conflicts of interest or financial ties to disclose.
Supporting information
Figure S1: Immunohistochemical findings (magnification ×100). SP‐A staining in primary tumor and first EEM are clearly positive, but no other clear positive findings.
CDX‐2: Caudal‐type homeobox protein‐2; CK: cytokeratin; EEM: Endotracheal/endobronchial metastasis; SP‐A: surfactant protein A.
References
- 1. Zhang Z, Mao Y, Chen H et al Endotracheal and endobronchial metastases in a patient with stage I lung adenocarcinoma. Ann Thorac Surg 2014; 97: e135–7. [DOI] [PubMed] [Google Scholar]
- 2. Chong S, Kim TS, Han J. Tracheal metastasis of lung cancer: CT findings in six patients. AJR Am J Roentgenol 2006; 186: 220–4. [DOI] [PubMed] [Google Scholar]
- 3. Martini N, Melamed MR. Multiple primary lung cancers. J Thorac Cardiovasc Surg 1975; 70: 606–12. [PubMed] [Google Scholar]
- 4. Loukeri AA, Kampolis CF, Ntokou A, Tsoukalas G, Syrigos K. Metachronous and synchronous primary lung cancers: Diagnostic aspects, surgical treatment, and prognosis. Clin Lung Cancer 2015; 16: 15–23. [DOI] [PubMed] [Google Scholar]
- 5. Nie Y, Gao W, Li N et al Relationship between EGFR gene mutation and local metastasis of resectable lung adenocarcinoma. World J Surg Oncol 2017; 15: 55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Schoenbaum S, Viamonte M. Subepithelial endobronchial metastases. Radiology 1971; 101: 63–9. [DOI] [PubMed] [Google Scholar]
- 7. Kiryu T, Hoshi H, Matsui E et al Endotracheal/endobronchial metastases: Clinicopathologic study with special reference to developmental modes. Chest 2001; 119: 768–75. [DOI] [PubMed] [Google Scholar]
- 8. Marchioni A, Lasagni A, Busca A et al Endobronchial metastasis: An epidemiologic and clinicopathologic study of 174 consecutive cases. Lung Cancer 2014; 84: 222–8. [DOI] [PubMed] [Google Scholar]
- 9. Segawa M, Kusajima Y, Nakamura H, Sugihara M, Saito K. Postoperative endotracheal metastasis of the peripheral adenocarcinoma of lung: A case report with review of the Japanese literature. Jpn J Lung Cancer 2000; 40: 633–7. [in Japanese]. [Google Scholar]
- 10. Mimae T, Tsutani Y, Miyata Y. Role of lymphatic invasion in the prognosis of patients with clinical node‐negative and pathologic node‐positive lung adenocarcinoma. J Thorac Cardiovasc Surg 2014; 147: 1820–6. [DOI] [PubMed] [Google Scholar]
- 11. Blum KS, Proulx ST, Luciani P, Leroux JC, Detmar M. Dynamics of lymphatic regeneration and flow patterns after lymph node dissection. Breast Cancer Res Treat 2013; 139: 81–6. [DOI] [PubMed] [Google Scholar]
- 12. Cui Y, Urschel JD, Petrelli NJ. The effect of cardiopulmonary lymphatic obstruction on heart and lung function. Thorac Cardiovasc Surg 2001; 49: 35–40. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1: Immunohistochemical findings (magnification ×100). SP‐A staining in primary tumor and first EEM are clearly positive, but no other clear positive findings.
CDX‐2: Caudal‐type homeobox protein‐2; CK: cytokeratin; EEM: Endotracheal/endobronchial metastasis; SP‐A: surfactant protein A.


