ABSTRACT
Background
There is a paucity of evidence regarding the role of food timing on cardiometabolic health and weight loss in adults.
Objectives
To determine whether late eating is cross-sectionally associated with obesity and cardiometabolic risk factors at baseline; and whether late eating is associated with weight loss rate and success following a weight loss intervention protocol. Also, to identify obesogenic behaviors and weight loss barriers associated with late eating.
Methods
Participants were recruited from a weight-loss program in Spain. Upon recruitment, the midpoint of meal intake was determined by calculating the midway point between breakfast and dinner times, and dietary composition was determined from diet recall. Population median for the midpoint of meal intake was used to stratify participants into early (before 14:54) and late (after 14:54) eaters. Cardiometabolic and satiety hormonal profiles were determined from fasting blood samples collected prior to intervention. Weekly weight loss and barriers were evaluated during the ∼19-wk program. Linear and logistic regression models were used to assess differences between late and early eaters in cardiometabolic traits, satiety hormones, obesogenic behaviors, and weight loss, adjusted for age, sex, clinic site, year of recruitment, and baseline BMI.
Results
A total of 3362 adults [mean (SD): age: 41 (14) y; 79.2% women, BMI: 31.05 (5.58) kg/m2] were enrolled. At baseline, no differences were observed in energy intake or physical activity levels between early and late eaters (P >0.05). Late eaters had higher BMI, higher concentrations of triglycerides, and lower insulin sensitivity compared with early eaters (all P <0.05) prior to intervention. In addition, late eaters had higher concentrations of the satiety hormone leptin in the morning (P = 0.001). On average, late eaters had an average 80 g lower weekly rate of weight loss [early, 585 (667) g/wk; late, 505 (467) g/wk; P = 0.008], higher odds of having weight-loss barriers [OR (95% CI): 1.22 (1.03, 1.46); P = 0.025], and lower odds of motivation for weight loss [0.81 (0.66, 0.99); P = 0.044] compared with early eaters.
Conclusion
Our results suggest that late eating is associated with cardiometabolic risk factors and reduced efficacy of a weight-loss intervention. Insights into the characteristics and behaviors related to late eating may be useful in the development of future interventions aimed at advancing the timing of food intake.
Keywords: chrono-nutrition, food timing, weight loss, cardiometabolic profiles, leptin, ghrelin, late eating
See corresponding editorial on page 5.
Introduction
The timing of food intake is emerging as an important predictor of overall health and a relevant risk factor for obesity in adults (1). Epidemiological evidence in healthy adults indicates that later food times is associated with higher odds of being overweight or obese (2, 3), decreased efficacy of weight-loss interventions (4), and increased risk of other adverse cardiometabolic health outcomes (5). The molecular mechanism linking late eating to adverse health outcomes is likely multifactorial and may be partly attributed to its contribution to circadian misalignment (6, 7), a state where environmental and behavioral cycles are out of synchrony with the endogenous circadian system (8). Indeed, previous studies have shown that acute circadian misalignment in the form of jet lag, shift work, and late meal timing, adversely impacts energy balance and glycemic control (7, 9–12).
With the growing public interest in understanding food timing as a novel dimension of nutrition and health, there remains a paucity of consistent evidence regarding its relation to obesity, cardiometabolic risk factors, and weight-loss success, particularly from large studies. Some findings suggest that the effect of late eating on obesity and health is mediated by its contribution to a higher energy intake (13, 14). However, the relevance of timing of food intake as an independent risk factor is supported by investigations of night shift workers who consume equivalent energy density diets as non-night shift workers yet have a higher risk of metabolic alterations (15, 16). Further confirmation of the adverse effect of late eating is critical for devising individualized food timing health-promoting strategies.
In addition, there remains large uncertainty in the characteristics and behaviors of late eaters. Early identification of obesogenic behaviors and anticipation of potential weight-loss barriers may improve the efficacy of weight-loss trials in vulnerable populations. For example common weight-loss barriers identified in other populations that may be targeted include stress eating, eating when bored, and overall low motivation to lose weight (17, 18). Recently, we described environmental and genetic factors that may contribute to late eating (1), but the exact characterization of late eaters undergoing weight loss remains unknown.
Our previous study in 420 adults demonstrated that late eating was associated with lower weight loss (4). Here, we aim to expand our earlier findings in a larger, nonoverlapping cohort of 3362 adults to determine whether late eating is cross-sectionally associated with obesity and cardiometabolic risk factors upon enrollment (i.e., baseline); and whether late eating is associated with weight-loss rate and success following a weight-loss intervention protocol. Furthermore, we aim to identify obesogenic behaviors and weight-loss barriers associated with late eating, which may promote persistent obesity, weight-loss failure, and future weight regain.
Methods
Participants
Participants were overweight or obese adults who were non-night shift workers from the Obesity, Nutrigenetics, Timing, and Mediterranean study (ONTIME; clinicaltrials.gov: NCT02829619) recruited across 6 weight-loss clinics in Spain. Participants were excluded if receiving treatment with thermogenic or lipogenic drugs; undergoing treatment with anxiolytic or antidepressant drugs; or having diabetes mellitus, chronic renal failure, hepatic diseases, or cancer diagnosis; diagnosed with bulimia or prone to binge eating. Participation was voluntary and conditional on written informed consent. All data collection procedures have been approved by the Committee of Research Ethics of the University of Murcia and were in accordance with good clinical practice.
All participants followed a standardized weight-loss program (Garaulet method©), which has been previously described (19). Briefly, participants attended 1 weekly 60-min therapy session in small support groups (n = 10 per group) during a weight-loss intervention period followed by a 5-mo maintenance period. The duration of the weight-loss intervention varied according to weight-loss goal [mean (95% CI): 18.7 (18.2, 19.3) wk duration]. The program was led by certified nutritionists and was based on the Mediterranean diet. Dietary energy was limited to 1200 to 1800 kcal per day for women and 1500 to 2000 kcal per day for men to induce an approximate loss of 0.5 to 1 kg per week, in order to achieve a total weight loss of 5–10% of the initial weight (20).
Dietary intake timing and composition
Upon enrollment (i.e., baseline), participants were asked, “On weekdays (or weekends), at what time do you usually have breakfast (lunch or dinner)?” and responses were in 30-min increments. Weighted weekly average of breakfast and dinner times were computed with 5/7 weighting for weekdays and 2/7 weighting for weekends. Midpoint of meal intake was determined by calculating the midpoint between the weekly averages for breakfast and dinner times (21, 22). Participants were then dichotomized by the population median into early (midpoint before 14:54) and late (midpoint after 14:54) eaters.
In addition, dietary intake was assessed using a single 24-h dietary recall where participants indicated type, quantity, and preparation of each recorded eating episode. Recalls were conducted from Monday through Friday and thus captured weekends (Sunday) and weekdays (from Monday to Thursday). Frequency of eating was determined as the count of eating episode reported during the 24-h period. In addition, 24-h and per meal energy intake and macronutrient composition were determined using the nutritional evaluation software program Nutrilet, based on Spanish food composition tables (23). From the 24-h dietary recalls, the intake of several food groups and dietary scores were also estimated including a Mediterranean Diet Score that is comprised of 9 food groups: 1) vegetables and greens; 2) pulses (lentils, beans, chickpeas, and peas); 3) fruit and nuts; 4) dairy products (milk, yogurt, and cheese); 5) cereals; 6) meat, meat derivatives, and eggs; 7) fish; 8) fat quality index: MUFAs to SFAs ratio; and 9) alcohol (24, 25). Other investigated food groups included soft drinks, which included carbonated and sweetened beverages.
Sleep traits, morningness–eveningness preference, and physical activity
Sleep duration was estimated by the following questions: “On weekdays (or weekends), at what time do you usually go to bed?” and “On weekdays (or weekends), at what time do you usually get up in the morning?” Sleep duration was determined as the difference between bed and wake times. As no participants worked irregular shifts, weighted weekly sleep duration was calculated as: [(weekday sleep duration × 5) + (weekend sleep duration × 2)]/7. In addition, the following question was asked regarding the number of awakenings during nocturnal sleep: “On weekdays (or weekends), how many times do you usually wake-up during the night?” and a weighted weekly awakenings per night was calculated as: [(weekday number of awakenings × 5) + (weekend number of awakenings × 2)]/7.
To define participants’ diurnal preference as morning or evening chronotypes, participants also completed the 19-item scale Morningness–Eveningness Questionnaire (MEQ) (26). MEQ scores were used to classify participants as: evening types when scores were <53 points, morning types when scores were >64 points, or intermediate types when scores were within the range of 53–64 points. In addition, participants completed the International Physical Activity Questionnaire that assesses physical activity over the past 7 d. This questionnaire has been previously validated in a Spanish population, and good correlation was obtained with accelerometer-derived physical activity estimates (27, 28). A total activity score capturing intensity and timing was calculated as Metabolic Units (METs) in minutes per week.
Body composition and cardiometabolic profiles
Participants were weighed upon enrollment (i.e., baseline) on a digital body weighing scale to the nearest 0.1 kg while barefoot and wearing light clothing at a clinical center. Height was assessed using a Harpenden digital stadiometer (with a rank of 0.7 to 2.05) while standing in a relaxed upright position with their head oriented in the Frankfurt plane. BMI was calculated as weight (kg)/height2 (m), and obesity was defined as BMI ≥30 kg/m2. In addition, waist circumference was measured at the level of the umbilicus. Body fat composition was determined with bioelectrical impedance, using the TANITA TBF-300 equipment (Tanita Corporation of America).
Participants were asked to fast for 8 h for their baseline visit and blood was collected between 08:00 and 08:30. Fasting glucose was determined in serum with the glucose oxidase method (29). Plasma concentrations of triglycerides, total cholesterol, and HDL cholesterol were determined using standard commercial kits (Roche Diagnostics GmbH), whereas LDL was estimated by the Friedewald equation. Using fasting glucose and insulin measures, HOMA-IR was calculated using the standard formula: fasting glucose (mmol/L) × fasting insulin (mIU/L)/22.5 (30). Arterial systolic and diastolic blood pressures were measured with a mercury sphygmomanometer. Morning appetite was also determined via survey with the question: “How is your appetite during the first hour after waking up in the morning?” with responses “very low,” “low,” “high,” and “very high.” Furthermore, in a subset of participants (n = 685), satiety and hunger hormones, leptin and ghrelin, were measured by radioimmunoassay (LincoResearch).
Weight-loss rate and weight-loss barriers survey
Weight loss was measured weekly during the intervention at the clinical center. Weight-loss rate was determined as the average grams of body weight lost per week during the intervention period. Weight-loss success was defined as those participants who had not dropped out of the weight-loss program and had lost ≥7% of their initial body weight or achieved a cumulative total weight loss of 5.8 kg (median) by the end of the intervention period, which varied across participants, and before starting the maintenance period.
In addition, during the fifth week of the weight-loss intervention, participants completed the Barriers to Weight Loss survey (19). The optional survey consisted of 29 questions across 6 sections (Supplemental Table 1): 1) meal recording, weight control, and weekly interviews; 2) eating habits; 3) portion size; 4) food and drink choice; 5) way and timing of eating; and 6) other obstacles to weight loss. Questions had 3 possible responses: “never,” “sometimes,” and “always,” which corresponded to specific points (Supplemental Table 1). A higher cumulative score generally reflects more barriers to weight loss. Scores were dichotomized to presence (score ≥1) or absence (score <1) of weight-loss barriers. Furthermore, weight-loss motivation was assessed with the question: “Have you lost your motivation to lose weight?” and dichotomized to yes/no.
Statistical analysis
Nonnormally distributed variables including fasting insulin, HOMA-IR, leptin, and ghrelin, were logarithmically transformed. Baseline analyses included all 3362 enrolled participants and weight-loss analyses were limited to the first 19 wk of treatment (mean duration of the weight-loss intervention period) and restricted to 2119 participants who had completed the weight-loss intervention. Linear regression models were performed to assess differences between late and early eaters in their baseline BMI (primary outcome) and other adiposity measures, cardiometabolic traits, and satiety hormones. Analyses were adjusted for age, sex, clinic site, year of recruitment, and BMI (for all outcomes except BMI). In sensitivity analyses, models were then further adjusted for the following potential confounders separately: meal frequency, dietary factors (soft drinks and dairy products), sleep duration, or MEQ score. Results are presented as β-coefficients and their corresponding 95% CIs. Moreover, logistic regression models were fitted to estimate the ORs and 95% CIs of late eating with 1) morning appetite (low compared with high), 2) baseline obesity (obese category compared with normal weight) and weight-loss success, and 3) obesogenic behaviors and dichotomized barriers score and motivation. Similarly, models were adjusted for age, sex, clinic site, year of recruitment, and baseline BMI (for all outcomes except obesity), and further adjusted for potential confounders in sensitivity analyses. We further performed analysis of covariance to assess differences between early and late eaters in their dietary intake, adjusted for age, sex, clinic site, and year of recruitment. Statistical analyses were conducted using SPSS 20.0 software (SPSS, IBM) and the STATA 15 statistical software (Stata Corporation). A 2-tailed P-value of <0.05 was considered statistically significant.
Results
Baseline characteristics and lifestyle traits
A total of 3362 overweight and obese adults [mean (SD): age: 41 y (14)(range 18, 84); 79.2% women, BMI: 31.0 (5.6) kg/m2; 51.4% obese] were enrolled (Table 1). The average clock times for breakfast, lunch, and dinner were 08:31, 14:36, and 21:21, respectively, and lunch comprised the highest relative energy density (39%), followed by dinner (28%) and breakfast (18%) (Table 1). The population median midpoint of intake was ∼15:00. As expected, late eaters reported having all meals at a later clock time than early eaters (all P <0.05), with the largest difference of over 1 h observed for the timing of breakfast followed by dinner with a 0.5-h difference. Except for soft drinks and dairy product intake, no other differences were evident between early and late eaters in dietary amount and quality and in the total and per meal energy density and macronutrient composition (Table 1, Supplemental Table 2).
TABLE 1.
Baseline characteristics and lifestyle traits of participants (n = 3362)
| Characteristic | Total | Early eaters (n = 1670) | Late eaters (n = 1692) | P value |
|---|---|---|---|---|
| Sex, % female | 79.2 | 77.5 | 80.8 | 0.02 |
| Age, y | 40.7 ± 13.5 | 41.2 ± 12.9 | 40.2 ± 14.1 | 0.02 |
| BMI, kg/m2 | 31.0 ± 5.6 | 30.7 ± 5.3 | 31.4 ± 5.9 | 0.001 |
| Dietary traits | ||||
| Energy intake, kcal | 1931.6 ± 706.5 | 1934.0 ± 709.3 | 1929.3 ± 703.9 | 0.99 |
| Protein, g | 82.6 ± 31.9 | 83.2 ± 32.7 | 81.9 ± 31.2 | 0.58 |
| Fat, g | 86.9 ± 45.8 | 86.1 ± 45.9 | 87.8 ± 45.7 | 0.29 |
| Carbohydrates, g | 197.1 ± 87.0 | 197.8 ± 86.2 | 196.1 ± 87.9 | 0.42 |
| Meal frequency | 4.1 ± 0.8 | 4.1 ± 0.8 | 4.0 ± 0.8 | <0.001 |
| Morning appetite, % high or very high | 40.8 | 45.3 | 35.8 | <0.001 |
| Meal timing | ||||
| Breakfast | 08:31 ± 0:58 | 07:51 ± 0:37 | 09:11 ± 0:49 | <0.001 |
| Lunch | 14:36 ± 0:34 | 14:32 ± 0:34 | 14:39 ± 0:33 | <0.001 |
| Dinner | 21:21 ± 0:37 | 21:02 ± 0:31 | 21: 39 ± 0:34 | <0.001 |
| Midpoint of intake | 14:56 ± 0:37 | 14:27 ± 0:20 | 15:25 ± 0:25 | <0.001 |
| Meal energy density, % total | ||||
| Breakfast | 18.3 ± 11.8 | 17.8 ± 11.5 | 18.9 ± 12.2 | 0.12 |
| Second breakfast | 10.1 ± 10.5 | 11.3 ± 10.4 | 8.8 ± 10.5 | <0.001 |
| Lunch | 38.8 ± 13.9 | 38.3 ± 13.4 | 39.3 ± 14.3 | 0.30 |
| Evening | 7.9 ± 10.0 | 7.6 ± 9.9 | 8.2 ± 10.0 | 0.43 |
| Dinner | 28.3 ± 13.5 | 28.0 ± 13.5 | 28.7 ± 13.5 | 0.28 |
| Sleep traits | ||||
| MEQ score | 52.4 ± 9.7 | 54.5 ± 9.3 | 50.2 ± 9.5 | <0.001 |
| Bedtime | 23:52 ± 0:52 | 23:34 ± 0:48 | 0:13 ± 0:50 | <0.001 |
| Waketime | 07:28 ± 0:54 | 07:06 ± 0:42 | 7:52 ± 0:56 | <0.001 |
| Sleep duration, h | 7.73 ± 1.02 | 7.65 ± 0.96 | 7.81 ± 1.07 | <0.001 |
| Nocturnal awakenings, n | 2.03 ± 1.13 | 1.93 ± 1.03 | 2.14 ± 1.22 | 0.004 |
| Physical activity level, METs | 3774.5 (5858.2) | >3807.2 (5145.4) | >3742.6 (6479.4) | 0.05 |
Values are mean ± SD. P values are derived from the Kruskall–Wallis test for all variables except sex (chi-square test) and BMI and age (student's t-test). Significant values are represented in bold. MEQ, Morningness–Eveningness Questionnaire; METs = Metabolic Equivalents.
Late eaters had lower MEQ scores (reflecting more evening preference) compared with early eaters. In addition, late eaters reported ∼39 min later bedtime, ∼46 min later wake time, ∼9 min longer sleep duration, and 0.21 higher number of nocturnal awakenings than early eaters (all P <0.05) (Table 1). Physical activity levels were similar between late and early eaters (Table 1).
Baseline adiposity and cardiometabolic profiles
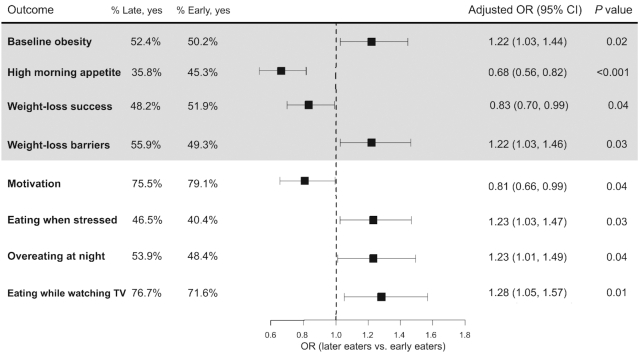
Adiposity measures, cardiometabolic disease risk factors, and morning satiety hormone concentrations differed between late and early eaters at baseline and prior to weight-loss intervention. Late eaters had a significantly higher baseline BMI (β-coefficient 0.80 kg/m 2; 95% CI: 0.44, 1.16 ; P value <0.001) (Table 2) and 22% [OR (95% CI): 1.22 (1.03, 1.44); P value = 0.02] higher odds of being obese compared with early eaters (Figure 1). In addition, body fat percentage (β-coefficient 0.47%; 95% CI: 0.04, 0.80; P value = 0.03) and waist circumference (β-coefficient 1.62 cm; 95% CI: 0.73, 2.50; P value <0.001) were higher in late compared with early eaters (Table 2). In sensitivity analyses, the associations observed for BMI and waist circumference did not change upon further adjustment for diet- and sleep-related covariates, however, the association for body fat percentage was attenuated (Table 3).
TABLE 2.
Cross-sectional associations between late eating and baseline adiposity traits, cardiometabolic traits, and satiety hormones in 3362 participants1
| Late versus early eaters | ||||
|---|---|---|---|---|
| Characteristic | Early eaters | Late eaters | β2 (95% CI) | P value |
| Adiposity traits | ||||
| BMI, kg/m2 | 30.7 ± 5.3 | 31.4 ± 5.9 | 0.80 (0.44, 1.16) | <0.001 |
| Body fat, % | 36.4 ± 6.7 | 37.1 ± 6.9 | 0.47 (0.04, 0.80) | 0.03 |
| Waist circumference, cm | 101.2 ± 14.8 | 102.3 ± 15.3 | 1.62 (0.73, 2.50) | <0.001 |
| Cardiometabolic traits | ||||
| Fasting glucose, mg/dL | 87.3 ± 15.5 | 87.1 ± 17.2 | 0.72 (−0.46, 1.90) | 0.23 |
| Fasting insulin,3 mIU/L | 7.3 ± 6.4 | 7.8 ± 7.0 | 0.03 (0.01, 0.05) | 0.005 |
| HOMA IR,3 | 1.66 ± 1.71 | 1.75 ± 1.87 | 0.03 (0.01, 0.06) | 0.003 |
| Triglycerides, mg/dL | 99.6 ± 52.7 | 103.1 ± 52.2 | 5.60 (1.67, 9.52) | 0.005 |
| Total cholesterol, mg/dL | 193.5 ± 37.9 | 190.0 ± 35.7 | −1.03 (−3.88, 1.83) | 0.48 |
| LDL-C, mg/dL | 116.2 ± 32.6 | 113.6 ± 31.2 | −1.40 (−3.90, 1.09) | 0.27 |
| HDL-C, mg/dL | 57.7 ± 15.1 | 56.1 ± 14.6 | −0.71 (−1.77, 0.35) | 0.19 |
| Systolic blood pressure, mmHg | 117.1 ± 14.6 | 116.9 ± 14.8 | 0.002 (−0.10, 0.11) | 0.97 |
| Diastolic blood pressure, mmHg | 72.8 ± 9.96 | 72.3 ± 10.3 | −0.02 (−0.10, 0.04) | 0.53 |
| Satiety hormone | ||||
| Leptin,3,4 ng/mL | 18.1 ± 13.7 | 20.2 ± 14.1 | 0.06 (0.03, 0.10) | 0.001 |
| Ghrelin,3,4 ng/mL | 1084.0 ± 1033.9 | 1077.5 ± 1008.5 | −0.02 (−0.06, 0.01) | 0.23 |
Values are mean ± SD.
Data are presented as mean difference (95% CI) for late eaters compared with early eaters derived from linear regression models. Significant values are represented in bold. Models are adjusted for age, sex, clinic site, year of recruitment, and BMI (except for adiposity outcomes).
Trait log transformed in analytical model.
n = 685.
FIGURE 1.
Significant associations between late eating and baseline obesity (n = 3362), weight-loss success at the end of the weight-loss program (n = 2119) and barriers and obesogenic behaviors determined from the Barriers to Weight Loss survey at the fifth week of treatment (n = 2154). Data are presented as OR (95% CI) for late eaters compared with early eaters. Logistic regression models are adjusted for age, sex, clinic site, year of recruitment, and baseline BMI (except for baseline obesity outcome).
TABLE 3.
Sensitivity analyses for the cross-sectional associations between late eating with baseline adiposity traits, cardiometabolic traits, and satiety hormones further adjusting for potential confounders: meal frequency, dietary factors, sleep duration, and morningness–eveningness score
| Model 1 | Model 2 | Model 3 | Model 4 | |||||
|---|---|---|---|---|---|---|---|---|
| Characteristic | β (95% CI) | P value | β (95% CI) | P value | β (95% CI) | P value | β (95% CI) | P value |
| Adiposity traits | ||||||||
| BMI, kg/m2 | 0.59 (0.14, 1.03) | 0.01 | 0.84 (0.39, 1.29) | <0.001 | 0.80 (0.40, 1.19) | <0.001 | 0.61 (0.15, 1.07) | 0.009 |
| Body fat, % | 0.28 (−0.24, 0.79) | 0.29 | 0.44 (−0.10, 0.97) | 0.11 | 0.35 (−0.12, 0.81) | 0.15 | 0.29 (−0.25, 0.82) | 0.30 |
| Waist circumference, cm | 1.35 (0.27, 2.43) | 0.01 | 1.69 (0.59, 2.79) | 0.003 | 1.53 (0.57, 2.49) | 0.002 | 1.01 (−0.11, 2.13) | 0.08 |
| Cardiometabolic traits | ||||||||
| Fasting glucose, mg/dL | 0.38 (−1.00, 1.76) | 0.59 | 0.83 (−0.61, 2.28) | 0.26 | 1.02 (−0.34, 2.39) | 0.14 | 0.59 (−0.79, 1.97) | 0.40 |
| Fasting insulin,1 mIU/L | 0.03 (0.006, 0.05) | 0.02 | 0.02 (−0.004, 0.05) | 0.10 | 0.02 (0.002, 0.05) | 0.03 | 0.02 (−0.004, 0.05) | 0.10 |
| HOMA-IR1 | 0.03 (0.006, 0.06) | 0.02 | 0.03 (−0.0003, 0.06) | 0.05 | 0.03 (0.007, 0.06) | 0.01 | 0.02 (−0.003, 0.05) | 0.09 |
| Triglycerides, mg/dL | 5.53 (0.90, 10.2) | 0.02 | 5.40 (0.23, 10.6) | 0.04 | 8.20 (3.72, 12.69) | <0.001 | 1.78 (−3.06, 6.62) | 0.47 |
| Total cholesterol, mg/dL | −1.69 (−5.07, 1.69) | 0.33 | −1.22 (−4.88, 2.45) | 0.51 | −0.07 (−3.26, 3.12) | 0.97 | −1.77 (−5.31, 1.78) | 0.33 |
| LDL-C, mg/dL | −1.97 (−4.93, 0.99) | 0.19 | −1.14 (−4.35, 2.07) | 0.48 | −1.49 (−4.29, 1.30) | 0.30 | −2.01 (−5.12, 1.09) | 0.20 |
| HDL-C, mg/dL | −0.56 (−1.85, 0.72) | 0.39 | −1.21 (−2.59, 0.17) | 0.09 | −0.42 (−1.62, 0.78) | 0.49 | 0.06 (−1.28, 1.39) | 0.93 |
| Systolic blood pressure, mmHg | −0.01 (−0.13, 0.11) | 0.83 | −0.01 (−0.14, 0.12) | 0.87 | −0.02 (−0.12, 0.09) | 0.77 | 0.04 (−0.09, 0.17) | 0.52 |
| Diastolic blood pressure, mmHg | −0.04 (−0.13, 0.04) | 0.29 | −0.03 (−0.12, 0.06) | 0.50 | −0.02 (−0.10, 0.06) | 0.63 | −0.01 (−0.10, 0.08) | 0.79 |
| Satiety hormone | ||||||||
| Leptin,1,2 ng/mL | 0.06 (0.009, 0.11) | 0.02 | 0.04 (−0.02, 0.10) | 0.16 | 0.03 (−0.03, 0.08) | 0.35 | 0.06 (0.02, 0.11) | 0.008 |
| Ghrelin,1,2 ng/mL | 0.006 (−0.04, 0.05) | 0.79 | 0.0008 (−0.04, 0.04) | 0.97 | −0.0009 (−0.04, 0.04) | 0.96 | −0.02 (−0.06, 0.02) | 0.43 |
Data are presented as mean difference (95% CI) for late eaters compared with early eaters derived from linear regression models. Significant values are represented in bold. All models are adjusted for age, sex, clinic site, year of recruitment, and BMI (except for adiposity outcomes), then further adjusted for additional covariates: model 1 further adjusted for meal frequency; model 2 further adjusted for dietary factors (soft drinks and dairy products); model 3 further adjusted for sleep duration; and model 4 further adjusted for MEQ score.
Trait log transformed in analytical model.
n = 685.
MEQ = Morningness–Eveningness Questionnaire.
At baseline, late eaters had higher fasting insulin (β-coefficient 0.03 mIU/L; 95% CI: 0.01, 0.05; P value = 0.005), HOMA-IR (β-coefficient 0.03; 95% CI: 0.01, 0.06; P value = 0.003), and triglycerides (β-coefficient 5.60 mg/dL; 95% CI: 1.67, 9.52; P value = 0.005) compared with early eaters (Table 2). No differences were observed in fasting glucose and blood pressure (Table 2). In a subset of 685 participants with data on appetite hormones, late eaters also had higher concentrations of the satiety hormone leptin in the morning compared with early eaters, but not the hunger hormone ghrelin (Table 2). In sensitivity analyses, differences in cardiometabolic risk factors and morning leptin concentrations remained significant after accounting for sleep- and diet-related covariates, but not after accounting for MEQ score (Table 3). Consistent with measured satiety hormones, compared with early eaters, late eaters reported being less likely to have a high appetite in the morning [OR (95% CI): 0.68 (0.56, 0.82); P <0.001] (Figure 1).
Weight-loss rate and weight-loss barriers
Among 2119 participants who completed the weight-loss intervention, weight-loss success was 17% lower among late eaters compared with early eaters [OR (95% CI): 0.83 (0.70, 0.99); P = 0.04] (Figure 1). On average, participants lost 537 (570) g per week, and late eaters had an average 80 g lower weekly rate of weight loss than early eaters [weekly weight loss: early, 585 (667) g; late, 505 (467) g; P = 0.008] (Figure 2). This weight-loss rate accounted for 1.520 kg less weight loss at 19 wk of the intervention. Each hourly delay in the midpoint of food intake was associated with 49 (21) g less weight loss per week.
FIGURE 2.
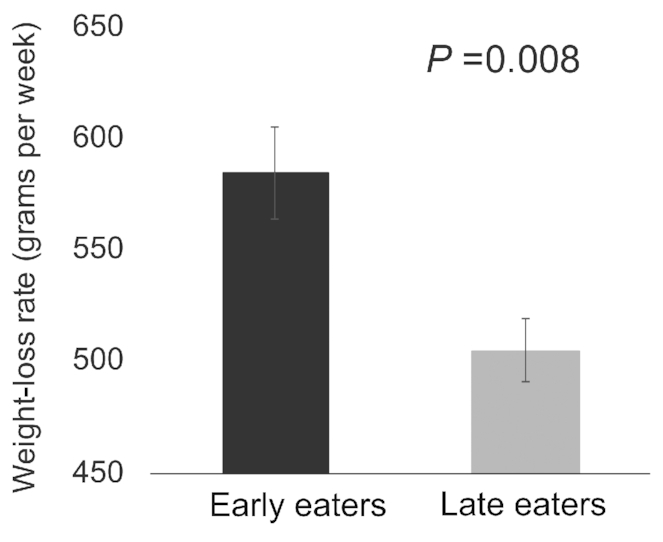
Average weekly weight-loss rate (in grams per week) in early and late eaters at the end of the weight-loss program (n = 2119). Data are mean and SE of the mean for early and late eaters. P value is from the student's t-test.
Based on responses from 2154 participants who completed the optional Barriers to Weight Loss survey, overall, late eaters had higher odds of facing weight-loss barriers [OR (95% CI): 1.22 (1.03, 1.46); P = 0.03] and lower odds of motivation for weight loss [OR (95% CI): 0.81 (0.66, 0.99); P = 0.04] compared with early eaters (Figure 1). Specifically, late eaters had higher odds of eating when stressed, overeating at night, and eating while watching TV, compared with early eaters (Figure 1). In sensitivity analyses, effect estimates remained largely unchanged following further adjustment for other potential confounders (Supplemental Table 3).
Discussion
In this large cohort of 3362 adults, we found that prior to weight-loss intervention, late eaters had higher baseline BMI, higher concentrations of triglycerides, and lower insulin sensitivity compared with early eaters. These associations remained significant after accounting for several lifestyle traits except for individual chronotype (MEQ score), an important determinant of food timing (1). Among 2119 participants who completed the weight-loss intervention, we found that late eaters had lower success and a lower weekly average rate of weight loss compared with early eaters. In assessing the characteristics of late eaters, we identified that late eaters were likely to have an evening chronotype and lower morning appetite. In addition, we observed that late eating was associated with several obesogenic behaviors that may hinder weight-loss success.
Our findings are consistent with human studies that have previously shown that late food timing is associated with obesity (31), hyperglycemia (32), dyslipidemia (33, 34), insulin sensitivity (35), and metabolic syndrome (36). We also found that the associations between late eating and higher concentrations of triglycerides and lower insulin sensitivity were independent of BMI. Furthermore, we identified no differences in total energy intake and several dietary components, and physical activity levels between late and early eaters, except for the intake of soft drink and dairy products, providing additional evidence to food timing as an independent risk factor and a novel dimension for cardiometabolic health.
The lower weight-loss success rate of 80 g less body weight per week in late eaters compared with early eaters is comparable to previous smaller weight-loss trials, including postbariatric surgery patients (4, 37). Although the weekly difference may appear small, it accounts for ∼1.5 kg difference in weight loss at the end of the treatment program. Presented differently, each hourly delay in the midpoint of meal intake was associated with ∼50 g less weight loss per week. Thus, a 3-h delay in the midpoint of meal intake resulting from delayed breakfast and dinner timing (i.e., 07:00 to 10:00 breakfast and 20:00 to 23:00 dinner), for instance, may contribute ≤ ∼150 g per week lower weight loss.
As expected, late eaters presented more obesogenic behaviors and other characteristics known to act as barriers for successful weight loss, likely impeding weight-loss success. Obesogenic behaviors included being prone to stress-related eating and eating at night while watching TV (38). Stress increases weight-loss trial attrition and is a risk factor for weight gain (19). In addition, when stressed, palatable foods may become increasingly rewarding (39, 40). Late eaters were also more likely to be less motivated, a critical characteristic that may hamper the initiation and maintenance of any behavior change, including maintaining a healthy diet (41, 42). Thus, future weight-loss strategies designed for late eaters should target perceived weight-loss barriers through motivational interviews or cognitive behavioral therapies (43). Late eating in the present study was also associated with higher concentrations of morning leptin contributing to low morning appetite. Thus, behavioral therapies may be focused on advancing dinner timing, rather than other meals (21), in order to enhance appetite in the next morning. The attenuation of the association between late eating and higher morning leptin by sleep duration and dietary factors points towards potential mechanisms mediating this relation.
Despite our large sample size and comprehensive cardiometabolic and lifestyle evaluation of our participants, our study has some limitations. In the present study, timings of eating episodes were recalled in response to 2 questions at baseline and may be susceptible to various biases and inaccuracies. In addition, we dichotomized our population into early and late eaters based on the midpoint of food intake. This approach differs from other studies that based their classification on the temporality of energy intake distribution (44, 45) or on the timing of consuming 50% of daily calories (7). Analogous to sleep midpoint [the midway point between sleep onset and wake-up times that reflects overall sleep timing (46)], the midpoint of meal intake is a heritable trait (64% heritability) that aims to reflect overall timing of meals (21). This metric shows strong phenotypic association with MEQ score (22), and substantial shared genetic architecture, and likely common biological pathways, with sleep timing and chronotype (21). In order to allow future comparisons across studies and enable pooled analyses across datasets, it is necessary to establish consensus with regards to the most suitable and scalable approach to quantify food timing. Despite our covariate adjustment for several sleep- and diet-related traits, we recognize that a general weakness of observational studies is a risk of bias due to residual confounding. Finally, genetic variants relevant to food timing were unaccounted for in our analyses, and future studies should investigate their specific role in the associations between late eating and obesity (21, 47).
Overall, our findings show that late meal timing was associated with reduced efficacy of weight-loss intervention, which is consistent with the notion that advancing the timing of meal intake may serve as a novel strategy for effective weight management (35, 48, 49). Insights into the characteristics and obesogenic behaviors related to late eating may be useful in the development of future individualized and successful weight-loss interventions.
Supplementary Material
ACKNOWLEDGEMENTS
The authors’ contributions were as follows–––the study was designed by HSD, PGA, JQ, AE, EM, FAJLS, and MG; PGA, EM, and MG: participated in acquisition and analysis of data; HSD, PGA, JQ, AE, EM, FAJLS, and MG: participated in interpretation of data; HSD, EM, and MG: wrote the manuscript and all co-authors reviewed, edited, read and approved the final manuscript. MG: is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. The authors report no conflicts of interest. FAJLS received speaker fees from Bayer Healthcare, Sentara Healthcare, Philips, Kellogg Company, Vanda Pharmaceuticals, and Pfizer.
Notes
Supported in part by The Spanish Government of Investigation, Development and Innovation (SAF2017-84135-R) including FEDER cofunding; The Autonomous Community of the Region of Murcia through the Seneca Foundation (20795/PI/18) and NIH (grant R01DK105072) granted to MG. JQ was supported in part by the American Diabetes Association (award 1-17-PDF-103) and by the NIH (grant R01DK102696). FAJLS was supported in part by the NIH (grants R01HL118601, R01DK099512, R01DK102696, R01DK105072, and R01HL140574). EM was supported by a Miguel Servet II grant (CPII19/00019) awarded by the Instituto de Salud Carlos III (ISCIII), Spanish Ministry of Science, Innovation and Universities, including FEDER cofunding. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Supplemental Tables 1–3 and Supplemental Figure 1 are available from the “Supplementary data” link in the online posting of the article and from the same link in the online table of contents at https://academic.oup.com/ajcn/.
Data described in the manuscript, code book, and analytic code will not be made available because approval has not been granted by study participants.
Abbreviations used: MEQ, Morningness-Eveningness Questionnaire; ONTIME, Obesity, Nutrigenetics, Timing, and Mediterranean study.
Contributor Information
Hassan S Dashti, Center for Genomic Medicine, Massachusetts General Hospital, Boston, MA, USA; Broad Institute, Cambridge, MA, USA; Department of Anesthesia, Critical Care and Pain Medicine, Massachusetts General Hospital and Harvard Medical School, Boston, MA, USA.
Puri Gómez-Abellán, Department of Physiology, University of Murcia, Murcia, Spain; IMIB-Arrixaca, Murcia, Spain.
Jingyi Qian, Medical Chronobiology Program, Division of Sleep and Circadian Disorders, Departments of Medicine and Neurology, Brigham and Women's Hospital, Boston, MA, USA; Division of Sleep Medicine, Department of Medicine, Harvard Medical School, Boston, MA, USA.
Alberto Esteban, Department of Physiology, University of Murcia, Murcia, Spain; IMIB-Arrixaca, Murcia, Spain.
Eva Morales, Department of Public Health Sciences, University of Murcia, Murcia, Spain; IMIB-Arrixaca, Murcia, Spain.
Frank A J L Scheer, Medical Chronobiology Program, Division of Sleep and Circadian Disorders, Departments of Medicine and Neurology, Brigham and Women's Hospital, Boston, MA, USA; Division of Sleep Medicine, Department of Medicine, Harvard Medical School, Boston, MA, USA.
Marta Garaulet, Department of Physiology, University of Murcia, Murcia, Spain; IMIB-Arrixaca, Murcia, Spain; Medical Chronobiology Program, Division of Sleep and Circadian Disorders, Departments of Medicine and Neurology, Brigham and Women's Hospital, Boston, MA, USA.
References
- 1. Dashti HS, Scheer F, Saxena R, Garaulet M. Timing of food intake: identifying contributing factors to design effective interventions. Adv Nutr. 2019;10:606–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hillyard TR, Mitchell CO, Crase DR, Clemens LE, Slawson DL, Watson JM. The effect of timing of food intake on body fatness. J Am Diet Assoc. 1999;99:A122. [Google Scholar]
- 3. Okada C, Imano H, Muraki I, Yamada K, Iso H. The association of having a late dinner or bedtime snack and skipping breakfast with overweight in Japanese women. J Obes. 2019;2019:2439571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Garaulet M, Gómez-Abellán P, Alburquerque-Béjar JJ, Lee Y-C, Ordovás JM, Scheer F. Timing of food intake predicts weight loss effectiveness. Int J Obes (Lond). 2013;37:604–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Maury E. Off the clock: from circadian disruption to metabolic disease. Int J Mol Sci. 2019;; 20:1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wehrens SMT, Christou S, Isherwood C, Middleton B, Gibbs MA, Archer SN, Skene DJ, Johnston JD. Meal timing regulates the human circadian system. Curr Biol. 2017;27:1768–1775.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. McHill AW, Phillips AJ, Czeisler CA, Keating L, Yee K, Barger LK, Garaulet M, Scheer FA, Klerman EB. Later circadian timing of food intake is associated with increased body fat. Am J Clin Nutr. 2017;106:ajcn161588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Morris CJ, Yang JN, Garcia JI, Myers S, Bozzi I, Wang W, Buxton OM, Shea SA, Scheer F. Endogenous circadian system and circadian misalignment impact glucose tolerance via separate mechanisms in humans. Proc Natl Acad Sci USA. 2015;112:E2225–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Garaulet M, Gómez-Abellán P. Timing of food intake and obesity: a novel association. Physiol Behav. 2014;134:44–50. [DOI] [PubMed] [Google Scholar]
- 10. Broussard JL, Cauter E V. Disturbances of sleep and circadian rhythms: novel risk factors for obesity. Curr Opin Endocrinol Diabetes Obes. 2016;23:353–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Qian J, Scheer F. Sex-dependent link between circadian misalignment and adiposity. Nat Rev Endocrinol. 2020;16:13–5. [DOI] [PubMed] [Google Scholar]
- 12. Qian J, Morris CJ, Caputo R, Garaulet M, Scheer F. Ghrelin is impacted by the endogenous circadian system and by circadian misalignment in humans. Int J Obes (Lond). 2019;43:1644–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Baron KG, Reid KJ, Horn LV, Zee PC. Contribution of evening macronutrient intake to total caloric intake and body mass index. Appetite. 2013;60:246–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Baron KG, Reid KJ, Kern AS, Zee PC. Role of sleep timing in caloric intake and BMI. Obesity. 2011;19:1374–81. [DOI] [PubMed] [Google Scholar]
- 15. Chellappa SL, Vujovic N, Williams JS, Scheer F. Impact of circadian disruption on cardiovascular function and disease. Trends Endocrinol Metab. 2019;30:767–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Mason IC, Qian J, Adler GK, Scheer F. Impact of circadian disruption on glucose metabolism: implications for type 2 diabetes. Diabetologia. 2020;63:462–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Arasu A, Moran LJ, Robinson T, Boyle J, Lim S. Barriers and facilitators to weight and lifestyle management in women with polycystic ovary syndrome: general practitioners’ perspectives. Nutrients. 2019;11:1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Sharifi N, Mahdavi R, Ebrahimi-Mameghani M. Perceived barriers to weight loss programs for overweight or obese women. Heal Promot Perspect. 2013;3:11–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Corbalán MD, Morales EM, Canteras M, Espallardo A, Hernández T, Garaulet M. Effectiveness of cognitive-behavioral therapy based on the Mediterranean diet for the treatment of obesity. Nutrition. 2009;25:861–9. [DOI] [PubMed] [Google Scholar]
- 20. NHLBI Obesity Education Initiative Expert Panel on the Identification E and T of O in A (US) Clinical Guidelines on the Identification, Evaluation, and Treatment of Overweight and Obesity in Adults. National Heart, Lung, and Blood Institute; 1998. [Google Scholar]
- 21. Lopez-Minguez J, Dashti HS, Madrid-Valero JJ, Madrid JA, Saxena R, Scheer FAJL, Ordoñana JR, Garaulet M. Heritability of the timing of food intake. Clin Nutr. 2019;38:767–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Vera B, Dashti HS, Gómez-Abellán P, Hernández-Martínez AM, Esteban A, Scheer F, Saxena R, Garaulet M. Modifiable lifestyle behaviors, but not a genetic risk score, associate with metabolic syndrome in evening chronotypes. Sci Rep. 2018;8:945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Mataix J, Mañas M, Llopis J, Martínez E. (Table of Composition of Spanish Foods) Tabla De Composición De Alimentos Españoles (in Spanish). 1995. [Google Scholar]
- 24. Tuny OM, Calle S, Torres JMÁ, Vives CC, Moreno ER, Moreiras GV. (Valoración de la dieta española de acuerdo al panel de consumo alimentario) Assessment of the Spanish diet according to the Food Consumption Panel (in Spanish). 2009. [Google Scholar]
- 25. Knoops KTB, de Groot LC, Fidanza F, Alberti-Fidanza A, Kromhout D, van Staveren WA. Comparison of three different dietary scores in relation to 10-year mortality in elderly European subjects: The HALE project. Eur J Clin Nutr. 2006;60:746–55. [DOI] [PubMed] [Google Scholar]
- 26. Horne JA, Ostberg O. A self-assessment questionnaire to determine morningness-eveningness in human circadian rhythms. Int J Chronobiol. 1976;4:97–110. [PubMed] [Google Scholar]
- 27. Roman-Viñas B, Serra-Majem L, Hagströmer M, Ribas-Barba L, Sjöström M, Segura-Cardona R. International physical activity questionnaire: reliability and validity in a Spanish population. Eur J Sport Sci. 2010;10:297–304. [Google Scholar]
- 28. Craig CL, Marshall AL, Sjöström M, Bauman AE, Booth ML, Ainsworth BE, Pratt M, Ekelund U, Yngve A, Sallis JF et al. International physical activity questionnaire: 12-country reliability and validity. Med Sci Sports Exerc. 2003;35:1381–95. [DOI] [PubMed] [Google Scholar]
- 29. Blaedel WJ, Uhl JM. Nature of materials in serum that interfere in the glucose oxidase-peroxidase-0-dianisidine method for glucose, and their mode of action. Clin Chem. 1975;21:119–24. [PubMed] [Google Scholar]
- 30. Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and β-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–9. [DOI] [PubMed] [Google Scholar]
- 31. Berg C, Lappas G, Wolk A, Strandhagen E, Torén K, Rosengren A, Thelle D, Lissner L. Eating patterns and portion size associated with obesity in a Swedish population. Appetite. 2009;52:21–6. [DOI] [PubMed] [Google Scholar]
- 32. Van Cauter E, Shapiro ET, Tillil H, Polonsky KS. Circadian modulation of glucose and insulin responses to meals: relationship to cortisol rhythm. Am J Physiol - Endocrinol Metab. 1992;262:E467–75. [DOI] [PubMed] [Google Scholar]
- 33. Yoshida J, Eguchi E, Nagaoka K, Ito T, Ogino K. Association of night eating habits with metabolic syndrome and its components: a longitudinal study. BMC Public Health. 2018;18:1366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Chen HJ, Chuang SY, Chang HY, Pan WH. Energy intake at different times of the day: its association with elevated total and LDL cholesterol levels. Nutr Metab Cardiovasc Dis. 2019;29:390–7. [DOI] [PubMed] [Google Scholar]
- 35. Bandín C, Scheer F, Luque AJ, Ávila-Gandía V, Zamora S, Madrid JA, Gómez-Abellán P, Garaulet M. Meal timing affects glucose tolerance, substrate oxidation and circadian-related variables: a randomized, crossover trial. Int J Obes (Lond). 2015;39:828–33. [DOI] [PubMed] [Google Scholar]
- 36. Kutsuma A, Nakajima K, Suwa K. Potential association between breakfast skipping and concomitant late-night-dinner eating with metabolic syndrome and proteinuria in the Japanese population. Scientifica. 2014;2014:253581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Ruiz-Lozano T, Vidal J, de Hollanda A, Scheer F, Garaulet M, Izquierdo-Pulido M. Timing of food intake is associated with weight loss evolution in severe obese patients after bariatric surgery. Clin Nutr. 2016;35:1308–14. [DOI] [PubMed] [Google Scholar]
- 38. Kim D, Hou W, Wang F, Arcan C. Factors affecting obesity and waist circumference among US adults. Prev Chronic Dis. 2019;16:180220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Widaman AM, Witbracht MG, Forester SM, Laugero KD, Keim NL. Chronic stress is associated with indicators of diet quality in habitual breakfast skippers. J Acad Nutr Diet. 2016;116:1776–84. [DOI] [PubMed] [Google Scholar]
- 40. Reutrakul S, Hood MM, Crowley SJ, Morgan MK, Teodori M, Knutson KL. The relationship between breakfast skipping, chronotype, and glycemic control in type 2 diabetes. Chronobiol Int. 2014;31:64–71. [DOI] [PubMed] [Google Scholar]
- 41. Bautista-Castaño I, Molina-Cabrillana J, Montoya-Alonso JA, Serra-Majem L. Variables predictive of adherence to diet and physical activity recommendations in the treatment of obesity and overweight, in a group of Spanish subjects. Int J Obes Relat Metab Disord. 2004;28:697–705. [DOI] [PubMed] [Google Scholar]
- 42. Lang A, Froelicher ES. Management of overweight and obesity in adults: behavioral intervention for long-term weight loss and maintenance. Eur J Cardiovasc Nurs. 2006;5:102–14. [DOI] [PubMed] [Google Scholar]
- 43. Armstrong MJ, Mottershead TA, Ronksley PE, Sigal RJ, Campbell TS, Hemmelgarn BR. Motivational interviewing to improve weight loss in overweight and/or obese patients: a systematic review and meta-analysis of randomized controlled trials. Obes Rev. 2011;12:709–23. [DOI] [PubMed] [Google Scholar]
- 44. Jakubowicz D, Barnea M, Wainstein J, Froy O. High caloric intake at breakfast vs. dinner differentially influences weight loss of overweight and obese women. Obesity (Silver Spring). 2013;21:2504–12. [DOI] [PubMed] [Google Scholar]
- 45. Versteeg RI, Ackermans MT, Nederveen AJ, Fliers E, Serlie MJ, la Fleur SE. Meal timing effects on insulin sensitivity and intrahepatic triglycerides during weight loss. Int J Obes (Lond). 2018;; 42:156–62. [DOI] [PubMed] [Google Scholar]
- 46. Duffy JF, Rimmer DW, Czeisler CA. Association of intrinsic circadian period with morningness-eveningness, usual wake time, and circadian phase. Behav Neurosci. 2001;115:895–9. [DOI] [PubMed] [Google Scholar]
- 47. Dashti HS, Merino J, Lane JM, Song Y, Smith CE, Tanaka T, McKeown NM, Tucker C, Sun D, Bartz TM et al. Genome-wide association study of breakfast skipping links clock regulation with food timing. Am J Clin Nutr. 2019;; 110:473–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Morris CJ, Garcia JI, Myers S, Yang JN, Trienekens N, Scheer F. The human circadian system has a dominating role in causing the morning/evening difference in diet-induced thermogenesis. Obesity (Silver Spring). 2015;23:2053–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Sutton EF, Beyl R, Early KS, Cefalu WT, Ravussin E, Peterson CM. Early time-restricted feeding improves insulin sensitivity, blood pressure, and oxidative stress even without weight loss in men with prediabetes. Cell. 2018;27:1212–1221.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.