Abstract
Exhibiting fear to non-threatening cues or contexts—generalized fear—is a shared characteristic of several anxiety disorders, which afflict women more than men. Female rats generalize contextual fear at a faster rate than males and this is due, in part, to actions of estradiol in the dorsal CA1 hippocampus (dCA1). To understand the mechanisms underlying estradiol’s effects on generalization, we infused estradiol into the anterior cingulate cortex (ACC) or ventral CA1 hippocampus (vCA1) of ovariectomized (OVX) female rats. Estradiol acts within the ACC, but not the vCA1, to promote generalized fear. We next examined if AMPA or NMDA receptor antagonists (NBQX, APV) infused into the dCA1 or the ACC of female rats could block generalized fear induced by systemic injections of estradiol. Immediate pre-testing infusions of NBQX or APV into either region eliminated estradiol-induced generalization. Specific blockade of GluN2B receptors with infusions of Ro 25–6981 into the dCA1 or ACC also eliminated generalized fear. Our results suggest that in addition to the dCA1, the ACC is an important locus for the effects of estradiol on fear generalization. Moreover, within these regions, AMPA and NMDA-GluN2B receptors are necessary for estradiol-induced generalization of fear responses, suggesting a critical involvement of glutamatergic transmission. Furthermore, we identified a novel role for GluN2B in mediating the effects of estradiol on generalized fear in female rats. These data potentially implicate GluN2B receptors in more general forms of memory retrieval inaccuracies, and form the foundation for exploration of glutamate receptor pharmacology for treatments of anxiety disorders involving generalization.
Keywords: Estradiol, Generalization, Glutamate, Hippocampus, Anterior cingulate cortex, Females
1. Introduction
Generalized fear— fear to non-threating contexts or cues— is a shared characteristic among nearly all anxiety disorders (Lissek, 2012; Lissek et al., 2008, 2014; Lissek et al., 2005), which are highly prevalent and disproportionally affect women (Kessler et al., 2005b; McLean et al., 2011). Although this sex bias is well-established, the underlying mechanisms remain unclear. Thus, as a means to elucidate the mechanisms of the sex differences in anxiety disorders, our work focuses on the neural underpinnings of fear generalization in both male and female rodents. Female rats generalize contextual fear at a faster rate than males and this effect is due, in part, to estradiol (Lynch et al., 2013). Administration of estradiol to females promotes generalized fear, however, estradiol reduces generalized responding in males (Lynch et al., 2016a), supporting a divergent role of estradiol in modulating fear memory processing in males and females. Mechanistically, the increased fear generalization observed in females occurs through activation of estrogen receptor-β (ERβ) in the dorsal CA1 hippocampus (dCA1) (Lynch et al., 2014, 2016c). In contrast, activation of ERα or ERβ can constrain fear generalization in male rats (Lynch et al., 2016a). These data have recently been corroborated, in part, by a study demonstrating that female rats generalize fear more readily than males, and this involves sex differences in dCA1 activity during memory retrieval (Keiser et al., 2017). Despite our recent progress in understanding the ER requirements involved in modulating generalized fear in males and females, the mechanisms downstream of ER activation remain unknown.
One well-documented effect of estradiol in the brain is its ability to modify neural circuits to enhance learning and memory through modulation of glutamatergic signaling. Classically, estradiol increases dendritic spine growth in the hippocampus through an NMDA receptor-dependent mechanism (Gould et al., 1990; McEwen, 2002; Woolley and McEwen, 1993, 1994). Estradiol also enhances hippocampal-dependent learning and long-term potentiation (Fernandez et al., 2008; Fortress et al., 2013; Gresack and Frick, 2006; Jasnow et al., 2006; Lewis et al., 2008; Morgan and Pfaff, 2002), and may do so through increased expression or modulation of AMPA and NMDA receptors (Foy, 2001; Smith and McMahon, 2005, 2006; Wong and Moss, 1992). For example, estradiol increases NMDA receptor mRNA and NMDA receptor binding within the dCA1 region of the hippocampus and enhances the sensitivity of pyramidal cells to NMDA receptor input (Gazzaley et al., 1996; Weiland, 1992; Woolley et al., 1997). In addition, the effect of estradiol to enhance long term potentiation (LTP) and learning are significantly attenuated when GluN2B-containing NMDA receptors are blocked (Smith et al., 2006) (Lewis et al., 2008; Vedder et al., 2013). Overall, estradiol can interact with glutamatergic signaling, particularly through AMPA and GluN2B-containing NMDA receptors, and such interaction is necessary for estradiol to enhance learning.
The vast majority of research implicating estradiol in learning and memory enhancements through increased glutamate signaling have predominantly focused on the acquisition or memory formation (Frick et al., 2018; Tuscher et al., 2014). Most of these studies administer estradiol before or shortly after training to modify the acquisition or consolidation period. However, our studies support a role for estradiol acting on memory retrieval, but not necessarily on memory consolidation (Lynch et al., 2014, 2016c). For instance, when estradiol was administered immediately after passive avoidance training, animals displayed generalization 24 h later, but not 7 days later (Lynch et al., 2014). A more enduring change in memory, or at least one that lasts 7 days, would be expected if estradiol acted through memory consolidation. In our paradigm, estradiol is administered 24 h after training, which is beyond what is traditionally considered as the synaptic consolidation period for rodents (Dudai, 2004, 2012). Rats are then tested 24 h after the estradiol treatment, typically outside of the rapid membrane-associated effects of estradiol (Vasudevan and Pfaff, 2008). Using this procedure, we consistently observe effects of estradiol on memory retrieval in male and female rats when testing for fear generalization (Lynch et al., 2016a, 2014, 2016c). Our previous work demonstrated that a loss of presynaptic inhibition and the resulting unrestricted glutamatergic signaling promotes contextual fear generalization in mice (Cullen et al., 2014; Lynch et al., 2016b). Given the powerful effects of estradiol to increase glutamate receptors and presynaptic release (Foy, 2001; Smejkalova and Woolley, 2010; Smith and McMahon, 2005; Wong and Moss, 1992; Woolley et al., 1997), we hypothesized that excessive glutamatergic signaling during retrieval represents a common neural pathway for generalization of contextual cues. Thus, the purpose of the present study was two-fold: 1) We first wanted to assess whether estradiol influences generalized contextual fear in regions outside of the dCA1. In particular, we targeted the anterior cingulate cortex (ACC) and the ventral CA1 region of the hippocampus (vCA1) as regions that express ERs and are critical for generalized fear (Cullen et al., 2014; Lynch et al., 2016b). 2) Within these regions and the dCA1, we examined if estradiol-induced generalization occurs through increased, and potentially excessive, glutamatergic signaling. In particular, we focused on AMPA and NMDA receptors in mediating the actions of estradiol on generalized fear in female rats.
2. Methods
2.1. Animals and housing conditions
All animals were adult ovariectomized (OVX) female Long-Evans rats approximately 90 days old provided by the breeding colony in the Department of Psychological Sciences at Kent State University. Rats were maintained on a 14:10 light:dark cycle. Food and water were available ad libitum throughout the experiment. Eleven days prior to beginning the experiment, all rats were ovariectomized and implanted with guide cannula through stereotaxic surgery then individually housed. All animal procedures were carried out in accordance with Kent State University Institutional Animal Care and Use (IACUC) guidelines and conducted in accordance with the National Research Council Guide for the Care and Use of Laboratory Animals, 8th Ed.
2.2. Surgical procedures
Female rats were anesthetized with isoflurane before being placed into a stereotaxic instrument for implantation of standard 22-gauge guide cannulas (Plastics One, Virginia) aimed at the dorsal CA1 region of the hippocampus (dCA1), the ventral CA1 region of the hippocampus (vCA1), or the anterior cingulate cortex (ACC). All coordinates were derived from (Paxinos and Watson, 1986). Perioperative analgesia was provided by subcutaneous (sc) administration of ketoprofen (5 mg/kg). The head was positioned in the stereotaxic instrument so that the skull was level between lambda and bregma. Rats were implanted unilaterally for the ACC (D/V: −1.75 mm; A/P: +1.5 mm, M/L: +0.4 mm) and bilaterally for the ventral CA1 hippocampus (5° angle, D/V: −5 mm, A/P: −5.8 mm, M/L: +5.8 mm) and dorsal CA1 hippocampus (14° angle, D/V: −2.9 mm, A/P-4 mm, M/L: +3.0 mm). Unilateral cannulations within the ACC aimed near the midline are sufficient to alter ACC function (Cullen et al., 2015). Immediately following cannulation, rats were bilaterally ovariectomized by dorsal incision, which was then closed using surgical staples. Rats were given 9 days to recover before handling and the start of the experiment.
2.3. Inhibitory avoidance procedure
Female rats were handled for 5 min a day, for two consecutive days, 9 days following surgeries. On day 12, rats were trained in a black/white inhibitory avoidance chamber (also known as passive avoidance) (52 × 30 × 35 cm, Inhibitory Avoidance Apparatus 7550, Ugo Basil, Comerio, Italy). For training, rats were brought into Context A (training context) held on the experimenter’s hand for 30 s, and then placed into the white side of the shuttle box. After 20 s, the door was raised and the latency to cross to the black side of the shuttle chamber was recorded. Upon crossing, the door closed and rats received a 2 s, 1.0 mA scrambled footshock. Ten seconds following the shock, rats were removed from the chamber and returned to the main colony. Rats were tested in either Context A (training) or Context B (neutral). Context A was a 1.6 × 2.33 m room with house fluorescent lights and contained bare white walls and no artificial scents or sounds and was cleaned with 70% Ethanol; Context B was a 1.83 × 2.74 m room that was lit by a 25-w red light bulb with posters on the walls. Context B had white noise (70db) and was cleaned with 2% quatricide. In each context, the experimenter wore different gloves (Rubber dish glove in A; vinyl lab glove in B) to handle rats and only a lab coat was worn for context A. The test procedure was identical to training except the sliding door remained open for a maximum of 540 s and no shocks were delivered. The latency to cross was recorded as the dependent measure of fear behavior/memory. Any rat that did not cross was given a score of 540 s. Upon crossing or at 540 s, rats were removed and returned to the main colony.
2.4. Locomotor behavior
Locomotor behavior following estradiol treatment was assessed using an open field paradigm. A circular testing arena used for open field measured 122 cm diameter. Twenty-four hours after estradiol injections, rats were placed into the arena and their behavior was recorded during a 9 min test. Total distance travelled, and time spent in the center vs the surround was assessed using AnyMaze 4.99 (Stoelting, Wood Dale, IL).
2.5. Drug administration
Estradiol benzoate (Cayman Chemicals) was administered either intracranially (10 μM dissolved in 50% DMSO/0.9% Saline) or subcutaneously (s.c.) (15 μg dissolved in sterile sesame oil; 0.1 ml injection,). The s.c. dose of estradiol provides a serum level of estradiol similar to the proestrus stage (Chang et al., 2009) and the intracranial concentration of estradiol modulates fear and induces generalization (Lynch et al., 2016c; Zeidan et al., 2011). For all experiments, estradiol was given 24-hr after passive avoidance training. Local intracranial infusions of estradiol were administered in a volume of 0.5 μl and administered at a rate of 0.1 μl/minute through 28-gauge infusion needles (Plastics One) connected to Hamilton syringes mounted on an infusion pump (Harvard Apparatus) via polyethylene tubing. After infusions were complete, needles remained in place for an extra minute before dummy cannula were replaced. Five minutes after the completion of the infusion, animals were returned to the main colony.
The AMPA receptor antagonist, NBQX (Tocris), was administered intracranially at a concentration of 7.8 mM and dissolved in 1 M NaOH. The NMDA receptor antagonist, APV (Cayman Chemicals), was administered intracranially at a concentration of 6.25 mM and dissolved in 0.9% sterile saline (Burman and Gewirtz, 2007; Hou et al., 2009; Walker and Davis, 1997; Walker et al., 2005). The GluN2B specific receptor antagonist, Ro 25–6981 hydrochloride hydrate (Sigma-Aldrich) was also administered intracranially at a concentration of 10.6 mM (4 μg/ul) dissolved in 0.9% saline, a concentration that blocks formation of contextual fear when infused into the ACC (Zhao et al., 2005). All intracranial infusions of glutamate receptor antagonists occurred twenty- hours after estradiol injections and were given in a volume of 0.5 μl, administered at a rate of 0.1 μl/minute through 28-gauge infusion needles (Plastics One) connected to Hamilton syringes mounted on an infusion pump (Harvard Apparatus) via polyethylene tubing. After infusions were complete, needles remained in place for an extra minute before dummy cannula were replaced. Five minutes after the completion of the infusion, animals were tested for memory retrieval in either the training or the neutral context.
2.6. Site verification
Cannula placement was verified using 0.5 μl infusions of xylene cyanol FF at 0.25% in saline followed by rapid decapitation. Brains were fresh frozen and sliced on a cryostat and slices were mounted and observed for correct placement using a Nikon TiS inverted microscope and analyzed using NIS Elements Software. Exclusion criteria include rats with unilateral infusions in the case of dCA1 and vCA1 infusions or infusions that completely missed the designated target.
2.7. Statistical analysis
Latency to cross in the training and neutral contexts was assessed using either factorial ANOVA or a 3 way ANOVA and Tukey’s post hoc analyses. Tukey’s post hoc were assessed regardless of significant interactions because of a priori assumptions based on our previous data examining generalization (Lynch et al., 2013, 2014, 2016a, 2016b, 2016c). Rats demonstrate significant generalization when they display similar latencies to cross compared to rats tested in the training context. In addition, estradiol-treated rats should demonstrate significantly longer latencies compared with vehicle-treated rats when tested in the neutral context. Additionally, only the following post hocs were reported for any 3-way interactions as they are the ones of most interest when assessing generalization: Veh-Veh Training vs Neutral; E2-Veh Training vs Neutral; E2-Veh Neutral vs Veh-Veh Neutral; E2-Veh Neutral vs E2-Antagonist Neutral; all comparisons in training context. Animals that had missed targets or were behavioral outliers were excluded from statistical analyses. Behavioral outliers included those rats that were one standard deviation away from the mean (Lynch et al., 2013, 2014; Lynch et al., 2016c). Statistical significance was set at p = 0.05. All data were graphed as mean ± S.E.M., and analyzed using GraphPad Prism 8.0 (GraphPad Software, La Jolla, CA). Effect sizes were calculated for completed experiments using G*Power 3.
3. Results
3.1. Estrogen receptors in the anterior cingulate cortex promote contextual fear generalization
In order to assess the role of the ACC in mediating the effects of estradiol on context fear generalization, infusions of estradiol or vehicle into the ACC were administered 24 h post training. Twenty-four hours after treatment, rats were tested for context generalization in a context-sensitive inhibitory avoidance procedure (Fig. 1B). We have previously demonstrated that estradiol administered s.c. or locally into the dCA1 promotes context fear generalization (Lynch et al., 2014, 2016c). Analyses revealed a significant main effect of context, (F(1,27) = 11.49, p < 0.01), no main effect for treatment, (F(1,27) = 1.57, p > 0.05), and no significant interaction term, (F(1,27)) = 3.21, p = 0.08), ŋp2 = .106). All vehicle treated animals that were tested in the training context had the maximum latency to cross of 540 s, therefore, no error bars are present in the figure. Tukey’s post hoc comparisons revealed that vehicle-treated rats did not generalize fear to the neutral context indicated by significantly longer latencies to cross in the training context compared to the neutral context (p < 0.01). Alternatively, estradiol-treated rats had similar latencies to cross in the training and neutral context (p > .05), and had longer latencies to cross in the neutral context than vehicle treated animals suggesting significant generalization of contexts (p < 0.05). Although not explicitly scored, the most frequent behavior observed during inhibitory avoidance testing was immobility while remaining on the light side of the shuttle box. This behavior is consistent with other experiments utilizing inhibitory avoidance (Lynch et al., 2013; Riccio et al., 1968; Rohrbaugh and Riccio, 1968; Zhou and Riccio, 1996). Together, these results suggest that estradiol acts within the ACC to promote context fear generalization in female rats (Fig. 1C). To ensure that estradiol administration alone does not influence locomotor behavior that would alter rats ability to cross from the light chamber to the dark chamber of the inhibitory avoidance apparatus, OVX rats were given s.c. injections of either estradiol, or vehicle and were tested for locomotor impairments for a total of 9 min in an open field paradigm twenty-four hours later (the same time they would be tested for inhibitory avoidance). Independent t-test analysis showed that estradiol-treated rats exhibit equivalent locomotor behavior compared to vehicle-treated rats as they both travelled a similar distance (t(6) = .69, p > .05) (Fig. 1E). Two way ANOVA analysis revealed a significant main effect of area of arena (center vs surround) (F(1,12) = 4067, p < .001). Tukey’s post hoc analyses also show that both groups spent more time in the surround vs the center of the arena (p < .05) (Fig. 1E). Together, these data suggest that estradiol acts within the ACC to promote context fear generalization without influencing locomotor behavior in a way that would affect latency to cross when rats were tested in the inhibitory avoidance apparatus.
Fig. 1.
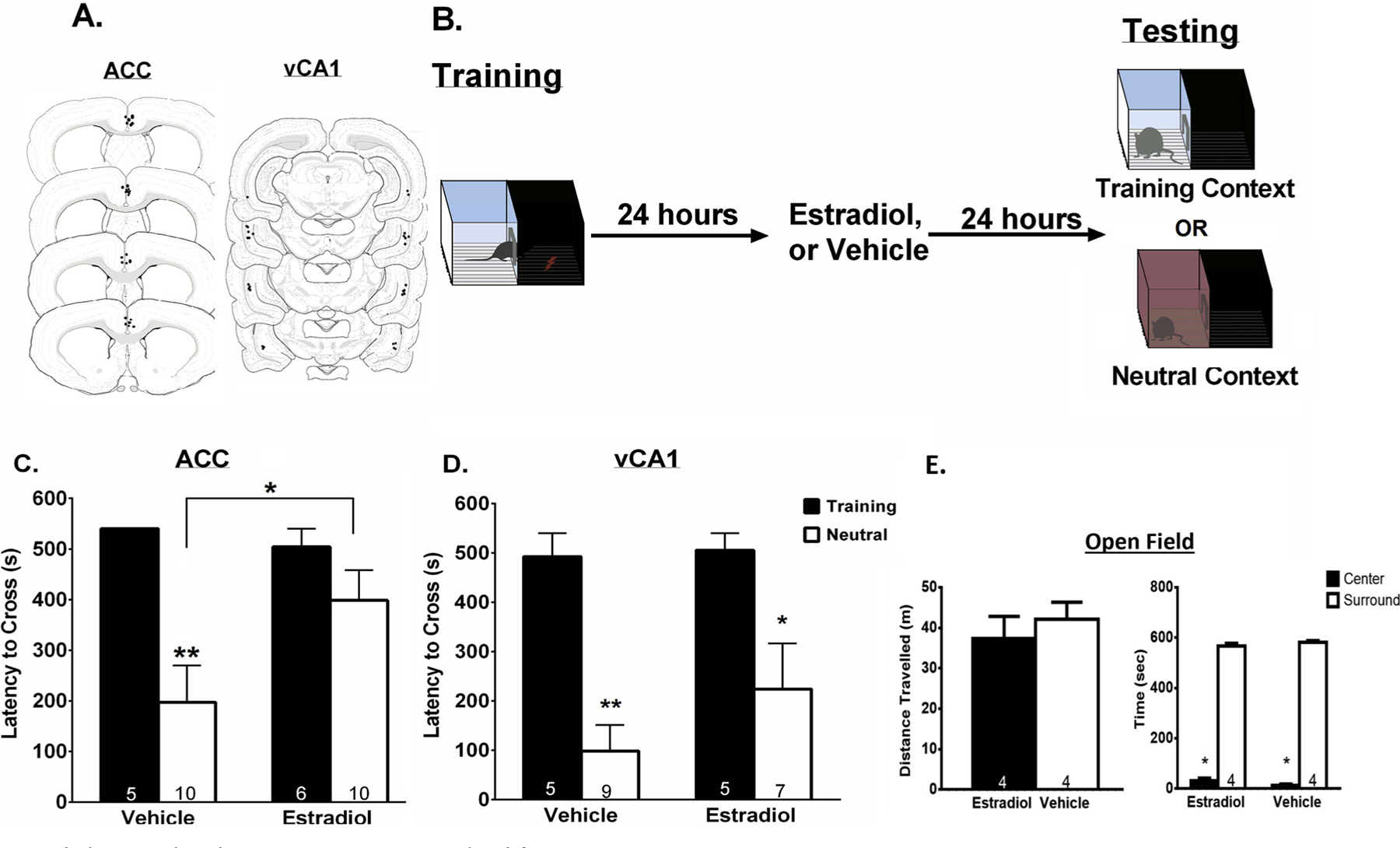
Estradiol acts within the ACC to promote generalized fear.
A. Local infusion site verification for the ACC and vCA1. Black dots represent infusion sites for one or more animals. Drawings are adapted from Swanson, (2018). B. Schematic of the experimental paradigm. All animals were trained in an inhibitory avoidance chamber and 24 h later received local infusion of either estradiol or vehicle. Animals were tested for generalization 24 h after infusions in either the training or the neutral context. C. Estradiol infusions into the ACC induces context generalization. Animals infused with estradiol displayed generalization as they had similar latencies to cross in the training and neutral context whereas vehicle animals had significantly shorter latencies to cross in the neutral than the training, suggesting context discrimination. D. Estradiol infusions into the vCA1 does not induce generalization. Vehicle- and estradiol-treated animals had significantly shorter latencies to cross in the neutral context than the training context, suggesting no generalization. E. Open field locomotor behavior. Estradiol and vehicle treated animals had similar distances traveled and spent equivalent amounts of time in the center and surround of the arena, suggesting estradiol treatment does not influence locomotion. Data are expressed as mean ± SEM *p < 0.05 **p < 0.01.
Next, to assess the role of vCA1 estrogen receptor activation on context fear generalization rats were infused with estradiol or vehicle 24 h after inhibitory avoidance training, and were then tested for generalization 24 h after the infusion (Fig. 1B). One animal was removed from analyses as a missed target. Analyses revealed a significant main effect of context (F(1,22) = 16.24, p < 0.001), a non-significant main effect of treatment (F(1,22) = 0.26, p > 0.05, and a non-significant interaction term (F(1,22) = 1.27 p > 0.05, ŋp2 = .029). Tukey’s multiple comparisons showed that vehicle-treated rats had significantly longer latencies to cross in the training context compared to the neutral context (p < 0.01), as did estradiol-treated rats (p < 0.05), suggesting no evidence of context generalization in either treatment group. Additionally, vehicle-treated and estradiol-treated rats had similar latencies to cross in the neutral context (p > 0.05). These data suggest that estradiol does not act within the vCA1 region of the hippocampus to promote generalized fear in female rats (Fig. 1D).
3.2. Dorsal hippocampal glutamate receptors mediate estradiol-induced fear generalization
Rats were given peripheral injections of estradiol or vehicle 24 h after training to assess the interaction between AMPA and NMDA receptors with estradiol in controlling generalized fear. Twenty-four hours after estradiol treatment, rats were tested for context generalization using the same inhibitory avoidance procedure described above. Five minutes prior to testing in the training or a neutral context, animals received infusions into the dCA1 of NBQX or APV (Fig. 2B). A total of 109 animals were used in this experiment and 14 of them were excluded from these analyses because they were identified as behavioral outliers or due to missed injections.
Fig. 2.
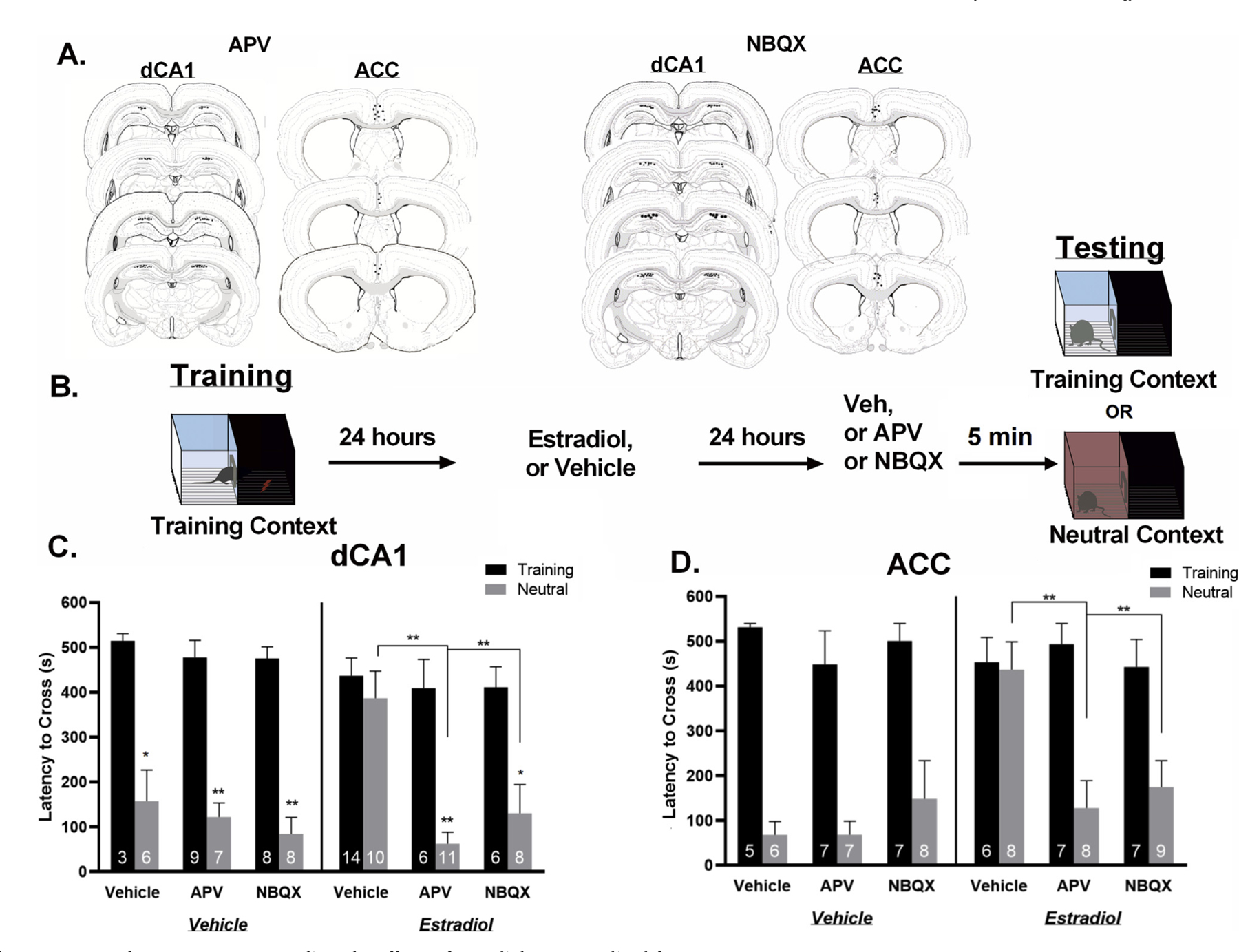
AMPA and NMDA receptors mediate the effects of estradiol on generalized fear.
A. Local infusion site verification for the dCA1 and ACC. Black dots represent infusion sites for one or more animals. Drawings are adapted from Swanson, (2018). B. Schematic of the experimental procedure. All animals were trained in an inhibitory avoidance chamber. Twenty-four hours after training animals received peripheral injections of either estradiol or vehicle. Twenty-four hours after injections animals received local infusions of either APV, NBQX, or vehicle and were then tested for generalization in either the training or the neutral context. C. Blocking AMPA or NMDA receptors within the dCA1 eliminates estradiol-induced generalization. Estradiol alone induced context generalization, but when either the NMDA antagonist (APV) or the AMPA antagonist (NBQX) were infused into the dCA1, estradiol-induced generalization was significantly attenuated. D. Estradiol treatment induced context generalization. Blocking either NMDA receptors with APV or AMPA receptors with NBQX in the ACC eliminated estradiol-induced generalization. Data are expressed as mean ± SEM *p < 0.05 **p < 0.01.
Three-way ANOVA analyses revealed that infusions of the NMDA receptor antagonist, APV, or the AMPA receptor antagonist, NBQX, into the dCA1, resulted in a significant main effect for context (F(1,84) = 102.3 p < 0.0001), a significant context X hormone treatment two way interaction (F(1,84) = 5.86, p < 0.05), but not a significant three way interaction term, (F(2,84 = 2.12, p = .13, ŋp2 = .225) (Fig. 2C). All Tukey’s post hoc analyses for comparisons between animals tested in the training context were not significant (p = ns). Analyses revealed that vehicle-vehicle treated rats had significantly longer latencies to cross in the training context when compared to the neutral context (p < 0.05) whereas estradiol-vehicle treated rats had similar latencies to cross in the training and the neutral context (p > 0.05). Additionally, estradiol-vehicle treated rats had significantly longer latencies to cross in the neutral context than vehicle-vehicle treated animals (p < 0.05) suggesting that based off our a priori assumptions, estradiol promotes generalized fear in females, replicating our previous results (Lynch et al., 2013, 2014; Lynch et al., 2016c). However, when estradiol-treated rats were subsequently administered a local infusion of APV in the dCA1 immediately prior to testing, they displayed significantly longer latencies to cross in the training context compared to the neutral context (p < 0.001) and significantly shorter latencies to cross in the neutral context compared to animals that were treated with estradiol and infused with vehicle (p < .001). APV had no effect on the latency to cross when rats were tested in the training context (p > 0.05) as they displayed similar latencies to cross to the other groups tested in the training context, suggesting that infusions of APV did not interfere with memory retrieval or affect locomotion. Furthermore, vehicle-treated rats given APV did not display any significant differences in latency to cross when compared with vehicle-vehicle animals in either context (p > 0.05). Fear generalization was also attenuated by infusions of NBQX— estradiol-treated rats that were subsequently infused with NBQX into the dCA1 immediately prior to testing had significantly shorter latencies to cross in the neutral context than those that were treated with estradiol and infused with vehicle (p < 0.05). NBQX infusions also had no effect on latencies to cross in the training context as they displayed similar latencies to cross when compared to the other groups tested in this context, suggesting no effect on context specific memory retrieval nor did it impact vehicle-treated animals (p > 0.05). Taken together, these data suggest that blocking AMPA or NMDA receptors with a low concentration of NBQX or APV, respectively, selectively attenuated estradiol-induced generalized fear without interrupting specific memory retrieval of the training context or affecting locomotion. Furthermore, these data demonstrate that AMPA and NMDA receptors within the dCA1 mediate the actions of estradiol on generalized fear responses. (Fig. 2C).
3.3. Anterior cingulate cortex glutamate receptors mediate estradiol-induced fear generalization
In the above experiments, we demonstrated that the ACC was an important locus for the effects of estradiol on generalized fear. Therefore, we next determined if estradiol influenced generalization through modulation of glutamate signaling in this region, similar to the results obtained for the dCA1. Using the same procedure described above, rats were trained in inhibitory avoidance and given hormone injections 24 h later. Rats were then administered APV or NBQX infusions 5 min before a 24 -h recall test in either the training or neutral context. A total of 105 animals were used in this experiment, and 19 animals were excluded from the analyses due to missed infusion targets or behavioral outliers. Three way ANOVA analyses revealed a significant main effect of context, (F(1,73) = 80.08, p < 0.0001), a significant context X hormone treatment two way interaction term, (F(1,73) = 6.96, p < 0.05), and a significant context X hormone treatment X ACC infusion three way interaction term, (F(2,73) = 3.52, p < 0.05, ŋp2 = .311) (Fig. 2D). Turkey’s post hoc analyses showed no generalization in vehicle-vehicle treated rats (p < 0.05) as they had significantly longer latencies to cross in the training context when compared to the neutral context. Additionally, estradiol-vehicle treated rats displayed significant generalization as they displayed similar latencies to cross in the training and neutral context (p > .05), and had significantly longer latencies to cross in the neutral context than vehicle-vehicle treated animals (p < 0.05). As with the previous experiment, fear generalization was attenuated by APV infusions into ACC. Post hoc analyses revealed that rats that were given estradiol and subsequently infused with APV immediately prior to testing displayed significantly shorter latencies to cross in the neutral context compared to rats that were given estradiol and infused with vehicle (p < 0.01), suggesting that blockade of NMDA receptors within the ACC eliminated generalized fear. Similar to our previous experiment, APV infusions did not alter latency to cross in the training context regardless of hormone treatment (p > .05) (Fig. 2D), suggesting that APV did not interfere with specific memory retrieval. These results suggest that estradiol promotes generalized fear through NMDA receptor activity within the ACC (Fig. 2D).
Fear generalization was also attenuated by NBQX infusions— post hoc analyses revealed that rats that were treated with estradiol and subsequently infused with NBQX immediately prior to testing had significantly shorter latencies to cross in the neutral context than rats given estradiol and received vehicle infusions (p < 0.05), suggesting that blockade of AMPA receptors attenuated generalized fear. Again, infusions of NBQX did not affect latency to cross in the training context of vehicle— (p > 0.05) or estradiol— (p > 0.05) treated rats, suggesting an effect restricted to generalized fear memory and not on specific memory retrieval. Together, these data suggest that AMPA and NMDA receptors within the ACC play an important role in mediating generalized fear in response to estradiol (Fig. 2C and 2D).
3.4. Dorsal hippocampal GluN2B-Containing NMDA receptors mediate generalized fear in female rats
Collectively, the results show that NMDA receptors and AMPA receptors mediate generalized fear in response to estradiol in female rats - blockade of both receptor subtypes eliminates fear responses in a neutral context without affecting specific memory retrieval of the training context (Fig. 2C and D). The enhancement of LTP following estradiol treatment is mainly due to actions on GluN2B-expressing NMDA receptors (Cyr et al., 2001; SSmith and McMahon, 2006; Smith et al., 2009). These receptors are implicated in memory consolidation (Rodrigues et al., 2001; Tang et al., 1999; Zhao et al., 2005). Thus, to determine if GluN2B-containing NMDA receptors mediate generalized fear in response to estradiol, we blocked GluN2B-containing receptors within the dCA1 or ACC using the antagonist, Ro 25–6981 (Ro 25). Experimental procedures were identical to those described above, but included pre-testing infusions of Ro 25 (Fig. 3B). A total of 93 animals were used in this experiment, and 13 animals were excluded as behavioral outliers or due to missed injection sites. Three-way ANOVA analyses revealed a significant main effect of context, (F(1,72) = 27.57, p < 0.0001), a significant dCA1 infusion X hormone treatment two way interaction (F(1,72) = 8.02, p < 0.01) and a significant context X dCA1 infusion X hormone treatment three way interaction term (F(1,72) = 5.76, p < 0.05, ŋp2 = 0.282) (Fig. 3C). Tukey’s Post hoc analyses revealed that vehicle-treated rats did not generalize because they had significantly longer latencies to cross in the training than neutral context (p < 0.05). However, estradiol treatment promoted generalized fear as rats displayed similar latencies to cross in the training and neural context (p > 0.05) and displayed significantly longer latencies to cross in the neutral context than vehicle-treated rats. When estradiol-treated rats were subsequently administered Ro 25 into the dCA1 immediately prior to testing, generalized fear was significantly attenuated as revealed by significantly shorter latencies to cross in the neutral context than rats that were treated with estradiol and given vehicle infusions (p < 0.05). Importantly, Ro 25 had no effects on memory retrieval in the training context for vehicle-treated (p > 0.05) or estradiol-treated (p > 0.05) rats, supporting recent findings that GluN2B-containing receptors do not play a role in specific context memory retrieval (Corcoran et al., 2011). Lastly, all post hoc comparisons between rats tested in the training context were not significant (p = ns). Thus, these data demonstrate that GluN2B-containing NMDA receptors specifically contribute to generalized recall in addition to their well-known role in memory consolidation. In addition, these data suggest that blocking GluN2B receptors within the dCA1 is sufficient to block the effects of estradiol-induced generalization (Fig. 3C).
Fig. 3.
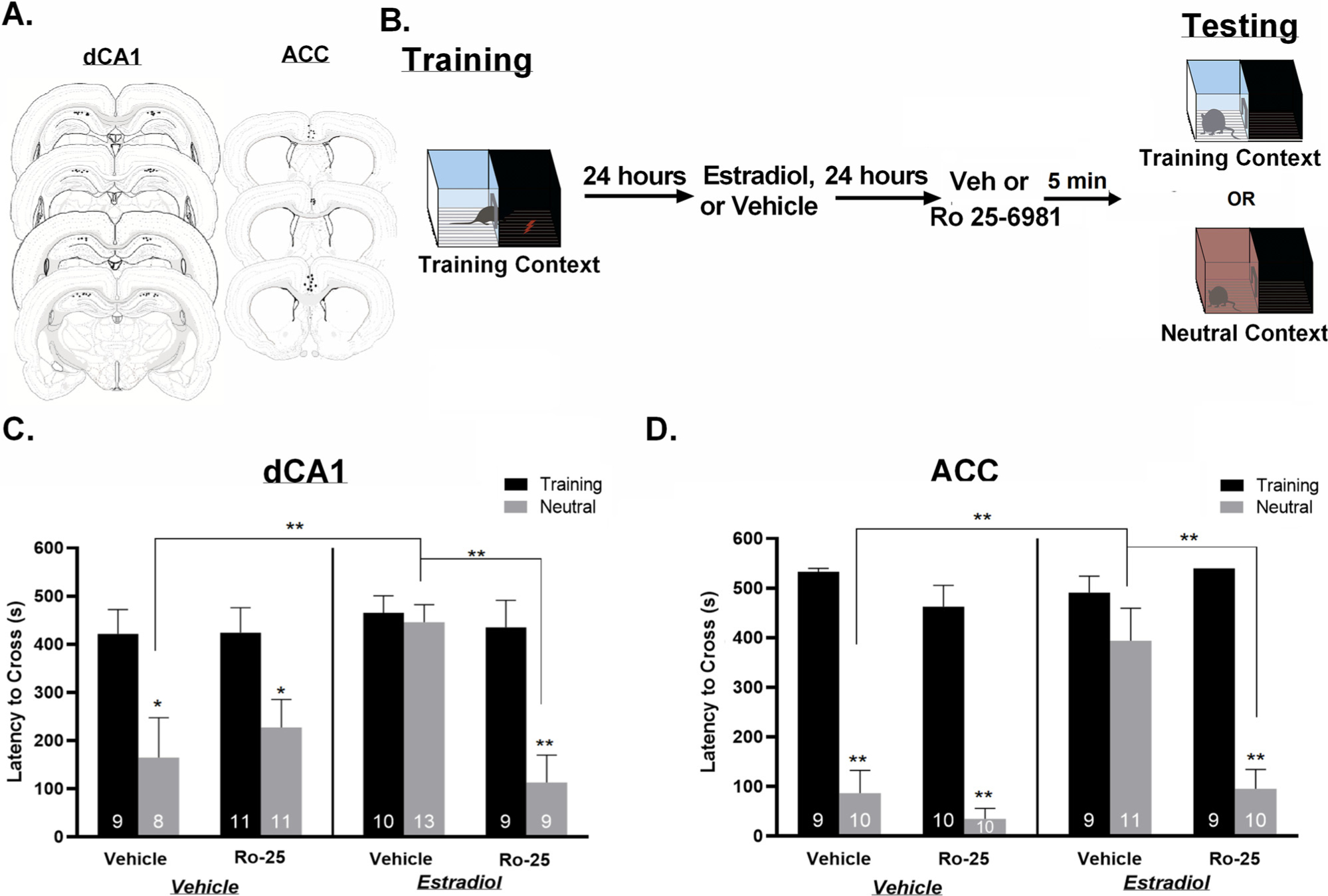
GluN2B receptors mediate the effects of estradiol on generalized fear.
A. Local infusion site verification for the dCA1 and ACC. Black dots represent infusion sites for one or more animals. Drawings are adapted from Swanson, (2018). B. Schematic of the experimental procedure. All animals were trained in an inhibitory avoidance chamber. Twenty-four hours after training animals received peripheral injections of either estradiol or vehicle. Twenty-four hours after injections animals received local infusions of either Ro 25–6981, or vehicle and were then tested for generalization in either the training or the neutral context. C. Estradiol treatment alone induced contextual fear generalization when compared to vehicle-treated animals. Estradiol-induced generalization was blocked when GluN2B receptors were blocked in the dCA1. D. Estradiol treatment induced contextual fear generalization when compared to vehicle-treated animals. Estradiol-induced generalization was reduced when GluN2B receptors were blocked in the ACC. Data are expressed as mean ± SEM *p < 0.05 **p < 0.01.
3.5. Anterior cingulate cortex GluN2B-Containing NMDA receptors mediate generalized fear in female rats
To determine if estradiol acts through the same mechanism in the ACC, we infused Ro 25 into the ACC and tested for context fear generalization. A total of 100 animals were used in this experiment and 20 were excluded due to missed infusion sites or behavioral outliers. Three-way ANOVA analyses revealed a significant main effect of context, (F(1,70) = 155.9, p < 0.001), a significant main effect of hormone treatment, (F(1,70) = 12.62, p < 0.001), a significant main effect of infusion (F(1,70) = 10.79, p < 0.01), a significant hormone treatment X context two way interaction term, (F(1,70) = 8.61, p < 0.001, ŋp2 = .284) and a significant hormone treatment X context X ACC infusion three way interaction, (F(1,70) = 10.44, p < 0.001, ŋp2 = .386 (Fig. 3D). Tukey’s Post hoc analyses revealed no generalization in vehicle-vehicle treated rats; they had significantly longer latencies to cross in the training context than the neutral context (p < 0.001). Estradiol-vehicle treated rats exhibited generalization as they displayed significantly longer latencies to cross in the neutral context than vehicle-vehicle treated rats (p < .05) and had similar latencies to cross in the training and neutral context (p > 0.05). Again, when estradiol-treated rats were administered Ro 25 immediately prior to testing, generalized fear was significantly attenuated (p < .01). Thus, rats that were treated with estradiol and then infused with Ro 25 displayed significantly shorter latencies to cross in the neutral context than rats that were treated with estradiol and given vehicle infusions (p < 0.001), suggesting that blockade of GluN2B-containing NMDA receptors significantly reduced generalization caused by estradiol. These results suggest that estradiol promotes generalization through actions on GluN2B-containing NMDA receptors within the ACC in addition to its actions within the dCA1 and its interaction with AMPA receptors (Fig. 3D).
4. Discussion
Previous work from our lab identified the dCA1 as an important locus for the effects of estradiol on generalized fear (Lynch et al., 2016c). Here, we show for the first time that the ACC also serves as an important locus for the actions of estradiol on generalized fear in female rats. However, unlike our previous findings supporting a critical role for the vCA1 in generalized fear in mice (Cullen et al., 2015; Lynch et al., 2016b), estradiol did not promote generalization when directly infused into this region. An additional aim of this study was to determine the role that glutamate receptors play in mediating the actions of estradiol on generalized fear. Through a series of experiments, we show that blocking NMDA or AMPA receptors within the dCA1 or ACC attenuates estradiol-induced generalization. In addition, we found that blocking GluN2B-containing NMDARs- traditionally thought to play a restricted role in memory consolidation- prior to testing also attenuated estradiol-induced generalization. The blockade of generalization was observed when GluN2B-containing receptors were inhibited in the dCA1 or ACC. These results suggest a critical involvement of glutamatergic transmission and a novel role for GluN2B-containing NMDARs in mediating the effects of estradiol on generalized fear in female rats.
Using local infusions of estradiol, we demonstrated that the ACC is an important locus of action for estradiol on fear generalization, in addition to the dCA1 hippocampus (Lynch et al., 2016c). Activity of ACC is implicated in remote memory and generalized fear memory in rodents and humans (Cullen et al., 2015; Frankland et al., 2004, 2006; Lissek et al., 2014; Ortiz et al., 2018; Suzuki et al., 2004). Thus, estradiol may recruit activity of the ACC to promote generalization. This idea is partly supported by studies showing that synaptic density within the ACC fluctuates throughout the estrus cycle with increased synaptic density and neuronal activity associated with higher estradiol levels, suggesting a critical role for estradiol in regulating ACC function (Hajszan et al., 2007; Hao et al., 2006; Wallace et al., 2006). Our later experiments reveal that estradiol promotes generalized fear potentially through increased glutamate signaling, which may ultimately lead to hyperreactivity within the ACC and its outputs to the amygdala. This is in line with our previous and current work demonstrating a critical role for ACC neuronal activity and its outputs to the amygdala in controlling generalized fear, regardless of the training-to-test interval (Cullen et al., 2015; Ortiz et al., 2018).
In addition to the ACC, we hypothesized that the vCA1 hippocampus would also be an important locus of action for the effects of estradiol on generalized fear. The ventral hippocampus is involved in emotion regulation more so than contextual processing, a task attributed to the dorsal hippocampus (Anagnostaras et al., 2002; Fanselow and Dong, 2010; Moser et al., 1995). However, studies show that the vCA1 plays an important role in Pavlovian fear conditioning that is likely to due to its connections with the amygdala (Cenquizca and Swanson, 2007; Kishi et al., 2006; Maren and Fanselow, 1995; Petrovich et al., 2001). In addition, activity of the vCA1 is also critical for fear generalization at a remote time point after fear training (Cullen et al., 2015). Contrary to our hypothesis, however, estradiol did not promote generalized fear when infused directly into the vCA1 region of the hippocampus. The reasons why estradiol promotes generalization when infused into the ACC, while failing to do so when infused into the vCA1 are not entirely clear, but it may have to do with the restricted role of the vCA1 in remote fear generalization (Ortiz et al., 2018). These data suggest that estradiol acts through mechanisms that are distinct from those that regulate remote generalization. For example, we consistently show the dorsal hippocampus is responsive to estradiol in promoting generalized fear (Lynch et al., 2013, 2014; Lynch et al., 2016c), and this region has recently been implicated in rapid generalization (Zhou et al., 2017). This raises an interesting question of whether estradiol could promote generalized fear locally in the vCA1 at remote time points after training. Alternatively, it may increase the rate at which this region begins to control generalization.
Estradiol enhances hippocampal LTP, synaptic plasticity, and improves hippocampal learning and memory—all of which are mediated through an interaction with glutamatergic signaling (Fortress et al., 2013; Lewis et al., 2008; Oberlander and Woolley, 2016; SSmejkalova and Woolley, 2010; Woolley and McEwen, 1992; WWoolley and McEwen, 1993; Woolley et al., 1997; Zhao et al., 2010). Using glutamate receptor antagonists, we specifically blocked estradiol-induced generalization without interrupting context-specific memory retrieval. Although the current work does not directly relate to learning and LTP per se, these data suggest that the downstream actions of estrogen receptor activation may involve similar alterations in glutamatergic signaling at the level of postsynaptic AMPA and NMDA receptors. Extensive work assessing the role of specific glutamate receptors reveals a connection with estradiol. For example, LTP is regulated by activation of AMPAR and NMDAR, which are both influenced by estradiol levels in female rats. More specifically, the increase in LTP following estradiol treatment is mediated by increased expression of GluN2B-containing NMDA receptors (Cyr et al., 2001; SSmith and McMahon, 2006; Smith et al., 2009). These studies show estradiol enhances the expression of GluN2B and increases glutamatergic transmission through modulation of these receptors (Smith and McMahon, 2006). Moreover, blockade of GluN2B attenuates estradiol-induced enhancements in LTP (Smith and McMahon, 2006)). More generally, transgenic mice overexpressing GluN2B display increased LTP and enhanced performance in hippocampal-dependent tasks, such as object recognition and conditioned fear learning (Tang et al., 2001). Although traditional studies have found a restricted role for GluN2B-containing NMDA receptors in memory consolidation (Rodrigues et al., 2001; Tang et al., 2001; Zhao et al., 2005), a few studies have demonstrated a critical role for GluN2B-containing NMDA receptors in some types of memory retrieval (Li et al., 2017; Mikics et al., 2017). Others, however, have found that GluN2B-containing NMDA receptors do not play a role in remote memory retrieval (Corcoran et al., 2011). The current data support the latter report suggesting that GluN2B-contianing NMDA receptors are not involved in specific context retrieval as we did not see a memory deficit in rats tested in the training context that were administered the GluN2B receptor antagonist Ro 25. GluN2B receptors show a slower offset decay than the other GluN subunits, making them a potential contributor for fear memory impairments, such as generalization (Monyer et al., 1994). Thus, evidence, including our own, reveal an important role for GluN2B-containing NMDA receptors in memory retrieval inaccuracies, such as when generalization occurs.
The current experiments suggest that estradiol-induced generalization is mediated through interactions with glutamate signaling. A possible mechanism for this interaction is estradiol may upregulate postsynaptic glutamate receptors, ultimately increasing glutamate signaling to promote generalized memory recall. Similar increases in glutamatergic signaling also produce contextual generalization for fear and non-fear tasks. For instance, genetic or pharmacological inactivation of presynaptic GABAb(1a) receptors, globally or within the dCA1, results in contextual fear generalization 24 h after training (Cullen et al., 2014; Lynch et al., 2016b). GABAb(1a) receptors are commonly found on presynaptic terminals of glutamatergic neurons, mediating glutamate release through presynaptic inhibition (Gassmann and Bettler, 2012). Genetic inactivation or pharmacological blockade of these receptors ultimately leads to an increased release of glutamate within the dCA1. Although mice are able to learn when GABAb(1a) receptors are inhibited, LTP is pushed beyond a functionally dynamic range (Guetg et al., 2009) making consolidation of specific contextual memories difficult. Although specific to consolidation of contextually precise memories, these data are in line with the current study observing a similar glutamatergic-based mechanism underlying how estradiol promotes generalized fear.
Recent evidence reveals a role for brain-derived estradiol in synaptic plasticity and learning and memory tasks (Lu et al., 2019; Tuscher et al., 2016). in vitro and in vivo work shows that estradiol is synthesized in the hippocampus and the cortex, regions that are involved in learning and memory (Hojo et al., 2004; Kretz et al., 2004; Remage-Healey et al., 2008). Additionally, locally synthesized estradiol is related to learning and memory tasks, such as object recognition (Tuscher et al., 2016). More specifically, infusions of letrozole into the hippocampus, which inhibits estradiol synthesis, impairs object recognition and spatial memory in ovariectomized mice (Tuscher et al., 2016). Another recent study utilized forebrain-neuron-specific aromatase knockout mice (FBN-ARO-KO) which lacks the ability to locally synthesize estradiol in the forebrain. FBN-ARO-KO mice have impairments in various hippocampally-dependent tasks, such as spatial reference memory, object recognition, and contextual fear conditioning (Lu et al., 2019). Together, these data point to a potential role of locally synthesized estradiol on synaptic plasticity and memory tasks. Although, not yet tested, we hypothesize that estradiol-induced generalization is not due to the presence of local estradiol synthesis as our data show that OVX rats do not generalize, suggesting that the presence of brain-derived estradiol is not sufficient alone to induce contextual fear generalization.
Previous work from our lab has shown that estradiol administration reduces generalization in gonadectomized males (Lynch et al., 2016a) through different receptor-dependent mechanisms compared to those in female rats. These data, support the growing evidence that estradiol has different modulatory effects in males and females on fear and memory processing. Currently, whether or not similar glutamatergic-centered mechanisms serve to constrain generalized fear in male rats remains unknown.
The evidence showing that women are more vulnerable than men to develop anxiety disorders is well-established (Kessler et al., 2005a; McLean et al., 2011). Additionally, women generalize fear cues more than men (Lonsdorf et al., 2015), suggesting that generalization may be a key contributing factor to the sex bias in anxiety disorders. Studies observing the neural mechanisms underlying these sex differences, however, are beneficial to the needed advancements of sex specific treatments. Given our findings here, we predict that estradiol acts within neural circuits that support memory recall to promote generalized fear to non-threatening contexts. In support of our previous neuroanatomical data (Cullen et al., 2015), we show that the ACC, in addition to the dCA1 (Lynch et al., 2016b, c), are important loci for regulating generalization that is modulated by the presence of estradiol. Further, we show that AMPA and NMDA receptors in general, as well as the specific GluN2B-containing NMDA receptors, are important mediators of estradiol-induced generalization. Although we did not demonstrate it here, the potential downstream effects of estradiol likely involves some increase in AMPA and/or NMDA receptor expression (Foy, 2001; SSmith and McMahon, 2005 Smith and McMahon, 2006); Wong and Moss, 1992). Additional pathways likely exist, and some recent work suggests that estradiol could act through various intracellular signaling mechanisms, such as ERK, P13k, and epigenetic alterations (Fan et al., 2010; Fernandez et al., 2008; Fortress et al., 2013, 2014; Lewis et al., 2008; Zhao et al., 2010). However, previous work from our lab has ruled out the influence of the ERK/MAPK signaling pathway as a mechanism involved in estradiol-induced generalization in female rats. Specifically, administration of the MAPK inhibitor, U0126, to animals that were given estradiol, failed to block fear generalization—suggesting this pathway is not required for estradiol-induced generalization in female rats. Given these data, and the timing of the effects of estradiol (days versus hours) we have been examining possible genomic mechanisms (Lynch et al., 2016c) to account for estradiol’s ability to modulate generalized fear. However, our data do not systematically rule out additional intracellular signaling pathways and post-translational modifications that may be influenced by estradiol. These findings and future work are necessary in order to better understand the sex differences seen in the prevalence rate of nearly all anxiety disorders and can provide a sturdy foundation for informing potential new and effective sex-specific treatments for sex-biased psychiatric disorders.
Acknowledgements
Special thanks to the animal care staff in the Department of Psychological Sciences animal facility. We also thank Dr. David Riccio for helpful guidance and comments during the completion of this work.
Funding and disclosure
Dr. Jasnow’s work was partially funded by a Whitehall Foundation Grant (#2012-12- 90). The Whitehall Foundation did not have any role in the design, collection, analysis, interpretation, writing, or manuscript submission processes for the experiments and data described herein. The authors have no conflicts of interest to declare.
Footnotes
Declarations of interest
The authors declare no competing or conflicts of interests.
References
- Anagnostaras SG, Gale GD, Fanselow MS, 2002. The hippocampus and Pavlovian fear conditioning: reply to Bast et al et al. Hippocampus 12 (4), 561–565. [DOI] [PubMed] [Google Scholar]
- Burman MA, Gewirtz JC, 2007. Hippocampal activity, but not plasticity, is required for early consolidation of fear conditioning with a short trace interval. Eur. J. Neurosci 25 (8), 2483–2490. [DOI] [PubMed] [Google Scholar]
- Cenquizca LA, Swanson LW, 2007. Spatial organization of direct hippocampal field CA1 axonal projections to the rest of the cerebral cortex. Brain Res. Rev 56 (1), 1–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang YJ, Yang CH, Liang YC, Yeh CM, Huang CC, Hsu KS, 2009. Estrogen modulates sexually dimorphic contextual fear extinction in rats through estrogen receptor β. Hippocampus 19 (11), 1142–1150. [DOI] [PubMed] [Google Scholar]
- Corcoran KA, Donnan MD, Tronson NC, Guzmán YF, Gao C, Jovasevic V, et al. , 2011. NMDA receptors in retrosplenial cortex are necessary for retrieval of recent and remote context fear memory. J. Neurosci 31 (32), 11655–11659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cullen PK, Dulka BN, Ortiz S, Riccio DC, Jasnow AM, 2014. GABA-mediated presynaptic inhibition is required for precision of long-term memory. Learn. Mem. (Cold Spring Harbor, NY) 21 (4), 180–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cullen PK, Gilman TL, Winiecki P, Riccio DC, Jasnow AM, 2015. Activity of the anterior cingulate cortex and ventral hippocampus underlie increases in contextual fear generalization. Neurobiol. Learn. Mem 124, 19–27. [DOI] [PubMed] [Google Scholar]
- Cyr M, Thibault C, Morissette M, Landry M, Di Paolo T, 2001. Estrogen-like activity of tamoxifen and raloxifene on NMDA receptor binding and expression of its subunits in rat brain. Neuropsychopharmacology 25 (2), 242–257. [DOI] [PubMed] [Google Scholar]
- Dudai Y, 2004. The Neurobiology of Consolidations, Or, How Stable is the Engram? Annu. Rev. Psychol 55 (1), 51–86. [DOI] [PubMed] [Google Scholar]
- Dudai Y, 2012. The restless engram: consolidations never end. Annu. Rev. Neurosci 35, 227–247. [DOI] [PubMed] [Google Scholar]
- Fan L, Zhao Z, Orr PT, Chambers CH, Lewis MC, Frick KM, 2010. Estradiol-induced object memory consolidation in middle-aged female mice requires dorsal hippocampal extracellular signal-regulated kinase and phosphatidylinositol 3-kinase activation. J. Neurosci 30 (12), 4390–4400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fanselow MS, Dong H-W, 2010. Are the dorsal and ventral hippocampus functionally distinct structures? Neuron 65 (1), 7–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez SM, Lewis MC, Pechenino AS, Harburger LL, Orr PT, Gresack JE, et al. , 2008. Estradiol-induced enhancement of object memory consolidation involves hippocampal extracellular signal-regulated kinase activation and membrane-bound estrogen receptors. J. Neurosci 28 (35), 8660–8667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fortress AM, Fan L, Orr PT, Zhao Z, Frick KM, 2013. Estradiol-induced object recognition memory consolidation is dependent on activation of mTOR signaling in the dorsal hippocampus. Learn. Mem 20 (3), 147–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fortress AM, Kim J, Poole RL, Gould TJ, Frick KM, 2014. 17β-Estradiol regulates histone alterations associated with memory consolidation and increases Bdnf promoter acetylation in middle-aged female mice. Learn. Mem 21 (9), 457–467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foy MR, 2001. 17β-estradiol: effect on CA1 hippocampal synaptic plasticity. Neurobiol. Learn. Mem 76 (3), 239–252. [DOI] [PubMed] [Google Scholar]
- Frankland PW, Bontempi B, Talton LE, Kaczmarek L, Silva AJ, 2004. The involvement of the anterior cingulate cortex in remote contextual fear memory. Science (New York, NY) 304 (5672), 881–883. [DOI] [PubMed] [Google Scholar]
- Frankland PW, Ding H-K, Takahashi E, Suzuki A, Kida S, Silva AJ, 2006. Stability of recent and remote contextual fear memory. Learn. Mem 13 (4), 451–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frick KM, Kim J, Koss WA, 2018. Estradiol and hippocampal memory in female and male rodents. Curr. Opin. Behav. Sci 23, 65–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gassmann M, Bettler B, 2012. Regulation of neuronal GABAB receptor functions by subunit composition. Nat. Rev. Neurosci 13 (6), 380. [DOI] [PubMed] [Google Scholar]
- Gazzaley AH, Weiland NG, McEwen BS, Morrison JH, 1996. Differential regulation of NMDAR1 mRNA and protein by estradiol in the rat hippocampus. J. Neurosci 16 (21), 6830–6838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould E, Woolley CS, Frankfurt M, McEwen BS, 1990. Gonadal steroids regulate dendritic spine density in hippocampal pyramidal cells in adulthood. J. Neurosci 10 (4), 1286–1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gresack JE, Frick KM, 2006. Post-training estrogen enhances spatial and object memory consolidation in female mice. Pharmacol. Biochem. Behav 84 (1), 112–119. [DOI] [PubMed] [Google Scholar]
- Guetg N, Seddik R, Vigot R, Turecek R, Gassmann M, Vogt KE, et al. , 2009. The GABAB1a isoform mediates heterosynaptic depression at hippocampal mossy fiber synapses. J. Neurosci 29 (5), 1414–1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajszan T, MacLusky NJ, Johansen JA, Jordan CL, Leranth C, 2007. Effects of androgens and estradiol on spine synapse formation in the prefrontal cortex of normal and testicular feminization mutant male rats. Endocrinology 148 (5), 1963–1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hao J, Rapp PR, Leffler AE, Leffler SR, Janssen WG, Lou W, et al. , 2006. Estrogen alters spine number and morphology in prefrontal cortex of aged female rhesus monkeys. J. Neurosci 26 (9), 2571–2578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hojo Y, Hattori T-a, Enami T, Furukawa A, Suzuki K, Ishii H-t, et al. , 2004. Adult male rat hippocampus synthesizes estradiol from pregnenolone by cytochromes P45017α and P450 aromatase localized in neurons. Proc. Natl. Acad. Sci. U. S. A 101 (3), 865–870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou Y.-y, Liu Y, Kang S, Yu C, Chi Z-q, Liu J-g, 2009. Glutamate receptors in the dorsal hippocampus mediate the acquisition, but not the expression, of conditioned place aversion induced by acute morphine withdrawal in rats. Acta Pharmacol. Sin 30 (10), 1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jasnow AM, Schulkin J, Pfaff DW, 2006. Estrogen facilitates contextual and cued fear, and increases corticotropin-releasing hormone mRNA expression in the central amygdala in female mice. Horm. Behav 49, 197–205. [DOI] [PubMed] [Google Scholar]
- Keiser AA, Turnbull LM, Darian MA, Feldman DE, Song I, Tronson NC, 2017. Sex differences in context fear generalization and recruitment of Hippocampus and amygdala during retrieval. Neuropsychopharmacology 42 (2), 397–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler RC, Berglund P, Demler O, Jin R, Merikangas KR, Walters EE, 2005a. Lifetime prevalence and age-of-onset distributions of dsm-iv disorders in the national comorbidity survey replication. Arch. Gen. Psychiatry 62 (6), 593–602. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Chiu WT, Demler O, Merikangas KR, Walters EE, 2005b. Prevalence, severity, and comorbidity of 12-month DSM-IV disorders in the National Comorbidity Survey Replication. Arch. Gen. Psychiatry 62 (6), 617–627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kishi T, Tsumori T, Yokota S, Yasui Y, 2006. Topographical projection from the hippocampal formation to the amygdala: a combined anterograde and retrograde tracing study in the rat. J. Comp. Neurol 496 (3), 349–368. [DOI] [PubMed] [Google Scholar]
- Kretz O, Fester L, Wehrenberg U, Zhou L, Brauckmann S, Zhao S, et al. , 2004. Hippocampal synapses depend on hippocampal estrogen synthesis. J. Neurosci 24 (26), 5913–5921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis MC, Kerr KM, Orr PT, Frick KM, 2008. Estradiol-induced enhancement of object memory consolidation involves NMDA receptors and protein kinase A in the dorsal hippocampus of female C57BL/6 mice. Behav. Neurosci 122 (3), 716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li YJ, Ping XJ, Qi C, Shen F, Sun LL, Sun XW, et al. , 2017. Re-exposure to morphine-associated context facilitated long-term potentiation in the vSUB-NAc glutamatergic pathway via GluN2B-containing receptor activation. Addict. Biol 22 (2), 435–445. [DOI] [PubMed] [Google Scholar]
- Lissek S, 2012. Toward an account of clinical anxiety predicated on basic, neurally mapped mechanisms of Pavlovian fear-learning: the case for conditioned over-generalization. Depress. Anxiety 29 (4), 257–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lissek S, Powers AS, McClure EB, Phelps EA, Woldehawariat G, Grillon C, et al. , 2005. Classical fear conditioning in the anxiety disorders: a meta-analysis. Behav. Res. Ther 43 (11), 1391–1424. [DOI] [PubMed] [Google Scholar]
- Lissek S, Biggs AL, Rabin SJ, Cornwell BR, Alvarez RP, Pine DS, et al. , 2008. Generalization of conditioned fear-potentiated startle in humans: experimental validation and clinical relevance. Behav. Res. Ther 46 (5), 678–687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lissek S, Bradford DE, Alvarez RP, Burton P, Espensen-Sturges T, Reynolds RC, et al. , 2014. Neural substrates of classically conditioned fear-generalization in humans: a parametric fMRI study. Soc. Cogn. Affect. Neurosci 9 (8), 1134–1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lonsdorf TB, Haaker J, Schumann D, Sommer T, Bayer J, Brassen S, et al. , 2015. Sex differences in conditioned stimulus discrimination during context-dependent fear learning and its retrieval in humans: the role of biological sex, contraceptives and menstrual cycle phases. J. Psychiatry Neurosci 40 (6), 368–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Y, Sareddy GR, Wang J, Wang R, Li Y, Dong Y, et al. , 2019. Neuron-Derived Estrogen Regulates Synaptic Plasticity and Memory. J. Neurosci 39 (15), 2792–2809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch J, Cullen PK, Jasnow AM, Riccio DC, 2013. Sex differences in the generalization of fear as a function of retention intervals. Learn. Mem 20 (11), 628–632. [DOI] [PubMed] [Google Scholar]
- Lynch JF, Dejanovic D, Winiecki P, Mulvany J, Ortiz S, Riccio DC, et al. , 2014. Activation of ERβ modulates fear generalization through an effect on memory retrieval. Horm. Behav 66 (2), 421–429. [DOI] [PubMed] [Google Scholar]
- Lynch JF 3rd, Vanderhoof T, Winiecki P, Latsko MS, Riccio DC, Jasnow AM, 2016a. Aromatized testosterone attenuates contextual generalization of fear in male rats. Horm. Behav 84, 127–135. [DOI] [PubMed] [Google Scholar]
- Lynch JF, Winiecki P, Gilman TL, Adkins JM, Jasnow AM, 2016b. Hippocampal GABAB(1a) receptors constrain generalized contextual fear. Neuropsychopharmacology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch JF, Winiecki P, Vanderhoof T, Riccio DC, Jasnow AM, 2016c. Hippocampal cytosolic estrogen receptors regulate fear generalization in females. Neurobiol. Learn. Mem 130, 83–92. [DOI] [PubMed] [Google Scholar]
- Maren S, Fanselow MS, 1995. Synaptic plasticity in the basolateral amygdala induced by hippocampal formation stimulation in vivo. J. Neurosci 15 (11), 7548–7564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen B, 2002. Estrogen actions throughout the brain. Recent Prog. Horm. Res 57, 357–384. [DOI] [PubMed] [Google Scholar]
- McLean CP, Asnaani A, Litz BT, Hofmann SG, 2011. Gender differences in anxiety disorders: prevalence, course of illness, comorbidity and burden of illness. J. Psychiatr. Res 45 (8), 1027–1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikics E, Toth M, Biro L, Bruzsik B, Nagy B, Haller J, 2017. The role of GluN2B-containing NMDA receptors in short- and long-term fear recall. Physiol. Behav 177, 44–48. [DOI] [PubMed] [Google Scholar]
- Monyer H, Burnashev N, Laurie DJ, Sakmann B, Seeburg PH, 1994. Developmental and regional expression in the rat brain and functional properties of four NMDA receptors. Neuron 12 (3), 529–540. [DOI] [PubMed] [Google Scholar]
- Morgan MA, Pfaff DW, 2002. Estrogen’s effects on activity, anxiety, and fear in two mouse strains. Behav. Brain Res 132 (1), 85–93. [DOI] [PubMed] [Google Scholar]
- Moser M-B, Moser EI, Forrest E, Andersen P, Morris R, 1995. Spatial learning with a minislab in the dorsal hippocampus. Proc. Natl. Acad. Sci 92 (21), 9697–9701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberlander JG, Woolley CS, 2016. 17β-estradiol acutely potentiates glutamatergic synaptic transmission in the Hippocampus through distinct mechanisms in males and females. J. Neurosci 36 (9), 2677–2690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortiz S, Latsko S, Maeson Fouty J., Dutta S, Adkins J, Jasnow A, 2018. Basolateral Amygdala Inputs From the Anterior Cingulate Cortex and Ventral Hippocampus Play a Selective Role in Contextual Fear Generalization in Prep.
- Petrovich GD, Canteras NS, Swanson LW, 2001. Combinatorial amygdalar inputs to hippocampal domains and hypothalamic behavior systems. Brain Res. Rev 38 (1–2), 247–289. [DOI] [PubMed] [Google Scholar]
- Remage-Healey L, Maidment NT, Schlinger BAJNn, 2008. Forebrain steroid levels fluctuate rapidly during social interactions. Nat. Neurosci 11 (11), 1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riccio DC, Rohrbaugh M, Hodges LAJDPTJotISfDP, 1968. Developmental aspects of passive and active avoidance learning in rats. Psychobiology 1 (2), 108–111. [Google Scholar]
- Rodrigues SM, Schafe GE, LeDoux JE, 2001. Intra-amygdala blockade of the NR2B subunit of the NMDA receptor disrupts the acquisition but not the expression of fear conditioning. J. Neurosci 21 (17), 6889–6896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohrbaugh M, Riccio DC, 1968. Stimulus generalization of learned fear in infant and adult rats. J. Comp. Physiol. Psychol 66 (2), 530–533. [DOI] [PubMed] [Google Scholar]
- Smejkalova T, Woolley CS, 2010. Estradiol acutely potentiates hippocampal excitatory synaptic transmission through a presynaptic mechanism. J. Neurosci 30 (48), 16137–16148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith CC, McMahon LL, 2005. Estrogen-induced increase in the magnitude of long-term potentiation occurs only when the ratio of NMDA transmission to AMPA transmission is increased. J. Neurosci 25 (34), 7780–7791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith CC, McMahon LL, 2006. Estradiol-induced increase in the magnitude of long-term potentiation is prevented by blocking NR2B-Containing receptors. J. Neurosci 26 (33), 8517–8522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith CC, Vedder LC, McMahon LL, 2009. Estradiol and the relationship between dendritic spines, NR2B containing NMDA receptors, and the magnitude of long-term potentiation at hippocampal CA3-CA1 synapses. Psychoneuroendocrinology 34, S130–S142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki A, Josselyn SA, Frankland PW, Masushige S, Silva AJ, Kida S, 2004. Memory reconsolidation and extinction have distinct temporal and biochemical signatures. J. Neurosci 24 (20), 4787–4795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang Y-P, Shimizu E, Dube GR, Rampon C, Kerchner GA, Zhuo M, et al. , 1999. Genetic enhancement of learning and memory in mice. Nature 401, 63. [DOI] [PubMed] [Google Scholar]
- Tang YP, Wang H, Feng R, Kyin M, Tsien JZ, 2001. Differential effects of enrichment on learning and memory function in NR2B transgenic mice. Neuropharmacology 41 (6), 779–790. [DOI] [PubMed] [Google Scholar]
- Tuscher JJ, Fortress AM, Kim J, Frick KM, 2014. Regulation of object recognition and object placement by ovarian sex steroid hormones. Behav. Brain Res [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuscher JJ, Szinte JS, Starrett JR, Krentzel AA, Fortress AM, Remage-Healey L, et al. , 2016. Inhibition of local estrogen synthesis in the hippocampus impairs hippocampal memory consolidation in ovariectomized female mice. Horm. Behav 83, 60–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasudevan N, Pfaff DW, 2008. Non-genomic actions of estrogens and their interaction with genomic actions in the brain. Front. Neuroendocrinol 29 (2), 238–257. [DOI] [PubMed] [Google Scholar]
- Vedder LC, Smith CC, Flannigan AE, McMahon LL, 2013. Estradiol-induced increase in novel object recognition requires hippocampal NR2B-containing NMDA receptors. Hippocampus 23 (1), 108–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker DL, Davis M, 1997. Double dissociation between the involvement of the bed nucleus of the stria terminalis and the central nucleus of the amygdala in startle increases produced by conditioned versus unconditioned fear. J. Neurosci 17 (23), 9375–9383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker DL, Paschall GY, Davis M, 2005. Glutamate receptor antagonist infusions into the basolateral and medial amygdala reveal differential contributions to olfactory vs. Context fear conditioning and expression. Learn. Mem 12 (2), 120–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace M, Luine V, Arellanos A, Frankfurt M, 2006. Ovariectomized rats show decreased recognition memory and spine density in the hippocampus and prefrontal cortex. Brain Res. 1126 (1), 176–182. [DOI] [PubMed] [Google Scholar]
- Weiland NG, 1992. Estradiol selectively regulates agonist binding sites on the N-methyl-D-aspartate receptor complex in the CA1 region of the hippocampus. Endocrinology 131 (2), 662–668. [DOI] [PubMed] [Google Scholar]
- Wong M, Moss RL, 1992. Long-term and short-term electrophysiological effects of estrogen on the synaptic properties of hippocampal CA1 neurons. J. Neurosci 12 (8), 3217–3225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolley CS, McEwen BS, 1992. Estradiol mediates fluctuation in hippocampal synapse density during the estrous cycle in the adult rat [published erratum appears in J Neurosci 1992 Oct; 12 (10): following table of contents]. J. Neurosci 12 (7), 2549–2554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolley CS, McEwen BS, 1993. Roles of estradiol and progesterone in regulation of hippocampal dendritic spine density during the estrous cycle in the rat. J. Comp. Neurol 336 (2), 293–306. [DOI] [PubMed] [Google Scholar]
- Woolley CS, McEwen BS, 1994. Estradiol regulates hippocampal dendritic spine density via an N-methyl-D-aspartate receptor-dependent mechanism. J. Neurosci 14 (12), 7680–7687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolley CS, Weiland NG, McEwen BS, Schwartzkroin PA, 1997. Estradiol increases the sensitivity of hippocampal CA1 pyramidal cells to NMDA receptor-mediated synaptic input: correlation with dendritic spine density. J. Neurosci 17 (5), 1848–1859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeidan MA, Igoe SA, Linnman C, Vitalo A, Levine JB, Klibanski A, et al. , 2011. Estradiol modulates medial prefrontal cortex and amygdala activity during fear extinction in women and female rats. Biol. Psychiatry 70 (10), 920–927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao M-G, Toyoda H, Lee Y-S, Wu L-J, Ko SW, Zhang X-H, et al. , 2005. Roles of NMDA NR2B subtype receptor in prefrontal long-term potentiation and contextual fear memory. Neuron 47 (6), 859–872. [DOI] [PubMed] [Google Scholar]
- Zhao Z, Fan L, Frick KM, 2010. Epigenetic alterations regulate estradiol-induced enhancement of memory consolidation. Proc. Natl. Acad. Sci. U. S. A 107 (12), 5605–5610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y, Riccio D, 1996. Manipulation of components of context: the context shift effect and forgetting of stimulus attributes. Learn. Motiv 27 (4), 400–407. [DOI] [PubMed] [Google Scholar]
- Zhou H, Xiong G-J, Jing L, Song N-N, Pu D-L, Tang X, et al. , 2017. The inter-hemispheric CA1 circuit governs rapid generalisation but not fear memory. Nat. Commun 8 (1), 2190. [DOI] [PMC free article] [PubMed] [Google Scholar]


