ABSTRACT
Background
There is evidence that low plasma vitamin B-12 and folate individually, as well as an imbalance of high folic acid and low vitamin B-12 status, may be associated with lower cognitive function.
Objectives
We examined dietary and plasma folate and vitamin B-12 status, and their interaction, in relation to cognitive function in a cohort of older Puerto Rican adults.
Methods
The design is cross-sectional, with 1408 participants from the Boston Puerto Rican Health Study (mean ± SD age: 57.1 ± 7.9 y). Cognitive function was assessed with a comprehensive test battery and a global composite score was derived. Plasma folate, vitamin B-12, and methylmalonic acid (MMA) were assessed in fasting blood samples.
Results
After adjusting for covariates, high plasma folate and high plasma vitamin B-12 were each positively associated with global cognitive score (β: 0.063; 95% CI: −0.0008, 0.127; P = 0.053 and β: 0.062; 95% CI: 0.009, 0.12; P = 0.023, respectively, for logged values, and β: 0.002; 95% CI: 0.00005, 0.004; P-trend = 0.044 and β: 0.00018; 95% CI: 0.00001, 0.0003; P-trend = 0.036, respectively, across tertiles). Nine percent of participants had vitamin B-12 deficiency (plasma vitamin B-12 < 148 pmol/L or MMA > 271 nmol/L), but none were folate deficient (plasma folate < 4.53 nmol/L). Deficient compared with higher vitamin B-12 was significantly associated with lower cognitive score (β: −0.119; 95% CI: −0.208, −0.029; P = 0.009). We could not examine the interaction for vitamin B-12 deficiency and high plasma folate, because there were too few individuals (<1% of the cohort) in this category to draw conclusions.
Conclusions
Low plasma vitamin B-12 and low plasma folate were each associated with worse cognitive function in this population. Vitamin B-12 deficiency was prevalent and clearly associated with poorer cognitive function. More attention should be given to identification and treatment of vitamin B-12 deficiency in this population. Additional, larger studies are needed to examine the effect of vitamin B-12 deficiency in the presence of high exposure to folic acid.
Keywords: vitamin B-12, folate, cognition, Puerto Rican adults, diet, folic acid
Introduction
The prevalence of cognitive impairment and dementia is growing with the aging of the US population. It has been estimated that dementia prevalence may range from 14% in those older than age 70 y, to 37% among those age 90 y and older (1, 2), The number of persons with dementia is expected to double every 20 y, resulting in an increase from 42.3 million individuals in 2020 to 81.1 million in 2040 (3). B-vitamins in particular have been associated with cognition, because they are critical for the synthesis of monoamine neurotransmitters and the formation of RBCs (4).
Vitamin B-12 deficiency has been associated with cognitive impairment (1, 5). Further, evidence suggests that vitamin B-12 inadequacy, which may develop owing to malabsorption, dietary inadequacy, or lack of intrinsic factor, may also be a risk factor (5, 6) for cognitive impairment. Vitamin B-12 deficiency is common among older populations, because the ability to absorb vitamin B-12 declines with the reduction in gastric cell function (6). The prevalence of subclinical vitamin B-12 deficiency has been shown to be high in older populations and in vegetarians (6).
Older adults with low folate status have been shown to be at higher risk of cognitive impairment and dementia (7, 8). A study reported that RBC folate was directly associated with cognitive
function score and, inversely, with dementia in elderly Latinos (7). A few studies have also raised the hypothesis that high exposure to folic acid may accelerate the effects of vitamin B-12 inadequacy (9, 10). In an analysis of national data, older adults with low vitamin B-12 status and high serum folate concentration had greater odds of cognitive impairment, whereas among those with normal vitamin B-12 status, high serum folate was found to be protective (9). Therefore, we sought to examine the associations between plasma folate, dietary intake of folic acid, vitamin B-12 status, and cognitive function, and to assess their interaction, in the Boston Puerto Rican Health Study.
Methods
Participants
This analysis was conducted within the Boston Puerto Rican Health Study, which included 1502 self-identified Puerto Rican adults, aged 45–75 y at baseline (2004–2009), residing in the Boston, MA metropolitan area. As described previously, study participants were recruited through door-to-door enumeration and community approaches (11). Study protocols were approved by the Institutional Review Board at Tufts Medical Center and by the University of Massachusetts Lowell.
Data collection
Trained bilingual interviewers administered questionnaires to participants in their homes (11). Many of the questionnaires and measures followed procedures used in the NHANES (12) and the MacArthur Studies of Successful Aging (13). Retraining and review sessions, including checks on scoring of tests and scales, were conducted periodically. Completed interviews were self- and peer-reviewed before data entry. Participants provided information on age and education and were asked to self-report whether they had been diagnosed with chronic conditions. Detailed information on prescription and over-the counter medications was collected by asking participants to show medication bottles. Frequency, history, and type of alcohol consumption and smoking were assessed. A physical activity score was calculated as the weighted sum of hours spent on activities during a typical 24-h period. Diabetes was defined as fasting glucose ≥126 mg/dL or use of diabetes medication. Genetic polymorphisms, including apolipoprotein E (APOE), were identified with applied Biosystems TaqMan SNP genotyping systems. Dietary intake was assessed with a semiquantitative FFQ, adapted and validated against 24-h recalls and several plasma measures such as vitamin B-12, vitamin E, and plasma carotenoids for this population. Based on the National Cancer Institute—Block format, the FFQ was revised to include foods, portion sizes, and recipes appropriate to this population (14–18).
Blood samples
Fasting blood samples (12-h) were drawn from participants by a certified phlebotomist on the morning after the interview, or as soon as possible thereafter, in the participant's home. Blood was collected into evacuated tubes containing EDTA and immediately centrifuged at 964 ×gat 4°C for 15 min. All tubes were shielded from light during specimen collection, processing, and handling. All samples were brought to the Nutrition Evaluation Laboratory at the Jean Mayer USDA Human Nutrition Research Center on Aging at Tufts University for further processing and storage. Samples were stored in cryogenic tubes at −80°C until further analysis. Plasma folate and vitamin B-12 concentrations were measured with a competitive, liquid-phase, ligand-labeled, protein-binding chemiluminescent assay with a commercially available kit from Diagnostics Products Corporation (IMMULITE 1000). Plasma pyridoxal-5-phosphate (PLP) was measured with a radioenzymatic assay on a Beckman LS 6500 Scintillation Counter. The intra- and interassay CVs (19) for each of these analytes were as follows: plasma PLP, 5% and 7%, respectively; plasma folate, 4.2% and 6.7%, respectively; and plasma vitamin B-12, 5.3% and 11.4%, respectively. Total homocysteine was measured with an improved method based on principles described by Araki and Sako (20). This analysis employed a C18 column on a Waters HPLC instrument equipped with a WISP automatic injector and attached to a fluorimeter (Krato from Applied Sciences Inc); detection was via a Perkin Elmer 650-15 Fluorescence Spectrophotometer (Perkin Elmer). Methylmalonic acid (MMA) in serum was determined after treatment with EDTA and protein precipitation solution by LC/MS/MS (API 5500 QTRAP, AB Sciex LLC; Agilent 1100 LC series, Agilent) according to Lakso et al. (21). MMA was measured with a range of 90–279 nmol/L, and intra- and interassay CVs were <10%. We defined deficient vitamin B-12 as plasma cobalamin <148 pmol/L or MMA > 271 nmol/L and deficient folate as <4.53 nmol/L, based on CDC recommendations (22), and high plasma folate as >59 nmol/L, based on a previously published cutoff defined by the 80th percentile in the 1999–2002 NHANES (9).
Cognitive function
Participants completed a battery of neuropsychological tests intended to assess various aspects of cognitive functioning. All cognitive testing was conducted in the participant's language of choice (98% in Spanish) at the participants’ home by trained interviewers, in consultation with a clinical neuropsychologist (TMS), and included the Mini-Mental State Examination as a measure of overall cognitive function (23); a 16-word list learning task for verbal memory; Digit Span forward and backward for attention and working memory (24); the Stroop test for processing speed, cognitive flexibility, and response to inhibition (24); clock drawing (25) and figure copying (26) for visual-spatial organization; and verbal fluency to assess verbal ability and executive function (24). Individual test scores were transformed to z scores and a global cognitive function composite score (GCS) was computed as the arithmetic mean of the individual z scores. The GCS was used as the outcome variable in this study, as done in prior work (27, 28). If a participant did not complete an individual test, the given score was imputed using the minimum z score of the same individual test for the rest of the cohort, unless the missing values were due to illiteracy, hearing impairment, or poor vision. In these cases, only the existing individual test values were averaged.
Statistical analysis
Statistical analyses were conducted using the SAS System for Windows (version 9.1; SAS Institute, Inc). Descriptive analyses were performed to examine baseline characteristics across tertiles of plasma vitamin B-12 and plasma folate, by age, sex/estrogen status (female postmenopausal with no estrogen use, female premenopausal or postmenopausal with estrogen use, or male), BMI (in kg/m2), physical activity score, level of education, APOE ε4 allele, diabetes status, vitamin B-6 (as PLP in nmol/L), smoking status (past, current, or never), and alcohol intake (heavy, moderate, or none). Multivariable linear regression models were used to estimate β coefficients and 95% CIs for the association between plasma folate and vitamin B-12 and GCS. Of 1502 participants, we excluded 67 participants owing to missing plasma vitamin B-12 or folate measures and 27 owing to missing data on other covariates, leaving a sample size of 1408 for analyses (Figure 1). In analyses of dietary folic acid intake, we excluded an additional 73 individuals who reported intakes of <600 kcal or >4800 kcal or had >12 line items blank on the FFQ, as having invalid dietary data, leaving an analytical sample of 1335 for those analyses.
FIGURE 1.
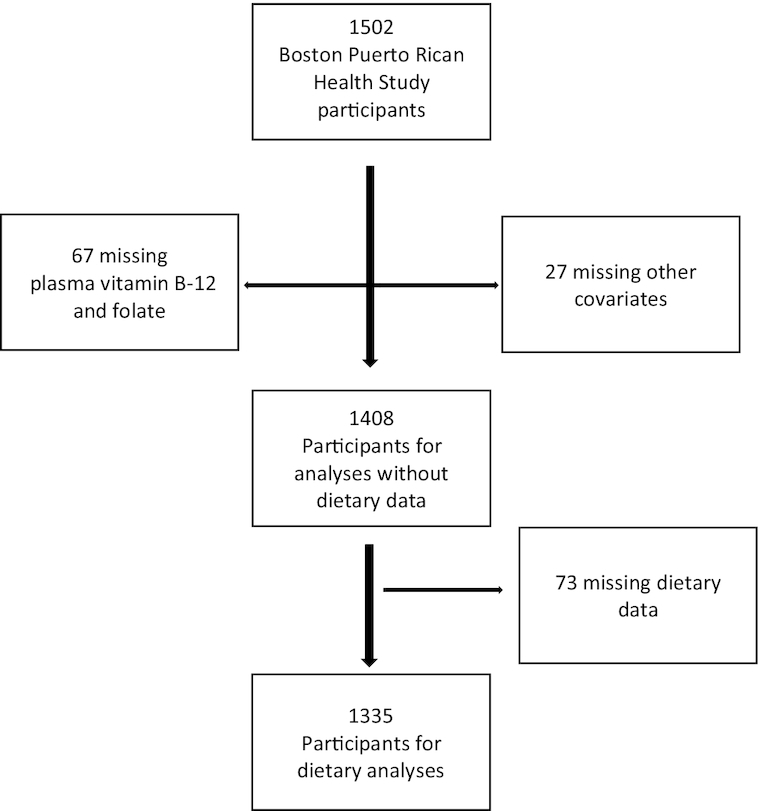
Participant flowchart.
Models were adjusted for variables previously observed to be associated with cognitive function. These included age, sex/estrogen status, BMI, physical activity score, education (less than high school, high school, or higher), diabetes (yes/no), smoking (past, current, or none), alcohol use (heavy, none, or moderate), and, when appropriate, energy intake. Additional models were adjusted also for APOE (ε4 carrier) and plasma PLP (nmol/L), as well as mutually adjusted for vitamin B-12 and folate. PLP was included as a confounder owing to its relation with the other B vitamins and homocysteine, and possible effects on cognitive function (29).
Results
The mean ± SD age of study participants at baseline was 57.1 ± 7.9 y and women comprised 70% of the sample (Table 1). The prevalence of diabetes was 40% and 48% had less than a high school education. Importantly, 124 of 1408 (9%) participants had vitamin B-12 deficiency, based on the CDC definition (plasma vitamin B-12 < 148 pmol/L or MMA > 271 nmol/L) (22), and none were folate deficient (<4.53 nmol/L). Following Morris et al. (10), based on NHANES data for high plasma folate (>59 nmol/L), 16.1% had high plasma folate. Although the true value may be underestimated with the FFQ, only 0.3% had folic acid intake above the upper limit (UL) of 1000 μg/d (Table 2).
TABLE 1.
Baseline characteristics, by tertile of plasma folate and vitamin B-12 status, among 1408 Boston Puerto Rican Health Study participants1
| Plasma folate, nmol/L | Plasma vitamin B-12, pmol/L | |||||||
|---|---|---|---|---|---|---|---|---|
| Tertile 1 | Tertile 2 | Tertile 3 | P | Tertile 1 | Tertile 2 | Tertile 3 | P | |
| Median | 26.3 | 39.2 | 58.5 | 223 | 344 | 596 | ||
| n | 467 | 473 | 468 | 474 | 468 | 466 | ||
| Age, y | 56.1 ± 7.6 | 57.1 ± 7.5 | 57.9 ± 7.3 | 0.001 | 56.8 ± 7.8 | 56.9 ± 7.3 | 57.2 ± 7.4 | 0.67 |
| Sex | <0.0001 | 0.63 | ||||||
| Male | 37.3 | 25.6 | 24.6 | 31.0 | 27.0 | 29.4 | ||
| Female pre- or postmenopausal with estrogen use | 14.0 | 14.6 | 12.0 | 13.4 | 14.6 | 12.7 | ||
| Female postmenopausal with no estrogen use | 48.8 | 59.8 | 63.5 | 55.5 | 58.7 | 57.8 | ||
| BMI | 31.9 ± 7.2 | 32.0 ± 6.6 | 31.7 ± 6.0 | 0.69 | 32.2 ± 6.9 | 31.9 ± 6.6 | 31.5 ± 6.3 | 0.19 |
| Physical activity2 | 31.2 ± 4.4 | 31.6 ± 5.0 | 31.9 ± 4.9 | 0.09 | 31.4 ± 4.7 | 31.8 ± 4.9 | 31.6 ± 4.8 | 0.44 |
| Current smoking | 32.8 | 19.2 | 20.5 | <0.0001 | 23.1 | 26.9 | 22.7 | 0.23 |
| Alcohol use | 0.57 | 0.51 | ||||||
| Nondrinker | 27.8 | 28.5 | 31.6 | 28.8 | 29.7 | 29.9 | ||
| Heavy drinker3 | 43.0 | 41.2 | 38.0 | 43.5 | 38.1 | 40.7 | ||
| Diabetes | 32.8 | 42.3 | 42.1 | 0.003 | 41.0 | 36.5 | 40.0 | 0.36 |
| ≤8th-grade education | 46.2 | 48.8 | 44.9 | 0.24 | 52.2 | 44.7 | 43.4 | 0.05 |
| APOE ε44 | 20.8 | 18.8 | 16.9 | 0.02 | 19.7 | 17.8 | 18.4 | 0.91 |
| Vitamin B-12, pmol/L | 346 ± 163 | 396 ± 216 | 457 ± 221 | <0.0001 | ||||
| Plasma folate, nmol/L | 38.2 ± 16.6 | 41.8 ± 17.2 | 48.8 ± 22.4 | <0.0001 | ||||
| PLP,5 nmol/L | 41.5 ± 38.9 | 53.0 ± 50.0 | 74.8 ± 77.9 | <0.0001 | 41.7 ± 28.6 | 52.5 ± 49.1 | 75.4 ± 83.1 | <0.0001 |
| Global cognitive z score | −0.050 ± 0.6 | −0.047 ± 0.6 | 0.034 ± 0.6 | 0.03 | −0.084 ± 0.6 | −0.015 ± 0.6 | 0.037 ± 0.5 | 0.004 |
| Supplement use | ||||||||
| Multivitamins | 11.8 | 18.6 | 28.2 | <0.0001 | 14.8 | 17.5 | 26.4 | <0.0001 |
| Folic acid | 0.43 | 1.3 | 4.7 | <0.0001 | 1.9 | 1.3 | 3.2 | 0.11 |
| Vitamin B-12 | 1.72 | 1.69 | 2.35 | 0.71 | 1.9 | 1.3 | 2.6 | 0.36 |
| B complex | 3.65 | 5.51 | 7.69 | 0.027 | 5.1 | 3.8 | 7.9 | 0.021 |
| Total folate intake, DFE | 662 ± 336 | 738 ± 398 | 860 ± 414 | <0.0001 | 715 ± 373 | 741 ± 381 | 807 ± 418 | 0.001 |
Values are means ± SDs or percentages unless otherwise indicated. APOE, apolipoprotein E; DFE, Dietary Folate Equivalents; PLP, pyridoxal-5-phosphate.
Physical activity score (range: 24.3–62.6), weighted for total time sleeping and lying down, sitting activity, light activity, moderate activity, and vigorous activity.
>1 drink/d for women or >2 drinks/d for men.
APOE ε4 carrier.
Vitamin B-6.
TABLE 2.
Vitamin B-12 and folate status among Puerto Rican adults1
| Vitamin B-12 status | |||
|---|---|---|---|
| Deficient2 | Not deficient | Total | |
| Plasma folate status | |||
| Deficient3 | 0 | 0 | 0 |
| Not deficient | 114 (8.1) | 1067 (75.8) | 1181 (83.9) |
| High4 | 10 (0.71) | 217 (15.4) | 227 (16.1) |
| Total | 124 (8.8) | 1284 (91.2) | 1408 (100) |
| Intake of folic acid, μg/d | |||
| ≤1000 | 118 (8.9) | 1213 (90.9) | 1331 (99.7) |
| >10005 | 0 | 4 (0.3) | 4 (0.3) |
| Total | 118 (8.9) | 1217 (91.1) | 1335 (100) |
n = 1408 for plasma; n = 1335 for dietary intake. Values are n (%).
Vitamin B-12 deficiency was defined as <148 pmol/L or MMA > 271 nmol/L.
Folate deficiency was defined as <4.53 nmol/L.
High plasma folate was defined as >59 nmol/L.
The upper limit for folic acid intake is >1000 μg/d.
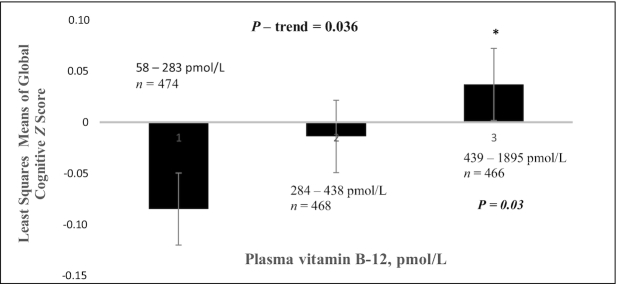
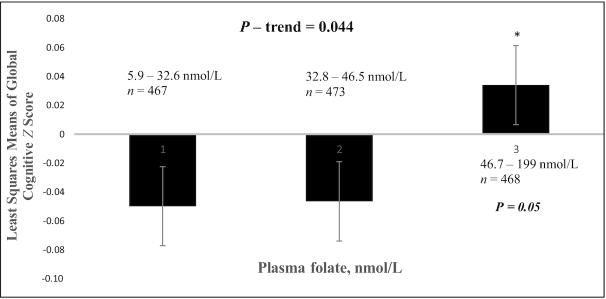
After adjusting for covariates [age, sex (postmenopausal women with no estrogen use, premenopausal or postmenopausal women with estrogen use, or male), APOE (ε4 carrier), BMI, physical activity score, diabetes status (yes/no), education level (less than high school, high school, or higher), smoking (past, current, or none), alcohol use (heavy, none, or moderate), and PLP (nmol/L)], we observed positive associations of both log plasma folate and vitamin B-12, modeled as linear variables, with GCS (β: 0.063; 95% CI: −0.0008, 0.127; P = 0.053 and β: 0.062; 95% CI: 0.009, 0.12; P = 0.023, respectively). Individuals in the highest tertile of plasma vitamin B-12 had significantly higher GCS than those in the lowest tertile, with a mean difference of 0.121 z score units (P = 0.033; P-trend = 0.036) (Figure 2). Individuals in the highest tertile of plasma folate also had higher GCS than those in the lowest tertile, with a mean difference of 0.083 z score units (P = 0.053; P-trend = 0.044) (Figure 3). Plasma folate, vitamin B-12, and PLP concentrations were intercorrelated in our participants, and further, those in the highest tertiles of plasma folate and of vitamin B-12 also had the highest plasma concentration of PLP (Table 1). Deficient vitamin B-12 status, relative to adequate vitamin B-12, was significantly associated with lower GCS (β: −0.119; 95% CI: −0.208, −0.029; P = 0.009) (Table 3). There were too few individuals with high folate and low vitamin B-12 status (<1% of the cohort) to test for interaction.
FIGURE 2.
Global cognitive function by tertile of plasma vitamin B-12, among 1408 Boston Puerto Rican Health Study participants at baseline. Adjusted for age (y), sex (premenopausal or postmenopausal female with estrogen use, female postmenopausal with no estrogen use, or male), BMI (in kg/m2), physical activity score, diabetes (yes/no), education level (≤8th grade, 9th–12th grade, or college), smoking (past, current, or none), alcohol use (heavy, none, or moderate), apoE ε4 carrier (yes/no), vitamin B-6 (pyridoxal-5-phosphate; nmol/L), and folate (nmol/L). *Significantly different from tertile 1 (P = 0.03).
FIGURE 3.
Global cognitive function by tertile of plasma folate, among 1408 Boston Puerto Rican Health Study participants at baseline. Adjusted for age (y), sex (premenopausal or postmenopausal female with estrogen use, female postmenopausal with no estrogen use, or male), BMI (in kg/m2), physical activity score, diabetes (yes/no), education level (≤8th grade, 9th–12th grade, or college), smoking (past, current, or none), alcohol use (heavy, none, or moderate), apoE ε4 carrier (yes/no), vitamin B-6 (pyridoxal-5-phosphate; nmol/L), and vitamin B-12 (pmol/L). *Significantly different from tertile 1 (P = 0.05).
TABLE 3.
Cognitive function (global z score) by plasma vitamin B-12 deficiency (yes/no) among 1408 Boston Puerto Rican Health Study participants at baseline1
| Vitamin B-12 | β (95% CI) | P-trend |
|---|---|---|
| Model 1 | −0.135 (−0.224, −0.047) | 0.003 |
| Model 2 | −0.128 (−0.217, −0.038) | 0.005 |
| Model 3 | −0.119 (−0.208, −0.029) | 0.009 |
1Vitamin B-12 deficiency was defined as <148 pmol/L or MMA > 271 nmol/L. Model 1: adjusted for age (y), sex (premenopausal or postmenopausal female with estrogen use, female postmenopausal with no estrogen use, or male), BMI (in kg/m2), physical activity score, diabetes (yes/no), education level (≤8th grade, 9th–12th grade, or college), smoking (past, current, or none), alcohol use (heavy, none, or moderate), and apoE ε4 carrier (yes/no). Model 2: Model 1 + plasma vitamin B-6 (pyridoxal-5-phosphate; nmol/L). Model 3: Model 2 + plasma folate (nmol/L).
Among the 10 individuals with high folate and low vitamin B-12, 7 were postmenopausal women, 3 were taking a folic acid supplement, 3 a B-complex, 2 multivitamins, and only 1 a vitamin B-12 supplement (Table 4). Interestingly, despite high plasma folate, the mean homocysteine concentration was significantly higher in this group than in those with adequate vitamin B-12 (Table 4).
TABLE 4.
Characteristics of 1408 Boston Puerto Rican Health Study participants, by vitamin B-12 deficiency and high plasma folate status1
| Deficient B-12/high folate | Deficient B-12/not high folate | Adequate B-12/high folate | Adequate B-12/not high folate | P | |
|---|---|---|---|---|---|
| n | 10 | 114 | 217 | 1067 | |
| Age, y | 58.8 ± 7.9 | 59.4 ± 7.8 | 59.07 ± 7.0 | 56.4 ± 7.4 | <0.0001 |
| Sex | 0.002 | ||||
| Male | 2 (20.0) | 43 (37.7) | 44 (20.3) | 321 (30.1) | |
| Pre- or postmenopausal female with estrogen use | 1 (10.0) | 7 (6.1) | 26 (12.0) | 156 (14.6) | |
| Postmenopausal female with no estrogen use | 7 (70.0) | 64 (56.1) | 147 (67.7) | 590 (55.3) | |
| Physical activity2 | 29.7 ± 3.6 | 30.7 ± 4.2 | 32.2 ± 5.4 | 31.5 ± 4.7 | 0.02 |
| PLP,3 nmol/L | 42.0 ± 21.2 | 34.9 ± 21.2 | 79.5 ± 67.4 | 54.2 ± 59.5 | <0.0001 |
| Homocysteine, μmol/L | 15.8 ± 14.7 | 12.7 ± 7.2 | 7.9 ± 2.8 | 8.8 ± 3.9 | <0.0001 |
| Global cognitive z score | −0.269 ± 1.1 | −0.224 ± 0.6 | 0.043 ± 0.5 | −0.009 ± 0.5 | <0.0001 |
| Multivitamin supplement | 2 (20.0) | 15 (13.1) | 75 (34.6) | 183 (17.1) | <0.0001 |
| Folic acid supplement | 3 (30.0) | 2 (1.8) | 13 (6.0) | 12 (1.1) | <0.0001 |
| Vitamin B-12 supplement | 1 (10.0) | 1 (0.9) | 5 (2.3) | 20 (1.9) | 0.23 |
| B complex supplement | 3 (30.0) | 5 (4.4) | 19 (8.8) | 52 (4.9) | 0.0008 |
Values are means ± SDs or n (%) unless otherwise indicated. Groups did not differ significantly in BMI, education, alcohol use, smoking, diabetes, or apoE ε4. Vitamin B-12 deficiency was defined as <148 pmol/L or MMA > 271 nmol/L. High plasma folate was defined as >59 nmol/L. PLP, pyridoxal-5-phosphate.
Physical activity score (range: 24.3–62.6), weighted for total time sleeping and lying down, sitting activity, light activity, moderate activity, and vigorous activity.
Vitamin B-6.
Discussion
As the population continues to age rapidly, dementia is of growing concern. Low concentrations of folate and vitamin B-12 appear to have important roles in the pathogenesis of cognitive impairment in the elderly population. Our findings support the hypothesis that higher plasma folate and vitamin B-12 concentrations are each associated with better GCSs, including linear associations above deficiency states. The β coefficients of 0.063 SD units for log plasma folate and 0.062 for log plasma vitamin B-12 may be comparable with 1 y of global cognitive decline among community-dwelling adults in their mid-70s, without dementia (30). These findings are consistent with other studies, including several studies showing significant associations of low folate and vitamin B-12 with accelerated cognitive decline (7, 29, 31–34) and development of Alzheimer disease (35). It should be noted that although individuals in the highest tertile of plasma vitamin B-12 also had the highest plasma concentrations of folate and PLP, a significant relation between vitamin B-12 and cognition remained after adjusting for the other 2. A recent study identified the presence of microstructural damage in the brains of patients with asymptomatic vitamin B-12 deficiency (36). Importantly, Feldman et al. (37) suggested that, if detected early enough, dementia caused by vitamin B-12 deficiency could be reversible (38). A randomized crossover study reported that whey protein isolate high in vitamin B-12 improved cognitive function in older Australians with low vitamin B-12 status (39).
Vitamin B-12 deficiency appears to be an important problem in this population, with 124 participants (9%) deficient. Malabsorption of vitamin B-12 is thought to be the most common cause of vitamin B-12 deficiency in older adults, because inflammation of the gastric mucosa increases with age and results in a reduction of stomach acid, which is required to cleave vitamin B-12 from protein (6). Vitamin B-12 is an important coenzyme for the methyl donation from 5-methyltetrahydrofolate to tetrahydrofolate and it plays an important role in methionine synthesis (40). A number of methylation reactions take place in the brain, including DNA-methylation, synthesis and degradation of neurotransmitters, and of membrane phospholipids. In addition, vitamin B-12 is essential in the maintenance of the myelin sheath of the central and peripheral nervous systems (41, 42). In either folate or vitamin B-12 deficiency, the methionine synthase reaction will be eventually impaired. Hyperhomocysteinemia in vitamin B-12 or folate deficiency is important as a sign of hypomethylation, for example, of DNA, RNA, myelin, phospholipids, or neurotransmitters. The result is reduced methionine synthesis, with subsequent lowering of the S-adenosyl methionine concentration (42). With insufficient vitamin B-12, methylmalonyl CoA (MMA) accumulates and may result in memory loss due to an accumulation of abnormal fatty acids in the membranes of neural tissue (5, 43). Therefore, vitamin B-12 is central in protecting cognitive function and against dementia in the aging population.
Plasma folate was also associated linearly with higher cognitive function in this population, despite the fact that none of the participants had folate deficiency based on the CDC cutoff point. Folate deficiency sometimes includes consideration of homocysteine. However, high homocysteine in this population was more likely due to the low vitamin B-12 and low PLP, because these were common, whereas none had deficient folate. Folate works with vitamin B-12 in the methionine synthase reaction important for the production of DNA and RNA in cells and tissues (4, 42). Several studies have previously identified a relation between plasma folate and cognitive function cross-sectionally (8, 44, 45) and longitudinally (5, 29, 46).
Since the introduction of folic acid in the American food supply in the 1990s via fortification of cereal grain products, along with the promotion of folic acid for protection against neural tube defects in women of childbearing age, exposure to folic acid has increased in the entire population. The UL for folic acid, established by the National Academies Food and Nutrition Board, is 1000 μg/d (47). With 400 μg in a typical vitamin supplement, 400 μg in a serving of fortified breakfast cereal, and an estimated 200 μg/d from fortified grains (47), it is easy to exceed this limit, raising concern about potential negative effects of high folic acid in combination with vitamin B-12 deficiency. In this cohort, few individuals exceeded 1000 μg/d (n = 4, 0.3%), although this may be underestimated due to the semiquantitative nature of the FFQ.
In this sample, there were too few individuals with vitamin B-12 deficiency and high plasma folate or folic acid intake above the UL, limiting the power to conduct interaction analysis. These results are, therefore, unable to support those reported from the Framingham study (5), as well as other studies, which showed more pronounced metabolic evidence of cognitive impairment with vitamin B-12 deficiency in the presence of high intakes of folic acid, than with lower intakes of folic acid (10, 48, 49). Among the small group with high folate and vitamin B-12 deficiency, supplement use was relatively high for both vitamin B-12 and folate, yet total homocysteine was high, which may suggest that this category contains individuals with vitamin B-12 malabsorption. However, the numbers were insufficient to draw conclusions and the evidence of an adverse impact of high folic acid exposure combined with low vitamin B-12 status on cognitive function must be further studied to evaluate the hypothesis that neurodegenerative consequences of vitamin B-12 deficiency may be exacerbated by high folic acid intake.
Strengths of our study include a comprehensive battery of culturally appropriate tests of cognition, and a thorough assessment of B vitamin status, diet, and relevant covariates. Limitations of our study include insufficient numbers of individuals in the combination of low vitamin B-12 and high folate or high folic acid intake, leading to inconclusive results. Reliance on the FFQ may have led us to underestimate the number of individuals with folic acid exposure > 1000 μg/d. Further limitations include the cross-sectional design, limiting our ability to examine the temporal evidence of cause–effect relations. This cohort study includes Puerto Rican older adults, with on average a lower education than that of the general population, limiting generalizability to other ethnicities and socioeconomic backgrounds.
In conclusion, low plasma vitamin B-12 and low plasma folate were each associated with worse global cognitive function in this high-risk population of Puerto Rican adults. Importantly, vitamin B-12 deficiency, which remained significantly associated with poorer cognitive function after full adjustment for confounders including other B vitamins, was prevalent and should be monitored in aging populations. Larger studies are needed to test the possible detrimental impact of a combination of high folate and vitamin B-12 deficiency status on cognitive decline.
ACKNOWLEDGEMENTS
We thank Xiyuan Zhang for data and statistical support.
The authors’ responsibilities were as follows—TB and KLT: contributed to the design; TB: conducted the data analysis and drafted the manuscript; TMS: contributed expertise regarding analyses of the cognitive function data; NP, J-SL, and KLT: provided analytical feedback and revised the manuscript; KLT: is the Principal Investigator of the Boston Puerto Rican Health Study and had primary responsibility for the final content; and all authors: read, contributed to the writing of, and approved the final manuscript. The authors report no conflicts of interest.
Notes
Supported by NIH grants P01 AG023394 (to KLT), P50 HL105185 (to KLT), and R01 AG055948 (to KLT).
Data Availability: Data described in the article, code book, and analytic code will be made available upon request pending application and approval.
Abbreviations used: APOE, apolipoprotein E; GCS, global cognitive function composite score; MMA, methylmalonic acid; PLP, pyridoxal-5-phosphate; UL, upper limit.
Contributor Information
Tahani Boumenna, Department of Public Health, University of Massachusetts Lowell, Lowell, MA, USA.
Tammy M Scott, Friedman School of Nutrition Science and Policy, Tufts University, Boston, MA, USA; Department of Psychiatry, School of Medicine, Tufts University, Boston, MA, USA.
Jong-Soo Lee, Department of Mathematical Sciences, University of Massachusetts Lowell, Lowell, MA, USA.
Natalia Palacios, Department of Public Health, University of Massachusetts Lowell, Lowell, MA, USA; Department of Nutrition, Harvard School of Public Health, Boston, MA, USA; Geriatric Research Education Clinical Center, Edith Nourse Rogers Memorial Veterans Hospital, Bedford, MA, USA.
Katherine L Tucker, Department of Biomedical and Nutritional Sciences, University of Massachusetts Lowell, Lowell, MA, USA.
References
- 1. Agnew-Blais JC, Wassertheil-Smoller S, Kang JH, Hogan PE, Coker LH, Snetselaar LG, Smoller JW. Folate, vitamin B-6, and vitamin B-12 intake and mild cognitive impairment and probable dementia in the Women's Health Initiative Memory Study. J Acad Nutr Diet. 2015;115:231–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Plassman BL, Langa KM, Fisher GG, Heeringa SG, Weir DR, Ofstedal MB, Burke JR, Hurd MD, Potter GG, Rodgers WL et al. Prevalence of dementia in the United States: the Aging, Demographics, and Memory Study. Neuroepidemiology. 2007;29:125–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ferri CP, Prince M, Brayne C, Brodaty H, Fratiglioni L, Ganguli M, Hall K, Hasegawa K, Hendrie H, Huang Y et al. Global prevalence of dementia: a Delphi consensus study. Lancet. 2005;366:2112–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Mahmood L. The metabolic processes of folic acid and vitamin B12 deficiency. J Health Res Rev. 2014;1:5–9. [Google Scholar]
- 5. Morris MS, Selhub J, Jacques PF. Vitamin B-12 and folate status in relation to decline in scores on the Mini-Mental State Examination in the Framingham Heart Study. J Am Geriatr Soc. 2012;60:1457–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. O'Leary F, Samman S. Vitamin B12 in health and disease. Nutrients. 2010;2:299–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ramos MI, Allen LH, Mungas DM, Jagust WJ, Haan MN, Green R, Miller JW. Low folate status is associated with impaired cognitive function and dementia in the Sacramento Area Latino Study on Aging. Am J Clin Nutr. 2005;82:1346–52. [DOI] [PubMed] [Google Scholar]
- 8. Riggs KM, Spiro A 3rd, Tucker K, Rush D. Relations of vitamin B-12, vitamin B-6, folate, and homocysteine to cognitive performance in the Normative Aging Study. Am J Clin Nutr. 1996;63:306–14. [DOI] [PubMed] [Google Scholar]
- 9. Morris MS, Jacques PF, Rosenberg IH, Selhub J. Folate and vitamin B-12 status in relation to anemia, macrocytosis, and cognitive impairment in older Americans in the age of folic acid fortification. Am J Clin Nutr. 2007;85:193–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Morris MS, Jacques PF, Rosenberg IH, Selhub J. Circulating unmetabolized folic acid and 5-methyltetrahydrofolate in relation to anemia, macrocytosis, and cognitive test performance in American seniors. Am J Clin Nutr. 2010;91:1733–44. [DOI] [PubMed] [Google Scholar]
- 11. Tucker KL, Mattei J, Noel SE, Collado BM, Mendez J, Nelson J, Griffith J, Ordovas JM, Falcon LM. The Boston Puerto Rican Health Study, a longitudinal cohort study on health disparities in Puerto Rican adults: challenges and opportunities. BMC Public Health. 2010;10:107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Chumlea WC, Guo SS, Wholihan K, Cockram D, Kuczmarski RJ, Johnson CL. Stature prediction equations for elderly non-Hispanic white, non-Hispanic black, and Mexican-American persons developed from NHANES III data. J Am Diet Assoc. 1998;98:137–42. [DOI] [PubMed] [Google Scholar]
- 13. Seeman TE, Charpentier PA, Berkman LF, Tinetti ME, Guralnik JM, Albert M, Blazer D, Rowe JW. Predicting changes in physical performance in a high-functioning elderly cohort: MacArthur Studies of Successful Aging. J Gerontol. 1994;49:M97–108. [DOI] [PubMed] [Google Scholar]
- 14. Ye X, Maras JE, Bakun PJ, Tucker KL. Dietary intake of vitamin B-6, plasma pyridoxal 5'-phosphate, and homocysteine in Puerto Rican adults. J Am Diet Assoc. 2010;110:1660–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Tucker KL, Bianchi LA, Maras J, Bermudez OI. Adaptation of a food frequency questionnaire to assess diets of Puerto Rican and non-Hispanic adults. Am J Epidemiol. 1998;148:507–18. [DOI] [PubMed] [Google Scholar]
- 16. Gao X, Martin A, Lin H, Bermudez OI, Tucker KL. α-Tocopherol intake and plasma concentration of Hispanic and non-Hispanic white elders is associated with dietary intake pattern. J Nutr. 2006;136:2574–9. [DOI] [PubMed] [Google Scholar]
- 17. Kwan LL, Bermudez OI, Tucker KL. Low vitamin B-12 intake and status are more prevalent in Hispanic older adults of Caribbean origin than in neighborhood-matched non-Hispanic whites. J Nutr. 2002;132:2059–64. [DOI] [PubMed] [Google Scholar]
- 18. Bermudez OI, Ribaya-Mercado JD, Talegawkar SA, Tucker KL. Hispanic and non-Hispanic white elders from Massachusetts have different patterns of carotenoid intake and plasma concentrations. J Nutr. 2005;135:1496–502. [DOI] [PubMed] [Google Scholar]
- 19. Camp VM, Chipponi J, Faraj BA. Radioenzymatic assay for direct measurement of plasma pyridoxal 5'-phosphate. Clin Chem. 1983;29:642–4. [PubMed] [Google Scholar]
- 20. Araki A, Sako Y. Determination of free and total homocysteine in human plasma by high-performance liquid chromatography with fluorescence detection. J Chromatogr B Biomed Sci Appl. 1987;422:43–52. [DOI] [PubMed] [Google Scholar]
- 21. Lakso HA, Appelblad P, Schneede J. Quantification of methylmalonic acid in human plasma with hydrophilic interaction liquid chromatography separation and mass spectrometric detection. Clin Chem. 2008;54:2028–35. [DOI] [PubMed] [Google Scholar]
- 22. Pfeiffer CM, Sternberg MR, Schleicher RL, Haynes BMH, Rybak ME, Pirkle JL. The CDC's Second National Report on Biochemical Indicators of Diet and Nutrition in the U.S. Population is a valuable tool for researchers and policy makers. J Nutr. 2013;143:938S–47S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Folstein MF, Folstein SE, McHugh PR. “Mini-mental state.” A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–98. [DOI] [PubMed] [Google Scholar]
- 24. Artiola I, Fortuny L, Romo DH, Heaton RK, Pardee RE III. Manual de normas y procedimientos para la bateria neuropsicologia. Tucson, AZ: M Press; 1999. [Google Scholar]
- 25. Wolf-Klein GP, Silverstone FA, Levy AP, Brod MS, Breuer J. Screening for Alzheimer's disease by clock drawing. J Am Geriatr Soc. 1989;37:730–4. [DOI] [PubMed] [Google Scholar]
- 26. Beery K. The Development Test of Visual-Motor Integration manual, revised. Cleveland, OH: Modern Curriculum Press; 1989. [Google Scholar]
- 27. Mattei J, Bigornia SJ, Sotos-Prieto M, Scott T, Gao X, Tucker KL. The Mediterranean diet and 2-year change in cognitive function by status of type 2 diabetes and glycemic control. Diabetes Care. 2019;42:1372–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Palacios N, Scott T, Sahasrabudhe N, Gao X, Tucker KL. Lower plasma vitamin B-6 is associated with 2-year cognitive decline in the Boston Puerto Rican Health Study. J Nutr. 2019;149:635–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Tucker KL, Qiao N, Scott T, Rosenberg I, Spiro A 3rd. High homocysteine and low B vitamins predict cognitive decline in aging men: the Veterans Affairs Normative Aging Study. Am J Clin Nutr. 2005;82:627–35. [DOI] [PubMed] [Google Scholar]
- 30. Jonker C, Schmand B, Lindeboom J, Havekes LM, Launer LJ. Association between apolipoprotein Eϵ4 and the rate of cognitive decline in community-dwelling elderly individuals with and without dementia. Arch Neurol. 1998;55:1065–9. [DOI] [PubMed] [Google Scholar]
- 31. Clarke R, Birks J, Nexo E, Ueland PM, Schneede J, Scott J, Molloy A, Evans JG. Low vitamin B-12 status and risk of cognitive decline in older adults. Am J Clin Nutr. 2007;86:1384–91. [DOI] [PubMed] [Google Scholar]
- 32. Moore E, Ames D, Carne R, Sanders K, Watters D. Cognitive impairment and vitamin B12: a review. Int Psychogeriatr. 2012;24:541–56. [DOI] [PubMed] [Google Scholar]
- 33. Smith AD, Refsum H. Vitamin B-12 and cognition in the elderly. Am J Clin Nutr. 2009;89:707S–11S. [DOI] [PubMed] [Google Scholar]
- 34. Oberlin BS, Tangney CC, Gustashaw KAR, Rasmussen HE. Vitamin B12 deficiency in relation to functional disabilities. Nutrients. 2013;5:4462–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Refsum H, Smith AD. Low vitamin B-12 status in confirmed Alzheimer's disease as revealed by serum holotranscobalamin. J Neurol Neurosurg Psychiatry. 2003;74:959–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Tak AZA, Dayan E, Bulut HT. Evaluation of diffusion tensor imaging changes and neurocognitive effects of asymptomatic vitamin B12 deficiency. Acta Neurol Belg. 2018;118:289–96. [DOI] [PubMed] [Google Scholar]
- 37. Feldman HH, Jacova C, Robillard A, Garcia A, Chow T, Borrie M, Schipper HM, Blair M, Kertesz A, Chertkow H. Diagnosis and treatment of dementia: 2. Diagnosis. Can Med Assoc J. 2008;178:825–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Health Quality Ontario Vitamin B12 and cognitive function: an evidence-based analysis. Ont Health Technol Assess Ser. 2013;13(23): 1–45. [PMC free article] [PubMed] [Google Scholar]
- 39. Zajac IT, Herreen D, Bastiaans K, Dhillon VS, Fenech M. The effect of whey and soy protein isolates on cognitive function in older Australians with low vitamin B12: a randomised controlled crossover trial. Nutrients. 2019;11:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Moretti R, Torre P, Antonello RM, Cattaruzza T, Cazzato G, Bava A. Vitamin B12 and folate depletion in cognition: a review. Neurol India. 2004;52:310–18. [PubMed] [Google Scholar]
- 41. Lindenbaum J, Healton EB, Savage DG, Brust JCM, Garrett TJ, Podell ER, Margell PD, Stabler SP, Allen RH. Neuropsychiatric disorders caused by cobalamin deficiency in the absence of anemia or macrocytosis. N Engl J Med. 1988;318:1720–8. [DOI] [PubMed] [Google Scholar]
- 42. Herrmann W, Obeid R. Causes and early diagnosis of vitamin B12 deficiency. Dtsch Arztebl Int. 2008;105:680–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Metz J. Cobalamin deficiency and the pathogenesis of nervous system disease. Annu Rev Nutr. 1992;12:59–79. [DOI] [PubMed] [Google Scholar]
- 44. Kim G, Kim H, Kim KN, Son JI, Kim SY, Tamura T, Chang N. Relationship of cognitive function with B vitamin status, homocysteine, and tissue factor pathway inhibitor in cognitively impaired elderly: a cross-sectional survey. J Alzheimers Dis. 2013;33:853–62. [DOI] [PubMed] [Google Scholar]
- 45. Elias MF, Robbins MA, Budge MM, Elias PK, Brennan SL, Johnston C, Nagy Z, Bates CJ. Homocysteine, folate, and vitamins B6 and B12 blood levels in relation to cognitive performance: the Maine-Syracuse study. Psychosom Med. 2006;68:547–54. [DOI] [PubMed] [Google Scholar]
- 46. Kado DM, Karlamangla AS, Huang MH, Troen A, Rowe JW, Selhub J, Seeman TE. Homocysteine versus the vitamins folate, B6, and B12 as predictors of cognitive function and decline in older high-functioning adults: MacArthur Studies of Successful Aging. Am J Med. 2005;118:161–7. [DOI] [PubMed] [Google Scholar]
- 47. NIH Office of Dietary Supplements (ODS) Folate fact sheet for health professionals. [Internet] Bethesda, MD: ODS; 2018; [updated 3 June, 2020; cited 15 September, 2020] Available from: https://ods.od.nih.gov/factsheets/Folate-HealthProfessional/#h10. [Google Scholar]
- 48. Tangney CC, Tang Y, Evans DA, Morris MC. Biochemical indicators of vitamin B12 and folate insufficiency and cognitive decline. Neurology. 2009;72:361–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Miller JW, Garrod MG, Allen LH, Haan MN, Green R. Metabolic evidence of vitamin B-12 deficiency, including high homocysteine and methylmalonic acid and low holotranscobalamin, is more pronounced in older adults with elevated plasma folate. Am J Clin Nutr. 2009;90:1586–92. [DOI] [PMC free article] [PubMed] [Google Scholar]