ABSTRACT
Background
Vitamins D and K, which are present in human brain, may have a role in neurodegenerative disease.
Objectives
Given the interest in measuring nutrient concentrations in archived brain samples, it is important to evaluate whether freezer storage time affects these concentrations. Therefore, we evaluated differences in vitamin D and vitamin K concentrations in human brain samples stored for various lengths of time.
Methods
Postmortem brain samples were obtained from 499 participants in the Rush Memory and Aging Project (mean age 92 y, 72% female). Concentrations of vitamins D and K and their metabolites were measured in 4 regions (midtemporal cortex, midfrontal cortex, cerebellum, anterior watershed white matter) using LC-MS/MS and HPLC, respectively. The predominant forms were 25-hydroxycholecalciferol [25(OH)D3] and menaquinone-4 (MK4). ANOVA was used to determine if concentrations differed according to storage time.
Results
The geometric mean of the mean 25(OH)D3 concentration (across 4 regions) in brains stored for 1.1 to 6.0 y did not differ from that in brains stored ≤1.0 y (all P ≥ 0.37), whereas 25(OH)D3 in brains stored >6.0 y was 31–40% lower (P ≤ 0.003). MK4 had similar results, with the geometric mean MK4 concentration in the brains stored ≥9.0 y being 48–52% lower than those in brains stored ≤1.0 y (P ≤ 0.012). The 25(OH)D3 and MK4 concentrations were positively correlated across all 4 regions (all Spearman ρ ≥ 0.79, P < 0.001).
Conclusions
25(OH)D3 and MK4 appear to be stable in brain tissue from older adults stored at -80°C for up to 6 and 9 y, respectively, but not longer. Freezer storage time should be considered in the design and interpretation of studies using archived brain tissue.
Keywords: Alzheimer disease and related dementias, vitamin D, vitamin K, storage, stability, brain
Introduction
Vitamin D and vitamin K are fat-soluble nutrients that have been implicated in Alzheimer disease and related dementias (ADRD). Vitamin D and the vitamin D receptor (VDR) are present in human brain tissue (1–3). In rodent experiments, 1,25-dihydroxyvitamin D3 upregulated neurotrophic factors (4), prevented excitotoxicity, and exerted anti-inflammatory activities in brain tissue (5). Vitamin K, which is also present in human brain tissue, predominantly in the form of menaquinone-4 (MK4) (6, 7), functions as an enzymatic cofactor for vitamin K–dependent proteins in cerebral tissue. In rodents, multiple vitamin K–dependent proteins are present in the cerebral cortex and other brain regions (8). Vitamin K is also involved in sphingolipid metabolism (9). Sphingolipids are important membrane constituents that have a role in cognitive behavior and are abundant in brain tissue. These mechanisms are supported by population-based studies that found vitamin D and vitamin K insufficiencies to be associated with an increased risk for AD/cognitive decline (10–15). It is therefore plausible for vitamin D and/or vitamin K to have a protective role in ADRD and cognitive decline. However, evidence would be strengthened by a broader evaluation of these vitamin metabolites in human brain tissue.
The establishment of biospecimen repositories that include storage of human brain tissue has facilitated translational research in ADRD (16). As interest in conducting analysis of archived brain samples increases, it is important to evaluate how storage conditions might affect the analyses of interest in human brain tissue. The objective of this study was to evaluate the influence of freezer storage time on vitamin D and vitamin K concentrations in human brain tissue stored for up to 13 y.
Methods
Study population
Postmortem brain samples were obtained from 499 participants in the Rush Memory and Aging Project (MAP), a clinical-pathologic prospective epidemiologic cohort study of residents of retirement communities and public housing in the Chicago area (17). At enrollment, all MAP participants were free of clinically diagnosed dementia and agreed to risk factor assessment, annual clinical evaluation, and brain donation at death. Samples obtained for this study were from participants who died between 2004 and 2019. The median (range) storage time of the brain samples was 4.9 (0.4–13.0) y. At autopsy and brain dissection, the tissues were immediately frozen and not allowed to thaw during the dissection. The tissues were stored at ˗80°C until analysis. The MAP study was approved by the Institutional Review Board of Rush University Medical Center. This ancillary study was additionally approved by the Institutional Review Board at Tufts University Health Sciences.
Vitamin D and vitamin K analyses
Cholecalciferol (D₃) and its metabolites, 25(OH)D3 and 1,25-dihydroxycholecalciferol [1,25(OH)2D3], were measured using LC-MS/MS in 4 brain regions [midtemporal cortex (MT), midfrontal cortex (MF), cerebellum (CR), and anterior watershed white matter (AWS)] as previously described (2). The lower limits of detection (LOD) for this assay are as follows: vitamin D3 0.06 pmol/g; 25(OH)D3 0.1 pmol/g, and 1,25(OH)2D3 0.06 pmol/g (2).
Phylloquinone (vitamin K1) and MK4 (a metabolite of phylloquinone and a form of vitamin K2) were extracted and purified from the MT, MF, CR, and AWS using established methods (18). To improve separation, a C30 column was used as described previously (19). The assay LOD for both phylloquinone and MK4 is 0.1 pmol/g. The details of the sample preparation and detection are described in the Supplemental Methods.
With regard to quality control procedures, there are currently no universal vitamin D and vitamin K standards available to use as controls for analyzing these nutrients in human brain tissue. Therefore, we used in-house controls (human brain samples or serum with known amounts of each vitamin) with each batch of 20 samples to monitor assay drift. Calibration and internal standards were injected every 6–10 samples. The CV values for inter-assay precision of D3, 25(OH)D3, and 1,25(OH)2D3 were characterized by relative SDs of 10.2%, 9.3%, and 10.0%, respectively. Similarly, the interassay precision values of MK4 and PK were 10.4% and 9.6%, respectively. All samples were analyzed randomly, not in sequence order of year received.
Statistical analyses
Our main statistical analyses focused on 25(OH)D3 and MK4 because these were the primary forms of vitamin D and vitamin K detected in the human brain samples. All statistical analyses were conducted using SAS v 9.4. The mean 25(OH)D3 and MK4 concentrations in the 4 regions were calculated [henceforth referred to as mean 25(OH)D3 and mean MK4]. Prior to analysis, the distributions of mean 25(OH)D3 and mean MK4 were natural-log transformed to improve normality. General linear models (proc glm) were used to evaluate differences in mean 25(OH)D3 and mean MK4 concentrations according to storage time. Storage time was categorized into 1-y increments, with the samples stored ≤1.0 y serving as the reference group. A Bonferroni corrected P value of <0.005 was considered statistically significant (0.05/10 comparisons). Because population-based studies indicate an increase in vitamin D supplement use in the United States over the course of this study (20), we adjusted for vitamin D supplement use in a sensitivity analysis. Unfortunately, information on supplement use was not available for participants whose brains were stored for >8 y. To verify that the association between 25(OH)D3 and storage time was independent of vitamin D supplement use, vitamin D supplement use was included as a covariate in a sensitivity analysis. In a separate sensitivity analysis, we adjusted the models for age at death to verify that the results were not age dependent. The differences in 25(OH)D3 and MK4 across storage time categories were evaluated in the separate MF, MT, CR, and AWS regions in secondary analyses following the same approach. Spearman rank order correlation coefficients were used to evaluate the correlations of 25(OH)D3 and MK4, respectively, among the 4 regions. The 25(OH)D3 and MK4 concentrations were compared across the 4 regions in the brain samples stored ≤6 y and ≤9 y, respectively, using a repeated measures mixed-effect models (proc mixed).
Results
This study utilized brain tissue samples from 499 subjects with a median (range) age at death of 92 (63–108) y. The majority (72%) were female.
The primary form of vitamin D detected in human brain tissue was 25(OH)D3. Although detected, D3 and 1,25(OH)2D3 were below the assay LOD in 22% and 58% of participants, respectively (Table 1). The geometric mean of the mean 25(OH)D3 concentration in the 4 brain regions in brains stored for 1.1–6.0 y did not differ from the 25(OH)D3 in brains stored ≤6.0 y (all P ≥ 0.37). However, the geometric mean of the mean 25(OH)D3 concentration in brains stored >6.0 y was significantly lower than that in the brains stored ≤1.0 y (P ≤ 0.003) (Figure 1A). When vitamin D supplement use was controlled for (using the 330 participants for whom information on supplement use was available), the association between mean 25(OH)D3 and storage time did not appreciably change (Supplemental Table 1). The brains stored 6.1–8.0 y had significantly less 25(OH)D3 than the brains stored ≤1.0 y.
TABLE 1.
Vitamin D and vitamin K concentrations in 4 brain regions of older adults in the MAP study1
| AWS | CR | MT | MF | Mean | % ND2 | |
|---|---|---|---|---|---|---|
| 25(OH)D3, pmol/g | 0.9 (ND-4.9) | 1.1 (ND-7.1) | 1.1 (ND-6.4) | 1.1 (ND-11.4) | 1.1 (ND-5.5) | 0 |
| 1,25(OH)2D3, pmol/g | ND (ND-0.8) | ND (ND-0.5) | ND (ND-0.3) | ND (ND-0.3) | ND (ND-0.3) | 58 |
| D3, pmol/g | 0.1 (ND-69.6) | 0.2 (ND-18.7) | 0.1 (ND-21.0) | 0.2 (ND-20.9) | 0.2 (ND-24.6) | 22 |
| MK4, pmol/g | 0.6 (ND-42.8) | 1.4 (ND-78.3) | 1.3 (ND-43.6) | 1.3 (ND-52.8) | 1.2 (ND-47.8) | 3 |
| Phylloquinone, pmol/g | ND (ND-4.8) | ND (ND-1.8) | ND (ND-2.1) | ND (ND-1.7) | ND (ND-2.3) | 83 |
Data are the median (range) of the mean concentrations of the 4 regions, n = 499 subjects. The LODs are as follows: 25(OH)D3: 0.1 pmol/g; 1,25(OH)2D3: 0.06 pmol/g; D3: 0.06 pmol/g; MK4: 0.1 pmol/g; and phylloquinone: 0.1 pmol/g. 1,25(OH)2D3, 1,25-dihydroxycholecalciferol; 25(OH)D3, 25-hydroxycholecalciferol; AWS, anterior watershed white matter; CR, cerebellum; D₃, cholecalciferol; LOD, lower limit of detection; MAP, Memory and Aging Project; MF, midfrontal cortex; MK4, menaquinone-4; MT, midtemporal cortex.
Percentage of participants with nondetectable (below the assay LOD) concentrations.
FIGURE 1.
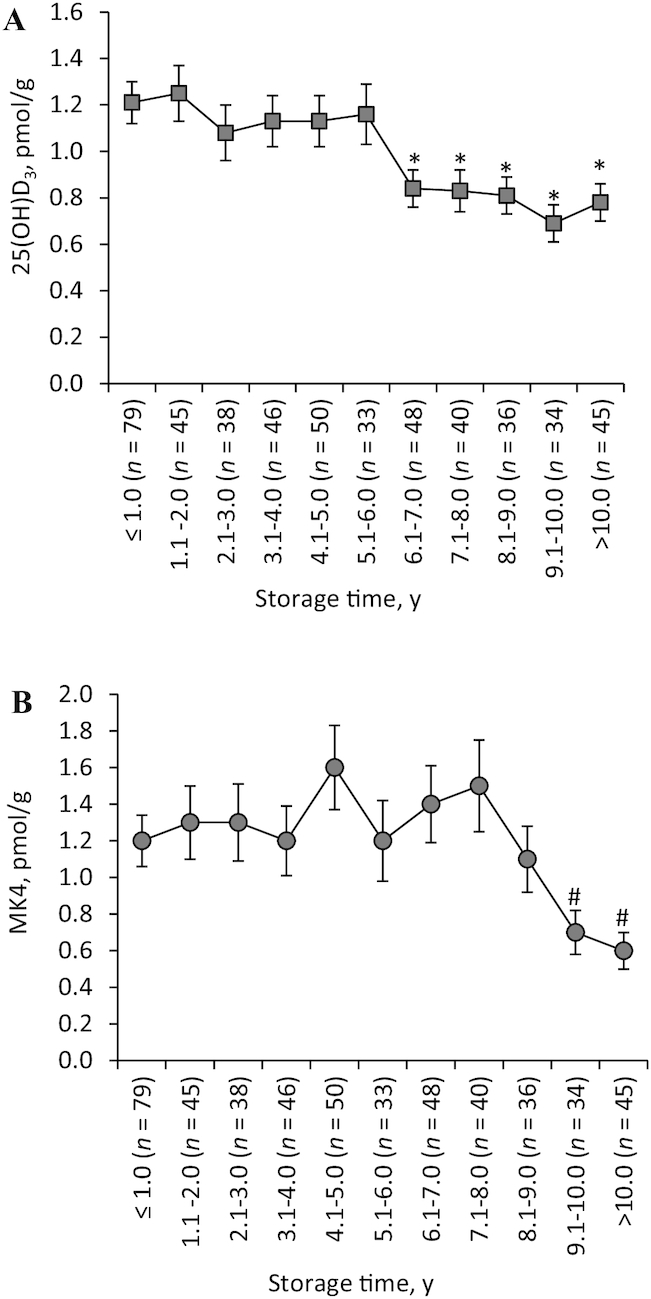
Brain 25(OH)D3 (A) and MK4 (B) concentrations across 4 regions of brains stored at -80°C for various periods of time from older adults in the MAP study. Values are geometric mean of the 4 brain regions means ± SEM. Significantly different from reference group (≤1.0 y storage time): *P ≤ 0.003; #P ≤ 0.001. 25(OH)D3, 25-hydroxycholecalciferol; MAP, Memory and Aging Project; MK4, menaquinone-4.
MK4 was the primary form of vitamin K detected in human brain. Phylloquinone was below the LOD in >80% of participants (Table 1). The geometric mean of the mean MK4 concentration in the 4 brain regions in brains stored for 1.1–9.0 y did not differ from the MK4 in brains stored ≤1.0 y (all P ≥ 0.11). However, the geometric mean MK4 concentration in the brains stored ≥9.0 y was significantly lower than the concentration in the brains stored ≤1.0 y (P ≤ 0.012) (Figure 1B). Similar results were obtained when the associations of 25(OH)D3 and MK4 with storage time were evaluated in the 4 regions separately (Supplemental Tables 2 and 3). Adjustment for age at death and sex did not change the results (data not shown).
The brain 25(OH)D3 and MK4 concentrations were positively correlated across all 4 regions, respectively, with and without adjustment for storage time (all Spearman ρ ≥0.79, P < 0.001) (Table 2). In the brains stored ≤6.0 y, the 25(OH)D3 concentrations in the MT, MF, and CR regions were similar, whereas the 25(OH)D3 in the AWS was significantly lower than in the other 3 regions (P < 0.001). In the brains stored ≤9.0 y, the MK4 concentrations in the MT, MF, and CR regions were similar, but the MK4 concentration in the AWS was significantly lower than concentrations in the other 3 regions (Table 3).
TABLE 2.
Spearman rank order correlations of 25(OH)D3 and MK4 between brain regions of older adults in the MAP study1
| 25(OH)D3 | MK4 | |||
|---|---|---|---|---|
| Unadjusted | Adjusted for storage time | Unadjusted | Adjusted for storage time | |
| AWS-CR | 0.89 | 0.89 | 0.79 | 0.80 |
| AWS-MF | 0.91 | 0.90 | 0.83 | 0.82 |
| AWS-MT | 0.90 | 0.89 | 0.82 | 0.82 |
| CR-MF | 0.93 | 0.92 | 0.87 | 0.88 |
| CF-MT | 0.94 | 0.94 | 0.89 | 0.89 |
| MF-MT | 0.95 | 0.94 | 0.90 | 0.90 |
All correlations are significant at P < 0.001. n = 499 subjects. 25(OH)D3, 25-hydroxycholecalciferol; AWS, anterior watershed; CR, cerebellum; MAP, Memory and Aging Project; MF, midfrontal cortex; MK4, menaquinone-4; MT, mid-temporal cortex.
TABLE 3.
25(OH)D3 and MK4 concentrations compared among 4 brain regions1
| 25(OH)D3, pmol/g store ≤6 y (n = 292) | MK4, pmol/g stored ≤9 y (n = 415) | |
|---|---|---|
| AWS | 1.0 (0.04)a | 0.6 (0.04)a |
| CR | 1.2 (0.04)b | 1.3 (0.08)b |
| MT | 1.2 (0.04)b | 1.2 (0.08)b |
| MF | 1.2 (0.04)b | 1.2 (0.08)b |
Values are geometric mean (SEM). Labeled means in a column without a common letter differ, P < 0.001. 25(OH)D3, 25-hydroxycholecalciferol; AWS, anterior watershed; CR, cerebellum; MF, midfrontal cortex; MK4, menaquinone-4; MT, midtemporal cortex.
Discussion
The goal of this study was to evaluate the association of freezer-storage time with vitamin D and vitamin K concentrations, 2 nutrients mechanistically linked to ADRD and cognitive decline (2, 5, 6, 8, 9, 21) in human brain tissue. The concentrations of 25(OH)D3, the primary form of vitamin D detected in human brain, were significantly lower in brains stored >6 y than in brains stored ≤1.0 y, suggesting that 25(OH)D3 was stable in samples stored frozen at -80°C for up to 6 y. The concentrations of MK4, the primary form of vitamin K detected in human brain, were significantly lower in brains stored >9.0 y than in brains stored ≤1.0 y, suggesting MK4 was stable in samples stored frozen at -80°C for up to 9 y. These findings highlight the importance of considering freezer storage time in the design and interpretation of studies using stored brain tissue samples to link brain concentrations of these vitamin metabolites to neurodegenerative diseases.
To the best of our knowledge, this is the first report on the association of freezer storage time with vitamin D and vitamin K concentrations in human brain tissue. In contrast to our findings in brain, 25(OH)D3 is reported to be stable in serum for up to 24 y stored frozen at −25°C and multiple freeze–thaw cycles (22–24). Unpublished data from our laboratory indicate that plasma phylloquinone is stable for ≥12 y in samples frozen at −80°C. The stability of nutrients in brain tissue may differ from stability in circulation because most nutrients are transported in serum/plasma bound to protein or lipoproteins. The brain is also a lipid-rich organ with barrier mechanisms required for normal functioning. Although the transport and storage of nutrients in brain tissue is not well understood, transport and metabolic considerations of nutrients could account for differences in the stability during freezer storage.
Our finding that MK4 was the predominant form of vitamin K in human brain is consistent with previous studies (6, 7). Although phylloquinone is the primary dietary and circulating form of vitamin K, its conversion to MK4 in brain tissue has been confirmed by use of rodent models and stable isotopes (25, 26). However, the human brain MK4 concentrations in our study were approximately 3–4 pmol/g lower than those reported by others (6, 7). This discrepancy may be related to study participant characteristics and/or analytical differences. We found that when a C18 column was used for separation of compounds of interest (18), there were coeluting peaks interfering with the MK4 peak on the chromatograms. When we modified the separation method to use a C30 column, the coeluting peaks that interfered with the MK4 peak were eliminated. The C30 column is designed for high-resolution separation of hydrophobic, long-chain compounds, such as MK4, and, based on our findings, is recommended for analysis of vitamin K forms in brain tissue. In a recently published study, serum phylloquinone was positively associated with cognitive function, but the MK4 brain concentration was not (6, 7). However, because the brain tissue samples used in this study were stored for >10 y before analysis, it is possible that the brain MK4 degraded during the storage time, which may have affected the results.
The 25(OH)D3 and MK4 concentrations were highly correlated among the 4 brain regions analyzed, respectively. However, the concentrations of both vitamin forms were significantly lower in the AWS than in the MF, MT, and CR. The AWS region sampled contained all white matter, whereas the other regions sampled contained grey matter. Little is known about the distribution of these nutrients in white or grey matter or in different brain regions. The AWS may be particularly susceptible to vascular diseases and vascular risk factors (27), whereas the MF and MT cortexes are the neocortical regions most commonly affected by AD pathology (28, 29). Additional research is needed to clarify the differential roles, if any, of nutrients in different brain regions.
Important strengths of this study are the large sample size and state-of-the-art laboratory methodology used to quantify the vitamin D and K forms in 4 separate regions of the human brain. Because 25(OH)D3 and MK4 were detected in nearly all of the brains analyzed, we were able to evaluate the effect of freezer storage time on these vitamin forms. A large number of participants had brain vitamin D3, 1,25(OH)2D3, and phylloquinone concentrations below the assay LOD, which challenged our ability to evaluate how long-term storage affects these vitamin forms. However, the likelihood of having concentrations of vitamin D3, 1,25(OH)2D3, and phylloquinone below the LOD did not appear to differ with storage time (data not shown). Information on vitamin D supplement use was not available for participants whose brains were stored for >8 y. However, our data suggest that vitamin D supplement use did not confound our main results. Information on vitamin K supplement use was not available on our participants. However, shifts in vitamin K supplement use would be less likely to affect the results because vitamin K supplement use has remained stable in the United States since 1999 (20). Circulating measures of vitamin D status and vitamin K status are also not currently available in MAP, which limits our understanding of how nutritional status of MAP participants is associated with the vitamin D and vitamin K brain concentrations.
In conclusion, the results of this study indicate concentrations of 25(OH)D3 and MK4 in human brain samples from older adults stored at −80°C are affected by duration of storage time. The design and interpretation of any research focused on elucidating the roles of these nutrients in neurodegenerative disease using banked brain tissue samples need to consider how long the samples have been stored. Moreover, as the use of stored brain tissue in translational ADRD research increases (16), it will be important to evaluate the stability of other biochemical measures over long-term freezer storage to ensure scientific rigor.
Supplementary Material
Acknowledgments
We acknowledge the critical contributions of the late Martha Clare Morris to the design of this study. The authors’ responsibilities were as follows—SLB: designed research; XF, GGD, WBP: conducted the research; MKS: performed statistical data analysis; XF, MKS: wrote the manuscript; TMH, JAS: provided essential materials; SLB: had primary responsibility for the final content; and all authors: read and approved the final manuscript.
Notes
Supported by the National Institute on Aging R01 AG051641 and the USDA Agricultural Research Service under Cooperative Agreement No. 58-1950-7-707. Any opinions, findings, conclusions, or recommendations expressed in this publication are those of the authors and do not necessarily reflect the views of the USDA.
Author disclosures: The authors report no conflicts of interest.
Supplemental Table 1–3 and supplemental method are available from the “Supplementary Data” link in the online posting of the article and from the same link in the online table of contents at https://academic.oup.com/jn.
Abbreviations used: 1,25(OH)2D3, 1,25-dihydroxycholecalciferol; 25(OH)D3, 25-hydroxycholecalciferol; ADRD, Alzheimer disease and related dementias; AWS: anterior watershed white matter; CR, cerebellum; D₃, cholecalciferol; LOD, lower limit of detection; MAP, Memory and Aging Project; MF, midfrontal cortex; MK4, menaquinone-4; MT, mid-temporal cortex; RSD, relative standard deviation; VDR, vitamin D receptor.
Contributor Information
Xueyan Fu, Jean Mayer US Department of Agriculture Human Nutrition Research Center on Aging at Tufts University, Boston, MA, USA.
M Kyla Shea, Jean Mayer US Department of Agriculture Human Nutrition Research Center on Aging at Tufts University, Boston, MA, USA.
Gregory G Dolnikowski, Jean Mayer US Department of Agriculture Human Nutrition Research Center on Aging at Tufts University, Boston, MA, USA.
William B Patterson, Jean Mayer US Department of Agriculture Human Nutrition Research Center on Aging at Tufts University, Boston, MA, USA.
Bess Dawson-Hughes, Jean Mayer US Department of Agriculture Human Nutrition Research Center on Aging at Tufts University, Boston, MA, USA.
Thomas M Holland, Rush Institute for Healthy Aging, Rush University, Chicago, IL, USA.
Julie A Schneider, Rush Institute for Healthy Aging, Rush University, Chicago, IL, USA.
Sarah L Booth, Jean Mayer US Department of Agriculture Human Nutrition Research Center on Aging at Tufts University, Boston, MA, USA.
References
- 1. Cui X, Gooch H, Petty A, McGrath JJ, Eyles D. Vitamin D and the brain: genomic and non-genomic actions. Mol Cell Endocrinol. 2017;453:131–43. [DOI] [PubMed] [Google Scholar]
- 2. Fu X, Dolnikowski GG, Patterson WB, Dawson-Hughes B, Zheng T, Morris MC, Holland TM, Booth SL. Determination of Vitamin D and its metabolites in human brain using an ultra-pressure LC-tandem mass spectra method. Curr Dev Nutr. 2019;3:nzz074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. King H, Rosenheim O, Webster TA. Vitamin D from sterols of mummified Egyptian brain. Biochem J. 1929;23:166–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Brown J, Bianco JI, McGrath JJ, Eyles DW. 1,25-dihydroxyvitamin D3 induces nerve growth factor, promotes neurite outgrowth and inhibits mitosis in embryonic rat hippocampal neurons. Neurosci Lett. 2003;343:139–43. [DOI] [PubMed] [Google Scholar]
- 5. Brewer LD, Thibault V, Chen KC, Langub MC, Landfield PW, Porter NM. Vitamin D hormone confers neuroprotection in parallel with downregulation of L-type calcium channel expression in hippocampal neurons. J Neurosci. 2001;21:98–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Thijssen HH, Drittij-Reijnders MJ. Vitamin K status in human tissues: tissue-specific accumulation of phylloquinone and menaquinone-4. Br J Nutr. 1996;75:121–7. [DOI] [PubMed] [Google Scholar]
- 7. Tanprasertsuk J, Ferland G, Johnson MA, Poon LW, Scott TM, Barbey AK, Barger K, Wang XD, Johnson EJ. Concentrations of circulating phylloquinone, but not cerebral menaquinone-4, are positively correlated with a wide range of cognitive measures: exploratory findings in centenarians. J Nutr. 2020;150:82–90. [DOI] [PubMed] [Google Scholar]
- 8. Ferland G. Vitamin K and the nervous system: an overview of its actions. Adv Nutr. 2012;3:204–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sundaram KS, Fan JH, Engelke JA, Foley AL, Suttie JW, Lev M. Vitamin K status influences brain sulfatide metabolism in young mice and rats. J Nutr. 1996;126:2746–51. [DOI] [PubMed] [Google Scholar]
- 10. Presse N, Shatenstein B, Kergoat MJ, Ferland G. Low vitamin K intakes in community-dwelling elders at an early stage of Alzheimer's disease. J Am Diet Assoc. 2008;108:2095–9. [DOI] [PubMed] [Google Scholar]
- 11. Shatenstein B, Kergoat MJ, Reid I. Poor nutrient intakes during 1-year follow-up with community-dwelling older adults with early-stage Alzheimer dementia compared to cognitively intact matched controls. J Am Diet Assoc. 2007;107:2091–9. [DOI] [PubMed] [Google Scholar]
- 12. Chouet J, Ferland G, Feart C, Rolland Y, Presse N, Boucher K, Barberger-Gateau P, Beauchet O, Annweiler C. Dietary vitamin K intake is associated with cognition and behaviour among geriatric patients: The CLIP Study. Nutrients. 2015;7:6739–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Feart C, Helmer C, Merle B, Herrmann FR, Annweiler C, Dartigues JF, Delcourt C, Samieri C. Associations of lower vitamin D concentrations with cognitive decline and long-term risk of dementia and Alzheimer's disease in older adults. Alzheimers Dement. 2017;13:1207–16. [DOI] [PubMed] [Google Scholar]
- 14. Morris MC, Wang Y, Barnes LL, Bennett DA, Dawson-Hughes B, Booth SL. Nutrients and bioactives in green leafy vegetables and cognitive decline: prospective study. Neurology. 2018;90:e214–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Chai B, Gao F, Wu R, Dong T, Gu C, Lin Q, Zhang Y. Vitamin D deficiency as a risk factor for dementia and Alzheimer's disease: an updated meta-analysis. BMC Neurol. 2019;19:284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. NCRAD History and Mission [cited 2020 9/9/2020] [Internet]; Available from: https://ncrad.iu.edu/. [Google Scholar]
- 17. Bennett DA, Schneider JA, Buchman AS, Barnes LL, Boyle PA, Wilson RS. Overview and findings from the Rush Memory and Aging Project. Curr Alzheimer Res. 2012;9:646–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Davidson KW, Sadowski JA. Determination of vitamin K compounds in plasma or serum by high-performance liquid chromatography using postcolumn chemical reduction and fluorimetric detection. Methods Enzymol. 1997;282:408–21. [DOI] [PubMed] [Google Scholar]
- 19. Fu X, Peterson JW, Hdeib M, Booth SL, Grusak MA, Lichtenstein AH, Dolnikowski GG. Measurement of deuterium-labeled phylloquinone in plasma by high-performance liquid chromatography/mass spectrometry. Anal Chem. 2009;81:5421–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kantor ED, Rehm CD, Du M, White E, Giovannucci EL. Trends in dietary supplement use among US adults from 1999–2012. JAMA. 2016;316:1464–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Fernandes de Abreu DA, Eyles D, Feron F. Vitamin D, a neuro-immunomodulator: implications for neurodegenerative and autoimmune diseases. Psychoneuroendocrinology. 2009;34(Suppl 1):S265–77. [DOI] [PubMed] [Google Scholar]
- 22. Agborsangaya C, Toriola AT, Grankvist K, Surcel HM, Holl K, Parkkila S, Tuohimaa P, Lukanova A, Lehtinen M. The effects of storage time and sampling season on the stability of serum 25-hydroxy vitamin D and androstenedione. Nutr Cancer. 2009;62:51–7. [DOI] [PubMed] [Google Scholar]
- 23. Antoniucci DM, Black DM, Sellmeyer DE. Serum 25-hydroxyvitamin D is unaffected by multiple freeze-thaw cycles. Clin Chem. 2005;51:258–61. [DOI] [PubMed] [Google Scholar]
- 24. Borai A, Khalil H, Alghamdi B, Alhamdi R, Ali N, Bahijri S, Ferns G. The pre-analytical stability of 25-hydroxyvitamin D: storage and mixing effects. J Clin Lab Anal. 2020;34:e23037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Al Rajabi A, Booth SL, Peterson JW, Choi SW, Suttie JW, Shea MK, Miao B, Grusak MA, Fu X. Deuterium-labeled phylloquinone has tissue-specific conversion to menaquinone-4 among Fischer 344 male rats. J Nutr. 2012;142:841–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Okano T, Shimomura Y, Yamane M, Suhara Y, Kamao M, Sugiura M, Nakagawa K. Conversion of phylloquinone (vitamin K1) into menaquinone-4 (vitamin K2) in mice: two possible routes for menaquinone-4 accumulation in cerebra of mice. J Biol Chem. 2008;283:11270–9. [DOI] [PubMed] [Google Scholar]
- 27. Minkner K, Lovblad KO, Yilmaz H, Alimenti A, Sekoranja L, Delavelle J, Sztajzel R, Rufenacht DA. White matter lesions in watershed territories studied with MRI and parenchymography: a comparative study. Neuroradiology. 2005;47:425–30. [DOI] [PubMed] [Google Scholar]
- 28. Bennett DA, Schneider JA, Bienias JL, Evans DA, Wilson RS. Mild cognitive impairment is related to Alzheimer disease pathology and cerebral infarctions. Neurology. 2005;64:834–41. [DOI] [PubMed] [Google Scholar]
- 29. Yang FM, Grigorenko A, Tommet D, Farias ST, Mungas D, Bennett DA, Jones RN, Crane PK. AD pathology and cerebral infarctions are associated with memory and executive functioning one and five years before death. J Clin Exp Neuropsychol. 2013;35:24–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


