ABSTRACT
Background
Serum 25-hydroxyvitamin D [25(OH)D] concentration is an indicator of vitamin D exposure, but it is also influenced by clinical characteristics that affect 25(OH)D production and clearance. Vitamin D is the precursor to 25(OH)D but is analytically challenging to measure in biological specimens.
Objectives
We aimed to develop and validate a liquid chromatography–tandem mass spectrometry (LC-MS/MS) method for quantification of vitamins D3 and D2 in serum and to explore the potential of circulating vitamin D as a biomarker of exposure in supplementation trials.
Methods
The method was validated using guideline C62-A from the Clinical and Laboratory Standards Institute and was applied in 2 pilot clinical trials of oral vitamin D3 supplementation. Pilot study 1 included 22 adults randomly assigned to placebo or 2000 IU/d. Blood was collected at baseline, 1, 3, 6, and 12 mo. Pilot study 2 included 15 adults randomly assigned to 2000 or 4000 IU/d. Blood and subcutaneous (SUBQ) adipose tissue were collected at baseline and 3 mo.
Results
In study 1, mean change (baseline to 3 mo) in serum vitamin D3 was −0.1 ng/mL in the placebo group and 6.8 ng/mL in the 2000 IU/d group (absolute difference: 6.9; 95% CI: 4.5, 9.3 ng/mL). In study 2, mean change (baseline to 3 mo) in serum vitamin D3 was 10.4 ng/mL in the 2000 IU/d group and 22.2 ng/mL in the 4000 IU/d group (fold difference: 2.15; 95% CI: 1.40, 3.37). Serum and adipose tissue vitamin D3 concentrations were correlated, and the dose-response of vitamin D3 in adipose mirrored that in serum.
Conclusions
We validated a sensitive, robust, and high-throughput LC-MS/MS method to quantify vitamins D3 and D2 in serum. Serum and SUBQ adipose tissue vitamin D3 concentrations increased proportionally to dose with 3 mo of daily supplementation.
These trials were registered at clinicaltrials.gov as NCT00552409 (pilot study 1) and NCT01477034 (pilot study 2).
Keywords: vitamin D, ergocalciferol, cholecalciferol, validation, Clinical and Laboratory Standards Institute, liquid-liquid extraction, liquid chromatography–tandem mass spectrometry
Introduction
Vitamin D occurs in 2 main forms, vitamin D3 (cholecalciferol) and vitamin D2 (ergocalciferol), and is an essential precursor to the steroid hormone 1,25-dihydroxyvitamin D [1,25(OH)2D]. Humans produce vitamin D3 in the epidermis upon exposure to UVB light and consume both forms (D3 and D2) in foods, beverages, and dietary supplements. Typical daily input from each of these routes and how this varies between individuals are unknown (1).
The first step in the activation of vitamin D is 25-hydroxylation to form 25-hydroxyvitamin D [25(OH)D]. This is catalyzed primarily by the cytochrome P450 family 2 subfamily R member 1 (CYP2R1) enzyme in the liver [the main site of 25(OH)D production] and to a lesser degree in other tissues (2). The enzyme CYP27B1 catalyzes hydroxylation of 25(OH)D to 1,25(OH)2D. CYP27B1 in the kidneys produces 1,25(OH)2D that acts in an endocrine fashion to help maintain extracellular calcium and phosphorus concentrations. Renal CYP27B1 is mainly regulated by parathyroid hormone and fibroblast growth factor 23, whereas in nonrenal cells, alternate mechanisms and availability of substrate dictate production of 1,25(OH)2D, which is then used locally (3, 4).
Serum 1,25(OH)2D concentration is held within a physiologic range and is not used to assess vitamin D nutritional status (4). Circulating 25(OH)D is used instead because it increases with UVB exposure and vitamin D intake and has a half-life of 2–3 wk (5). The parent vitamin D has a shorter plasma half-life (0.5–5 d) than 25(OH)D (6). This has limited clinical interest in circulating vitamin D and limited efforts to overcome technical challenges to its measurement. One of the few studies that assessed serum vitamin D concentration during supplementation indicated that it may respond linearly to the dose of vitamin D administered daily (7). This contrasts with the dose-response of serum 25(OH)D to vitamin D intake, which appears to be steeper at low doses and approach a plateau as dose increases (5). These observations raise the possibility that the serum vitamin D concentration may add value as a biomarker of vitamin D intake. Furthermore, parent vitamin D may have biological significance as a substrate for nonrenal cells and storage in adipose tissue (8), but limited availability of sensitive, specific, and high-throughput assays for vitamin D has made it difficult to address these hypotheses. Whereas liquid chromatography–tandem mass spectrometry (LC-MS/MS) is preferred and routine for measurement of serum 25(OH)D in clinical labs (9), only a few LC-MS/MS methods for vitamins D3 and/or D2 in serum or plasma have been described (10–14) and none were validated in a fashion expected of clinical laboratory methods.
We present the validation of a new LC-MS/MS method for quantifying vitamins D3 and D2 in human serum/plasma according to guideline C62-A from the Clinical and Laboratory Standards Institute (CLSI). We used the assay in 2 pilot clinical trials of vitamin D3 supplementation and here describe the serum vitamin D3 and 25(OH)D3 (25-hydroxycholecalciferol) responses to supplementation. One of these studies collected subcutaneous (SUBQ) adipose tissue, and we also report the concentrations of vitamin D3 and 25(OH)D3 in these tissue samples.
Methods
Study population
Specimens were collected during 2 pilot clinical trials of oral vitamin D3 supplementation conducted in Seattle, Washington. The Institutional Review Board at the University of Washington approved study procedures, and all participants provided written informed consent.
Pilot study 1 (NCT00552409) was conducted between March 2008 and June 2010 and was designed to obtain preliminary data on vitamin D3 supplementation for prevention of diabetic kidney disease. Eligibility was restricted to adults with type 2 diabetes, estimated glomerular filtration rate ≥60 mL · min−1 · 1.73 m−2, and serum 25(OH)D concentration <30 ng/mL (<75 nmol/L), who agreed to avoid use of nonstudy vitamin D supplements and tanning salons during the study period. Potential participants were identified through computerized queries at clinics within the University of Washington Medicine system. Investigational Drug Services at the University of Washington Medical Center generated the random sequence, allocated participants to treatment, and dispensed study supplements. Twenty-two participants were randomly assigned in blocks of 2, stratified by clinic of origin, to 12 mo of 2000 IU vitamin D3/d or matching placebo in a 1:1 ratio. Supplements (Carlson Laboratories) were dispensed every 3 mo, and adherence was monitored by pill counts when the pill bottles were exchanged at study visits. Participants and the study team were blinded to treatment assignment while the study was ongoing. Blood was collected at baseline and after 1, 3, 6, and 12 mo of supplementation. Supplemental Figure 1 depicts the pilot study 1 participant flow.
Pilot study 2 (NCT01477034) was conducted between January 2012 and June 2013 and was designed to obtain preliminary data on vitamin D3 supplementation for reduction of adipose tissue inflammation in obesity. Participants were recruited from the community. Eligibility was restricted to adults with a serum 25(OH)D concentration between 7 and 20 ng/mL (17.5 and 50 nmol/L), BMI ≥25 kg/m2, no history of recent or extreme weight change, and no chronic medical conditions, including diabetes. Eligible participants agreed to avoid use of nonstudy vitamin D supplements and tanning salons during the study period. The random allocation sequence of treatments “A” and “B” was generated by a study investigator. Study supplements (Carlson Laboratories) were supplied to 2 clinical research staff members unaffiliated with the study. The supplement bottles were labeled to conceal treatment identity, then returned to the study team. Seventeen participants were randomly assigned by study personnel to 3 mo of 2000 or 4000 IU vitamin D3/d in a 1:1 ratio. Study personnel dispensed the supplements to participants monthly and adherence was monitored by pill counts and participant daily logs. Participants and the study team were blinded to treatment assignment while the study was ongoing. Blood and SUBQ adipose tissue were collected at baseline and 3 mo. Adipose tissue was obtained by needle aspiration biopsy ∼50 mm lateral of the umbilicus. Two participants (1 from each arm) withdrew before the 3-mo visit, and their baseline data were excluded from analyses. Supplemental Figure 2 depicts the pilot study 2 participant flow.
Because both were pilot studies, neither was powered for formal hypothesis testing, and no results were published previously.
Quantification of vitamin D3 and vitamin D2 in serum
The Supplemental Methods provide a detailed description of the method used to quantify vitamins D3 and D2 in serum. Briefly, 200 µL of human serum samples, calibrators, or quality control material was mixed with 200 µL 1 M NaOH, incubated in a deep-well plate before spiking with 200 µL internal standards (d6-vitamin D3 and d6-vitamin D2 at 5 ng/mL in methanol), and then extracted with 1 mL 90:10 n-heptane:ethyl acetate (pilot study 1) or 1 mL 50:50 n-heptane:methyl tert-butyl ether (MTBE) (pilot study 2). The organic layer was removed from each well as described previously (15) and dried under nitrogen. The residue was derivatized with 100 μL 0.5 g/L 4-phenyl-1,2,4-triazoline-3,5-dione (PTAD) in acetonitrile. After 15 min, 100 μL LC/MS-grade water was added to quench the derivatizing agent.
Quantification of vitamin D3 and 25(OH)D3 in adipose tissue
The Supplemental Methods provide a detailed description of the method used to quantify vitamin D3 and 25(OH)D3 in adipose tissue. Briefly, saponification was performed by combining ∼10 mg SUBQ adipose tissue with 200 µL 0.9 g/dL saline and 200 µL 1 M NaOH in a deep-well plate, mixing on a vortex, and incubating at 95°C with agitation for 1 h. A volume of 200 µL internal standards in methanol was added before extraction with 1 mL 50:50 n-heptane:MTBE. The organic layer was removed from each well as described previously (15) and dried under nitrogen. To remove residual salts, this residue was reconstituted in 250 µL acetonitrile, transferred to a new plate, and redried. The resulting residue was derivatized with 50 µL 0.5 g/L PTAD in acetonitrile. After 15 min, 70 µL LC/MS-grade water was added to quench the derivatizing agent.
Chromatography and MS
For the quantification of vitamin D3 and 25(OH)D3 in adipose tissue, a volume of 40 µL derivatized extract was analyzed by LC-MS/MS as previously described (16), with the addition of transitions for vitamin D3 and its internal standard (listed in the Supplemental Methods). For the quantification of vitamins D3 and D2 in serum, a volume of 40 µL was analyzed by LC-MS/MS using a different reversed-phase liquid chromatographic column (1.9 μm × 50 mm × 2.1 mm, 140 Å Pentafluorophenyl-propyl (PFPP) ultra-high pressure liquid chromatography (UPLC) column; Restek). Three synonymous transitions were monitored for each analyte and internal standard to improve the precision of the assay (Supplemental Table 1). Instrument-specific cone and collision parameters were derived experimentally (Supplemental Table 2). The chromatographic method was not designed to separate R and S isomers of derivatized vitamins D3 and D2.
Calibration and quality control
Calibrators for the quantification of vitamin D in human serum contained vitamins D2 (Medical Isotopes) and D3 (Sigma Aldrich) at 0.096, 0.48, 0.96, 28.8, and 96 ng/mL. The 2 lowest-concentration calibrators were made using FBS (Gibco), whereas the remaining calibrators were made using MSG4000 (Golden West Biologicals). Calibrators for the quantification of vitamin D3 and 25(OH)D3 in adipose tissue contained 10, 25, 50, 100, 500, and 1000 pg of each analyte, which were prepared in methanol and spiked into a mixture of saponified adipose tissues.
Quantification of hydroxylated vitamin D metabolites in serum
Serum concentrations of 1,25(OH)2D2 and 1,25(OH)2D3 were quantified using immunoaffinity extraction and LC-MS/MS, and serum concentrations of 24,25-dihydroxyvitamin D3 [24,25(OH)2D3], 25(OH)D2, and 25(OH)D3 were quantified using liquid-liquid extraction and LC-MS/MS, as previously described (16).
Data analyses
Study outcomes included changes in serum and SUBQ adipose tissue vitamin D3 and 25(OH)D3 concentrations and bivariate associations among analyte concentrations. Bivariate associations were estimated with the Pearson correlation coefficient when both variables were normally distributed or the Spearman rank correlation coefficient when ≥1 variable was skewed. Within-person variation in serum vitamin D3 concentration and 25(OH)D3 concentration over time was estimated by computing intraclass correlation coefficients (ICCs) using repeated measurements from pilot study 1. Linear mixed-effects models with a random subject effect were used to estimate changes in analyte concentrations between baseline and 3 mo; contrasts were made between treatment groups within the same pilot study. Estimates and associated CIs for the change between baseline and 3 mo within group and for the absolute difference in change between groups were extracted from the linear mixed model; estimates and associated CIs for the fold difference in change between groups were obtained through bootstrap methods with 2000 replicates. Mixed models were restricted to the baseline to 3-mo period to facilitate comparison between the pilot studies and because results from pilot 1 indicated that serum vitamin D3 and 25(OH)D3 concentrations had reached a new steady state by 3 mo of supplementation. Serum vitamin D3 concentrations below the lower limit of the measuring interval (LLMI) [which included 11 baseline observations (5 in pilot study 1, 6 in pilot study 2) and 17 follow-up observations in the placebo group] were assigned a value of 0.07 ng/mL (half the LLMI). Analyses were conducted with R version 3.6.2 (R Foundation for Statistical Computing).
Results
Validation of the new method for quantification of vitamins D3 and D2 in serum included evaluation of specificity and selectivity, ion suppression, carryover, LLMI, linearity, imprecision, interference, and trueness according to CLSI guideline C62-A. The LLMI was selected to ensure that the assay could reliably quantify a doubling of the concentration (i.e., total imprecision <36%) and was determined to be 0.130 and 0.137 ng/mL for vitamins D2 and D3, respectively (Supplemental Tables 3, 4). There was minimal influence from background noise, common interferences in human samples (namely hyperproteinemia, hypertriglyceridemia, hyperbilirubinemia, uremia, and ex vivo hemolysis), and isobaric molecules (Supplemental Tables 5–7). By comparing samples drawn into different types of phlebotomy tubes, evaluating for ion suppression in T-infusion experiments, and assessing the parallelism of the response in sample-mixing and spike-recovery samples, the assay was shown to be free from significant matrix effects that could cause bias in the assay of individual samples (Supplemental Table 8, Supplemental Figures 3, 4). The analytes were determined to be stable in the primary samples during storage, after freezing and thawing of the primary samples, and while queued on the analyzer after sample preparation (Supplemental Tables 8–10). The assay was linear from the LLMI up to the highest calibrator tested, 130 ng/mL (Supplemental Figure 5).
Pilot 1 participants were aged 61 ± 11 y (mean ± SD) and mostly males (64%), with type 2 diabetes (Table 1). Pilot 2 participants were aged 35 ± 10 y and mostly females (73%), with overweight or obesity but no other chronic medical conditions (Table 1). Mean ± SD serum 25(OH)D3 concentration at baseline was 18 ± 8 ng/mL in pilot study 1 and 13 ± 4 ng/mL in pilot study 2. Mean serum vitamin D3 concentration at baseline was <1.3 ng/mL in all treatment groups. Mean serum vitamin D2 concentration was <0.1 ng/mL and mean serum 25(OH)D2 concentration was ≤2 ng/mL across groups and time points, indicating exposure to vitamin D2 was minimal throughout the studies. For this reason, concentrations of vitamin D2 and D2 metabolites are not reported hereafter. In pilot 1, serum calcium and phosphorus were within normal limits at baseline and were not affected by supplementation. Serum calcium and phosphorus concentrations were not measured in pilot 2.
TABLE 1.
Baseline characteristics of participants in 2 pilot clinical trials of vitamin D3 supplementation1
| Pilot 1 | Pilot 2 | |||
|---|---|---|---|---|
| Placebo | 2000 IU/d | 2000 IU/d | 4000 IU/d | |
| n | 11 | 11 | 8 | 7 |
| Age, y | 61.0 ± 13 | 60.6 ± 10 | 30.3 ± 9.5 | 40.3 ± 8.3 |
| Male sex | 7 (64) | 7 (64) | 2 (25) | 2 (29) |
| Race | ||||
| White/Caucasian | 6 (55) | 9 (82) | 7 (87) | 3 (43) |
| Black/African American | 1 (9) | 2 (18) | 1 (13) | 2 (29) |
| Asian | 2 (18) | None | None | 1 (14) |
| Hispanic/Latino | 1 (9) | None | None | 1 (14) |
| Native Hawaiian/Pacific Islander | 1 (9) | None | None | None |
| Serum 25(OH)D3, ng/mL | 20.2 ± 8.7 | 16.4 ± 7.2 | 12.5 ± 3.7 | 12.9 ± 4.6 |
| Serum vitamin D3, ng/mL | 0.42 [0.17–0.92] | 0.23 [0.13–0.50] | 0.17 [0.14–0.28] | 0.07 [0.07–0.32] |
| Serum 1,25(OH)2D3, pg/mL | 29.4 ± 15 | 32.6 ± 10 | 32.1 ± 5.8 | 35.0 ± 6.1 |
| Serum 24,25(OH)2D3, ng/mL | 2.68 ± 1.4 | 2.22 ± 1.6 | 1.01 ± 0.45 | 1.03 ± 0.72 |
| Serum PTH, pg/mL | 43.1 ± 19 | 60.4 ± 21 | 35.4 ± 10 | 51.4 ± 10 |
| BMI, kg/m2 | 35.3 ± 8.0 | 39.9 ± 8.7 | 31.8 ± 5.3 | 36.6 ± 7.6 |
| T2DM diagnosis | 11 (100) | 11 (100) | None | None |
Values are means ± SDs, n (%), or medians [IQRs] unless otherwise indicated. SI conversion factors: 25(OH)D3, 1 ng/mL = 2.5 nmol/L; vitamin D3, 1 ng/mL = 2.6 nmol/L; 24,25(OH)2D3, 1 ng/mL = 2.4 nmol/L; 1,25(OH)2D3, 1 pg/mL = 2.4 pmol/L. PTH, parathyroid hormone; T2DM, type 2 diabetes mellitus; 1,25(OH)2D3, 1,25-dihydroxyvitamin D3; 24,25(OH)2D3, 24,25-dihydroxyvitamin D3; 25(OH)D3, 25-hydroxyvitamin D3.
Figure 1 depicts the time courses of the serum vitamin D3 and 25(OH)D3 concentrations over 12 mo in pilot 1. In the vitamin D3 treatment group (2000 IU/d), serum vitamin D3 concentration increased and reached a plateau by 1 mo. Serum 25(OH)D3 concentration increased at a slower rate and reached a plateau at 3 mo. The ICCs of serum vitamin D3 and 25(OH)D3 were 0.51 and 0.63, respectively, in the placebo group and 0.51 and 0.88, respectively, in the 2000 IU/d group (excluding baseline data). Mean change (95% CI) in serum vitamin D3 between baseline and 3 mo was −0.1 (−1.8, 1.6) ng/mL in the placebo group and 6.8 (5.1, 8.5) ng/mL in the 2000 IU/d group (absolute difference: 6.9; 95% CI: 4.5, 9.3 ng/mL) (Table 2). Mean change (95% CI) in serum 25(OH)D3 between baseline and 3 mo was −2.0 (−8.2, 4.1) ng/mL in the placebo group and 20.8 (14.7, 26.9) ng/mL in the 2000 IU/d group (absolute difference: 22.8; 95% CI: 14.2, 31.5 ng/mL).
FIGURE 1.
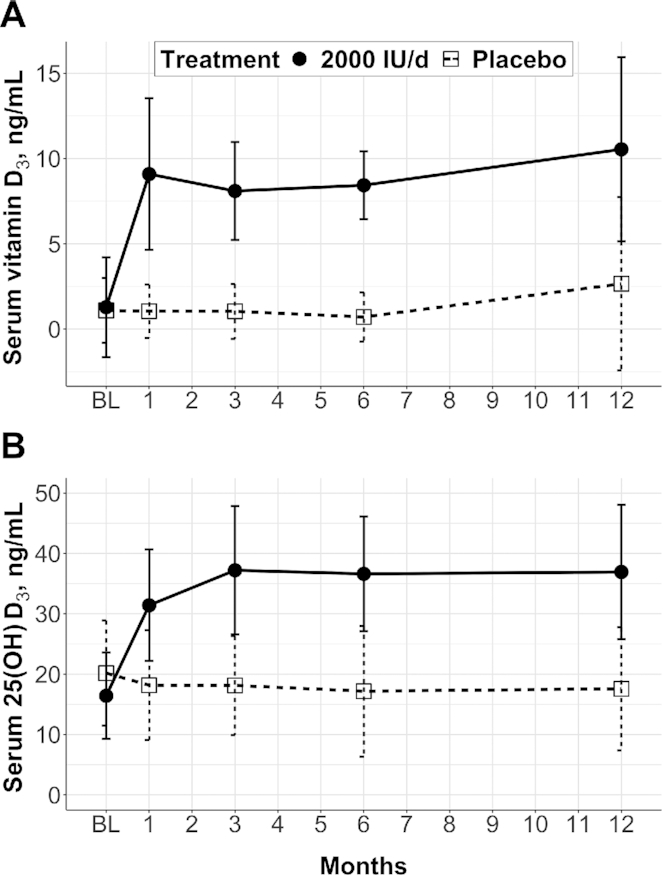
Time courses of serum vitamin D3 concentration (A) and serum 25(OH)D3 concentration (B) in the placebo and vitamin D3 (2000 IU/d) groups in pilot study 1. Data are means ± SDs; n = 11/group, 104 observations total, and 6 missing observations (2 at month 1, 1 at month 6, and 3 at month 12). SI conversion factors: vitamin D3, 1 ng/mL = 2.6 nmol/L; 25(OH)D3, 1 ng/mL = 2.5 nmol/L. BL, baseline; 25(OH)D3, 25-hydroxyvitamin D3.
TABLE 2.
Changes in serum vitamin D3, 25(OH)D3, 24,25(OH)2D3, and 1,25(OH)2D3 concentrations from baseline to 3 mo in 2 pilot clinical trials of vitamin D3 supplementation1
| Vitamin D3, ng/mL | 25(OH)D3, ng/mL | 24,25(OH)2D3, ng/mL | 1,25(OH)2D3, pg/mL | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Baseline | 3-mo | Mean change from baseline to 3 mo | Baseline | 3-mo | Mean change from baseline to 3 mo | Baseline | 3-mo | Mean change from baseline to 3 mo | Baseline | 3-mo | Mean change from baseline to 3 mo | |
| Pilot 1 | ||||||||||||
| Placebo (n = 11) | 1.1 ± 1.9 | 1.0 ± 1.6 | −0.1 (−1.8, 1.6) | 20.2 ± 8.7 | 18.1 ± 8.2 | −2.0 (−8.2, 4.1) | 2.7 ± 1.4 | 2.6 ± 1.8 | −0.1 (−1.1, 0.9) | 29.4 ± 15.0 | 31.6 ± 12.2 | 2.3 (−5.7, 10.3) |
| 2000 IU/d (n = 11) | 1.3 ± 2.9 | 8.1 ± 2.9 | 6.8 (5.1, 8.5) | 16.4 ± 7.2 | 37.2 ± 10.6 | 20.8 (14.7, 26.9) | 2.2 ± 1.6 | 6.0 ± 2.0 | 3.8 (2.8, 4.8) | 32.6 ± 10.2 | 37.1 ± 14.3 | 4.5 (−3.4, 12.5) |
| Absolute difference | 6.9 (4.5, 9.3) | 22.8 (14.2, 31.5) | 3.9 (2.5, 5.3) | 2.3 (−9.0, 13.6) | ||||||||
| Pilot 2 | ||||||||||||
| 2000 IU/d (n = 8) | 0.2 ± 0.2 | 10.6 ± 5.8 | 10.4 (6.0, 14.8) | 12.5 ± 3.7 | 30.4 ± 6.5 | 17.9 (13.7, 22.1) | 1.0 ± 0.5 | 3.8 ± 1.5 | 2.8 (1.9, 3.7) | 32.1 ± 5.8 | 40.8 ± 7.3 | 8.6 (2.4, 14.8) |
| 4000 IU/d (n = 7) | 0.4 ± 0.7 | 22.6 ± 7.4 | 22.2 (17.5, 26.9) | 12.9 ± 4.6 | 37.9 ± 5.6 | 25.0 (20.5, 29.5) | 1.0 ± 0.7 | 5.1 ± 1.5 | 4.1 (3.1, 5.0) | 35.0 ± 6.1 | 37.3 ± 8.8 | 2.3 (−4.3, 8.9) |
| Absolute difference | 11.8 (5.4, 18.3) | 7.1 (0.9, 13.3) | 1.2 (−0.1, 2.6) | −6.3 (−15.4, 2.7) | ||||||||
| Fold difference | 2.15 (1.40, 3.37) | 1.40 (1.05, 1.83) | 1.44 (0.97, 2.20) | 0.28 (−0.57, 1.41) | ||||||||
Values are means ± SDs or means (95% CIs). Linear mixed-effects models were used to estimate change between baseline and 3 mo within group, absolute difference in change between groups, and associated CIs. Estimates and CIs for the fold difference in change between groups were obtained through bootstrap methods with 2000 replicates. SI conversion factors: vitamin D3, 1 ng/mL = 2.6 nmol/L; 25(OH)D3, 1 ng/mL = 2.5 nmol/L; 24,25(OH)2D3, 1 ng/mL = 2.4 nmol/L; 1,25(OH)2D3, 1 pg/mL = 2.4 pmol/L. 1,25(OH)2D3, 1,25-dihydroxyvitamin D3; 24,25(OH)2D3, 24,25-dihydroxyvitamin D3; 25(OH)D3, 25-hydroxyvitamin D3.
Dose-response effects were assessed within pilot 2, which tested 2 levels of vitamin D3 supplementation. Mean change (95% CI) in serum vitamin D3 between baseline and 3 mo was 10.4 (6.0, 14.8) ng/mL in the 2000 IU/d group and 22.2 (17.5, 26.9) ng/mL in the 4000 IU/d group (fold difference: 2.15; 95% CI: 1.40, 3.37), indicating the serum vitamin D3 response was proportional to dose (Table 2). Likewise, mean change in SUBQ adipose tissue vitamin D3 was ∼2-fold greater in the 4000 than in the 2000 IU/d group (fold difference: 2.15; 95% CI: 1.34, 4.10) (Table 3). By contrast, mean change (95% CI) in serum 25(OH)D3 was 17.9 (13.7, 22.1) ng/mL in the 2000 IU/d group and 25.0 (20.5, 29.5) ng/mL in the 4000 IU/d group (fold difference: 1.40; 95% CI: 1.05, 1.83) (Table 2), and the mean adipose tissue 25(OH)D3 response was also less than proportional to dose (fold difference: 1.73; 95% CI: 1.18, 2.79) (Table 3). Similarly, the serum 24,25(OH)2D3 response was only 44% greater in the 4000 than in the 2000 IU/d group (fold difference: 1.44; 95% CI: 0.97, 2.17) (Table 2). In both pilots, change in serum 1,25(OH)2D3 concentration did not differ between treatment groups (Table 2).
TABLE 3.
Changes in subcutaneous adipose tissue vitamin D3 and 25(OH)D3 concentrations from baseline to 3 mo in a pilot clinical trial of vitamin D3 supplementation1
| Vitamin D3, pg/mg | 25(OH)D3, pg/mg | |||||
|---|---|---|---|---|---|---|
| Baseline | 3-mo | Mean change from baseline to 3 mo | Baseline | 3-mo | Mean change from baseline to 3 mo | |
| 2000 IU/d (n = 8) | 10.8 ± 4.6 | 21.3 ± 9.3 | 10.6 (5.0, 16.1) | 1.1 ± 0.3 | 2.5 ± 0.8 | 1.4 (0.9, 1.8) |
| 4000 IU/d (n = 7) | 9.1 ± 7.8 | 31.8 ± 14.4 | 22.6 (16.7, 28.6) | 1.1 ± 0.3 | 3.5 ± 0.3 | 2.4 (1.9, 2.8) |
| Absolute difference | 12.1 (4.0, 20.2) | 1.0 (0.3, 1.7) | ||||
| Fold difference | 2.15 (1.34, 4.10) | 1.73 (1.18, 2.79) | ||||
Values are means ± SDs or means (95% CIs). Data are from pilot study 2. Linear mixed-effects models were used to estimate change between baseline and 3 mo within group, absolute difference in change between groups, and associated CIs. Estimates and CIs for the fold difference in change between groups were obtained through bootstrap methods with 2000 replicates. SI conversion factors: vitamin D3, 1 pg/mg = 2.6 nmol/kg; 25(OH)D3, 1 pg/mg = 2.5 nmol/kg. 25(OH)D3, 25-hydroxyvitamin D3.
Combining data from both pilots, the serum vitamin D3 concentration never exceeded 1 ng/mL when serum 25(OH)D3 concentration was <16 ng/mL, whereas there was much more variability in the serum vitamin D3 concentration when serum 25(OH)D3 was >20 ng/mL (Figure 2). This threshold was evident at baseline and in the placebo group throughout follow-up, indicating that the threshold was not mediated by supplementation. After ≥3 mo of treatment with 2000 or 4000 IU/d, all participants achieved a serum 25(OH)D3 concentration >20 ng/mL, and Figure 2 suggests that, in this context, the association between serum vitamin D3 and 25(OH)D3 varied by dose.
FIGURE 2.
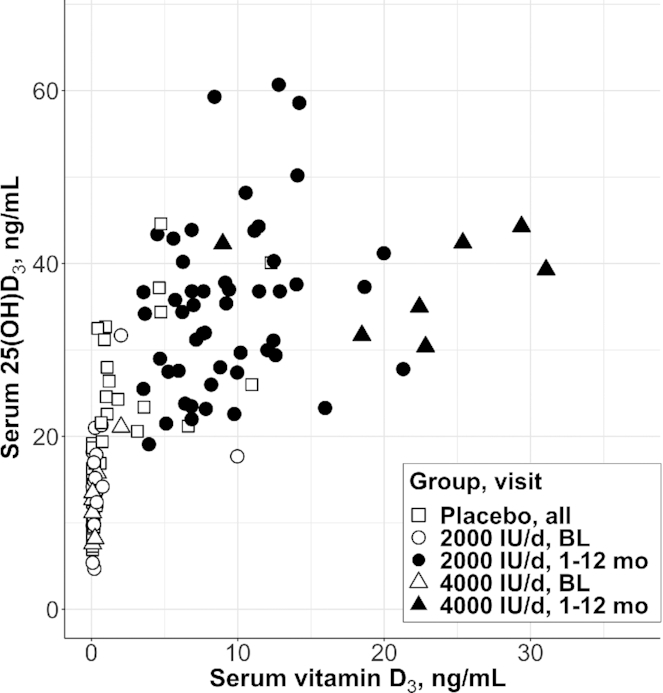
Serum 25(OH)D3 concentration compared with serum vitamin D3 concentration at baseline and in the placebo group, i.e., in the unsupplemented state (open shapes), and after 1, 3, 6, or 12 mo of supplementation (closed shapes) in 2 pilot clinical trials of vitamin D3 supplementation. Data are individual participant data (placebo, n = 11; 2000 IU/d, n = 19; 4000 IU/d, n = 7), all observations. SI conversion factors: vitamin D3, 1 ng/mL = 2.6 nmol/L; 25(OH)D3, 1 ng/mL = 2.5 nmol/L. BL, baseline; 25(OH)D3, 25-hydroxyvitamin D3.
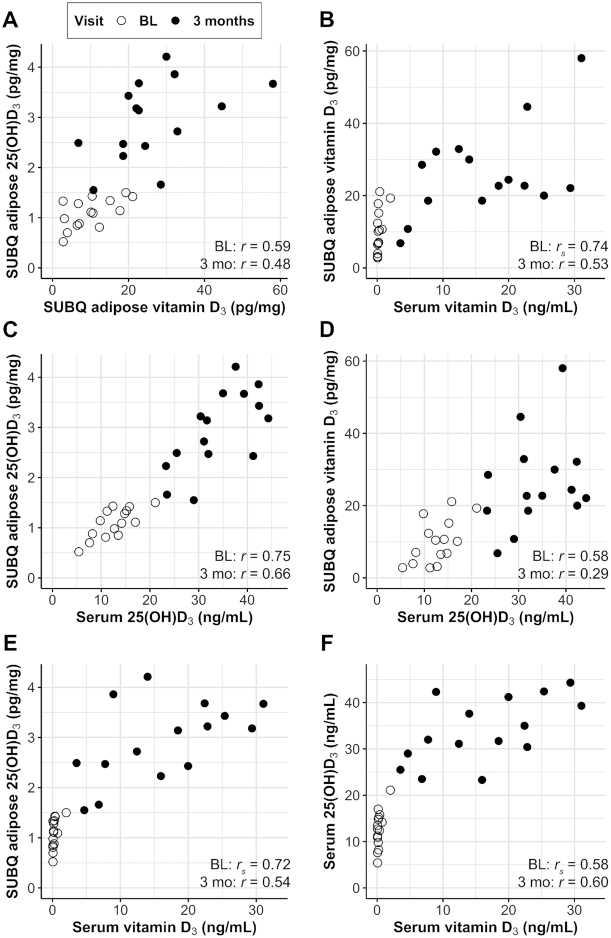
Adipose tissue vitamin D3 and 25(OH)D3 were positively correlated at baseline (r = 0.59, P = 0.02) and 3 mo (r = 0.48, P = 0.07), and there was ∼10 times more vitamin D3 than 25(OH)D3 in the adipose tissue at both time points (Figure 3A). Some participants had notable concentrations of adipose tissue vitamin D3 at baseline despite very low serum vitamin D3 concentrations. Nevertheless, there was a significant Spearman rank correlation between serum and adipose tissue vitamin D3 at baseline (Spearman r = 0.74, P < 0.01) and Pearson correlation between serum and adipose tissue vitamin D3 at 3 mo (r = 0.53, P = 0.04) (Figure 3B). Adipose tissue 25(OH)D3 and serum 25(OH)D3 were strongly correlated at both visits (baseline: r = 0.75, P < 0.01; 3 mo: r = 0.66, P < 0.01) (Figure 3C). Adipose tissue vitamin D3 was related to serum 25(OH)D3 at baseline (r = 0.58, P = 0.02) but not after 3 mo of supplementation (r = 0.29, P = 0.30) (Figure 3D).
FIGURE 3.
Serum and SUBQ adipose tissue concentrations of vitamin D3 and 25(OH)D3 at baseline (open circles) and after 3 mo of supplementation (closed circles) with 2000 IU/d (n = 8) or 4000 IU/d (n = 7) of vitamin D3 in pilot study 2. For each visit separately, bivariate associations were estimated with r when both variables were normally distributed or rs when ≥1 variable was skewed. (A) Adipose tissue 25(OH)D3 compared with adipose tissue vitamin D3; (B) adipose tissue vitamin D3 compared with serum vitamin D3; (C) adipose tissue 25(OH)D3 compared with serum 25(OH)D3; (D) adipose tissue vitamin D3 compared with serum 25(OH)D3; (E) adipose tissue 25(OH)D3 compared with serum vitamin D3; (F) serum 25(OH)D3 compared with serum vitamin D3. SI conversion factors: vitamin D3, 1 ng/mL = 2.6 nmol/L, 1 pg/mg = 2.6 nmol/kg; 25(OH)D3, 1 ng/mL = 2.5 nmol/L, 1 pg/mg = 2.5 nmol/kg. BL, baseline; SUBQ, subcutaneous; 25(OH)D3, 25-hydroxyvitamin D3.
Discussion
We developed and validated an LC-MS/MS method to quantify serum concentrations of vitamins D3 and D2 and applied it in 2 pilot studies of daily vitamin D3 supplementation. With supplementation, we found that the serum vitamin D3 concentration increased proportionally to dose and reached a plateau by 1 mo of treatment. The bivariate relation of serum vitamin D3 and 25(OH)D3 exhibited a threshold at a serum 25(OH)D3 concentration of ∼20 ng/mL (50 nmol/L), which also is the concentration that indicates adequate vitamin D status to support bone health in most individuals according to the National Academy of Medicine (5). Serum vitamin D3 concentration was minimal when serum 25(OH)D3 concentration was <20 ng/mL and more variable when serum 25(OH)D3 exceeded this threshold. Our data support the hypothesis that the 25-hydroxylation of vitamin D3 follows nonlinear kinetics with respect to vitamin D3 concentration (17, 18), even at modest doses <4000 IU/d, possibly owing to capacity-limited formation or another mechanism. In addition, and perhaps most importantly, the serum vitamin D3 rather than 25(OH)D3 concentration was more strongly correlated with the vitamin D3 concentration in adipose tissue during supplementation.
Historically, serum vitamin D concentration was determined by competitive protein binding assay (CPBA) or HPLC (7, 19–24). These methods have limitations, such as low specificity in the case of CPBA, or limited throughput (25). MS is the preferred analytical method for quantitation of small molecules, such as vitamin D, because it offers high analytical sensitivity, specificity, and reproducibility. A handful of LC-MS/MS methods for measurement of vitamins D2 and/or D3 in serum or plasma have been described in the past decade but have not been applied extensively (10–14). Our method meets performance expectations of the CLSI guideline and, with an LLMI of 0.13–0.14 ng/mL and throughput of 96 samples/d, enables analysis of serum vitamin D concentration in large clinical studies.
A few studies have documented the serum vitamin D3 response to daily supplementation, and the results from our pilot studies are concordant with their findings. For example, in pilot study 1, serum vitamin D3 reached a plateau within 1 mo of treatment and serum 25(OH)D3 reached a plateau later at 3 mo. The finding of a shorter time to steady state is consistent with the shorter plasma half-life of vitamin D3 than of 25(OH)D3 (24, 26). In addition, the mean serum vitamin D3 response to supplementation was proportional to dose. Specifically, treatment with 2000 IU/d led to an increment of 6.8–10.4 ng/mL and 4000 IU/d led to an increment of 22.2 ng/mL. This dose-response is relatively consistent with previously observed serum vitamin D3 increments of 5, 53, or 340 ng/mL after 8 wk of treatment with 1000, 10,000, or 50,000 IU vitamin D3/d, respectively (7). By contrast, we found the serum 25(OH)D3 response to supplementation was less than proportional to dose, which is consistent with some (5, 27) but not all (28) previous meta-analyses that determined the dose-response of serum 25(OH)D concentration to vitamin D intake is nonlinear.
Such a nonlinear relation could arise from multiple mechanisms. For example, vitamin D3 binding to CYP2R1 could become saturated at high doses, yielding capacity-limited 25(OH)D formation kinetics (29). Alternatively, elimination of 25(OH)D by enzymes like CYP24A1 could be induced with increasing exposure to vitamin D3, although this mechanism seems less likely given the strong linear relation between 24,25(OH)2D3 and 25(OH)D3 and the lack of effect of supplementation on 1,25(OH)2D3, which is the most important inducer of CYP24A1 expression. Another possible mechanism involves negative cooperativity in the enzymatic formation of 25(OH)D via allosteric binding of a second molecule of vitamin D or a downstream metabolite, but such complex kinetics were not evident in previous experiments (29–31). Finally, it is possible that increasing hepatic exposure to vitamin D and its metabolites causes downregulation of CYP2R1.
Very little vitamin D3 was detected in the circulation of study participants who had a serum 25(OH)D3 concentration <20 ng/mL (50 nmol/L). It seems that any recent input of vitamin D3 in these individuals was small enough to be rapidly converted to 25(OH)D3. Interestingly, a previous study found a biphasic relation between steady-state serum vitamin D3 and 25(OH)D3 concentrations. At low concentrations, small increases in vitamin D3 were associated with large but steadily decreasing increases in 25(OH)D3 until the relation became linear once 25(OH)D3 concentration surpassed 35 ng/mL (88 nmol/L) (24). Although these 2 thresholds may seem quite different, direct comparison between our study and the previous is difficult owing to differences in the assay methods and calibration schemes, the different doses used in each study, and the exclusion of baseline samples from the previous publication [i.e., few data points included a serum 25(OH)D concentration <20 ng/mL after participants had received 18–20 wk of oral vitamin D treatment or UVB treatment]. Of interest, several studies have shown that the serum 25(OH)D response to vitamin D supplementation was attenuated when baseline serum 25(OH)D concentration was >16 (32) or >20 ng/mL (33).
One of the main strengths of our study, in contrast to previous studies (34–39), is our ability to compare serum and adipose concentrations of vitamin D and 25(OH)D in the same people (pilot study 2). We found that serum and SUBQ adipose tissue 25(OH)D3 concentrations were strongly positively correlated at baseline and 3 mo into supplementation. Serum and adipose vitamin D3 concentrations were more moderately correlated during supplementation, but the dose-response of vitamin D3 in adipose tissue after 3 mo of treatment mirrored the dose-response of vitamin D3 in serum. Interestingly, at baseline, when vitamin D status was low, adipose tissue vitamin D3 was positively correlated with serum 25(OH)D3, but after 3 mo of supplementation, adipose vitamin D3 was not significantly related to serum 25(OH)D3. These results suggest that when participants had little to no recent vitamin D input, adipose tissue was supplying the plasma with vitamin D3 for conversion to 25(OH)D3. This proposition is supported by recent findings from Martinaityte et al. (39) who showed that adipose vitamin D stores acquired during supplementation may attenuate the decline in serum 25(OH)D concentration after discontinuation of vitamin D treatment.
The main limitations of this study are the small sample size and exploratory nature. We describe mean analyte responses to supplementation but did not investigate sources of interindividual variation owing to the sample size. However, fat mass likely influences serum and adipose concentrations of vitamin D, and this should be addressed in future research. Future research that characterizes circulating vitamin D3 concentration should also evaluate within-person fluctuation during daily supplementation, the effect of timing of blood collection, and the plasma half-life after termination of extended oral dosing. As mentioned, this study was exploratory but contributes to the very limited literature documenting vitamin D concentrations in blood and fat.
In summary, we applied a modern LC-MS/MS method to the study of archived samples from 2 pilot clinical trials of vitamin D3 supplementation that demonstrate the potential importance of serum vitamin D concentration as a biomarker of actual vitamin D exposure in response to supplementation. Our study results suggest that the serum vitamin D concentration may be a useful measure of adherence in supplementation trials. More broadly, our study results suggest that additional work to assess vitamin D concentrations in blood and other tissues will improve our understanding of vitamin D homeostasis in humans.
Supplementary Material
ACKNOWLEDGEMENTS
The authors’ responsibilities were as follows—ANH, IHdB, and MK: designed the research; ANH, IHdB, MK, DVR, TJL, HP, KEF-S, JNK, GC, IL, DKH, and KH: acquired the data; CMB and LRZ: conducted the statistical data analyses; CMB, ANH, IHdB, MK, SH, and KET: interpreted the data; CMB, ANH, IHdB, MK, and KET: wrote the paper; ANH: had primary responsibility for the final content; and all authors: critically read and approved the final manuscript. The authors report no conflicts of interest.
Notes
Supported by National Heart, Lung, and Blood Institute grants T32HL007028 (to CMB) and R01HL096875 (to IHdB); NIH grants KL2RR025015 (to IHdB), UL1TR002319 (to IHdB), and P30DK035816 (to MK and ANH); and National Institute of Diabetes and Digestive and Kidney Diseases grants P30DK017047 (to IHdB and ANH), R01DK099199 (to IHdB), and R01DK088762 (to IHdB).
MK is a member of the Journal's Editorial Board.
Supplemental Methods, Supplemental Tables 1–10, and Supplemental Figures 1–5 are available from the “Supplementary data” link in the online posting of the article and from the same link in the online table of contents at https://academic.oup.com/ajcn/.
Data Availability: Data described in the article, code book, and analytic code will be made available upon request pending application and approval.
Abbreviations used: CLSI, Clinical and Laboratory Standards Institute; CPBA, competitive protein binding assay; CYP2R1, cytochrome P450 family 2 subfamily R member 1; CYP24A1, cytochrome P450 family 24 subfamily A member 1; CYP27B1, cytochrome P450 family 27 subfamily B member 1; ICC, intraclass correlation coefficient; LC-MS/MS, liquid chromatography–tandem mass spectrometry; LLMI, lower limit of the measuring interval; MTBE, methyl tert-butyl ether; PTAD, 4-phenyl-1,2,4-triazoline-3,5-dione; SUBQ, subcutaneous; 1,25(OH)2D, 1,25-dihydroxyvitamin D; 24,25(OH)2D3, 24,25-dihydroxyvitamin D3; 25(OH)D, 25-hydroxyvitamin D.
Contributor Information
Cora M Best, Department of Laboratory Medicine, University of Washington, Seattle, WA, USA; Kidney Research Institute, University of Washington, Seattle, WA, USA.
Devon V Riley, Department of Laboratory Medicine, University of Washington, Seattle, WA, USA.
Thomas J Laha, Department of Laboratory Medicine, University of Washington, Seattle, WA, USA.
Hannah Pflaum, Department of Laboratory Medicine, University of Washington, Seattle, WA, USA.
Leila R Zelnick, Kidney Research Institute, University of Washington, Seattle, WA, USA; Department of Medicine, University of Washington, Seattle, WA, USA.
Simon Hsu, Kidney Research Institute, University of Washington, Seattle, WA, USA; Department of Medicine, University of Washington, Seattle, WA, USA.
Kenneth E Thummel, Department of Pharmaceutics, University of Washington, Seattle, WA, USA.
Karen E Foster-Schubert, Department of Medicine, University of Washington, Seattle, WA, USA; VA Puget Sound Healthcare System, Seattle, WA, USA.
Jessica N Kuzma, Cancer Prevention Program, Division of Public Health Sciences, Fred Hutchinson Cancer Research Center, Seattle, WA, USA.
Gail Cromer, Cancer Prevention Program, Division of Public Health Sciences, Fred Hutchinson Cancer Research Center, Seattle, WA, USA.
Ilona Larson, Cancer Prevention Program, Division of Public Health Sciences, Fred Hutchinson Cancer Research Center, Seattle, WA, USA.
Derek K Hagman, Cancer Prevention Program, Division of Public Health Sciences, Fred Hutchinson Cancer Research Center, Seattle, WA, USA.
Kelly Heshelman, Cancer Prevention Program, Division of Public Health Sciences, Fred Hutchinson Cancer Research Center, Seattle, WA, USA.
Mario Kratz, Department of Medicine, University of Washington, Seattle, WA, USA; Cancer Prevention Program, Division of Public Health Sciences, Fred Hutchinson Cancer Research Center, Seattle, WA, USA; Department of Epidemiology, University of Washington, Seattle, WA, USA.
Ian H de Boer, Kidney Research Institute, University of Washington, Seattle, WA, USA; Department of Medicine, University of Washington, Seattle, WA, USA; Department of Epidemiology, University of Washington, Seattle, WA, USA.
Andrew N Hoofnagle, Department of Laboratory Medicine, University of Washington, Seattle, WA, USA; Kidney Research Institute, University of Washington, Seattle, WA, USA; Department of Medicine, University of Washington, Seattle, WA, USA.
References
- 1. Heaney RP, Armas LA. Quantifying the vitamin D economy. Nutr Rev. 2015;73(1):51–67. [DOI] [PubMed] [Google Scholar]
- 2. Cheng JB, Motola DL, Mangelsdorf DJ, Russell DW. De-orphanization of cytochrome P450 2R1: a microsomal vitamin D 25-hydroxilase. J Biol Chem. 2003;278(39):38084–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Meyer MB, Benkusky NA, Kaufmann M, Lee SM, Onal M, Jones G, Pike JW. A kidney-specific genetic control module in mice governs endocrine regulation of the cytochrome P450 gene Cyp27b1 essential for vitamin D3 activation. J Biol Chem. 2017;292(42):17541–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Chun RF, Shieh A, Gottlieb C, Yacoubian V, Wang J, Hewison M, Adams JS. Vitamin D binding protein and the biological activity of vitamin D. Front Endocrinol. 2019;10:718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Institute of Medicine Committee to Review Dietary Reference Intakes for Vitamin D and Calcium Ross AC, Taylor CL, Yaktine AL, Del Valle HB, Dietary Reference Intakes for calcium and vitamin D. Washington (DC): National Academies Press (US) National Academy of Sciences; 2011. [PubMed] [Google Scholar]
- 6. Schoenmakers I, Jones KS. Pharmacology and pharmacokinetics. : Feldman D, Pike JW, Bouillon R, Giovanucci E, Goltzman D, Hewison M, editors. Vitamin D. London, United Kingdom: Elsevier; 2018. p. 635–61. [Google Scholar]
- 7. Barger-Lux MJ, Heaney RP, Dowell S, Chen TC, Holick MF. Vitamin D and its major metabolites: serum levels after graded oral dosing in healthy men. Osteoporos Int. 1998;8(3):222–30. [DOI] [PubMed] [Google Scholar]
- 8. Hollis BW, Wagner CL. Clinical review: the role of the parent compound vitamin D with respect to metabolism and function: why clinical dose intervals can affect clinical outcomes. J Clin Endocrinol Metab. 2013;98(12):4619–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. van den Ouweland JM, Vogeser M, Bächer S. Vitamin D and metabolites measurement by tandem mass spectrometry. Rev Endocr Metab Disord. 2013;14(2):159–84. [DOI] [PubMed] [Google Scholar]
- 10. Adamec J, Jannasch A, Huang J, Hohman E, Fleet JC, Peacock M, Ferruzzi MG, Martin B, Weaver CM. Development and optimization of an LC-MS/MS-based method for simultaneous quantification of vitamin D2, vitamin D3, 25-hydroxyvitamin D2 and 25-hydroxyvitamin D3. J Sep Science. 2011;34(1):11–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Binkley N, Lappe J, Singh RJ, Khosla S, Krueger D, Drezner MK, Blank RD. Can vitamin D metabolite measurements facilitate a “treat-to-target” paradigm to guide vitamin D supplementation?. Osteoporos Int. 2015;26(5):1655–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Burild A, Frandsen HL, Jakobsen J. Simultaneous quantification of vitamin D3, 25-hydroxyvitamin D3 and 24,25-dihydroxyvitamin D3 in human serum by LC-MS/MS. Scand J Clin Lab Invest. 2014;74(5):418–23. [DOI] [PubMed] [Google Scholar]
- 13. Contractor P, Gandhi A, Solanki G, Shah PA, Shrivastav PS. Determination of ergocalciferol in human plasma after Diels-Alder derivatization by LC–MS/MS and its application to a bioequivalence study. J Pharm Anal. 2017;7(6):417–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Abu Kassim NS, Shaw PN, Hewavitharana AK. Simultaneous determination of 12 vitamin D compounds in human serum using online sample preparation and liquid chromatography-tandem mass spectrometry. J Chromatogr A. 2018;1533:57–65. [DOI] [PubMed] [Google Scholar]
- 15. Hoofnagle AN, Laha TJ, Donaldson TF. A rubber transfer gasket to improve the throughput of liquid-liquid extraction in 96-well plates: application to vitamin D testing. J Chromatogr B. 2010;878(19):1639–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Laha TJ, Strathmann FG, Wang Z, de Boer IH, Thummel KE, Hoofnagle AN. Characterizing antibody cross-reactivity for immunoaffinity purification of analytes prior to multiplexed liquid chromatography–tandem mass spectrometry. Clin Chem. 2012;58(12):1711–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ocampo-Pelland AS, Gastonguay MR, French JF, Riggs MM. Model-based meta-analysis for development of a population-pharmacokinetic (PPK) model for vitamin D3 and its 25OHD3 metabolite using both individual and arm-level data. J Pharmacokinet Pharmacodyn. 2016;43(2):191–206. [DOI] [PubMed] [Google Scholar]
- 18. Hollis BW, Wagner CL, Drezner MK, Binkley NC. Circulating vitamin D3 and 25-hydroxyvitamin D in humans: an important tool to define adequate nutritional vitamin D status. J Steroid Biochem Mol Biol. 2007;103(3–5):631–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hollis BW. Comparison of equilibrium and disequilibrium assay conditions for ergocalciferol, cholecalciferol and their major metabolites. J Steroid Biochem. 1984;21(1):81–6. [DOI] [PubMed] [Google Scholar]
- 20. Chen TC, Turner AK, Holick MF. A method for the determination of the circulating concentration of vitamin D. J Nutr Biochem. 1990;1(5):272–6. [DOI] [PubMed] [Google Scholar]
- 21. Armas LA, Hollis BW, Heaney RP. Vitamin D2 is much less effective than vitamin D3 in humans. J Clin Endocrinol Metab. 2004;89(11):5387–91. [DOI] [PubMed] [Google Scholar]
- 22. Armas LA, Dowell S, Akhter M, Duthuluru S, Huerter C, Hollis BW, Lund R, Heaney RP. Ultraviolet-B radiation increases serum 25-hydroxyvitamin D levels: the effect of UVB dose and skin color. J Am Acad Dermatol. 2007;57(4):588–93. [DOI] [PubMed] [Google Scholar]
- 23. Heaney RP, Davies KM, Chen TC, Holick MF, Barger-Lux MJ. Human serum 25-hydroxycholecalciferol response to extended oral dosing with cholecalciferol. Am J Clin Nutr. 2003;77(1):204–10. [DOI] [PubMed] [Google Scholar]
- 24. Heaney RP, Armas LA, Shary JR, Bell NH, Binkley N, Hollis BW. 25-Hydroxylation of vitamin D3: relation to circulating vitamin D3 under various input conditions. Am J Clin Nutr. 2008;87(6):1738–42. [DOI] [PubMed] [Google Scholar]
- 25. Norman AW. From vitamin D to hormone D: fundamentals of the vitamin D endocrine system essential for good health. Am J Clin Nutr. 2008;88(2):491S–9S. [DOI] [PubMed] [Google Scholar]
- 26. Dawson-Hughes B. Vitamin D deficiency in adults: definition, clinical manifestations, and treatment. : Drezner MK, Rosen CJ, Mulder JE, editors. UpToDate. Waltham, MA: UpToDate; 2018; [accessed 21 October, 2020]. Available from: https://www.uptodate.com/contents/vitamin-d-deficiency-in-adults-definition-clinical-manifestations-and-treatment. [Google Scholar]
- 27. EFSA Panel on Dietetic Products, Nutrition and Allergies (NDA) Dietary reference values for vitamin D. EFSA J. 2016;14(10):e04547. [Google Scholar]
- 28. Cashman KD, Ritz C, Kiely M, Odin C. Improved dietary guidelines for vitamin D: application of individual participant data (IPD)-level meta-regression analyses. Nutrients. 2017;9(5):469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Cheng CYS, Kim T-K, Jeayeng S, Slominski AT, Tuckey RC. Properties of purified CYP2R1 in a reconstituted membrane environment and its 25-hydroxylation of 20-hydroxyvitamin D3. J Steroid Biochem Mol Biol. 2018;177:59–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Shinkyo R, Sakaki T, Kamakura M, Ohta M, Inouye K. Metabolism of vitamin D by human microsomal CYP2R1. Biochem Biophys Res Commun. 2004;324(1):451–7. [DOI] [PubMed] [Google Scholar]
- 31. Strushkevich N, Usanov SA, Plotnikov AN, Jones G, Park HW. Structural analysis of CYP2R1 in complex with vitamin D3. J Mol Biol. 2008;380(1):95–106. [DOI] [PubMed] [Google Scholar]
- 32. Chung M, Balk EM, Brendel M, Ip S, Lau J, Lee J, Lichtenstein A, Patel K, Raman G, Tatsioni A et al. Vitamin D and calcium: a systematic review of health outcomes. Evid Rep Technol Assess (Full Rep). 2009(183):1–420. [PMC free article] [PubMed] [Google Scholar]
- 33. Black LJ, Seamans KM, Cashman KD, Kiely M. An updated systematic review and meta-analysis of the efficacy of vitamin D food fortification. J Nutr. 2012;142(6):1102–8. [DOI] [PubMed] [Google Scholar]
- 34. Mawer EB, Backhouse J, Holman CA, Lumb GA, Stanbury SW. The distribution and storage of vitamin D and its metabolites in human tissues. Clin Sci. 1972;43(3):413–31. [DOI] [PubMed] [Google Scholar]
- 35. Blum M, Dolnikowski G, Seyoum E, Harris SS, Booth SL, Peterson J, Saltzman E, Dawson-Hughes B. Vitamin D3 in fat tissue. Endocrine. 2008;33(1):90–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Beckman LM, Earthman CP, Thomas W, Compher CW, Muniz J, Horst RL, Ikramuddin S, Kellogg TA, Sibley SD. Serum 25(OH) vitamin D concentration changes after Roux-en-Y gastric bypass surgery. Obesity. 2013;21(12):E599–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Piccolo BD, Dolnikowski G, Seyoum E, Thomas AP, Gertz ER, Souza EC, Woodhouse LR, Newman JW, Keim NL, Adams SH et al. Association between subcutaneous white adipose tissue and serum 25-hydroxyvitamin D in overweight and obese adults. Nutrients. 2013;5(9):3352–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Didriksen A, Burild A, Jakobsen J, Fuskevåg OM, Jorde R. Vitamin D3 increases in abdominal subcutaneous fat tissue after supplementation with vitamin D3. Eur J Endocrinol. 2015;172(3):235–41. [DOI] [PubMed] [Google Scholar]
- 39. Martinaityte I, Kamycheva E, Didriksen A, Jakobsen J, Jorde R. Vitamin D stored in fat tissue during a 5-year intervention affects serum 25-hydroxyvitamin D levels the following year. J Clin Endocrinol Metab. 2017;102(10):3731–8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.