ABSTRACT
Background
Women's dietary diversity and quality are limited in low- and middle-income countries (LMICs). Nutrition-sensitive interventions that promote food crop diversity and women's access to income could improve diets and address the double burden of malnutrition in LMICs.
Objectives
We examined the associations among food crop diversity and women's income-earning activities with women's diet quality, as well as effect modification by access to markets, in the context of small-holder food production in rural Tanzania.
Methods
Data from a cross-sectional study of 880 women from Rufiji, Tanzania, were analyzed. Women's dietary intake was assessed using a food frequency questionnaire. The prime diet quality score (PDQS; 21 food groups; range, 0–42), a unique diet-quality metric for women that captures the healthy and unhealthy aspects of diet, was computed. Generalized estimating equation linear models were used to evaluate the associations of food crop diversity and women's income-earning activities with PDQS, while controlling for socio-economic factors.
Results
Maternal overweight (24.3%) and obesity (13.1%) were high. The median PDQS was 19 (IQR, 17–21). Households produced 2.0 food crops (SD ± 1.0) yearly. Food crop diversity was positively associated with PDQS (P < 0.001), but the association was strengthened by proximity to markets (P for interaction = 0.02). For women living close (<1.1 km) to markets, producing 1 additional food crop was associated with a 0.67 (95% CI, 0.22–1.12) increase in PDQS, versus a 0.40 (95% CI, 0.24–0.57) increase for women living farther away. The PDQS increased with women's salaried employment (estimate, 0.96; 95% CI, 0.26–1.67).
Conclusions
Household food production may interact with access to markets for sales and purchases, while nonfarm income also improves women's diet quality in rural Tanzania. Programs to improve women's diet quality should consider improving market access and women's access to income (source of empowerment), in addition to diversifying production.
Keywords: maternal diet quality, prime diet quality score, PDQS, Tanzania, food crop diversity, market food diversity, distance to market, women's access to income, production diversity, food systems
Introduction
Food systems are a key influencer of diet quality and can play an important role in addressing the double-burden of malnutrition—that is, both undernutrition and overnutrition - in low- and middle-income countries (LMICs) (1–3). Diet-related diseases are a leading cause of morbidity and have been associated with 11 million deaths globally (4–6). In many LMICs, women's diets are characterized by consumption of staple grains and low intake of animal-source foods (ASF), fruits, and vegetables and, consequently, poor micronutrient intake (7–9). In Tanzania, only 46% of rural women meet minimum dietary diversity (i.e., consume at least 5 food groups out of 10 daily), a measure of micronutrient adequacy (10, 11), and women's intake of protein, fruits, and vegetables is low (12). The consequences of poor dietary intake for women are dire. Globally, 500 million women are anemic and 15% of all women are vitamin A deficient, while 10% are underweight (BMI < 18.5) and 40% are overweight or obese (BMI ≥ 25), with the highest burden of undernutrition in LMICs (13–15).
Nutrition-sensitive interventions in agriculture are critical for addressing poor diets in LMICs. Diversification of household agricultural production is a strategy to improve women's diets (16, 17), and provides a direct pathway to improved consumption through increased availability of diverse foods for home consumption. Agricultural production also indirectly improves diets, as it is a source of livelihood for rural farmers and provides income to purchase nutrient-dense foods for women (16, 18). Previous studies have shown small, positive associations between production diversity and household and maternal dietary diversity; however, how these relationships have been measured has been inconsistent, and associations vary with geographic regions (16, 19–24).
The relationship between crop production diversity and women's diet quality is complex. The literature is not clear as to which pathway through which diversified production leads to diet change (i.e., consumption of own produced food vs. using income from sales of crops) is more important, and few studies have evaluated this. Studies suggest that the subsistence production pathway (i.e., diversified production that contributes directly to own consumption) is the stronger pathway in most contexts (22, 24). Other studies have found that the magnitude of the association between income from market sales and diet is larger than that of diversified production for own consumption and diet (25, 26), arguing that increased agricultural income is also a primary way in which households can diversify their diets (27). Conversely, agricultural income can increase purchasing power to potentially purchase unhealthy or processed foods (28). Further, the functionality of local markets, the diversity of foods sold, and distance to markets may determine a household's access to nutrient-rich foods for purchase and whether foods produced on-farm are sold or consumed (29). Finally, literature shows that women's decision-making on the use of income from agriculture and off-farm employment may influence diet quality by increasing women's ability to act on their nutrition knowledge (30, 31). It is unclear how these factors interact to inform women's diets in rural Tanzania.
We focus on women's diets over men's because in rural LMICs women are often more nutritionally vulnerable than men and at greater risk of micronutrient and macronutrient deficiencies, given the biologic demands of pregnancy and women's often poorer social status (32, 33). In addition, women's nutrition status prior to and during pregnancy has an impact on the nutrition status of their offspring (33). Quality women's diets have a key role in preventing micronutrient and other deficiencies and optimizing nutrition for women and their infants (34).
Dietary diversity (DD), a proxy for micronutrient adequacy, has been used in previous studies as a measure for diet quality. However, DD does not assess other aspects of diet quality, including consumption of unhealthy foods and caloric moderation (35, 36). There is limited knowledge on diet quality and its determinants among women living in rural Tanzania. It is important to determine whether agriculture production is associated with women's diet quality, given recent nutrition transition and dietary changes in Tanzania and the potential consequences of poor-quality diets (37–39). We used the prime diet quality score (PDQS), an index that considers consumption of healthy and unhealthy foods associated with diet-related diseases, as a measure of diet quality in Tanzania (34)(13).
The objective of this study was to determine the associations between food crop diversity, women's income-earning activities, and access to and quality of food markets with women's diet quality (PDQS) in a cross-sectional study in rural Tanzania. We evaluated for modification of these associations by access to food markets. We hypothesized that greater food crop diversity and women's access to income would enhance diet quality and that market access and diversity had differential effects on observed associations.
Methods
Study design and population
The study population participated in a cluster-randomized, prospective Homestead Agriculture and Nutrition (HANU) project set in the rural Rufiji district in Eastern Tanzania (ClinicalTrials.gov NCT03311698). Out of 16 villages within the Rufiji Health and Demographic Surveillance System, 10 were randomly selected, pair-matched based on location and other factors, and randomly assigned to the intervention or control arms (40). Participants were women enrolled in the study conducted to evaluate the association of integrated homestead food production with maternal and child health and nutrition. We analyzed data from 880 women participating in a midline assessment conducted from August to October 2017. The midline study included an extensive dietary intake assessment, unlike the study baseline. Baseline data collection was also before differences in dietary intake and quality would have occurred. Details of the parent study are published elsewhere (40).
The eligibility criteria for the study included households with a woman of reproductive age (18–49 years) with at least 1 child aged 6–36 months and access to land for vegetable production. Study participants provided informed consent. Study research assistants used semi-structured questionnaires to collect data on household demographics and socio-economic status, maternal anthropometric data, dietary intake, hemoglobin levels, and household agriculture production for the study.
Exposure variables
The main exposure, food crop diversity, was determined by classifying all crops grown by households (self-reported) in the previous year into 7 groups based on the FAO's Minimum Dietary Diversity for Women (MDD-W) index. The 7 crop groups included in the score were 1) grains, roots and tubers, and plantains; 2) legumes (beans, peas, and lentils); 3) nuts and seeds; 4) vitamin A−rich dark green vegetables; 5) other vitamin A−rich fruits and vegetables; 6) other vegetables; and 7) other fruits. We excluded animal-source foods (dairy; meats, poultry, and fish; and eggs). A similar classification has been used elsewhere (23, 41). Food crop diversity was determined as the sum of food crop groups produced by the household in the previous year. Additionally, we considered including livestock production in the computation of production diversity in a sensitivity analysis and created an 8 food-group variable instead of the original (7 food-group) indicator that excludes animal-source foods.
An alternative method of evaluating the diversity of on-farm production is to use a measure of species richness (24). Crop species richness has been used as an indicator of diversity of on-farm production in previous studies (22, 23, 42). We assessed crop species' richness as the number of food crop species produced by households in the previous year, out of 37 food crops. Cash crop diversity was evaluated as the number of cash crops grown by the household in the previous year out of 3 crops: cashew, sesame, and cotton. We determined the main cash crops based on the study data, and sesame was the most commonly sold food crop (31.5%), while cashew was sold by 9.4% of households. We also determined that cotton was a cash crop based on literature (43).
Market food diversity (MFD) was determined based on the diversity of foods sold in smaller, local markets using the 10 food groups for the MDD-W (including ASF). A similar approach has been taken in previous research in Ethiopia (41). A total of 27 key informants selected by project staff in collaboration with local leaders in each village recalled foods sold in their local markets in the previous year, and all foods were classified into the 10 food groups. MFD was determined as the total number of food groups sold in the market based on each informant's recall. We computed a median score of MFD based on available informants per village. MFD was calculated at the village level and each household from the village was assigned the median MFD.
We calculated distance to market for each study household to 2 larger, regional markets that had GPS coordinates using the Stata program. The 2 larger markets identified by informants in the study were Kibiti market for Kibiti A, Kibiti B, Mchukwi A, and Mchukwi B villages and Ikwiriri market for Umwe North, Umwe South, Mgomba North, Mgomba South, Ikwiriri South, and Ikwiriri Centre villages. MFD was calculated for local markets closer to the respondents’ villages, and the distance to markets was calculated for 2 larger regional markets. A similar approach of referencing 2 types of markets has been taken in another study (44).
We considered women's participation in nonfarm economic activities and women's receipt of wages or salaried employment. We asked women whether they participated in nonfarm economic activities (yes/no) or wage and salary employment (yes/no). Finally, a livestock diversity score was defined as the number of livestock species owned by the household.
Study outcome
The primary outcome of the study, women's diet quality, was assessed using the PDQS, a food group−based dietary score based on self-reported women's dietary intake. Women's dietary intake was assessed using a food frequency questionnaire (FFQ). The FFQ was composed of a list of 79 common local foods and was validated in the Tanzanian context (45). Women were asked to recall foods consumed in the previous month and the frequency of consumption of the foods, with options of frequency of consumption of 0 times in a month, 1–3 times per month, 1 time per week, 2–4 times per week, 5–6 times per week, 1 time per day, 2–3 times per day, 4–5 times per day and 6 or more times per day.
Foods consumed by women in the previous month, as reported by the FFQ, were classified into 21 food groups for the PDQS. Foods were classified into 14 healthy food groups [dark green leafy vegetables, other vitamin A−rich vegetables (including carrots), cruciferous vegetables, other vegetables, whole citrus fruits, other fruits, fish, poultry, legumes, nuts, low-fat dairy, whole grains, eggs, and liquid vegetable oils] included in the PDQS (13, 46). In addition, foods were classified into 7 unhealthy food groups (red meat, processed meats, refined grains and baked goods, sugar-sweetened beverages, desserts and ice cream, fried foods obtained away from home, and potatoes) (13, 46). We made the following adaptations to the score: 1) red and orange fruits and vegetables were included in the “other vitamin A−rich fruits and vegetables” category, in place of “carrots” as a food group; and 2) a “roots and tubers” group was used in place of the “potatoes” group from the original score. We categorized maize flour−based products as refined grains. Processed meat intake and liquid vegetable oil intake were not measured in the study and low-fat dairy intake could not be ascertained; therefore, all women were assigned low intakes for these groups, meaning 2 points for processed meats and 0 points for liquid vegetable oil and low-fat dairy intake. These adaptions were made in order to conform to local food consumption patterns.
Points were assigned for consumption of healthy food groups as 0–1 serving/week (0 points), 2–3 servings/week (1 point), and ≥4 servings/week (2 points). Scoring for unhealthy food groups was assigned as: 0–1 serving/week (2 points), 2–3 servings/week (1 point), and ≥4 servings/week (0 points) (47). Points for each food group were summed to give an overall score.
The PDQS has been used as measure of diet quality in studies in developed countries and has been associated with a higher risk of chronic diseases, including cardiovascular disease and diabetes (13, 46, 48, 49).
Ethics
Approval for the study was provided by the Ifakara Health Institute independent research board and the medical research council committee of the National Institutes of Medical Research in Tanzania and by the Harvard TH Chan School of Public Health (Boston) institutional review board. Written informed consent was obtained from all enrolled women.
Analysis
We described the prevalence of the household demographic characteristics and individual characteristics using means and standard deviations for continuous data, and frequencies for categorical data. We also described the frequency of household production of food crops, the prevalence of sales of food crops, and consumption of PDQS foods by women in the study population using frequencies.
We used generalized estimating equation (GEE) linear models with exchangeable correlation (50), controlling for clustering by village pair, to evaluate the associations of food crop diversity and women's participation in nonfarm economic activities or employment with diet quality in the study. We adjusted for assignment to intervention (homestead garden/control) in the parent study and the cluster study design. In a sub-analysis, we further evaluated the association of production of specific food groups (grains, white roots and tubers, and plantains; legumes; nuts and seeds; vitamin A−rich dark green vegetables; other vitamin A−rich fruits and vegetables; other vegetables; and, other fruits) with the PDQS. In a secondary analysis, we also evaluated the association of alternative measures of on-farm diversity, crop species richness, and cash crop diversity with the PDQS. We evaluated for effect modification of associations by 1) MFD; 2) market access; and 3) sale of crops (sold at least 1 food crop in the previous year).
We selected confounders based on associations with the outcome in univariate models at P < 0.20. Potential confounders included maternal education (no/primary, secondary, tertiary), wealth index (quintiles), parity (0, 1–2, ≥3 children), family size (continuous), women's age (18–24 years, 25–34 years, ≥35 years), BMI (<18.5, 18.5–24.9, ≥25 kg/m2), marital status (married/not married), food expenditure (continuous), weekly income (log, continous), farm size (continuous), and sale of food crops (sold at least 1 food crop in the previous year). We controlled for BMI as a proxy for energy intake, which could be a potential confounder. The missing-indicator method was used to account for missing covariate data.
In a sensitivity analysis, we evaluated women's dietary diversity according to the MDD-W criteria outlined above. Food consumed by women was categorized into 10 food groups. We calculated a daily frequency of consumption for all food groups. If her frequency of consumption of a food group was 1 or more times daily, a woman was considered to have consumed the food group. Dietary diversity score (DDS) was computed as the total number of food groups consumed by a woman. We evaluated the association of food crop diversity with the DDS. The analyses were conducted using SAS version 9.4.
Results
We analyzed data from 870 women. We excluded 10 women under 18 years or aged 50 years or older from the analysis. Table 1 presents the baseline characteristics of the study population. The mean age (± SD) for women in the study was 31.5 (± 7.7) years. The mean BMI (± SD) for women in the study was 24.4 (± 5.0), and the prevalences of underweight, overweight, and obesity were 6.8%, 24.3%, and 13.1%, respectively. Maternal education was low, with 91.2% of women having primary school education or less; men's education was also low, with 85.6% of men having primary school education or less. The mean household size (± SD) was 6.5 (±2.6). Only 32 households (4.0%) owned goats, and 271 (31.1%) owned chickens. The median MFD score was 7.5 (IQR, 7.5–8.0) food groups and the median distance traveled to the nearest local market was 1.1 (IQR, 0.8–1.7) km. There were 136 women (15.7%) that reported participating in salaried employment and 252 women (29.0%) who participated in nonfarm income activities.
TABLE 1.
Background characteristics of study women in Tanzania
| Characteristic | N | Values |
|---|---|---|
| Sociodemographic | ||
| Maternal age (years) | 868 | 31.5 ± 7.7 |
| 18–24 | — | 200 (23.0) |
| 25–34 | — | 354 (40.8) |
| ≥35 | — | 314 (36.2) |
| Marital status | 868 | — |
| Married | — | 672 (77.4) |
| Single, divorced or widowed | — | 196 (22.6) |
| BMI | 862 | — |
| Mean ± SD | — | 24.4 ± 5.0 |
| Underweight, BMI <18.5 | — | 59 (6.7) |
| Normal weight, BMI 18.5–24.99 | — | 481 (55.8) |
| Overweight, BMI 25–29.99 | — | 209 (24.3) |
| Obese, BMI ≥30 | — | 113 (13.1) |
| Maternal education | 868 | — |
| None | — | 293 (33.8) |
| Primary school | — | 499 (57.5) |
| Secondary school or higher | — | 76 (8.8) |
| Paternal education | 689 | — |
| None | — | 169 (24.5) |
| Primary school | — | 421 (61.1) |
| Secondary school or higher | — | 99 (14.4) |
| Parity | 868 | — |
| 1 child or none | — | 166 (19.1) |
| 2 or more children | — | 702 (80.9) |
| Household | ||
| Size of household | 868 | 6.5 ± 2.6 |
| Wealth quintile score | 868 | — |
| First/lowest | — | 173 (19.9) |
| Second | — | 231 (26.6) |
| Third | — | 109 (12.6) |
| Fourth | — | 182 (21.0) |
| Fifth/highest | — | 173 (19.9) |
| Food expenditure, Tanzanian shillings | 821 | 7327 ± 4166 |
| Plot size, acres | 711 | 3.4 ± 3.7 |
| Ownership of livestock | ||
| Chickens | 288 | 9.3 ± 14.4 |
| Goats | 288 | 1.0 ± 3.6 |
| Sold at least 1 crop | 870 | 442 (50.8) |
| Women's participation in nonfarm economic activities | 868 | 252 (29.0) |
| Woman received wages or salaried employment | 868 | 136 (15.7) |
| Distance to market, km | 878 | 1.1 [0.8–1.7] |
| Market food diversity | 870 | 7.5 [7.5–8.0] |
| Food crop diversity | 870 | 2.0 [1.0–3.0] |
| PDQS median | 870 | 19.0 [17.0–21.0] |
Values are mean ± SD, median [IQR], or frequency (percent). N = 870. Abbreviation: PDQS, prime diet quality score.
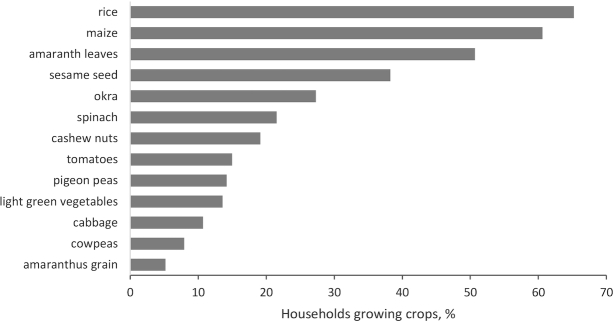
Most households produced grains (80.6% of all participants) and dark green vegetables (51.0%). The most common crops produced by households in the study were rice (65.2%), maize (60.6%), amaranthus leaves (50.7%), and sesame (38.3%; Figure 1). Food crop diversity was low, with 2.4 food groups (± 1.5) produced in the previous year out of a maximum of 7 crop groups. Approximately 49.2% of the study households did not report selling crops in the previous year. On average, 31.5% of all households reported selling sesame, 11.2% reported selling amaranthus leaves, and 9.4% reported selling cashew nuts (results not shown).
FIGURE 1.
Percentage of study households growing crops in the previous year.
The median PDQS for women was 19 (IQR, 17–21; possible maximum score is 42; Table 1). Table 2 presents the distribution of foods consumed by women using the PDQS classification. Most women consumed at least 4 servings/week of other vegetables (97.1%), fish (89.4%), legumes (81.6%), and dark green leafy vegetables (62.3%). Alternatively, the consumption of refined grains was high (100%), as was the consumption of foods in the roots and tubers group (including potatoes; 82.7%).
TABLE 2.
Percent of women reporting consumption of prime diet quality score food groups in rural Tanzania
| 0–1 serving/wk | 2–3 servings/wk | ≥4 servings/wk | |
|---|---|---|---|
| Healthy foods | 0 point | 1 point | 2 points |
| Cruciferous vegetables | 706 (81.3) | 133 (15.3) | 29 (3.3) |
| Dark leafy green vegetables | 137 (15.8) | 190 (21.9) | 541 (62.3) |
| Eggs | 839 (96.7) | 26 (3.0) | 3 (0.3) |
| Fish | 5 (0.6) | 87 (10.0) | 776 (89.4) |
| Legumes | 46 (5.3) | 114 (13.1) | 708 (81.6) |
| Liquid vegetable oils1 | 870 (100) | 0 (0) | 0 (0) |
| Low fat dairy1 | 870 (100) | 0 (0) | 0 (0) |
| Nuts | 793 (91.4) | 61 (7.0) | 14 (1.6) |
| Other vegetables | 5 (0.6) | 20 (2.3) | 853 (97.1) |
| Other vitamin A–rich vegetables, incl. carrots | 469 (54.0) | 208 (24.0) | 191 (22.0) |
| Other whole fruits | 397 (46.7) | 244 (28.1) | 227 (26.2) |
| Poultry | 818 (94.2) | 40 (4.6) | 10 (1.2) |
| Whole citrus fruits | 324 (37.3) | 295 (34.0) | 249 (28.7) |
| Whole grains | 532 (61.3) | 209 (24.1) | 127 (14.6) |
| Unhealthy foods | 2 points | 1 point | 0 points |
| Desserts and ice cream2 | 190 (21.9) | 307 (35.4) | 371 (42.7) |
| Fried foods away from home | 830 (95.6) | 33 (3.8) | 5 (0.6) |
| Potatoes3 | 29 (3.3) | 121 (13.9) | 718 (82.7) |
| Processed meat1 | 870 (100) | 0 (0) | 0 (0) |
| Red meats | 777 (89.5) | 83 (9.6) | 8 (0.9) |
| Refined grains, baked goods4 | 0 (0) | 0 (0) | 868 (100) |
| Sugar-sweetened beverages | 566 (65.2) | 193 (22.2) | 109 (12.6) |
Values are frequencies (percent).
Processed meat intake, low-fat dairy, and liquid vegetable oil were not measured in the study; therefore, all women were assigned low intake for these groups. Low intake was 2 points for processed meats and 0 points for low-fat dairy and liquid vegetable oil.
The desserts and ice cream group includes cakes, doughnuts, rice cake, honey, and ice cream.
A roots and tubers group was used in place of a “potatoes” group in the original score. This category includes potatoes, sweet potatoes, and taro.
Maize flour–based products are included as refined grains.
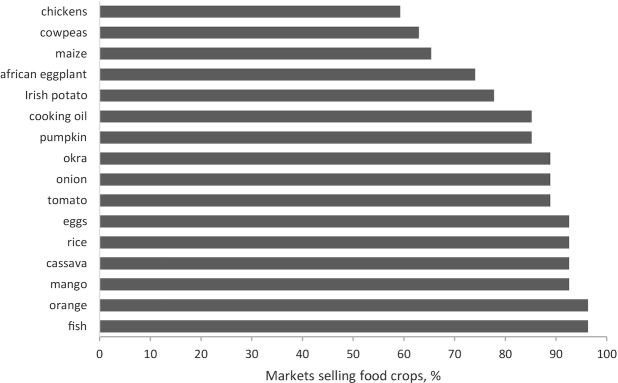
All 27 market respondents indicated that their markets sold starchy staples, other fruits, and meats and few markets sold dairy or nuts and seeds (<23% of markets). The foods most commonly sold in the local markets in the previous year included fish, oranges, mangoes, cassava, rice, eggs, tomatoes, onions, okra, pumpkins, and cooking oil (Figure 2).
FIGURE 2.
Percentage of markets selling common foods in the previous year based on 27 key informant interviews.
Table 3 shows the association of food crop diversity with PDQS. In multivariate models, for every food group produced, women's PDQS increased by 0.47 (95% CI, 0.27–0.67). MFD was positively associated with PDQS (estimate, 0.50; 95% CI, 0.06–0.94), while distance to local markets was negatively associated with PDQS (estimate, -0.27; 95% CI, -0.39 to -0.14) in adjusted models. Women who were employed had a 0.96 (95% CI, 0.26–1.67) higher PDQS compared to women who were not employed. Further, we found that selling at least 1 food crop was negatively associated with the PDQS (estimate, -0.88; 95% CI, -1.17 to -0.58), and found no association between livestock diversification at the farm level with the PDQS. When we introduced interaction variables, the association of production diversity with PDQS varied by distance to market (P for interaction = 0.02) in multivariate models. Figure 3 shows that for women living close to a market (under a median distance of 1.1 km), production of an additional food crop was associated with a 0.67 (95% CI, 0.22–1.12) increase in PDQS, compared to a 0.40 (95% CI, 0.24–0.57) increase for women living further from markets.
TABLE 3.
Association of food crop diversity with prime diet quality score among women in rural Tanzania
| Prime diet quality score | ||
|---|---|---|
| Univariate1 | Adjusted model2 | |
| Food crop diversity score | 0.32 (0.19–0.44)*** | 0.47 (0.27–0.67)*** |
| Livestock diversity score | 0.27 (0.08–0.47)* | −0.07 (−0.38–0.24) |
| Women's participation in off-farm activities | ||
| Women's participation in nonfarm economic activities | 0.60 (0.22–0.98)** | 0.47 (−0.02–0.96) |
| Women's participation in wage/salary employment | 0.87 (0.43–1.32)*** | 0.96 (0.26–1.67)* |
| Market participation | ||
| Sold crops | 0.09 (−0.06–0.24) | −0.88 (−1.17−0.58)*** |
| Market food diversity score | 0.81 (0.29–1.32)** | 0.50 (0.06–0.94)* |
| Distance to market | −0.10 (−0.20–0.01) | −0.27 (−0.39–0.14)*** |
*P < 0.05, **P < 0.01, ***P < 0.001. Values are estimates (95% CIs). GEE linear models with exchangeable correlation, controlling for clustering by village pair, were used to evaluate the association of agriculture production diversity with maternal diet quality.
Univariate models are shown. Abbreviations: GEE, generalized estimating equation; HANU, Homestead Agriculture and Nutrition.
Adjusted model controls for treatment (HANU/control), maternal age (15–24 years, 25–34 years, ≥35 years), maternal education (none, primary, secondary, and higher), parity (0–2, ≥3), wealth index (quintiles), land size (acres), weekly income (log), livestock diversity score, women's participation in nonfarm economic activities, receiving wages or salary, household sold at least 1 food crop in last year, maternal BMI categories, and market food diversity score and distance to market.
FIGURE 3.
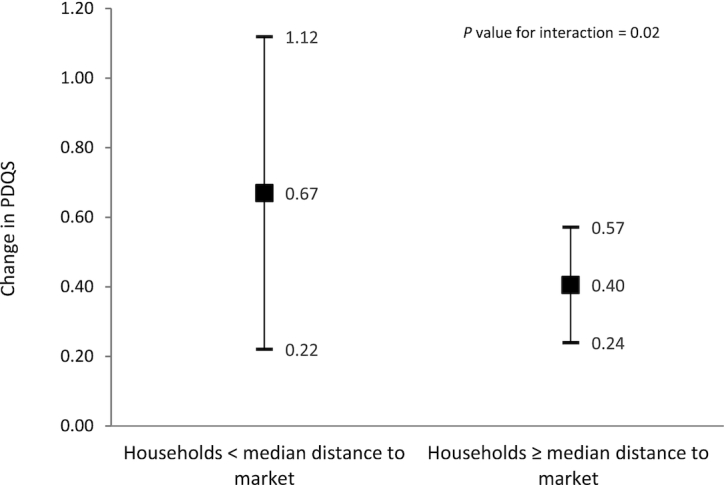
Association of food crop diversity with PDQS among women in rural Tanzania, stratified by distance to markets. Error bars are 95% CIs. The median distance to market is 1.1 kilometers. GEE linear models with exchangeable correlation, controlling for clustering by village pair, were used to evaluate the association of food crop diversity with maternal diet quality. Stratified models were restricted to women whose households were nearer than the median distance to their nearest market or whose household was at a median distance to market or further. The models control for treatment (HANU/control), maternal age (15–24 years, 25–34 years, ≥35 years), maternal education (none, primary, secondary, and higher), parity (0–2, ≥3), wealth index (quintiles), land size (acres), livestock diversity score, women's participation in nonfarm economic activities, women receiving wages or salary, household sold at least 1 food crop in last year, maternal BMI categories, and market food diversity score. For women whose households were nearer to markets, production diversity had a higher estimated association with diet quality compared to women who were further away from the market. Abbreviations: GEE, generalized estimating equation; HANU, Homestead Agriculture and Nutrition; PDQS, prime diet quality score.
We considered an analysis of the association of an 8 food-group production diversity score (including livestock production) with the PDQS in a sensitivity analysis. We found that for every food group produced, women's PDQS increased by 0.44 (95% CI, 0.24–0.64; Supplemental Table 1).
In another sensitivity analysis, we evaluated the association of food crop diversity with DDS. We found that in multivariate models, for every food group produced, women's DDS was higher by 0.14 (95% CI, 0.02–0.26; Supplemental Table 2). Additionally, livestock diversity (estimate, 0.37; 95% CI, 0.23–0.51) and women's participation in nonfarm economic activities (estimate, 0.36; 95% CI, 0.20–0.52) were positively associated with the DDS. Distance to a local market (estimate, -0.19; 95% CI, −0.22 to −0.16) and sale of food crops (estimate, −0.36; 95% CI, −0.54 to −0.18) were negatively associated with DDS (Supplemental Table 2).
Table 4 shows the association of production of food crops with PDQS. The production of crops in the nuts and seeds group (sesame, cashew, and groundnuts) was associated with a 0.72 (95% CI, 0.22–1.22) point increase in women's PDQS. The production of crops in the “other vitamin A−rich fruits and vegetables” group (carrots, mangoes, orange sweet potatoes, pawpaw fruits, and pumpkin) was associated with a 1.40 (95% CI, 0.72–2.07) point increase in the PDQS, and production of vitamin A−rich dark green vegetables (green leafy vegetables, spinach, and amaranthus leaves) was associated with a 0.69 (95% CI, 0.37–1.01) point increase in the PDQS in adjusted models that controlled for production of other food groups. In the analysis for DDS, we found that production of crops in the “other vegetables” group was associated with a higher DDS (estimate, 0.37; 95% CI, 0.22–0.51; Supplemental Table 3).
TABLE 4.
Association of production of food crops with prime diet quality score among women in rural Tanzania
| Univariate model | Adjusted model1,2 | |
|---|---|---|
| Staples and grains | 0.57 (0.02–1.11)* | 0.24 (−0.12–0.61) |
| Other vitamin A–rich fruits and vegetables3 | 0.81 (−0.09−1.72) | 1.40 (0.72–2.07)*** |
| Other fruit | 0.72 (−0.39−1.83) | −0.50(−1.08−0.09) |
| Nuts and seeds3 | 0.50 (0.32–0.68)*** | 0.72 (0.22–1.22)** |
| Vitamin A–rich dark green vegetables3 | 0.64 (−0.17–1.45) | 0.69 (0.37–1.01)*** |
| Other vegetables | 1.00 (0.30–1.69)** | 0.59 (−0.04–1.21) |
| Beans and peas | 0.22 (−0.12−0.56) | 0.10 (−0.39−0.58) |
*P < 0.05, **P < 0.01, ***P < 0.001. Values are estimates (95% CIs).
GEE linear models with exchangeable correlation, controlling for clustering by village pair, were used to evaluate the association of production of food groups with maternal diet quality. Abbreviations: GEE, generalized estimating equation; HANU, Homestead Agriculture and Nutrition; PDQS, prime diet quality score.
Results are from a multivariate model that includes all food groups produced and covariates.
Adjusted model includes production of all food groups and controls for treatment (HANU/control), maternal age (years), maternal education (none, primary, secondary, and higher), parity (0–2, ≥3), wealth index (quintiles), land size (acres), livestock diversity score, women's participation in nonfarm economic activities, receiving wages or salary, household selling at least 1 food crop, maternal BMI categories, and market food diversity score and distance to market.
Production of other vitamin A–rich vegetables is associated with a 1.40-unit increase in PDQS score; nut and seed production is associated with a 0.72 increase in score; and vitamin A–rich dark green vegetable production is associated with a 0.69 increase in PDQS.
We evaluated crop species richness as an alternative measure of production diversity. In adjusted models that controlled for cash crop diversity and other factors, we found a significant, positive association between crop species richness and PDQS. Production of an additional food crop was associated with a 0.31 (95% CI, 0.18–0.44) unit increase in the PDQS in fully adjusted models (results not shown). MFD was positively associated with the PDQS (estimate, 0.57; 95% CI, 0.02–1.12), while distance to a local market was negatively associated (estimate, −0.23; 95% CI, −0.36 to −0.09) with the PDQS in adjusted models (results not shown). We found evidence of effect modification in the association of crop species richness with PDQS by the sale of food crops (P for interaction < 0.001). Among households that sold at least 1 crop, the production of an additional food crop was associated with a 0.48 (95% CI, 0.37–0.60) increase in the PDQS, versus no association with the PDQS (estimate, 0.00; 95% CI, −0.18 to 0.17) for women from households that did not sell any crops (Figure 4).
FIGURE 4.
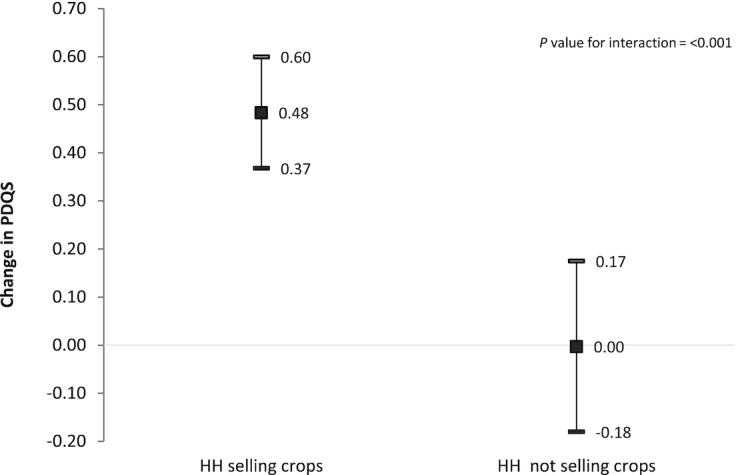
Association of crop species richness with PDQS among women in rural Tanzania, stratified by sale of food crops. Error bars are 95% CIs. GEE linear models with exchangeable correlation, controlling for clustering by village pair, were used to evaluate the association of crop species richness with maternal diet quality. Stratified models were restricted to women whose households sold at least 1 food crop or households that did not sell food crops in the previous year. The models control for treatment (HANU/control), maternal age (years), maternal education (none, primary, secondary, and higher), parity (0–2, ≥3), wealth index (quintiles), land size (acres), livestock diversity score, women's participation in nonfarm economic activities, receiving wages or salary, maternal BMI categories, market food diversity score, and distance to market. The association of crop species richness with PDQS is stronger among women from households that sold food crops. Abbreviations: GEE, generalized estimating equation; HH, household; PDQS, prime diet quality score.
Discussion
In this study, we found a positive association between food crop diversity and women's diet quality, as measured by the PDQS in rural Tanzania. Distance to a market was a modifier of this association, since food crop diversity was associated with the PDQS with a larger effect estimate for women residing close to a market compared to women who lived further from a market. Women's participation in wage or salaried employment was positively associated with the PDQS. Finally, diversified food markets were associated with a higher PDQS, while living further away from a market was associated with a lower PDQS.
Previous studies in LMICs, including in Tanzania, have found positive associations between production diversity and maternal DD (23, 24, 26, 41, 42, 44, 51–55). In this study, we found a small increase in the PDQS with increasing food crop diversity. On average, the production of an additional food group was associated with a 0.47 (95% CI, 0.27–0.67) unit increase in the PDQS (Table 3). In a sensitivity analysis, we also found an association of a smaller magnitude between food crop diversity and DDS (estimate, 0.14; 95% CI, 0.02–0.26; Supplemental Table 2). This implies that a large increase in food crop diversity might be needed to substantially improve the PDQS (or DD) through this pathway, a finding that is consistent with previous studies (23, 24, 44). A recent review reported that under 20% of studies found an association between production diversity and DD (55). It should be noted that the associations observed are consistent in magnitude and direction when comparing across the same metrics. However, because the types of metrics used to evaluate these associations to date have varied widely, there has been inconsistency in findings across different models (55). There has also been a bias in the literature toward studies from sub-Saharan Africa, limiting the generalizability of the overall literature.
Other studies have shown stronger associations when species diversity is used a measure of production diversity (25, 26). Our study found a positive association between crop species richness and the PDQS; however, our findings indicate a stronger association for food crop diversity. These mixed results could also be attributed to limitations in study designs and differences in the indicators of production diversity and DD used (24, 56).
Household participation in markets, size and diversification of local markets, physical distance to markets, and economic access to markets can play a critical role in determining what is consumed at the household level (41). We found that distance to markets was negatively associated with the PDQS, implying that households living farther from a market had lower PDQS results. We also found evidence of interaction by distance to markets in associations between food crop diversity and PDQS. Sibhatu et al. (26) similarly found negative associations in a multi-country study, but found limited evidence of mediation by distance to markets. Another study found that decreasing distance to markets was more positively associated with DD than producing 1 additional food group (44). The role of markets is, however, complex. Markets are important for diets because they allow households to generate income from farm production and can increase household demand for quality diets (57). Markets can also supply a diversity of foods to satisfy demand for quality diets (57). Conversely, increased access to markets can lead to increased access to refined and processed foods. In this study in a rural setting in Tanzania, we found that selling food crops was negatively associated with maternal diet quality. However, markets remain important in this setting, and it is feasible that when markets offer a variety of foods, households are more likely to purchase and consume them.
We found that diverse food markets were associated with greater diet quality. The magnitude of the estimate of association was similar to that for food crop diversity, indicating a potentially important contribution of market foods to diet quality. Given that the food crop diversity measure includes fewer food groups (excludes ASFs), it is important to note that we did not expect to find similar associations, speaking to the equivalent importance of production diversity in comparison to market diversity for diets in this context. As a sensitivity analysis, we included livestock in the computation of production diversity scores and found that results for associations with the PDQS were similar to the original food crop diversity (excluding ASF) score. This finding is reassuring for our analysis using food crop diversity that is presented in this study.
Our findings have implications for interventions to improve diet quality, as they show that the food environment is important for maternal diet quality even in rural locations. Previous studies have shown similar associations with women's DD (19, 22, 29, 41, 58). We suggest that programs aiming to improve maternal diet quality in rural farming households consider also actions to increase access to markets (decreasing distance to) and promoting the development of functional rural markets. In addition, evidence suggests that households with better access to markets have more diverse children's diets (59).
We also considered women's access to and use of income resources, and the effect of these resources on women's ability to access quality diets (30). We found that women who participated in salaried employment were more likely to have higher diet quality, with estimated effects of higher magnitude than the effects of food crop diversity, and this association was independent of wealth status. We believe that women's employment and participation in off-farm labor may contribute to increasing women's access to and control of income and enhance their empowerment in our study. Our findings for women's employment are consistent with other studies that show that women's empowerment (or its components; e.g. input in credit decisions) is positively associated with women's DD (60–63).
There are several ways that off-farm income may affect diets. First, it diversifies income for women and households, providing additional resources for the purchase of food, and has been associated with improved household food security (64). Secondly, women's decision-making power over productive assets and the use of income from agriculture and off-farm employment can serve as an enabler for women to act on their nutrition knowledge (30, 31). Studies show that income under women's control is more likely to be directed to food purchases (24). Male control of farming revenue has been negatively associated with dietary quality (65, 66). Additionally, income from off-farm activities can also be used by households to enhance farm production, increase purchases of farm inputs, or meet health and other needs, with benefits to nutrition (67).
We found that production of “other vitamin A−rich fruits and vegetables” was positively associated with the PDQS, and its coefficient was nearly twice as large as that for nuts and seeds production. Significant associations were also found for the production of vitamin A−rich dark green vegetables, whose production was promoted under the homestead gardening intervention. Sales of vegetables and fruits were not prevalent in this study. This observation suggests that the production of vegetables and fruits for consumption by the household may have been important for supporting maternal diet quality. While nuts and seeds production—the most commonly produced of which is sesame, a cash crop—were positively associated with PDQS, the income pathway to an improved PDQS may be relatively less important. Finally, while cashew nuts were grown in the area, qualitative data from the study suggests that they are considered an “exotic food”; therefore, they are not often consumed. One study suggested that production of non-cereal food groups was associated with an increase in their consumption (68). Evidence also suggests that the subsistence pathway may be important in farms with low agricultural biodiversity (24). In this study, food crop diversity was low, with a mean (± SD) of 2 (± 2) food groups produced. It is plausible that in this study, which was conducted in the context of a homestead agriculture intervention, households consumed what they produced on-farm: for example, amaranthus leaves and spinach (Table 2). However, amaranthus was sold by 11% of the households; therefore, it may have had a dual role in households by also contributing to income.
This study found a positive association between crop species richness and PDQS, and evidence of effect modification by sale of food crops. In households where food crops were sold, the association of crop species richness with PDQS was stronger. However, we also observed in this study that there was a negative association between selling crops and the PDQS. These findings suggest that for households that were producing surplus or crops for sale, crop species richness is important for women's diet quality, as the additional income is not translated to increases in purchases of healthy foods. It could also be that the additional income is translated to purchases of unhealthy foods. Alternatively, households that did not sell crops may not be producing a surplus or may be poorer and, thus, have poorer diet quality.
This study adds to the literature by evaluating the association of food crop diversity with maternal diet quality using a unique dietary metric that captures the healthy and unhealthy aspects of diet simultaneously. Studies in LMICs have often assessed dietary diversity, which measures micronutrient adequacy (36), as a proxy for women's diet quality. However, DD does not fully account for rapid nutrition transition, shifts in diet patterns, and increased consumption of unhealthy and processed foods, including in low-resource settings such as rural Tanzania (38, 69, 70). The PDQS considers consumption of unhealthy saturated fats, consumption of refined grains, and excess consumption of red and processed meats that have been associated with an increased risk of chronic diseases, including coronary heart disease and hypertension, in high-income settings (13, 47, 71). In another study, we have also recently found an association between the PDQS and poor pregnancy outcomes in urban Tanzanian women (34). We observed relatively high prevalences of overweight (24.3%) and obesity (13.1%) in our study population, and this supports the use of the PDQS in this setting, given its relevance for double-burden settings. Our study provides evidence of poor diet quality in rural Tanzania and clarifies the role of food crop diversity in maternal diet quality in this context.
This study has several strengths. We assessed various measures of agriculture production diversity and related them to maternal diet quality using the PDQS, a novel tool that has been validated in developed country locations. Our study provides some of the first measures of diet quality in a rural population in Tanzania and explores potential determinants. There are several limitations related to the study. The cross-sectional design of the study limits our ability to make causal inference. We controlled for known confounders for the associations evaluated; however, a prospective study is required to evaluate these associations in the future. The study did not assess women's caloric intake, which could confound associations between production diversity and PDQS, where diet quality could be a proxy for energy intake. However, we mitigated for this by adjusting for BMI in order to adjust for excess or insufficient caloric intake by study participants. However, there may still be residual confounding, which may affect our findings. In this study, we present food crop diversity based on the number of crops grown over the previous year, and the PDQS based on a recall of consumption over the previous month. Previous studies have taken a similar approach, with these measured at nonoverlapping time points (23, 72, 73). We believe this approach is reasonable in our study, given that we are interested in evaluating the overall role of crop diversity in all agricultural seasons and how it relates to maternal diet quality. Finally, this study was conducted within the context of a homestead gardening intervention, and eligibility for the intervention included access to land for vegetable production, which can limit generalizability of the study.
In conclusion, this research examines the complexity of pathways from food systems to improved nutrition outcomes among women. Our findings indicate that household food production may act both with access to markets for sale and purchase and with access to nonfarm income activities (nonfarm determinants) in its association with women's diet quality among Tanzanian women. Policies and programs to improve women's diet quality should consider aspects of market access and women's access to off-farm income, in addition to diversifying household crop production. Further, it is imperative that nutrition programs consider overall diet quality for women in LMICs, including in rural locations, in addition to measures of dietary diversity.
Supplementary Material
Acknowledgments
The authors’ responsibilities were as follows – IM: conceived of and designed the study, analyzed the data, and drafted the manuscript; WWF: was the principal investigator for the parent study, conceived of the study, designed the study, interpreted the data, and guided revisions of the manuscript; MMB, ALB, CRC, DM, SB, RAN, PW, SG: designed the study, interpreted the data, and guided revisions of the draft manuscript; HM, JK: were co-investigators for the study and participated in the study implementation, interpreted the data, and guided revisions of the manuscript; and all authors read and approved the final manuscript.
Notes
This study was funded by the IZUMI Foundation.
Author disclosures: IM was supported by the Feed the Future Innovation Lab for Nutrition at Tufts University in Boston, MA, which is funded by the United States Agency for International Development (award AID-OAA-L-10-00006). DM was supported by the Fogarty International Center of the National Institutes of Health under award number D43 TW007886/TW/FIC NIH HHS/United States. MMB was supported by the Aker Scholarship. All the other authors report no conflicts of interest.
Supplemental Tables 1–3 are available from the “Supplementary data” link in the online posting of the article and from the same link in the online table of contents at https://academic.oup.com/ajcn/.
Abbreviations used: DD, dietary diversity; DDS, dietary diversity score; GEE, generalized estimating equation; HANU, Homestead Agriculture and Nutrition; LMICs, low- and middle-income countries; MDD-W, minimum dietary diversity for women; MFD, market food diversity; PDQS, prime diet quality score.
Contributor Information
Isabel Madzorera, Department of Global Health and Population, Harvard TH Chan School of Public Health, Boston, MA, USA.
Mia M Blakstad, Department of Global Health and Population, Harvard TH Chan School of Public Health, Boston, MA, USA.
Alexandra L Bellows, Department of International Health (Human Nutrition), Johns Hopkins Bloomberg School of Public Health, Baltimore, MD, USA.
Chelsey R Canavan, Department of Global Health and Population, Harvard TH Chan School of Public Health, Boston, MA, USA.
Dominic Mosha, Ifakara Health Institute, Dar es Salaam, Tanzania.
Sabri Bromage, Department of Nutrition, Harvard TH Chan School of Public Health, Boston, MA, USA.
Ramadhani A Noor, Department of Global Health and Population, Harvard TH Chan School of Public Health, Boston, MA, USA.
Patrick Webb, Friedman School of Nutrition Science and Policy, Tufts University, Boston, MA, USA.
Shibani Ghosh, Friedman School of Nutrition Science and Policy, Tufts University, Boston, MA, USA.
Joyce Kinabo, Department of Food Science Technology, Nutrition and Consumer Sciences, Sokoine University of Agriculture, Morogoro, Tanzania.
Honorati Masanja, Ifakara Health Institute, Dar es Salaam, Tanzania.
Wafaie W Fawzi, Department of Global Health and Population, Harvard TH Chan School of Public Health, Boston, MA, USA; Department of Nutrition, Harvard TH Chan School of Public Health, Boston, MA, USA; Department of Epidemiology, Harvard TH Chan School of Public Health, Boston, MA, USA.
References
- 1. Popkin BM, Corvalan C, Grummer-Strawn LM. Dynamics of the double burden of malnutrition and the changing nutrition reality. Lancet North Am Ed. 2020;395:65–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Popkin BM. Relationship between shifts in food system dynamics and acceleration of the global nutrition transition. Nutr Rev. 2017;75:73–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Serra-Majem L. HLPE: nutrition and food systems. A report by the High Level Panel of Experts on Food Security and Nutrition. Nutrition and Food Systems, Rome. 2017. [Google Scholar]
- 4. Haddad L, Hawkes C, Waage J, Webb P, Godfray C, Toulmin C. Global Panel on Agriculture and Food Systems for Nutrition. Food systems and diets: Facing the challenges of the 21st century. London, UK. 2016. [Google Scholar]
- 5. Stanaway JD, Afshin A, Gakidou E, Lim SS, Abate D, Abate KH, Abbafati C, Abbasi N, Abbastabar H, Abd-Allah Fet al. Global, regional, and national comparative risk assessment of 84 behavioural, environmental and occupational, and metabolic risks or clusters of risks for 195 countries and territories, 1990-2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet North Am Ed. 2018;392:1923–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Global Burden of Disease Study Collaborators . Health effects of dietary risks in 195 countries, 1990-2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet North Am Ed. 2019;393:1958–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Yeneabat T, Adugna H, Asmamaw T, Wubetu M, Admas M, Hailu G, Bedaso A, Amare T. Maternal dietary diversity and micronutrient adequacy during pregnancy and related factors in East Gojjam Zone, Northwest Ethiopia, 2016. BMC Pregnancy Childbirth. 2019;19:173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lee SE, Talegawkar SA, Merialdi M, Caulfield LE. Dietary intakes of women during pregnancy in low- and middle-income countries. Public Health Nutr. 2013;16:1340–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lander RL, Hambidge KM, Westcott JE, Tejeda G, Diba TS, Mastiholi SC, Khan US, Garces A, Figueroa L, Tshefu Aet al. Pregnant women in four low-middle income countries have a high prevalence of inadequate dietary intakes that are improved by dietary diversity. Nutrients. 2019;11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Justus O, Afari-Sefa V, Lukumay P, Dubois T. Determinants of dietary diversity and the potential role of men in improving household nutrition in Tanzania. PLOS One. 2017;12:e0189022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Women's Dietary Diversity Project (WDDP) Study Group . Development of a dichotomous indicator for population-level assessment of dietary diversity in women of reproductive age. Curr Dev Nutr. 2017;1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lukmanji Z, Hertzmark E, Spiegleman D, Fawzi WW. Dietary patterns, nutrient intake, and sociodemographic characteristics in HIV-infected Tanzanian pregnant women. Ecol Food Nutr. 2013;52:34–62. [DOI] [PubMed] [Google Scholar]
- 13. Fung TT, Isanaka S, Hu FB, Willett WC. International food group-based diet quality and risk of coronary heart disease in men and women. Am J Clin Nutr. 2018;107:120–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Daru J, Zamora J, Fernández-Félix BM, Vogel J, Oladapo OT, Morisaki N, Tunçalp O, Torloni MR, Mittal S, Jayaratne Ket al. Risk of maternal mortality in women with severe anaemia during pregnancy and post partum: A multilevel analysis. Lancet Glob Health. 2018;6:e548–e54. [DOI] [PubMed] [Google Scholar]
- 15. Black RE, Victora CG, Walker SP, Bhutta ZA, Christian P, de Onis M, Ezzati M, Grantham-Mcgregor S, Katz J, Martorell Ret al. Maternal and child undernutrition and overweight in low-income and middle-income countries. Lancet North Am Ed. 2013;382:427–51. [DOI] [PubMed] [Google Scholar]
- 16. Sibhatu KT, Krishna VV, Qaim M. Production diversity and dietary diversity in smallholder farm households. Proc Natl Acad Sci USA. 2015;112:10657–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. ZimmererKS, de Haan S. Agrobiodiversity and a sustainable food future. Nature Plants. 2017;3:(4);17047. [DOI] [PubMed] [Google Scholar]
- 18. Gillespie S, van den Bold M. Agriculture, food systems, and nutrition: Meeting the challenge. Glob Chall. 2017;1:1600002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Koppmair S, Kassie M, Qaim M. Farm production, market access and dietary diversity in Malawi. Public Health Nutr. 2017 Feb;20:325–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Bellon MR, Ntandou-Bouzitou GD, Caracciolo F. On-farm diversity and market participation are positively associated with dietary diversity of rural mothers in Southern Benin, West Africa. PLOS One. 2016;11:e0162535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Jones AD, Creed-Kanashiro H, Zimmerer KS, de Haan S, Carrasco M, Meza K, Cruz-Garcia GS, Tello M, Plasencia Amaya F, Marin RMet al. Farm-level agricultural biodiversity in the Peruvian Andes is associated with greater odds of women achieving a minimally diverse and micronutrient adequate diet. J Nutr. 2018;148:1625–37. [DOI] [PubMed] [Google Scholar]
- 22. Jones AD. On-farm crop species richness is associated with household diet diversity and quality in subsistence- and market-oriented farming households in Malawi. J Nutr. 2017;147:86–96. [DOI] [PubMed] [Google Scholar]
- 23. Bellows AL, Canavan CR, Blakstad MM, Mosha D, Noor RA, Webb P, Kinabo J, Masanja H, Fawzi WW. The relationship between dietary diversity among women of reproductive age and agricultural diversity in rural Tanzania. Food Nutr Bull. 2020;41:(1):50−60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Jones AD. Critical review of the emerging research evidence on agricultural biodiversity, diet diversity, and nutritional status in low- and middle-income countries. Nutr Rev. 2017;75:769–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Sibhatu K, Qaim M. Farm production diversity and dietary quality: Linkages and measurement issues. Food Sec. 2018;10:47–59. [Google Scholar]
- 26. Sibhatu KT, Krishna VV, Qaim M. Production diversity and dietary diversity in smallholder farm households. Proc Natl Acad Sci USA. 2015;112:10657–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Rajendran S, Afari-Sefa V, Shee A, Bocher T, Bekunda M, Dominick I, Lukumay PJ. Does crop diversity contribute to dietary diversity? Evidence from integration of vegetables into maize-based farming systems. Agric Food Secur. 2017;6:1–13. [Google Scholar]
- 28. Herforth A, Ahmed S. The food environment, its effects on dietary consumption, and potential for measurement within agriculture-nutrition interventions. Food Sec. 2015;7:505–20. [Google Scholar]
- 29. Bellon MR, Ntandou-Bouzitou GD, Caracciolo F. On-farm diversity and market participation are positively associated with dietary diversity of rural mothers in Southern Benin, West Africa. PLOS One. 2016;11:e0162535–e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Madzorera I, Fawzi W. Women empowerment is central to addressing the double burden of malnutrition. EClinicalMedicine. 2020;20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Maligalig R, Demont M, Umberger WJ, Peralta A. Off-farm employment increases women's empowerment: Evidence from rice farms in the Philippines. J Rural Studies. 2019;71:62–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Black REP, Victora CGP, Walker SPP, Bhutta ZAP, Christian PP, de Onis MMD, Ezzati MP, Grantham-McGregor SP, Katz JP, Martorell RPet al. Maternal and child undernutrition and overweight in low-income and middle-income countries. Lancet North Am Ed. 2013;382:427–51. [DOI] [PubMed] [Google Scholar]
- 33. Gernand AD, Schulze KJ, Stewart CP, West KP, Christian P. Micronutrient deficiencies in pregnancy worldwide: Health effects and prevention. Nat Rev Endocrinol. 2016;12:274–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Madzorera I, Isanaka S, Wang M, Msamanga GI, Urassa W, Hertzmark E, Duggan C, Fawzi WW. Maternal dietary diversity and dietary quality scores in relation to adverse birth outcomes in Tanzanian women. Am J Clin Nutr. 2020;112(3):695–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Preedy VR, Hunter L-A, Patel VB. Diet quality: An evidence-based approach. Volume 2. 1st ed. New York, NY: Springer New York, Imprint, Humana; 2013. [Google Scholar]
- 36. Arimond M, Wiesmann D, Becquey E, Carriquiry A, Daniels MC, Deitchler M, Fanou-Fogny N, Joseph ML, Kennedy G, Martin-Prevel Yet al. Simple food group diversity indicators predict micronutrient adequacy of women's diets in 5 diverse, resource-poor settings. J Nutr. 2010;140:(11):2059S–69S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Keding GB, Msuya JM, Maass BL, Krawinkel MB. Dietary patterns and nutritional health of women: The nutrition transition in rural Tanzania. Food Nutr Bull. 2011;32:218–26. [DOI] [PubMed] [Google Scholar]
- 38. Keding G. Nutrition transition in rural Tanzania and Kenya. World Rev Nutr Diet. 2016;115:68–81. [DOI] [PubMed] [Google Scholar]
- 39. Steyn NP, McHiza ZJ. Obesity and the nutrition transition in sub-Saharan Africa. Ann NY Acad Sci. 2014;1311:88–101. [DOI] [PubMed] [Google Scholar]
- 40. Mosha D, Canavan CR, Bellows AL, Blakstad MM, Noor RA, Masanja H, Kinabo J, Fawzi W. The impact of integrated nutrition-sensitive interventions on nutrition and health of children and women in rural Tanzania: Study protocol for a cluster-randomized controlled trial. BMC Nutr. 2018;4:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Ambikapathi R, Gunaratna NS, Madzorera I, Passarelli S, Canavan CR, Noor RA, Madzivhandila T, Sibanda S, Abdelmenan S, Tadesse AWet al. Market food diversity mitigates the effect of environment on women's dietary diversity in the Agriculture to Nutrition (ATONU) study, Ethiopia. Public Health Nutr. 2019;22:2110–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Jones AD, Shrinivas A, Bezner-Kerr R. Farm production diversity is associated with greater household dietary diversity in Malawi: Findings from nationally representative data. Food Policy. 2014;46:1–12. [Google Scholar]
- 43. Anderson K, Distortions to agricultural incentives: A global perspective, 1955–2007. The World Bank; 2009. [Google Scholar]
- 44. Koppmair S, Kassie M, Qaim M. Farm production, market access and dietary diversity in Malawi. Public Health Nutr. 2017;20:325–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Zack RM, Irema K, Kazonda P, Leyna GH, Liu E, Gilbert S, Lukmanji Z, Spiegelman D, Fawzi W, Njelekela Met al. Validity of an FFQ to measure nutrient and food intakes in Tanzania. Public Health Nutr. 2018;21:2211–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Gicevic S, Gaskins AJ, Fung TT, Rosner B, Tobias DK, Isanaka S, Willett WC. Evaluating pre-pregnancy dietary diversity vs. dietary quality scores as predictors of gestational diabetes and hypertensive disorders of pregnancy. PLOS One. 2018;13:e0195103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Fung TT, Isanaka S, Hu FB, Willett WC. International food group-based diet quality and risk of coronary heart disease in men and women. Am J Clin Nutr. 2018;107:120–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Alvarez-Alvarez I, Toledo E, Lecea O, Salas-Salvadó J, Corella D, Buil-Cosiales P, Zomeño MD, Vioque J, Martinez JA, Konieczna Jet al. Adherence to a priori dietary indexes and baseline prevalence of cardiovascular risk factors in the PREDIMED-Plus randomised trial. Eur J Nutr. 2020;59:1219–32. [DOI] [PubMed] [Google Scholar]
- 49. Fung TT, Bromage S, Li Y, Bhupathiraju S, Batis C, Fawzi W, Holmes M, Stampfer M, Hu FB, Willett WC. A global diet quality index and risk of type 2 diabetes in U.S. women, Curr. Dev. Nutr. 2020;4:1401. [Google Scholar]
- 50. Liang K, Zeger S. A comparison of two bias-corrected covariance estimators for generalized estimating equations. Biometrika. 1986;73:13–22. [Google Scholar]
- 51. Jones AD, Creed-Kanashiro H, Zimmerer KS, de Haan S, Carrasco M, Meza K, Cruz-Garcia GS, Tello M, Plasencia Amaya F, Marin RMet al. Farm-level agricultural biodiversity in the Peruvian Andes is associated with greater odds of women achieving a minimally diverse and micronutrient adequate diet. J Nutr. 2018;148:1625–37. [DOI] [PubMed] [Google Scholar]
- 52. Kumar N, Harris J, Rawat R. If they grow it, will they eat and grow? Evidence from Zambia on agricultural diversity and child undernutrition. J Dev Stud. 2015;51:1060–77. [Google Scholar]
- 53. Murendo C, Nhau B, Mazvimavi K, Khanye T, Gwara S. Nutrition education, farm production diversity, and commercialization on household and individual dietary diversity in Zimbabwe. Food Nutr Res. 2018;62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Chegere MJ, Stage J. Agricultural production diversity, dietary diversity and nutritional status: Panel data evidence from Tanzania. World Dev. 2020;129:104856. [Google Scholar]
- 55. Sibhatu KT, Qaim M. Review: Meta-analysis of the association between production diversity, diets, and nutrition in smallholder farm households. Food Policy. 2018;77:1–18. [Google Scholar]
- 56. Berti PR. Relationship between production diversity and dietary diversity depends on how number of foods is counted. Proc Natl Acad Sci USA. 2015;112:E5656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Zanello G, Shankar B, Poole N. Buy or make? Agricultural production diversity, markets and dietary diversity in Afghanistan. Food Policy. 2019;87:101731. [Google Scholar]
- 58. Bellon M, Ntandou-Bouzitou G, Caracciolo F. On-farm diversity and market participation are positively associated with dietary diversity of rural mothers in Southern Benin, West Africa. PLOS One. 2016;11:e0162535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Abay K, Hirvonen K. Does market access mitigate the impact of seasonality on child growth? Panel data evidence from Northern Ethiopia. J Dev Stud. 2017;53:1414–29. [Google Scholar]
- 60. Sraboni E, Quisumbing A. Women's empowerment in agriculture and dietary quality across the life course: Evidence from Bangladesh. Food Policy. 2018;81:21–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Galiè A, Teufel N, Girard AW, Baltenweck I, Dominguez-Salas P, Price MJ, Jones R, Lukuyu B, Korir L, Raskind Iet al. Women's empowerment, food security and nutrition of pastoral communities in Tanzania. Global Food Security. 2019;23:125–34. [Google Scholar]
- 62. Malapit H, Kadiyala S, Quisumbing A, Cunningham K, Tyagi P. Women's empowerment mitigates the negative effects of low production diversity on maternal and child nutrition in Nepal. J Dev Stud. 2015;51:1097–123. [Google Scholar]
- 63. Malapit HJL, Quisumbing AR. What dimensions of women's empowerment in agriculture matter for nutrition in Ghana?. Food Policy. 2015;52:54–63. [Google Scholar]
- 64. Babatunde RO, Qaim M. Impact of off-farm income on food security and nutrition in Nigeria. Food Policy. 2010;35:303–11. [Google Scholar]
- 65. Chege CGK, Andersson CIM, Qaim M. Impacts of supermarkets on farm household nutrition in Kenya. World Dev. 2015;72:394–407. [Google Scholar]
- 66. Fischer E, Qaim M. Gender, agricultural commercialization, and collective action in Kenya. Food Sec. 2012;4:441–53. [Google Scholar]
- 67. Bjornlund H, Zuo A, Wheeler SA, Parry K, Pittock J, Mdemu M, Moyo M. The dynamics of the relationship between household decision-making and farm household income in small-scale irrigation schemes in southern Africa. Agric Water Manage. 2019;213:135–45. [Google Scholar]
- 68. Kennedy E, Kershaw M, Coates J. Food systems: Pathways for improved diets and nutrition. Curr Dev Nutr. 2018;2:(9). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Popkin BM. The nutrition transition: An overview of world patterns of change. Nutr Rev. 2004;62:S140–3. [DOI] [PubMed] [Google Scholar]
- 70. Snyder J, Ijumba C, Tschirley D, Reardon T. Local response to the rapid rise in demand for processed and perishable foods: Results of an inventory of processed food products in Dar es Salaam; 2015. [Google Scholar]
- 71. Gicevic S, Gaskins AJ, Fung TT, Rosner B, Tobias DK, Isanaka S, Willett WC. Evaluating pre-pregnancy dietary diversity vs. dietary quality scores as predictors of gestational diabetes and hypertensive disorders of pregnancy. PLOS One. 2018;13:e0195103–e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Gupta S, Sunder N, Pingali PL. Market access, production diversity, and diet diversity: Evidence from India. Food Nutr Bull. 2020;41:167–85. [DOI] [PubMed] [Google Scholar]
- 73. Adubra L, Savy M, Fortin S, Kameli Y, Kodjo NE, Fainke K, Mahamadou T, Le Port A, Martin-Prevel Y. The minimum dietary diversity for women of reproductive age (MDD-W) indicator is related to household food insecurity and farm production diversity: Evidence from rural Mali. Curr Dev Nutr. 2019;3(3):nzz002. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.