ABSTRACT
Background
Studies have reported a protective relation to cognitive decline with long-term intake of total and individual dietary carotenoids. However, the underlying mechanisms have not yet been clearly established in humans.
Objectives
To evaluate the prospective association between intakes of total and individual carotenoids and risk of incident Alzheimer dementia (AD) and explore the underlying neuropathological basis.
Methods
Among 927 participants from the Rush Memory and Aging Project who were free from AD at baseline and were followed up for a mean of 7 y, we estimated HRs for AD using Cox proportional hazards models by intakes of energy-adjusted carotenoids. Brain AD neuropathology was assessed in postmortem brain autopsies among 508 deceased participants. We used linear regression to assess the association of carotenoid intake with AD-related neuropathology.
Results
Higher intake of total carotenoids was associated with substantially lower hazard of AD after controlling for age, sex, education, ApoE-ε4, participation in cognitively stimulating activities, and physical activity level. Comparing the top and bottom quintiles (median intake: 24.8 compared with 6.7 mg/d) of total carotenoids, the multivariate HR (95% CI) was 0.52 (0.33, 0.81), P-trend < 0.01. A similar association was observed for lutein-zeaxanthin, a weaker linear inverse association was observed for β-carotene, and a marginally significant linear inverse association was found for β-cryptoxanthin. Among the deceased participants, consumers of higher total carotenoids (top compared with bottom tertile, 18.2 compared with 8.2 mg/d) had less global AD pathology (b: −0.10; SE = 0.04; P-trend = 0.01). For individual carotenoids, lutein-zeaxanthin and lycopene were inversely associated with brain global pathology, whereas lutein-zeaxanthin showed additional inverse associations with AD diagnostic score, neuritic plaque severity, and neurofibrillary tangle density and severity.
Conclusions
Our findings support a beneficial role of total carotenoid consumption, in particular lutein/zeaxanthin, on AD incidence that may be related to the inhibition of brain β-amyloid deposition and fibril formation.
Keywords: dietary carotenoids, Alzheimer dementia, neuropathology, prospective cohort study, cognitive function
Introduction
Alzheimer disease, the most common form of dementia, is projected to influence more than 8 million older adults in the United States in the next decade, as a consequence of rapid population aging and the lack of effective means to prevent the disease (1–7).
Carotenoids are naturally occurring pigments found in red, yellow, orange, and dark green fruits and vegetables. The following 6 carotenoids account for almost all of the carotenoids found in human blood and brain: lutein, zeaxanthin, lycopene, α-carotene, β-carotene, and β-cryptoxanthin (8–10). Given their antioxidative and anti-inflammatory properties and their presence in the brain (11, 12), the role of carotenoids in preventing or treating neurodegenerative diseases, including Alzheimer disease, is of specific interest.
A growing literature suggests that carotenoids play a role in the prevention of cognitive decline and Alzheimer disease (13–15). Several long-term observational studies and randomized controlled trials have reported a protective relation to cognitive decline with long-term intake of total and individual dietary carotenoids (16–19). However, the underlying mechanisms have not yet been clearly established in humans.
We previously reported a linear association of lutein/zeaxanthin and β-carotene (marginally) with slower cognitive decline in a prospective study of 960 participants followed up over a mean of 4.7 y from the Memory and Aging Project (MAP) (20). With additional information on Alzheimer dementia (AD) clinical diagnosis and brain neuropathology, the present study was performed to provide more detailed insights into the roles of total and individual carotenoids in the development of AD.
Methods
Study design
The study was conducted among participants of the Rush MAP, a study of volunteers living in retirement communities and senior public housing units in the Chicago area. The ongoing open cohort study began in 1997 and includes annual clinical neurological examinations, as previously described (21). Beginning in 2004, the MAP study participants were invited to complete FFQs. As of January 2019, 2096 older persons had enrolled in the MAP study, 80 died, and 159 withdrew before the diet study began. Of these 1857 participants, 1068 completed the dietary questionnaires, 927 of whom had at least a baseline and 1 follow-up neuropsychological assessment by 2018 and were clinically determined not to have AD at the baseline. During this period, 508 of the participants died and autopsies were performed (Supplementary Figure 1). The Institutional Review Board of Rush University Medical Center approved the study, and all participants gave written informed consent.
Dietary assessment
Dietary intake was assessed with a 144-item semiquantitative FFQ (SFFQ) that was validated for use in older Chicago residents (22). Participants reported how often, on average, they had consumed a standard portion size of each food over the previous 12 mo at each annual clinical evaluation. Daily intakes of specific nutrients were obtained by multiplying the nutrient content of the individual food items by the frequency of consumption and summing over all items. SFFQs with plausible energy intake (500–3800 kcal/d for women and 700–4000 kcal/d for men) and <61 blank food items were included in the analysis. For AD incidence, intakes of carotenoids from the first SFFQ were used to best represent long-term diet before symptoms related to disease onset. In the cross-sectional analyses of postmortem AD neuropathology, carotenoid intake was averaged over all available valid SFFQs as the best representation of intake premortem. Dietary intake of carotenoids included β-carotene, α-carotene, lutein-zeaxanthin, lycopene, and β-cryptoxanthin. Each participant's nutrient intakes were adjusted for total energy intake using the residual method within sex (23). In a validation study of 232 randomly selected CHAP (Chicago Health and Aging Project) participants, the original cohort for the MAP study, the average energy-adjusted Pearson correlation coefficient was 0.46 for comparative validity between nutrient intake levels on the SFFQ and the dietary recalls, without accounting for the random error in the dietary recall assessments (22). In linear regression models adjusted for age, sex, race, total energy intake, and total plasma cholesterol and triglyceride concentrations for 56 of these participants, the partial correlation between plasma concentration and intake amounts was 0.43 for β-carotene and 0.46 for β-cryptoxanthin, without accounting for the random error in the plasma biomarker measurements (24). Of the carotenoids, only β-carotene was regularly included in vitamin supplements throughout the dietary reporting period; thus, we evaluated supplemental β-carotene separately. Average total energy intake and major nutrients from the dietary questionnaires were also calculated.
Assessment of AD
Clinical diagnosis of probable AD was determined at each annual evaluation as previously described (25). Briefly, the AD diagnosis was made by an experienced clinician using data from a structured neurological examination and medical history, and cognitive performance testing, and with the assistance of an algorithmically based rating of cognitive impairment. The AD diagnosis was based on criteria of the joint working group of the NINCDS-ADRDA (National Institute of Neurological and Communicative Disorders and Stroke and the Alzheimer's Disease and Related Disorders Association) (26), which requires a history of cognitive decline and impairment in memory and at least 1 other cognitive domain. According to these diagnostic criteria, there were 237 incident cases of AD during the follow-up from 2004 to 2018. A board-certified neurologist blinded to the dietary assessment data reviewed all clinical evaluation data before making clinical diagnoses of neurological diseases.
Assessment of brain AD-related neuropathology
The methods for brain autopsies and pathologic evaluations have been described in detail elsewhere (27–29). Briefly, slabs from 1 cerebral hemisphere were placed in a −80°C freezer. Slabs from the contralateral hemisphere were fixed in 4% paraformaldehyde, and then dissected tissue samples from specific brain regions were embedded in paraffin blocks, cut into 6-micron sections, and mounted on slides. The mean ± SD postmortem interval was 8.3 ± 7.4 h.
AD neuropathology, including diffuse amyloid plaques (using immunohistochemistry), neuritic plaques (plaques most closely associated with neuronal injury), and neurofibrillary tangles were identified by use of modified Bielschowsky silver–stained 6-micron sections in multiple cortical regions (entorhinal cortex proper, hippocampus, superior frontal cortex, dorsolateral prefrontal cortex, inferior temporal cortex, angular gyrus cortex, anterior cingulate cortex, and calcarine cortex). Raw counts (greatest density in 1mm2 area) of the neuritic and diffuse plaques and tangles were standardized in each region and averaged across regions to create 3 summary scores that were then averaged to yield a global measure of AD pathology. We also analyzed 2 established semiquantitative measures of AD pathology, the Consortium to Establish a Registry for Alzheimer's Disease (CERAD) neuritic plaque density (30), and Braak neurofibrillary tangle stage (31). CERAD scores ranged from 1 (no neuritic plaques) to 4 (frequent neuritic plaques). Braak staging scores ranged from 0 (no tangles in any region) to 6 (frequent tangles across multiple regions). Another variable describes the level of AD pathology based on National Institute on Aging (NIA)-Reagan criteria (32) with scores ranging from 1 (low AD) to 4 (high AD). A board-certified neuropathologist blinded to age and clinical data made the neuropathology diagnoses.
Assessment of other covariates
Information on covariates of interest in the analyses, including lifestyle factors and medical history, was obtained from structured interview questions and measurements at the participants’ baseline clinical evaluations. Age (y) was computed from self-reported birth date and date of the baseline cognitive assessment. Education (y) was self-reported as years of regular schooling. Apolipoprotein E (ApoE) genotyping was performed using high-throughput sequencing, as previously described (33–35). Participation in cognitively stimulating activities was computed as the average frequency rating, based on a 5-point scale, of different activities (e.g., reading, playing games, writing letters, visiting the library) (36). Physical activity (h per wk) was computed from self-reported minutes spent within the previous 2 wk on 5 activities: walking for exercise, yard work, calisthenics, biking, and water exercise (37). Depressive symptoms (number) were assessed by a modified 10-item version of the Center for Epidemiological Studies depression scale (38). BMI (kg/m2) was computed from measured weight and height and modeled as 2 indicator variables, BMI: ≤20 and ≥30. Hypertension history was determined by self-reported medical diagnosis, measured blood pressure (average of 2 measurements ≥160 mmHg systolic or ≥90 mmHg diastolic), or current use of hypertensive medications. Myocardial infarction history was based on self-reported medical diagnosis or use of cardiac glycosides (e.g., lanoxin or digitoxin). Diabetes history was determined by self-reported medical diagnosis or current use of diabetic medications. Medication use was based on interviewer inspection of agents brought to clinic. Clinical diagnosis of stroke was based on clinician review of self-reported history, neurological examination, and cognitive testing history (39).
Statistical analyses
We used proportional hazards models to calculate HRs and 95% CIs for the relations between intakes of total and individual carotenoids and time (y) to diagnosis of AD. The proportionality of hazards assumption was satisfied by using the Schoenfeld's residuals method and the time-varying covariates method. Intakes of carotenoids were divided into quintiles. We first examined the relations in separate age-adjusted and basic adjusted models. The basic model included potential confounders with the most established evidence for association with AD: age (y), sex, education (y), participation in cognitively stimulating activities, physical activity, and ApoE-ε4 (at least 1 allele compared with none). Further analyses added the following covariates to the basic adjusted models: 1) cardiovascular conditions, which have high likelihoods of mediating the dietary effects on dementia, and 2) depression and weight measures, which may act as effect mediators, but also have complex cause and effect relations with dementia. A complete data approach was used in the analyses; therefore, the extended model may have a smaller sample size (n = 853–927), depending on the availability of the additionally added covariates. To examine if the observed associations were independent of other nutrients and lifestyle factors, we conducted further analyses adjusting for smoking status, multivitamin use, folate, vitamin B12, vitamin E, and DHA. We also report the P value for linear trend based on a categorical variable of the quintiles scored at the quintile median. To reduce the potential influence of reverse causation, we conducted sensitivity analyses by eliminating cases that occurred within the first 2 y of follow-up. Effect modification was investigated for the total and individual carotenoids and each covariate by including a multiplicative term between the carotenoids and the potential effect modifier in the basic-adjusted model and testing at P < 0.05.
Among 508 deceased participants with brain neuropathologic outcomes, we used linear regression models to investigate the relations of the carotenoids to AD neuropathologic outcomes (square root scale of global AD pathology, NIA Reagan score, square root scale of overall amyloid level, CERAD score, square root scale of neurofibrillary tangles density, and Braak score) (40). Intakes of carotenoids were divided into tertiles. Models were adjusted for age at death, sex, and education. All analyses were conducted using SAS software, version 9.3 (SAS Institute Inc.).
Results
A total of 237 incident cases of AD developed over an average follow-up of 7 y in the sample of 927 MAP participants with an average age of 81 y at study baseline. The mean time to AD diagnosis from the date that diet was assessed was 6.6 y (range: 1–14 y). Participants in the lowest quintile of carotenoid intake had higher energy consumption, more depression symptoms, more diabetes and stroke diagnoses, and lower levels of physical activity, and were less likely to have a BMI ≤20 kg/m2 (Table 1).
TABLE 1.
Baseline characteristics of MAP participants1
| Total carotenoid intake | ||||
|---|---|---|---|---|
| Baseline characteristic | Overall (n = 927) | Q1 (median = 6.7 mg/d, n = 185) | Q3 (median = 13.2 mg/d, n = 186) | Q5 (median = 24.8 mg/d, n = 186) |
| Age, mean ± SD, y | 81 ± 7 | 82 ± 7 | 81 ± 7 | 80 ± 7 |
| Males, % | 25.1 | 24.9 | 27.4 | 23.7 |
| Education, mean ± SD, y | 15 ± 3 | 14 ± 3 | 15 ± 3 | 15 ± 3 |
| ApoE-ε4, % | 21.6 | 23.2 | 27.4 | 18.8 |
| Total energy intake, kcal/d | 1734 ± 539 | 1930 ± 694 | 1721 ± 514 | 1570 ± 417 |
| Cognitive activity frequency, rating | 3.2 ± 0.6 | 3.1 ± 0.7 | 3.3 ± 0.6 | 3.3 ± 0.7 |
| Physical activity, h/wk | 3.4 ± 3.6 | 2.6 ± 3.5 | 3.4 ± 3.7 | 4.3 ± 4.1 |
| Depressive symptoms, n | 1.0 ± 1.6 | 1.3 ± 1.7 | 1.0 ± 1.3 | 0.9 ± 1.5 |
| BMI, % (n = 897) | ||||
| ≤20 | 3.8 | 2.3 | 2.7 | 5.5 |
| ≥30 | 24.5 | 24.9 | 19.7 | 26.5 |
| Medical condition, % | ||||
| Diabetes | 12.9 | 17.8 | 7.0 | 13.4 |
| Hypertension (n = 920) | 69.0 | 67.2 | 69.4 | 67.8 |
| Myocardial infarction | 16.2 | 17.3 | 18.3 | 14.5 |
| Stroke (n = 853) | 11.5 | 15.8 | 8.7 | 9.3 |
1Values are means ± SDs or percentages. ApoE, apolipoprotein E; MAP, Memory and Aging Project; Q, quintile.
Total carotenoids intake was significantly associated, linearly and statistically, with lower risk of developing AD in the age-adjusted model (Table 2). In the basic model adjusted for age, sex, education, ApoE-ε4, physical activity, and participation in cognitively stimulating activities, participants in the top quintile of total carotenoids (median intake: 24.8 mg/d) had a 48% (HR: 0.52; 95% CI: 0.33, 0.81; P-trend < 0.01) lower rate of developing AD than participants in the lowest quintile (median intake: 6.7 mg/d). The results were similar after further adjusting for cardiovascular conditions (including diabetes, hypertension, stroke, and myocardial infarction), depressive symptoms, and low or high BMI. The associations were also similar after further adjusting for intakes of alcohol, vitamin E, folate, vitamin B12, and DHA, and use of multivitamins. Moreover, when further adjusted for the Mediterranean diet score or the DASH dietary score, which were previously linked to the dementia outcomes in our study, the associations remained similar (data not shown). After eliminating cases that occurred within the first 2 y of follow-up, we observed similar findings between total carotenoids (top compared with bottom quintile) and AD incidence (HR: 0.60; 95% CI: 0.36, 1.00; P < 0.05). In further analyses, we did not find evidence for effect modification by age, ApoE-ε4 status, education, physical activity, obesity, and smoking. However, statistically significant interactions were observed for sex; carotenoid intake was found to be more protective in men than in women (P-interaction = 0.02) in the stratified analysis by sex using quintiles for the total population (Supplementary Figure 2).
TABLE 2.
Proportional HR and 95% CI of estimated effects of total carotenoids on time to incident AD in age-adjusted (n = 927; 237 AD cases) and basic-adjusted* models in MAP participants over a mean 7- follow-up, 2004–20181
| Total carotenoids | Q1 (median = 6.7 mg/d, n = 185) | Q2 (median = 10.3 mg/d, n = 185) | Q3 (median = 13.2 mg/d, n = 186) | Q4 (median = 16.7 mg/d, n = 185) | Q5 (median = 24.8 mg/d, n = 186) | P-trend |
|---|---|---|---|---|---|---|
| Person-y | 1142 | 1200 | 1275 | 1149 | 1397 | |
| AD incident cases, n | 61 | 47 | 42 | 56 | 31 | |
| Age adjusted | 1 (Referent) | 0.81 (0.56, 1.19) | 0.70 (0.47, 1.04) | 0.89 (0.62, 1.28) | 0.47 (0.31, 0.73) | 0.002 |
| Basic adjusted2 | 1 (Referent) | 0.93 (0.63, 1.36) | 0.73 (0.49, 1.09) | 1.00 (0.69, 1.45) | 0.52 (0.33, 0.81) | 0.009 |
| Basic-adjusted + cardiovascular conditions | 1 (Referent) | 0.98 (0.65, 1.47) | 0.70 (0.46, 1.07) | 1.03 (0.70, 1.50) | 0.54 (0.34, 0.85) | 0.015 |
| Basic-adjusted + BMI, depression | 1 (Referent) | 0.97 (0.65, 1.43) | 0.70 (0.46, 1.05) | 1.00 (0.69, 1.45) | 0.52 (0.33, 0.82) | 0.008 |
Values are HRs (95% CIs) or n unless otherwise indicated. AD, Alzheimer dementia; ApoE, apolipoprotein E; MAP, Memory and Aging Project; Q, quintile.
Basic-adjusted model included terms for age, sex, education, ApoE-ε4 (any), participation in cognitively stimulating activities, and physical activity.
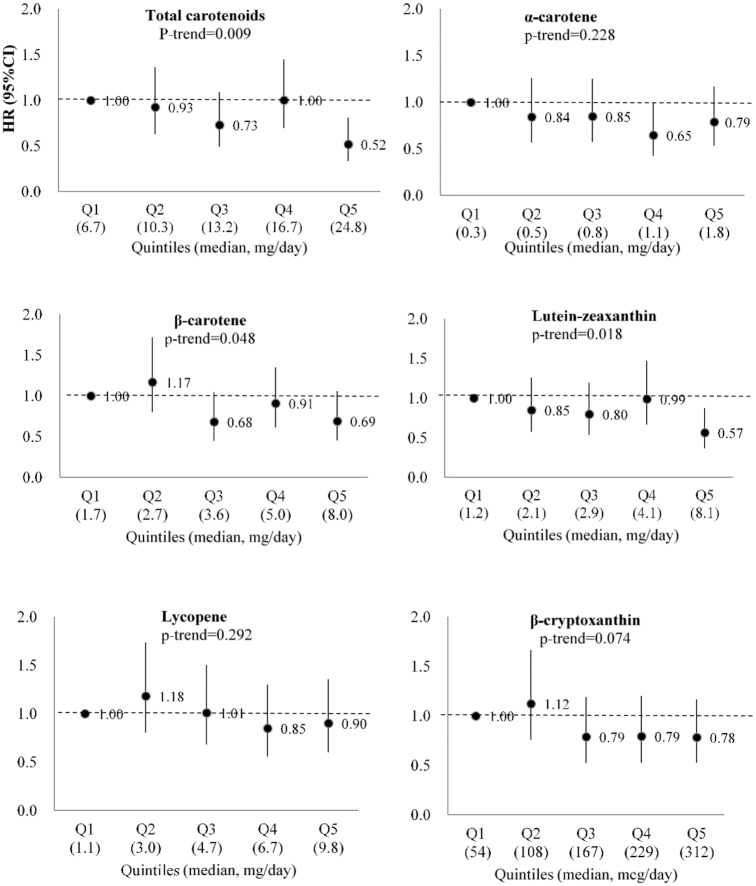
In the analyses for individual carotenoids, greater intake of lutein/zeaxanthin was associated with lower risk of developing AD in the age-adjusted model and the basic model (Figure 1 and Supplementary Table 1). Comparing the highest compared with the lowest quintiles of lutein/zeaxanthin, the HR for AD was 0.57 (95% CI: 0.37, 0.87; P-trend = 0.02) in the basic model. A significant linear inverse association was observed for dietary β-carotene, and a marginally significant linear inverse association was found for β-cryptoxanthin in the basic model. The linear association for β-cryptoxanthin became significant after further adjustment of BMI and depression level. No significant associations were observed for α-carotene, lycopene, and use of β-carotene supplement. Intakes of α-carotene and lutein-zeaxanthin were highly correlated with β-carotene (Spearman correlation, r = 0.81 and 0.84, respectively) (Supplementary Table 2). In our study population, the major food source of carotenoids included tomatoes, tomato juice, tomato sauce, carrots, yams/sweet potatoes, squash, kale, and spinach. Lutein/zeaxanthin were found to be highly related to vegetables, especially dark-green leafy vegetables, in this cohort (Supplementary Table 3).
FIGURE 1.
HR for Alzheimer dementia across increasing quintiles of total and individual carotenoid intakes (n = 927; 237 AD cases) in basic-adjusted models in MAP participants over a mean 7 y follow-up, 2004–2018. Footnote: Multivariate model was adjusted for age, sex, education, ApoE-ε4 (any), participation in cognitively stimulating activities, and physical activity. AD, Alzheimer dementia; ApoE, apolipoprotein E; MAP, Memory and Aging Project.
In the analyses with brain AD pathology, dietary intakes of total carotenoids, lutein, and lycopene were each linearly associated with less global AD pathology (P values < 0.05). In particular, total carotenoid consumers (top compared with bottom quintile) had less global AD pathology (b = −0.10, SE = 0.04, P-trend = 0.01), lower AD diagnostic score (NIA-Reagan classification: b = −0.17, SE = 0.08, P-trend = 0.03) and lower CERAD score (neuritic plaque severity: b = −0.29, SE = 0.12, P-trend = 0.02) in separate models adjusted for age at death, sex, and education (Table 3). For individual carotenoids, participants with higher intake of lutein-zeaxanthin (top compared with bottom quintile) had less global AD pathology (b = −0.12, P-trend < 0.01), lower AD diagnostic score (b = −0.20, P-trend = 0.02), lower CERAD score (neuritic plaque severity: b = −0.31, P-trend = 0.02), lower neurofibrillary tangles density (b = −0.32, P-trend = 0.04), and lower Braak stage (neurofibrillary tangles stage: b = −0.41, P-trend < 0.01). Significant associations were also observed for higher lycopene intake with less global AD pathology (b = −0.09, P- trend = 0.02) (Table 4). No significant associations were observed for the other carotenoids.
TABLE 3.
Effect estimates and P values for total carotenoids with AD neuropathology based on adjusted linear regression models (AD neuropathology) among 508 deceased MAP participants, 2004–20181
| Linear regression models of AD neuropathology | ||||||||
|---|---|---|---|---|---|---|---|---|
| Total carotenoids (energy-adjusted) | n | Median, µg/d | Global AD pathology | AD diagnostic score2 | Overall amyloid level | Neuritic plaque density3 | Neurofibrillary tangles density | Neurofibrillary tangles stage4 |
| Point estimate (standard error) | ||||||||
| Tertile 1 | 168 | 8185 | Referent | Referent | Referent | Referent | Referent | Referent |
| Tertile 2 | 173 | 12,715 | −0.06 (0.04) | −0.13 (0.08) | −0.11 (0.13) | −0.21 (0.12) | −0.14 (0.14) | −0.11 (0.13) |
| Tertile 3 | 167 | 18,221 | −0.10 (0.04) | −0.17 (0.08) | −0.21 (0.13) | −0.29 (0.12) | −0.17 (0.15) | −0.20 (0.13) |
| P-trend | 0.01 | 0.03 | 0.12 | 0.02 | 0.26 | 0.13 | ||
Models adjusted for age at death, sex, and education. AD, Alzheimer dementia; CERAD, Consortium to Establish a Registry for Alzheimer's Disease; MAP, Memory and Aging Project; NIA, National Institute on Aging.
NIA Reagan score.
CERAD score.
Braak Score.
TABLE 4.
Effect estimates and P values for individual carotenoids with AD neuropathology based on adjusted linear regression models (AD neuropathology) among 508 deceased MAP participants, 2004–20181
| Linear regression models of AD neuropathology point estimate, P for linear trend | ||||||||
|---|---|---|---|---|---|---|---|---|
| Individual carotenoids | N | Median (mcg/d) | Global AD pathology | AD diagnostic score2 | Overall amyloid level | Neuritic plaque density3 | Neurofibrillary tangles density | Neurofibrillary tangles stage |
| α-carotene (energy- adjusted), µg/d | ||||||||
| Tertile 1 | 168 | 405 | Referent | Referent | Referent | Referent | Referent | Referent |
| Tertile 2 | 173 | 817 | −0.01 | −0.02 | 0 | −0.07 | 0.10 | −0.01 |
| Tertile 3 | 167 | 1475 | −0.02 | −0.08 | −0.05 | −0.22 | −0.01 | −0.06 |
| P-trend | 0.50 | 0.27 | 0.70 | 0.07 | 0.83 | 0.66 | ||
| β-carotene (energy adjusted), µg/d | ||||||||
| Tertile 1 | 168 | 2063 | Referent | Referent | Referent | Referent | Referent | Referent |
| Tertile 2 | 173 | 3693 | −0.04 | −0.02 | −0.1 | −0.09 | −0.05 | −0.07 |
| Tertile 3 | 167 | 5986 | −0.04 | −0.10 | −0.04 | −0.17 | −0.14 | −0.17 |
| P-trend | 0.39 | 0.18 | 0.84 | 0.19 | 0.34 | 0.19 | ||
| Lutein-zeaxanthin (energy adjusted), µg/d | ||||||||
| Tertile 1 | 168 | 1487 | Referent | Referent | Referent | Referent | Referent | Referent |
| Tertile 2 | 173 | 2833 | −0.11* | −0.16* | −0.21 | −0.27* | −0.27* | −0.29* |
| Tertile 3 | 167 | 5434 | −0.12* | −0.20* | −0.21 | −0.31* | −0.32* | −0.41* |
| P-trend | <0.01* | 0.02* | 0.17 | 0.02* | 0.04* | <0.01* | ||
| Lycopene (energy adjusted), µg/d | ||||||||
| Tertile 1 | 168 | 2027 | Referent | Referent | Referent | Referent | Referent | Referent |
| Tertile 2 | 173 | 4728 | −0.04 | −0.05 | −0.02 | −0.06 | −0.04 | −0.07 |
| Tertile 3 | 167 | 7314 | −0.09* | −0.10 | −0.17 | −0.19 | −0.01 | 0.00 |
| P-trend | 0.02* | 0.17 | 0.19 | 0.13 | 0.95 | 0.96 | ||
| β-cryptoxanthin (energy-adjusted), mcg/d | ||||||||
| Tertile 1 | 168 | 76 | Referent | Referent | Referent | Referent | Referent | Referent |
| Tertile 2 | 173 | 166 | 0.04 | −0.01 | 0.14 | 0.09 | −0.01 | −0.18 |
| Tertile 3 | 167 | 267 | −0.04 | −0.13 | −0.10 | −0.18 | −0.15 | −0.12 |
| P-trend | 0.25 | 0.09 | 0.40 | 0.13 | 0.28 | 0.40 | ||
Models adjusted for age at death, sex, and education. AD, Alzheimer dementia; CERAD, Consortium to Establish a Registry for Alzheimer's Disease; MAP, Memory and Aging Project; NIA, National Institute on Aging.
NIA Reagan score.
CERAD score.
p-value significant at 0.05.
Discussion
In this cohort of 927 elderly Midwestern residents with an average of 7 y of follow-up, higher intake of total carotenoids (median intake of 24.8 compared with 6.7 mg/d) was associated with a 48% reduction in the rate of AD. The association was independent of other healthy lifestyle behaviors, cardiovascular-related conditions, dietary fats, and other dietary antioxidant nutrients. Similar associations were observed for lutein/zeaxanthin, and a weaker association was observed for β-carotene and β-cryptoxanthin. Among 508 deceased participants, higher total carotenoid consumers, in particular higher consumers of lutein/zeaxanthin and lycopene, had less AD-related neuropathology.
These findings extend results of an earlier study in this cohort (20), based on a shorter follow-up time (average of 4.7 y), which indicated that higher consumption of lutein-zeaxanthin and β-carotene was significantly associated with slower cognitive decline. With the advantage of additional information on AD clinical diagnosis over a longer follow-up period, we additionally observed a strong inverse association of intake of total carotenoids and lutein-zeaxanthin with risk of AD. Some associations were also observed for β-carotene and β-cryptoxanthin. Furthermore, the results are consistent with several long-term observational studies reporting apparent protective effect on cognitive decline and AD with intake of lutein (16, 17) and β-carotene (17, 18), with follow-up times of 7 to >17 y. Placebo-controlled randomized studies also suggest that supplementation with β-carotene (50 mg every other day for 18 y among healthy men) (19), lutein, or lutein plus zeaxanthin (12 mg/d for 1 y among elderly women and men) may have beneficial cognitive effects (41, 42). In our analyses of specific carotenoids, we found strong and robust inverse associations between lutein/zeaxanthin intake and development of AD. For the highest compared with the lowest lutein/zeaxanthin intake, the corresponding mean intakes of green leafy vegetables were around 1.3 servings/d compared with 0.1 serving/d. Potential beneficial roles for β-carotene and β-cryptoxanthin were also suggested. Taken together, substantial evidence suggests that dietary carotenoids have protective associations against the AD development, and this effect may not be limited to a single carotenoid.
Our study is one of a few that have reported an apparent beneficial role of carotenoids with a wide spectrum of brain AD-related neuropathology in humans. In addition to the strong inverse associations of total carotenoids, lutein/zeaxanthin, and lycopene with global AD pathology score, higher intakes of total carotenoids and lutein/zeaxanthin were significantly associated with less severe neurofibrillary tangle pathology. Moreover, lutein/zeaxanthin intake was inversely associated with the amyloid plaque severity and neurofibrillary tangle density. The findings are supported by laboratory studies showing that carotenoids may protect the brain against pathology through their antioxidant properties (43, 44) and anti-inflammatory functions by interacting with inflammatory signaling cascades (45). Individual carotenoids may also act through other mechanisms relevant to brain function. Among them, lutein has received particular interest as a major carotenoid specifically present in the macula and the brain (46). The present study provides strong additional evidence to support the protective role of carotenoids in the development of neurodegeneration. More research is needed to establish and confirm the brain-protective effects of specific carotenoids.
The present study has a number of strengths. The prospective study design, long-term follow-up, and careful control of various potential confounders minimize selection bias and reverse causation and provide relatively valid estimates of associations. Moreover, the diagnosis of AD was based on annual neuropsychological testing and structured clinical neurological evaluations by clinicians. The dietary intake of carotenoids was assessed with a comprehensive SFFQ that was validated in older community-dwelling Chicago residents. These features reduce the potential for biased and random misclassification of disease status and diet exposures in the analyses. The average dietary intakes calculated from multiple years further dampen within-subject variation and best represent habitual diet. Last but not least, all postmortem neuropathology measures were quantified by experienced and trained examiners blinded to all clinical data. The high autopsy rate reduces selection bias, and the neuropathology measurements based on averaged assessments over brain regions help to minimize bias due to measurement error. The study also has limitations. The primary limitation is the observational study design, such that the study findings may be subject to residual and unmeasured confounding by other disease and lifestyle factors. However, the estimates of dietary associations on AD remained similar after statistical adjustment for many important risk factors for AD, suggesting that confounding is not a likely explanation for the observed associations. Second, the subjective measure of dietary intake levels may be subject to errors. However, the dietary tool was validated with biochemical and other dietary assessments, and random errors would be unlikely to create the strong associations that we observed. Another limitation is the possibility that incident cases may have undergone dietary changes at the time of baseline assessment as a result of preclinical disease. We investigated this issue by reanalyzing the data after eliminating cases that occurred within the first 2 y of follow-up, and we observed similar findings between total carotenoids and AD incidence. Moreover, the most plausible confounding factors are other constituents of the foods high in the carotenoids that we studied. These constituents include numerous phytochemicals that alone or in combination could exert neuroprotective effects. For this reason, caution is warranted in the interpretation of our findings. However, the fact that β-carotene (19) and lutein (47), respectively, as an isolated substance, influenced cognitive function in randomized trials supports a causal interpretation of our findings. Finally, our findings were observed among primarily white and well-educated Midwestern elderly adults in the United States, and therefore, the findings may not be generalized to other populations.
This prospective study supports a potential beneficial role of carotenoids in AD development and AD neuropathology, possibly through the inhibition of brain β-amyloid deposition and fibril formation. Our findings most strongly support benefits of lutein-zeaxanthin. Carotenoids are promising dietary bioactives: further evidence from long-term randomized trials would be desirable for a definitive determination that dietary carotenoids protect against the development of AD.
Supplementary Material
ACKNOWLEDGEMENTS
We dedicate this paper to Dr. Martha Clare Morris, who passed away peacefully following a battle with cancer before the paper was submitted for publication. Dr. Martha played an essential role in producing this paper and is greatly missed.
The authors’ contributions were as follows—MCM, CY: designed the analysis; YW: performed the statistical analyses; CY and HC: interpreted the data and drafted and revised the manuscript; MCM: supervised the data analysis and interpretation; MCM, CY: had the primary responsibility for the study final content; all authors: critically reviewed the manuscript and approved the final draft; and all authors: read and approved the final manuscript. Author disclosures: The authors report no conflicts of interest.
Notes
Supported by the National Institutes of Health (R01AG031553 and R01AG17917). The funding agencies had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Supplementary Tables 1–3 and Supplementary Figures 1 and 2 are available from the “Supplementary data” link in the online posting of the article and from the same link in the online table of contents at https://academic.oup.com/ajcn/.
Data Availibility: Data described in the manuscript, code book, and analytic code will be made available upon request pending application and approval (https://www.rushu.rush.edu/research/departmental-research/memory-and-aging-project).
Abbreviations used: AD, Alzheimer dementia; CERAD, Consortium to Establish a Registry for Alzheimer's Disease; MAP, Memory and Aging Project; NIA, National Institute on Aging; SFFQ, semiquantitative food frequency questionnaire.
Contributor Information
Changzheng Yuan, Department of Big Data and Health Science, Zhejiang University School of Public Health, Hangzhou, Zhejiang, China; Department of Nutrition, Harvard TH Chan School of Public Health, Boston, MA, USA; Channing Division of Network Medicine, Brigham and Women's Hospital and Harvard Medical School, Boston, MA, USA.
Hui Chen, Department of Big Data and Health Science, Zhejiang University School of Public Health, Hangzhou, Zhejiang, China.
Yamin Wang, Rush Institute for Healthy Aging, Rush University Medical Center, Chicago, IL, USA.
Julie A Schneider, Rush Alzheimer's Disease Center, Rush University Medical Center, Chicago, IL, USA.
Walter C Willett, Department of Nutrition, Harvard TH Chan School of Public Health, Boston, MA, USA; Channing Division of Network Medicine, Brigham and Women's Hospital and Harvard Medical School, Boston, MA, USA; Department of Epidemiology, Harvard TH Chan School of Public Health, Boston, MA, USA.
Martha Clare Morris, Rush Institute for Healthy Aging, Rush University Medical Center, Chicago, IL, USA.
Reference
- 1. Schaller S, Mauskopf J, Kriza C, Wahlster P, Kolominsky-Rabas PL. The main cost drivers in dementia: a systematic review. Int J Geriatr Psychiatry. 2015;30(2):111–29. [DOI] [PubMed] [Google Scholar]
- 2. Hebert LE, Weuve J, Scherr PA, Evans DA. Alzheimer disease in the United States (2010–2050) estimated using the 2010 census. Neurology. 2013;80(19):1778–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Wimo A, Winblad B, Jonsson L. The worldwide societal costs of dementia: estimates for 2009. Alzheimers Dement. 2010;6(2):98–103. [DOI] [PubMed] [Google Scholar]
- 4. Alzheimer's Association 2014 Alzheimer's disease facts and figures. Alzheimers Dement. 2014;10(2):e47–92. [DOI] [PubMed] [Google Scholar]
- 5. Satizabal CL, Beiser AS, Chouraki V, Chene G, Dufouil C, Seshadri S. Incidence of dementia over three decades in the Framingham Heart Study. N Engl J Med. 2016;374(6):523–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. WHO Dementia fact sheet [Internet]. Available from: http://wwwwhoint/mediacentre/factsheets/fs362/en/2016. [Google Scholar]
- 7. Grillner S, Ip N, Koch C, Koroshetz W, Okano H, Polachek M, Poo M-m, Sejnowski TJ. Worldwide initiatives to advance brain research. Nat Neurosci. 2016;19(9):1118–22. [Internet]. Available from: http://www.nature.com/neuro/journal/v19/n9/abs/nn.4371.html#supplementary-information. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Crews H, Alink G, Andersen R, Braesco V, Holst B, Maiani G, Ovesen L, Scotter M, Solfrizzo M, van den Berg R et al. . A critical assessment of some biomarker approaches linked with dietary intake. Br J Nutr. 2001;86(1):S5–35. [DOI] [PubMed] [Google Scholar]
- 9. Parker RS. Carotenoids in human blood and tissues. J Nutr. 1989;119(1):101–4. [DOI] [PubMed] [Google Scholar]
- 10. Ames BN. Prolonging healthy aging: Longevity vitamins and proteins. Proc Natl Acad Sci USA. 2018;115(43):10836–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Johnson EJ, Vishwanathan R, Johnson MA, Hausman DB, Davey A, Scott TM, Green RC, Miller LS, Gearing M, Woodard J et al. . Relationship between serum and brain carotenoids, α-tocopherol, and retinol concentrations and cognitive performance in the oldest old from the Georgia centenarian study. J Aging Res. 2013;951786(10):9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Cho KS, Shin M, Kim S, Lee SB. Recent advances in studies on the therapeutic potential of dietary carotenoids in neurodegenerative diseases. Oxid Med Cell Longev. 2018;2018:4120458 doi:10.1155/2018/4120458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Crichton GE, Bryan J, Murphy KJ. Dietary antioxidants, cognitive function and dementia—a systematic review. Plant Foods Hum Nutr. 2013;68(3):279–92. [DOI] [PubMed] [Google Scholar]
- 14. Morris MC. Nutrition and risk of dementia: overview and methodological issues. Ann NY Acad Sci. 2016;1367(1):31–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ames BN. Prolonging healthy aging: longevity vitamins and proteins. Proc Natl Acad Sci USA. 2018;115:10836 doi:10.1073/pnas.1809045115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Devore EE, Kang JH, Stampfer MJ, Grodstein F. The association of antioxidants and cognition in the nurses' health study. Am J Epidemiol. 2012;177(1):33–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Morris MC, Wang Y, Barnes LL, Bennett DA, Dawson-Hughes B, Booth SL. Nutrients and bioactives in green leafy vegetables and cognitive decline: prospective study. Neurology. 2018;90(3):e214–e22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wengreen HJ, Munger RG, Corcoran CD, Zandi P, Hayden KM, Fotuhi M, Skoog I, Norton MC, Tschanz J, Breitner JC et al. . Antioxidant intake and cognitive function of elderly men and women: the Cache County Study. J Nutr Health Aging. 2007;11(3):230–7. [PubMed] [Google Scholar]
- 19. Grodstein F, Kang JH, Glynn RJ, Cook NR, Gaziano JM. A randomized trial of beta carotene supplementation and cognitive function in men: the Physicians' Health Study II. Arch Intern Med. 2007;167(20):2184–90. [DOI] [PubMed] [Google Scholar]
- 20. Morris MC, Wang Y, Barnes LL, Bennett DA, Dawson-Hughes B, Booth SL. Nutrients and bioactives in green leafy vegetables and cognitive decline. Neurology. 2018;90(3):e214–e22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Bennett DA, Schneider JA, Buchman AS, Barnes LL, Boyle PA, Wilson RS. Overview and findings from the Rush Memory and Aging Project. Curr Alzheimer Res. 2012;9(6):646–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Morris MC, Tangney CC, Bienias JL, Evans DA, Wilson RS. Validity and reproducibility of a food frequency questionnaire by cognition in an older biracial sample. Am J Epidemiol. 2003;158(12):1213–7. [DOI] [PubMed] [Google Scholar]
- 23. Willett WC, Howe GR, Kushi LH. Adjustment for total energy intake in epidemiologic studies. Am J Clin Nutr. 1997;65(4 Suppl):1220S–8S.; discussion 9S–31S. [DOI] [PubMed] [Google Scholar]
- 24. Tangney CC, Bienias JL, Evans DA, Morris MC. Reasonable estimates of serum vitamin E, vitamin C, and β-Cryptoxanthin are obtained with a food frequency questionnaire in older black and white adults. J Nutr. 2004;134(4):927–34. [DOI] [PubMed] [Google Scholar]
- 25. Bennett DA, Schneider JA, Aggarwal NT, Arvanitakis Z, Shah RC, Kelly JF, Fox JH, Cochran EJ, Arends D, Treinkman AD et al. . Decision rules guiding the clinical diagnosis of Alzheimer's disease in two community-based cohort studies compared to standard practice in a clinic-based cohort study. Neuroepidemiology. 2006;27(3):169–76. [DOI] [PubMed] [Google Scholar]
- 26. McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer's disease. Report of the NINCDS‐ADRDA Work Group* under the auspices of Department of Health and Human Services Task Force on Alzheimer's Disease. Neurology. 1984;34(7):939 doi:10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- 27. Bennett DA, Schneider JA, Arvanitakis Z, Kelly JF, Aggarwal NT, Shah RC, Wilson RS. Neuropathology of older persons without cognitive impairment from two community-based studies. Neurology. 2006;66(12):1837–44. [DOI] [PubMed] [Google Scholar]
- 28. Bennett DA, Wilson RS, Schneider JA, Evans DA, Aggarwal NT, Arnold SE, Cochran EJ, Berry-Kravis E, Bienias JL. Apolipoprotein E epsilon4 allele, AD pathology, and the clinical expression of Alzheimer's disease. Neurology. 2003;60(2):246–52. [DOI] [PubMed] [Google Scholar]
- 29. Bennett DA, Schneider JA, Tang Y, Arnold SE, Wilson RS. The effect of social networks on the relation between Alzheimer's disease pathology and level of cognitive function in old people: a longitudinal cohort study. Lancet Neurol. 2006;5(5):406–12. [DOI] [PubMed] [Google Scholar]
- 30. Mirra SS, Heyman A, McKeel D, Sumi SM, Crain BJ, Brownlee LM, Vogel FS, Hughes JP, van Belle G, Berg L. The Consortium to Establish a Registry for Alzheimer's Disease (CERAD). Part II. Standardization of the neuropathologic assessment of Alzheimer's disease. Neurology. 1991;41(4):479–86. [DOI] [PubMed] [Google Scholar]
- 31. Braak H, Braak E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol. 1991;82(4):239–59. [DOI] [PubMed] [Google Scholar]
- 32. Hyman BT, Trojanowski JQ, Consensus recommendations for the postmortem diagnosis of Alzheimer's disease. The National Institute on Aging, and Reagan Institute Working Group on Diagnostic Criteria for the Neuropathological Assessment of Alzheimer's Disease. Neurobiol Aging. 1997;18(4 Suppl):S1–2. [PubMed] [Google Scholar]
- 33. Hixson JE, Vernier DT. Restriction isotyping of human apolipoprotein E by gene amplification and cleavage with HhaI. J Lipid Res. 1990;31(3):545–8. [PubMed] [Google Scholar]
- 34. Evans DA, Bennett DA, Wilson RS, Bienias JL, Morris MC, Scherr PA, Hebert LE, Aggarwal N, Beckett LA, Joglekar R et al. . Incidence of Alzheimer disease in a biracial urban community: Relation to apolipoprotein E allele status. JAMA Neurol. 2003;60(2):185–9. [DOI] [PubMed] [Google Scholar]
- 35. Wenham PR, Price WH, Blandell G. Apolipoprotein E genotyping by one-stage PCR: Lancet. 1991;337(8750):1158–9. [DOI] [PubMed] [Google Scholar]
- 36. Wilson RS, Barnes LL, Krueger KR, Hoganson G, Bienias JL, Bennett DA. Early and late life cognitive activity and cognitive systems in old age. J Int Neuropsychol Soc. 2005;11(4):400–7. [PubMed] [Google Scholar]
- 37. McPhillips JB, Pellettera KM, Barrett-Connor E, Wingard DL, Criqui MH. Exercise patterns in a population of older adults. Am J Prev Med. 1989;5(2):65–72. [PubMed] [Google Scholar]
- 38. Kohout FJ, Berkman LF, Evans DA, Cornoni-Huntley J. Two shorter forms of the CES-D (Center for Epidemiological Studies Depression) depression symptoms index. J Aging Health. 1993;5(2):179–93. [DOI] [PubMed] [Google Scholar]
- 39. Bennett DA. Secular trends in stroke incidence and survival, and the occurrence of dementia. Stroke. 2006;37(5):1144–5. [DOI] [PubMed] [Google Scholar]
- 40. Bennett DA, Buchman AS, Boyle PA, Barnes LL, Wilson RS, Schneider JA. Religious orders study and rush memory and aging project. J Alzheimers Dis. 2018;64(s1):S161–S89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Renzi-Hammond LM, Bovier ER, Fletcher LM, Miller LS, Mewborn CM, Lindbergh CA, Baxter JH, Hammond BR. Effects of a lutein and zeaxanthin intervention on cognitive function: A randomized, double-masked, placebo-controlled trial of younger healthy adults. Nutrients. 2017;9(11):1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Lindbergh CA, Renzi-Hammond LM, Hammond BR, Terry DP, Mewborn CM, Puente AN, Miller LS. Lutein and zeaxanthin influence brain function in older adults: a randomized controlled trial. J Int Neuropsychol Soc. 2018;24(1):77–90. [DOI] [PubMed] [Google Scholar]
- 43. Rafnsson SB, Dilis V, Trichopoulou A. Antioxidant nutrients and age-related cognitive decline: a systematic review of population-based cohort studies. Eur J Nutr. 2013;52(6):1553–67. [DOI] [PubMed] [Google Scholar]
- 44. Dias IH, Polidori MC, Li L, Weber D, Stahl W, Nelles G, Grune T, Griffiths HR. Plasma levels of HDL and carotenoids are lower in dementia patients with vascular comorbidities. J Alzheimers Dis. 2014;40(2):399–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Morris MC, Tangney CC, Wang Y, Sacks FM, Barnes LL, Bennett DA, Aggarwal NT. MIND diet slows cognitive decline with aging. Alzheimer's Dement. 2015;11(9):1015–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Johnson EJ. Role of lutein and zeaxanthin in visual and cognitive function throughout the lifespan. Nutr Rev. 2014;72(9):605–12. [DOI] [PubMed] [Google Scholar]
- 47. Nouchi R, Suiko T, Kimura E, Takenaka H, Murakoshi M, Uchiyama A, Aono M, Kawashima R. Effects of lutein and astaxanthin intake on the improvement of cognitive functions among healthy adults: a systematic review of randomized controlled trials. Nutrients. 2020;12(3):617 doi:10.3390/nu12030617. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.