ABSTRACT
Background
The role of nutrition in the primary prevention of peripheral artery disease (PAD), the third leading cause of atherosclerotic cardiovascular disease, is undetermined. Flavonoids may attenuate atherosclerosis and therefore persons who consume flavonoid-rich foods may have a lower risk of developing PAD.
Objectives
We aimed to examine the association between flavonoid intake and PAD hospitalizations and investigate if the association differs according to established risk factors for PAD.
Methods
Baseline data from 55,647 participants of the Danish Diet, Cancer, and Health Study without PAD, recruited from 1993 to 1997, were cross-linked with Danish nationwide registries. Flavonoid intake was calculated from FFQs using the Phenol-Explorer database. Associations were examined using multivariable-adjusted restricted cubic splines based on Cox proportional hazards models.
Results
After a median [IQR] follow-up time of 21 [20–22] y, 2131 participants had been hospitalized for any PAD. The association between total flavonoid intake and total PAD hospitalizations was nonlinear, reaching a plateau at ∼750–1000 mg/d. Compared with the median flavonoid intake in quintile 1 (174 mg/d), an intake of 1000 mg/d was associated with a 32% lower risk of any PAD hospitalization (HR: 0.68; 95% CI: 0.60, 0.77), a 26% lower risk of atherosclerosis (HR: 0.74; 95% CI: 0.62, 0.88), a 28% lower risk of an aneurysm (HR: 0.72; 95% CI: 0.59, 0.88), and a 47% lower risk of a hospitalization for other peripheral vascular disease (HR: 0.53; 95% CI: 0.42, 0.67). A higher total flavonoid intake was also significantly associated with a lower incidence of revascularization or endovascular surgery and lower extremity amputation. The association between total flavonoid intake and PAD hospitalizations differed according to baseline smoking status, alcohol intake, BMI, and diabetes status.
Conclusions
Ensuring the adequate consumption of flavonoid-rich foods, particularly in subpopulations prone to the development of atherosclerosis, may be a key strategy to lower the risk of PAD.
Keywords: nutrition, peripheral artery disease, primary prevention, cohort study, lifestyle
Introduction
Peripheral artery disease (PAD) is the atherosclerotic obstruction, or ischemia, of arteries and most commonly manifests in the lower extremities (1). PAD prevalence rises steeply with age; in high-income countries, such as Denmark, PAD prevalence is <7% for persons in their fifties, >10% for persons in their sixties, and >18% for persons in their eighties (2). In 2010, >200 million adults worldwide had PAD (2). With the aging of the global population, it is likely that PAD will become increasingly common (2). PAD is associated with cardiovascular disease morbidity and mortality (3) and the most common symptom of PAD, intermittent claudication, can induce considerable suffering and limit walking ability (4). Furthermore, severe PAD can lead to limb ischemia and amputations of the lower extremities. The public health significance of PAD calls for evidence-based primary prevention strategies.
The role that nutrition plays in the primary prevention of PAD is currently uncharacterized. Flavonoids, polyphenolic compounds found ubiquitously in plant-derived foods and beverages such as fruits, vegetables, dark chocolate, tea, and red wine (5), have been shown to improve NO bioavailability and endothelial function and decrease blood pressure and inflammation (6). The main risk factors for PAD are age, smoking, and diabetes mellitus. They are thought to contribute to PAD development by an increase in inflammation, increase in arterial stiffness, decrease in NO, increase in oxidative stress, and decrease in vasodilation (7). Thus, flavonoid-rich foods could be important moderators of PAD. Although there is some evidence that higher flavonoid intakes are associated with a lower incidence of PAD (8), larger, longer-term epidemiological studies are needed to further examine potential associations.
We have previously shown that higher total flavonoid intakes are associated with lower atherosclerotic cardiovascular disease hospitalizations, and, more specifically, lower PAD hospitalizations (9). However, unexplored areas include whether the prevalence of risk factors modifies the association between flavonoids and incidence of PAD hospitalization; associations of individual flavonoid subclasses and individual flavonoid compounds with PAD; associations of flavonoids with subtypes of PAD such as atherosclerosis, aneurysm, embolism or thrombosis, and other peripheral vascular diseases (PVDs); and associations of flavonoids with PAD-related procedures such as revascularizations or endovascular surgery, and amputations.
Therefore, the primary aim of this study was to investigate the association of total flavonoid and flavonoid subclass intakes with PAD hospitalizations in the Danish Diet, Cancer, and Health cohort. Secondary aims were to investigate whether these associations differed according to the presence of risk factors for PAD.
Methods
Study population
Participants were recruited from the greater areas of Copenhagen and Aarhus between 1993 and 1997 as part of the Danish Diet, Cancer, and Health study. Of the 57,053 participants recruited, 56,468 completed an FFQ and were without a cancer diagnosis before enrollment. All Danish residents are assigned a unique and permanent number allowing cross-linking of participants to nationwide registers. The following databases were cross-linked to the cohort: the Civil Registration System, the Integrated Database for Labor Market Research, and the Danish National Patient Register, which contains information on all hospital admissions in Denmark since 1978. For all admissions this includes 1 primary diagnosis and ≥1 secondary diagnoses defined by the International Classification of Diseases (ICD): the 8th revision (ICD-8) until 1993 and the 10th revision (ICD-10) from 1994 (10). Participants were excluded if they had improbable energy intakes [<2092 kJ/d (<500 kcal/d) and >20,920 kJ/d (>5000 kcal/d)] (n = 202), or if they had missing covariates or were extreme outliers (n = 218). Finally, participants were excluded if they had any prevalent PAD (n = 401): a prior diagnosis of atherosclerosis (ICD-8: 440, 414; ICD-10: I70), aortic aneurysm (ICD-8: 441; ICD-10: I71), other aneurysm (ICD-8: 442; ICD-10: I72), other PVD (ICD-8: 443; ICD-10: I73), or arterial embolism and thrombosis (ICD-8: 444; ICD-10: I74) (Supplemental Figure 1).
This study was approved by the Danish Data Protection Agency (Ref no. 2012-58-0004; I-Suite nr: 6357; VD-2018-117).
Exposures
Exposures were intakes of total flavonoids, flavonoid subclasses, and individual flavonoid compounds with mean intakes > 5 mg/d. The calculations of flavonoid intake we used have been described previously (11). Briefly, estimates of the flavonoid content of all foods and beverages in the FFQ were obtained from the Phenol-Explorer database (12). Because the average intakes of isoflavones, dihydrochalcones, dihydroflavonols, and chalcones were <5 mg/d, they were not included in the individual subclass analyses. Total flavonoid intake was calculated by summing each of the 219 flavonoid compounds.
Study outcomes
The primary outcome was first-time PAD hospitalization. PAD hospitalization was defined as hospitalization with a primary or secondary diagnosis code for atherosclerosis, aneurysm (aortic and other), arterial embolism or thrombosis, or other PVD (ICD-8 or ICD-10 as aforementioned). Other PVD diagnoses were primarily unspecified PVD: more specifically, intermittent claudication. Secondary outcomes were atherosclerosis, aneurysm (aortic and other), arterial embolism or thrombosis, or other PVD, discretely. These ICD codes for PAD hospitalization have a positive predictive value of 81.2% in the Danish National Patient Register (13).
PAD-related procedures
To assess the association between flavonoid intake and PAD-related procedures, we obtained the following procedure codes from the Danish National Patient Register: major amputation of the lower extremities (above ankle), and vascular reconstruction and endovascular procedures of the central or lower extremity peripheral arteries (Supplemental Table 1). Because the Danish National Patient Register only started recording procedure codes in 1996, data were left truncated to 1 January, 1996. As such, for this outcome analysis, 66 participants were excluded because they died, and 9 participants were excluded because they developed PAD, before 1 January, 1996 (n = 55,572).
Validated case analysis
To verify the registry-based outcomes, we re-examined associations using only medically reviewed and validated cases (ICD-10: I702, I702A, I739A, I739B, I739C), with follow-up between August 1994 and December 2009. Validation was undertaken by scrutinizing all medical records using prespecified criteria for a diagnosis of PAD, as described in more detail previously (13). Owing to prior diagnosis of validated PAD, a further 7 participants were excluded in this analysis (n = 55,640).
Covariates
Data on sex, age, education, smoking habits, alcohol consumption, daily activity, and diet were obtained from questionnaires completed by participants upon study enrollment. Smoking status was defined as “current smoker” if the participant indicated that they currently smoked daily, “previous smoker” if the participant indicated that they smoked daily for ≥1 y at any stage of their life, and “never smoker” if the participant was neither a current nor a previous smoker, as defined above. Anthropometry and total cholesterol were measured at the study centers. Mean annual income over 5 y (defined as household income after taxation and interest, using the value of the Danish currency in 2015) was used to represent socioeconomic status. ICD-8 and ICD-10 codes were used to determine prevalent chronic kidney disease, chronic obstructive pulmonary disease (COPD), ischemic heart disease, ischemic stroke, heart failure, atrial fibrillation, and cancers (Supplemental Table 2). For hypertension and diabetes mellitus, self-reported data were used owing to underreporting of these diagnoses in the Danish National Patient Register (14).
Statistical analysis
Participants were followed from the date of enrollment until the date of PAD hospitalization, death, emigration, or end of follow-up (August 2017), whichever came first. Nelson–Aalen plots of cumulative incidence for PAD with a competing risk of death, as well as by quintiles of total flavonoid intake, were computed. Quintiles were derived separately for each exposure variable. Multivariable Cox proportional hazards models were used to investigate relations between the exposures of interest and all outcomes, fitting a separate model for each exposure of interest and outcome combination. To allow the association between the exposure and outcome to be nonlinear, the modeling of continuous exposure variables was carried out using restricted cubic splines using the “rms” R package with the rcs() function [see (15) for a practical description of this]. For presentation purposes (but not for modeling), quintiles of the exposure variable were generated and the median value of each quintile was calculated. HRs calculated from each of the aforementioned fitted models, relative to a reference value of the median of the first quintile of the relevant exposure variable, were plotted against the exposure variable, with 95% confidence bands provided. Using these values, HRs were calculated from the fitted models comparing the median of each quintile to the reference value of the median in quintile 1, and tabulated with 95% CIs. Individuals with intakes >4 SDs above the mean were excluded from the spline analysis. Four models of adjustment were used: 1) minimally adjusted: age and sex; 1b) multivariable-adjusted: age, sex, BMI, smoking status (current/former/never), physical activity (total daily metabolic equivalent), pure alcohol intake (g/d), and socioeconomic status (income); 2) multivariable-adjusted including total energy intake and potential dietary confounders: all variables in Model 1b plus energy intake (kJ/d) and intakes (g/d) of fish, red meat, processed meat, PUFAs, MUFAs, and SFAs; and 3) multivariable-adjusted including covariates that may be on the causal pathway: all variables in Model 1b plus hypertension (yes/no), hypercholesterolemia (yes/no), and prevalent disease (stroke, ischemic heart disease, heart failure, diabetes, COPD, chronic kidney disease, and cancer; entered into the model separately). Covariates were chosen a priori to the best of our knowledge of potential confounders of flavonoid intake and PAD. Cox proportional hazards assumptions were tested using log-log plots of the survival function against time and assessed for parallel appearance. All deaths were censored rather than treated as a competing risk (16). Because the presence of major chronic diseases may influence the diet, as it was captured at baseline, and result in a bias, we conducted a sensitivity analysis where all participants with a comorbidity at baseline were excluded. In addition, owing to no censoring in this cohort, standard logistic regression models were used to obtain the 20-y absolute risk estimates of PAD. For these analyses, a binary outcome indicating the presence of PAD hospitalization during 20 y of follow-up was used. Unless indicated by the stratification variable, these estimates are for a nonsmoking participant, aged 56 y, with a BMI (in kg/m2) of 25.5, a total daily metabolic equivalent score of 56, with a mean household income of 394,701–570,930 DKK/y, and an alcohol intake of 13 g/d. Emerging evidence suggests that flavonoids may afford greater protection to those with lifestyle habits placing them at a higher risk of cardiovascular disease (9); to investigate whether the associations differed according to established risk factors for PAD, firstly, P values for interaction terms were obtained from likelihood ratio tests of Cox proportional hazards models with and without the interaction term and, secondly, all analyses were stratified by risk factors for PAD (17) for which data had been collected at baseline (smoking status, alcohol intake, BMI, sex, diabetes status, cholesterol concentrations, and hypertension status). Because there is potential for residual confounding, when stratifying by smoking status [never smoker or ever smoker (current or previous smoker)], alcohol intake, and BMI, the corresponding continuous variables (smoking pack-years, alcohol intake, and BMI, respectively) were included in the model where appropriate. We chose stratification cutoffs of 20 g pure alcohol per day and a BMI of 30 as done previously in this cohort (11). All analyses were undertaken using STATA/IC 14.2 (StataCorp LLC) and R statistics (R Core Team, 2019) (18). Statistical significance was set at P ≤ 0.05 (2-tailed) for all tests.
Results
This population of 55,647 Danish residents, with a median [IQR] age of 56 [52–60] y at entry, had a median [IQR] follow-up of 21 [20–22] y. During a maximum of 23 y of follow-up, 2131 individuals were hospitalized for any form of PAD. For PAD subtypes, 993 were hospitalized for atherosclerosis, 800 for an aneurysm, 161 for an embolism or thrombosis, and 653 for other PVD. Some participants received >1 PAD diagnosis. During follow-up, 799 participants underwent revascularization or endovascular surgery and 242 had a lower extremity amputation. Furthermore, 12,738 participants died from any cause without a PAD hospitalization. Supplemental Figure 2 shows the cumulative incidence of PAD hospitalizations and death without a PAD hospitalization.
Baseline characteristics
Compared with participants with the lowest flavonoid intakes, those with the highest habitual intakes were more likely to be female, have a lower BMI, be more physically active, have a higher degree of education, and have a higher income, and were less likely to have ever smoked or to be hypercholesterolemic. Furthermore, they tended to eat more fish, dietary fiber, fruits, and vegetables, and eat less red and processed meat (Table 1).
TABLE 1.
Baseline characteristics of the study population1
| Total population (n = 55,647) | Total flavonoid intake quintiles | |||||
|---|---|---|---|---|---|---|
| Characteristic | Q1 (n = 11,130) | Q2 (n = 11,129) | Q3 (n = 11,130) | Q4 (n = 11,129) | Q5 (n = 11,129) | |
| Total flavonoid intake, mg/d | 496 [287–805] | 174 [128–213] | 321 [287–357] | 496 [443–549] | 727 [660–805] | 1202 [1025–1436] |
| Sex, male | 26,429 (47.5) | 6417 (57.7) | 5693 (51.2) | 5290 (47.5) | 4948 (44.5) | 4081 (36.7) |
| Age, y | 56 [52–60] | 56 [52–60] | 56 [52–60] | 56 [52–60] | 56 [52–60] | 55 [52–59] |
| BMI, kg/m2 | 25.5 [23.3–28.2] | 26.1 [23.8–28.9] | 25.9 [23.6–28.5] | 25.6 [23.3–28.3] | 25.3 [23.2–27.9] | 24.9 [22.7–27.4] |
| MET score | 56.5 [37.0–84.8] | 51.0 [32.0–78.0] | 55.5 [36.3–84.0] | 57.3 [38.0–85.0] | 58.3 [38.5–87.0] | 60.0 [39.8–88.5] |
| Smoking status | ||||||
| Never | 19,628 (35.3) | 2741 (24.6) | 3740 (33.6) | 3983 (35.8) | 4444 (39.9) | 4720 (42.4) |
| Former | 16,027 (28.8) | 2673 (24.0) | 3017 (27.1) | 3243 (29.1) | 3563 (32.0) | 3531 (31.7) |
| Current | 19,992 (35.9) | 5716 (51.4) | 4372 (39.3) | 3903 (35.1) | 3122 (28.1) | 2879 (25.9) |
| Education, y | ||||||
| ≤7 | 18,248 (32.8) | 5066 (45.5) | 4222 (37.9) | 3555 (31.9) | 3006 (27.0) | 2399 (21.6) |
| 8–10 | 25,677 (46.1) | 4876 (43.8) | 5234 (47.0) | 5326 (47.9) | 5266 (47.3) | 4975 (44.7) |
| ≥11 | 11,694 (21.0) | 1182 (10.6) | 1669 (15.0) | 2244 (20.2) | 2850 (25.6) | 3749 (33.7) |
| Mean household income, DKK/y | ||||||
| ≤394,700 | 13,753 (24.7) | 3293 (29.6) | 2713 (24.4) | 2685 (24.1) | 2560 (23.0) | 2502 (22.5) |
| 394,701–570,930 | 13,914 (25.0) | 3241 (29.1) | 2993 (26.9) | 2693 (24.2) | 2582 (23.2) | 2405 (21.6) |
| 570,931–758,297 | 13,965 (25.1) | 2918 (26.2) | 3004 (27.0) | 2879 (25.9) | 2604 (23.4) | 2560 (23.0) |
| >758,297 | 14,015 (25.2) | 1678 (15.1) | 2419 (21.7) | 2872 (25.8) | 3383 (30.4) | 3663 (32.9) |
| Hypertensive | 9006 (16.2) | 1779 (16.0) | 1832 (16.5) | 1833 (16.5) | 1808 (16.2) | 1754 (15.8) |
| Hypercholesterolemic | 4088 (7.3) | 877 (7.9) | 808 (7.3) | 833 (7.5) | 848 (7.6) | 722 (6.5) |
| Comorbidities | ||||||
| Diabetes | 1152 (2.1) | 266 (2.4) | 216 (1.9) | 246 (2.2) | 215 (1.9) | 209 (1.9) |
| Heart failure | 200 (0.4) | 48 (0.4) | 50 (0.4) | 36 (0.3) | 37 (0.3) | 29 (0.3) |
| Atrial fibrillation | 274 (0.5) | 55 (0.5) | 55 (0.5) | 59 (0.5) | 49 (0.4) | 56 (0.5) |
| Ischemic heart disease | 2101 (3.8) | 546 (4.9) | 404 (3.6) | 424 (3.8) | 383 (3.4) | 344 (3.1) |
| Ischemic stroke | 734 (1.3) | 206 (1.9) | 140 (1.3) | 135 (1.2) | 125 (1.1) | 128 (1.2) |
| COPD | 844 (1.5) | 219 (2.0) | 185 (1.7) | 155 (1.4) | 156 (1.4) | 129 (1.2) |
| CKD | 199 (0.4) | 42 (0.4) | 32 (0.3) | 44 (0.4) | 41 (0.4) | 40 (0.4) |
| Cancer | 241 (0.4) | 52 (0.5) | 42 (0.4) | 58 (0.5) | 33 (0.3) | 56 (0.5) |
| Medication use | ||||||
| Insulin treated | 374 (0.7) | 76 (0.7) | 64 (0.6) | 82 (0.7) | 81 (0.7) | 71 (0.6) |
| Antihypertensive | 6794 (12.2) | 1339 (12.0) | 1403 (12.6) | 1381 (12.4) | 1350 (12.1) | 1321 (11.9) |
| Statin | 1021 (1.8) | 240 (2.2) | 205 (1.8) | 208 (1.9) | 206 (1.9) | 162 (1.5) |
| HRT | ||||||
| Never | 15,885 (28.5) | 2594 (23.3) | 3031 (27.2) | 3258 (29.3) | 3248 (29.2) | 3754 (33.7) |
| Current | 8780 (15.8) | 1291 (11.6) | 1559 (14.0) | 1687 (15.2) | 1999 (18.0) | 2244 (20.2) |
| Former | 4521 (8.1) | 819 (7.4) | 842 (7.6) | 887 (8.0) | 928 (8.3) | 1045 (9.4) |
| NSAID | 17,998 (32.6) | 3482 (31.5) | 3496 (31.6) | 3626 (32.8) | 3606 (32.6) | 3788 (34.3) |
| Aspirin | 7009 (12.6) | 1362 (12.2) | 1343 (12.1) | 1430 (12.8) | 1382 (12.4) | 1492 (13.4) |
| Dietary characteristics | ||||||
| Energy, kcal | 2271 [1878–2717] | 2060 [1681–2485] | 2213 [1844–2629] | 2330 [1944–2768] | 2375 [1988–2828] | 2373 [1975–2842] |
| Total fish intake, g/d | 38 [25–55] | 33 [22–49] | 38 [25–54] | 39 [27–57] | 41 [28–59] | 40 [27–57] |
| Red meat intake, g/d | 78 [57–107] | 80 [58–108] | 81 [59–110] | 80 [58–110] | 78 [57–107] | 72 [52–99] |
| Processed meat intake, g/d | 25 [14–40] | 28 [17–45] | 26 [15–42] | 25 [14–40] | 23 [14–38] | 20 [11–34] |
| Dietary fiber intake, g/d | 20 [16–25] | 17 [13–20] | 19 [16–23] | 21 [17–25] | 22 [18–27] | 23 [19–29] |
| Total carbohydrate intake, g/d | 246 [201–297] | 213 [174–256] | 239 [199–285] | 253 [208–303] | 264 [217–316] | 267 [218–323] |
| SFA, g/d | 31 [24–39] | 29 [23–37] | 31 [24–39] | 32 [24–40] | 32 [25–41] | 32 [24–41] |
| PUFA, g/d | 13 [10–17] | 12 [9–16] | 13 [10–17] | 14 [10–18] | 14 [11–18] | 14 [10–18] |
| MUFA, g/d | 27 [21–35] | 26 [20–34] | 27 [21–35] | 28 [22–35] | 28 [22–35] | 27 [21–34] |
| Fruit intake, g/d | 172 [95–282] | 88 [45–142] | 162 [98–239] | 193 [114–301] | 225 [140–360] | 240 [141–390] |
| Vegetable intake, g/d | 162 [105–231] | 115 [72–171] | 150 [100–212] | 168 [114–236] | 185 [127–254] | 196 [136–272] |
| Alcohol intake, g/d | 13 [6–31] | 11 [3–24] | 13 [6–25] | 15 [6–34] | 14 [7–32] | 13 [6–32] |
1Values are medians [IQRs] or n (%), unless otherwise indicated. CKD, chronic kidney disease; COPD, chronic obstructive pulmonary disease; DKK, Danish Krone; HRT, hormone replacement therapy; MET, metabolic equivalent; NSAID, nonsteroidal anti-inflammatory drug.
Associations between total flavonoid intake and PAD-related hospitalizations and procedures
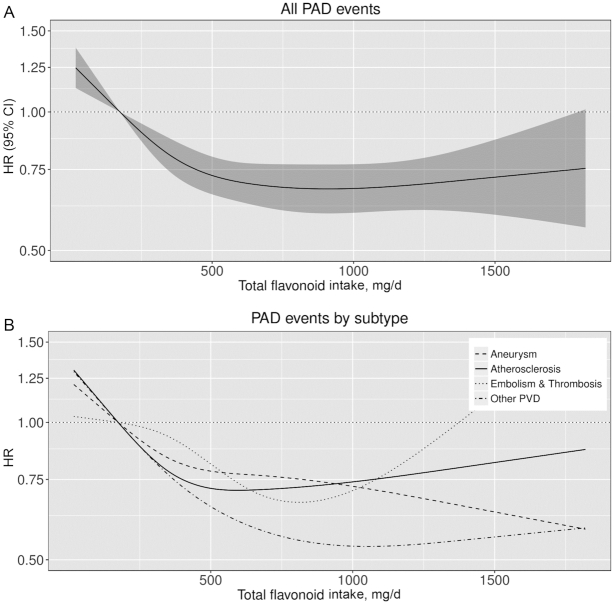
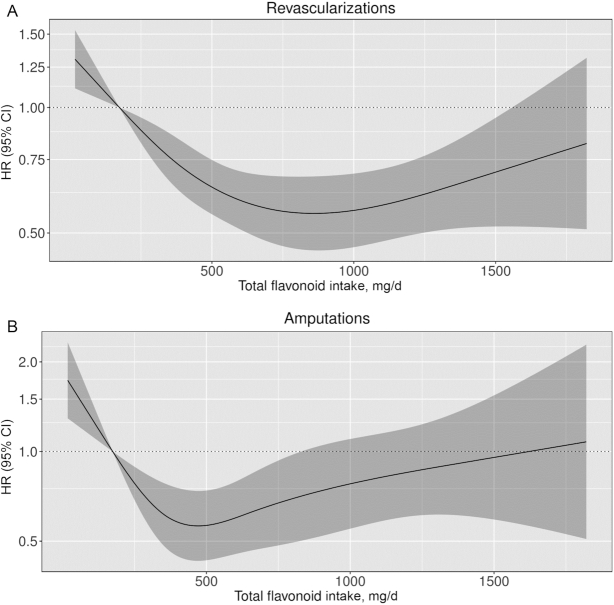
The association between total flavonoid intake and total PAD hospitalizations was nonlinear; restricted cubic splines showed a threshold of the inverse association at ∼750–1000 mg/d (Figure 1). Compared with a total flavonoid intake of 174 mg/d (median intake in quintile 1) and after multivariable adjustments (Model 1b), an intake of 1000 mg/d was associated with a 32% lower risk of a hospitalization for PAD (HR: 0.68; 95% CI: 0.60, 0.77). Figure 1 and Supplemental Table 3 show associations between total flavonoid intake and PAD hospitalization subtypes. A total flavonoid intake of 1000 mg/d was associated with a 26% lower risk of a hospitalization for atherosclerosis (HR: 0.74; 95% CI: 0.62, 0.88), a 28% lower risk of a hospitalization for an aneurysm (HR: 0.72; 95% CI: 0.59, 0.88), a nonsignificant 27% lower risk of a hospitalization for an arterial embolism or thrombosis (HR: 0.73; 95% CI: 0.46, 1.13), and a 47% lower risk of a hospitalization for other PVD (HR: 0.53; 95% CI: 0.42, 0.67). The association between total flavonoid intake and PAD-related procedures appeared to be “U-shaped” (Figure 2). Compared with a total flavonoid intake of 174 mg/d, the lowest risks were seen for intakes of 1000 mg/d for revascularizations/endovascular surgery (HR: 0.57; 95% CI: 0.46, 0.69), and 500 mg/d for amputations (HR: 0.56; 95% CI: 0.43, 0.74), after multivariable adjustments (Model 1b). Associations were subtly strengthened, in that HRs became more extreme, in a sensitivity analysis excluding all participants with a comorbidity at baseline (n = 4779 excluded; n = 50,868 remaining in the analysis; data not shown).
FIGURE 1.
Cubic spline curves describing the association between total flavonoid intake and both total PAD hospitalization events (A) and subtypes of PAD (B) (n = 55,647). HRs and 95% CIs are based on Cox proportional hazards models adjusted for age, sex, BMI, smoking status, physical activity, socioeconomic status (income), and alcohol intake (Model 1b) and are comparing the specific amount of flavonoid intake (horizontal axis) with the median intake for participants in the lowest intake quintile (174 mg/d). Compared with a total flavonoid intake of 174 mg/d, an intake of 1000 mg/d was associated with a 32% lower risk of PAD (HR: 0.68; 95% CI: 0.60, 0.77), a 26% lower risk of atherosclerosis (HR: 0.74; 95% CI: 0.62, 0.88), a 28% lower risk of an aneurysm (HR: 0.72; 95% CI: 0.59, 0.88), a nonsignificant 27% lower risk of an arterial embolism or thrombosis (HR: 0.73; 95% CI: 0.46, 1.13), and a 47% lower risk of a hospitalization for other PVD (predominantly intermittent claudication) (HR: 0.53; 95% CI: 0.42, 0.67). PAD, peripheral artery disease; PVD, peripheral vascular disease.
FIGURE 2.
Cubic spline curves describing the association between total flavonoid intake and total peripheral artery disease–related procedures [revascularizations/endovascular surgery (A) and amputations (B)] in 55,572 participants of the Danish Diet, Cancer, and Health cohort. HRs and 95% CIs are based on Cox proportional hazards models adjusted for age, sex, BMI, smoking status, physical activity, socioeconomic status (income), and alcohol intake (Model 1b) and are comparing the specific amount of flavonoid intake (horizontal axis) with the median intake for participants in the lowest intake quintile (174 mg/d). Compared with a total flavonoid intake of 174 mg/d, an intake of 1000 mg/d was associated with a 43% lower risk of revascularizations/endovascular surgery (HR: 0.57; 95% CI: 0.46, 0.69), and an intake of 500 mg/d was associated with a 44% lower risk of an amputation (HR: 0.56; 95% CI: 0.43, 0.74).
Associations between flavonoid subclass intakes and PAD-related hospitalizations
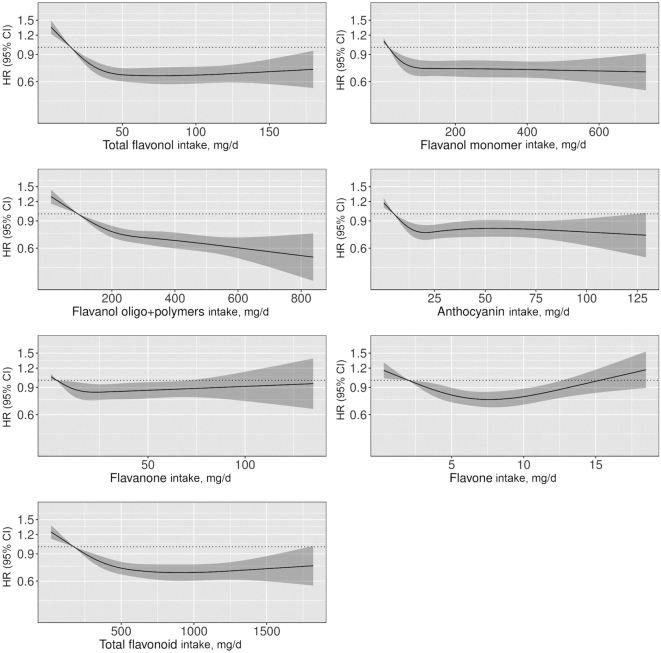
For all flavonoid subclasses, after multivariable adjustments, participants in quintiles 2–5 had a lower risk of a PAD hospitalization than participants in quintile 1 (Table 2). HRs after adjustments for covariates that are both confounders and on the causal pathway (Model 3) are presented in Supplemental Table 4 but did not differ substantively. Associations were strongest for the flavonol and flavanol oligo + polymers subclasses where participants in quintile 5 had a 32% (Q5 compared with Q1 HR: 0.68; 95% CI: 0.60, 0.77) and 33% (Q5 compared with Q1 HR: 0.67; 95% CI: 0.59, 0.77) lower risk of a PAD hospitalization, respectively, after multivariable adjustments (Model 1b) (Table 2). All subclasses were nonlinearly associated with PAD hospitalizations, with thresholds occurring at varying amounts of intake (Figure 3).
TABLE 2.
HRs of PAD hospitalizations by quintiles of flavonoid and flavonoid subclass intakes1
| Flavonoid intake quintiles | |||||
|---|---|---|---|---|---|
| Q1 (n = 11,130) | Q2 (n = 11,129) | Q3 (n = 11,130) | Q4 (n = 11,129) | Q5 (n = 11,129) | |
| Total flavonoids | |||||
| Events, n | 648 | 471 | 404 | 324 | 284 |
| Intake,2 mg/d | 174 [6–251] | 321 [251–395] | 496 [395–602] | 727 [602–909] | 1202 [909–3552] |
| HR (95% CI) | |||||
| Model 1 | Ref. | 0.71 (0.65, 0.76) | 0.55 (0.50, 0.61) | 0.49 (0.44, 0.55) | 0.47 (0.42, 0.53) |
| Model 1b | Ref. | 0.83 (0.77, 0.89) | 0.73 (0.66, 0.80) | 0.69 (0.61, 0.77) | 0.69 (0.61, 0.78) |
| Model 2 | Ref. | 0.85 (0.79, 0.92) | 0.77 (0.70, 0.85) | 0.74 (0.65, 0.83) | 0.75 (0.66, 0.86) |
| Flavonols | |||||
| Events, n | 699 | 443 | 385 | 321 | 283 |
| Intake,2 mg/d | 15 [0–21] | 26 [21–32] | 39 [32–50] | 66 [50–83] | 116 [83–251] |
| HR (95% CI) | |||||
| Model 1 | Ref. | 0.70 (0.66, 0.75) | 0.54 (0.49, 0.60) | 0.46 (0.41, 0.51) | 0.46 (0.40, 0.51) |
| Model 1b | Ref. | 0.81 (0.76, 0.87) | 0.70 (0.63, 0.77) | 0.65 (0.58, 0.73) | 0.68 (0.60, 0.77) |
| Model 2 | Ref. | 0.82 (0.76, 0.88) | 0.72 (0.65, 0.80) | 0.69 (0.61, 0.78) | 0.71 (0.62, 0.81) |
| Flavanol monomers | |||||
| Events, n | 670 | 451 | 398 | 333 | 279 |
| Intake,2 mg/d | 14 [0–21] | 30 [21–46] | 67 [46–116] | 261 [116–282] | 473 [282–916] |
| HR (95% CI) | |||||
| Model 1 | Ref. | 0.83 (0.79, 0.87) | 0.62 (0.55, 0.69) | 0.48 (0.43, 0.54) | 0.49 (0.44, 0.56) |
| Model 1b | Ref. | 0.91 (0.87, 0.96) | 0.79 (0.70, 0.88) | 0.71 (0.61, 0.80) | 0.73 (0.65, 0.83) |
| Model 2 | Ref. | 0.93 (0.88, 0.98) | 0.83 (0.73, 0.93) | 0.78 (0.69, 0.89) | 0.77 (0.68, 0.88) |
| Flavanol oligo + polymers | |||||
| Events, n | 683 | 452 | 382 | 322 | 292 |
| Intake,2 mg/d | 92 [0–136] | 179 [136–217] | 255 [217–303] | 360 [303–434] | 537 [434–2254] |
| HR (95% CI) | |||||
| Model 1 | Ref. | 0.66 (0.61, 0.72) | 0.54 (0.50, 0.59) | 0.49 (0.44, 0.55) | 0.45 (0.40, 0.51) |
| Model 1b | Ref. | 0.80 (0.74, 0.87) | 0.71 (0.65, 0.78) | 0.67 (0.60, 0.74) | 0.67 (0.59, 0.77) |
| Model 2 | Ref. | 0.82 (0.75, 0.89) | 0.75 (0.68, 0.82) | 0.72 (0.64, 0.81) | 0.69 (0.61, 0.78) |
| Anthocyanins | |||||
| Events, n | 566 | 392 | 364 | 424 | 385 |
| Intake,2 mg/d | 5 [0–10] | 13 [10–17] | 20 [17–24] | 36 [24–53] | 71 [53–397] |
| HR (95% CI) | |||||
| Model 1 | Ref. | 0.66 (0.60, 0.72) | 0.57 (0.52, 0.64) | 0.63 (0.56, 0.70) | 0.70 (0.62, 0.79) |
| Model 1b | Ref. | 0.81 (0.74, 0.88) | 0.76 (0.68, 0.85) | 0.79 (0.71, 0.88) | 0.81 (0.72, 0.92) |
| Model 2 | Ref. | 0.84 (0.77, 0.92) | 0.80 (0.72, 0.90) | 0.83 (0.74, 0.94) | 0.85 (0.75, 0.97) |
| Flavanones | |||||
| Events, n | 523 | 420 | 398 | 351 | 439 |
| Intake,2 mg/d | 3 [0–6] | 9 [6–13] | 18 [13–26] | 32 [26–49] | 70 [49–564] |
| HR (95% CI) | |||||
| Model 1 | Ref. | 0.82 (0.76, 0.89) | 0.70 (0.62, 0.80) | 0.69 (0.61, 0.77) | 0.77 (0.68, 0.86) |
| Model 1b | Ref. | 0.92 (0.85, 0.99) | 0.85 (0.75, 0.96) | 0.83 (0.74, 0.93) | 0.90 (0.80, 1.01) |
| Model 2 | Ref. | 0.93 (0.86, 1.01) | 0.88 (0.78, 1.00) | 0.89 (0.79, 1.00) | 0.94 (0.82, 1.06) |
| Flavones | |||||
| Events, n | 554 | 456 | 376 | 341 | 404 |
| Intake,2 mg/d | 2 [0–3] | 4 [3–4] | 5 [4–6] | 7 [6–9] | 11 [9–51] |
| HR (95% CI) | |||||
| Model 1 | Ref. | 0.70 (0.63, 0.77) | 0.63 (0.57, 0.70) | 0.59 (0.53, 0.65) | 0.63 (0.56, 0.71) |
| Model 1b | Ref. | 0.85 (0.77, 0.94) | 0.80 (0.72, 0.89) | 0.76 (0.68, 0.85) | 0.81 (0.72, 0.91) |
| Model 2 | Ref. | 0.89 (0.80, 0.98) | 0.85 (0.76, 0.95) | 0.81 (0.72, 0.91) | 0.90 (0.79, 1.02) |
HRs (95% CIs) for PAD hospitalizations during 23 y of follow-up, obtained from restricted cubic splines based on Cox proportional hazards models. Model 1 adjusted for age and sex; Model 1b adjusted for age, sex, BMI, smoking status, physical activity, alcohol intake, and socioeconomic status (income); Model 2 adjusted for all covariates in Model 1b plus energy intake and intakes of fish, red meat, processed food, PUFAs, MUFAs, and SFAs. PAD, peripheral artery disease.
Values are median [range].
FIGURE 3.
HRs based on cubic spline curves to describe the association between flavonoid subclass intakes (mg/d) and total peripheral artery disease hospitalizations among participants of the Danish Diet, Cancer, and Health cohort (n = 55,647). HRs and 95% CIs are based on Cox proportional hazards models adjusted for age, sex, BMI, smoking status, physical activity, socioeconomic status (income), and alcohol intake (Model 1b) and are comparing the specific amount of flavonoid intake (horizontal axis) with the median intake for participants in the lowest intake quintile.
Associations between major flavonoid compound intakes and PAD-related hospitalizations
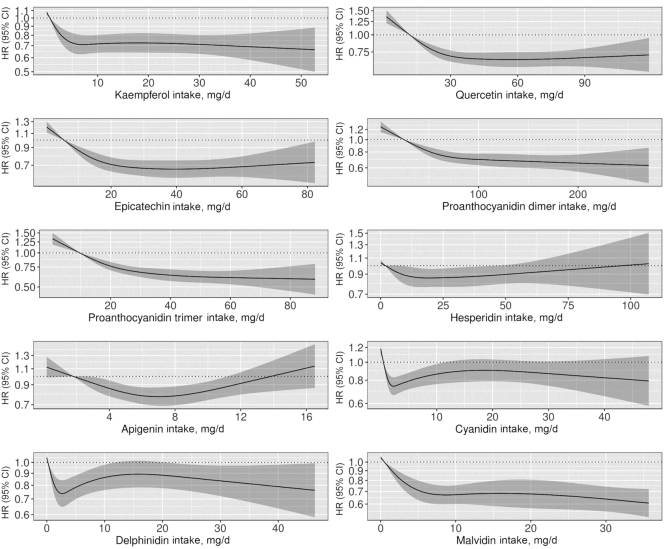
Table 3 and Figure 4 present associations between all individual flavonoid compounds with mean intakes > 5 mg/d and PAD hospitalizations. For the flavanol monomer subclass, we present only the results for epicatechin because the flavanol monomers are derived primarily from the same food sources, and thus have a very high correlation (Pearson's correlations ranged from 0.89 to 1.00) and very similar HRs. Intakes above those in quintile 1 for all individual flavonoid compounds were associated with a lower risk of a PAD hospitalization after multivariable adjustments.
TABLE 3.
HRs of PAD hospitalizations by quintiles of flavonoid compound intakes1
| Flavonoid intake quintiles | |||||
|---|---|---|---|---|---|
| Q1 (n = 11,130) | Q2 (n = 11,129) | Q3 (n = 11,130) | Q4 (n = 11,129) | Q5 (n = 11,129) | |
| Flavonols | |||||
| Kaempferol | |||||
| Events, n | 674 | 451 | 388 | 335 | 283 |
| Intake,2 mg/d | 1 [0–1] | 2 [1–3] | 4 [3–8] | 18 [8–20] | 33 [20–68] |
| HR (95% CI) | |||||
| Model 1 | Ref. | 0.82 (0.78, 0.86) | 0.57 (0.51, 0.65) | 0.50 (0.44, 0.56) | 0.47 (0.41, 0.54) |
| Model 1b | Ref. | 0.89 (0.85, 0.94) | 0.74 (0.65, 0.84) | 0.73 (0.64, 0.82) | 0.70 (0.62, 0.80) |
| Model 2 | Ref. | 0.91 (0.87, 0.96) | 0.79 (0.70, 0.89) | 0.78 (0.68, 0.88) | 0.76 (0.67, 0.87) |
| Quercetin | |||||
| Events, n | 689 | 446 | 404 | 312 | 280 |
| Intake,2 mg/d | 12 [0–16] | 20 [16–24] | 29 [24–37] | 46 [37–58] | 78 [58–168] |
| HR (95% CI) | |||||
| Model 1 | Ref. | 0.70 (0.66, 0.76) | 0.55 (0.50, 0.60) | 0.47 (0.42, 0.53) | 0.45 (0.40, 0.51) |
| Model 1b | Ref. | 0.81 (0.75, 0.87) | 0.70 (0.64, 0.77) | 0.66 (0.59, 0.74) | 0.67 (0.59, 0.76) |
| Model 2 | Ref. | 0.82 (0.76, 0.88) | 0.72 (0.65, 0.80) | 0.70 (0.62, 0.79) | 0.71 (0.63, 0.82) |
| Flavanol monomers | |||||
| Epicatechin | |||||
| Events, n | 666 | 480 | 396 | 308 | 281 |
| Intake,2 mg/d | 6 [0–9] | 12 [9–15] | 19 [15–25] | 31 [25–39] | 53 [39–155] |
| HR (95% CI) | |||||
| Model 1 | Ref. | 0.69 (0.64, 0.75) | 0.54 (0.49, 0.60) | 0.47 (0.42, 0.52) | 0.45 (0.40, 0.51) |
| Model 1b | Ref. | 0.82 (0.76, 0.88) | 0.72 (0.65, 0.79) | 0.67 (0.60, 0.75) | 0.67 (0.59, 0.76) |
| Model 2 | Ref. | 0.84 (0.78, 0.91) | 0.76 (0.68, 0.84) | 0.72 (0.64, 0.81) | 0.73 (0.64, 0.83) |
| Flavanol oligo + polymers | |||||
| Procyanidin dimers | |||||
| Events, n | 674 | 447 | 389 | 324 | 297 |
| Intake,2 mg/d | 25 [0–37] | 49 [38–62] | 78 [62–94] | 113 [94–138] | 177 [138–510] |
| HR (95% CI) | |||||
| Model 1 | Ref. | 0.68 (0.63, 0.73) | 0.54 (0.49, 0.59) | 0.50 (0.45, 0.56) | 0.47 (0.41, 0.53) |
| Model 1b | Ref. | 0.81 (0.75, 0.88) | 0.72 (0.65, 0.79) | 0.69 (0.62, 0.78) | 0.66 (0.58, 0.75) |
| Model 2 | Ref. | 0.84 (0.77, 0.91) | 0.76 (0.68, 0.83) | 0.74 (0.66, 0.83) | 0.72 (0.63, 0.82) |
| Procyanidin trimers | |||||
| Events, n | 656 | 458 | 355 | 371 | 291 |
| Intake,2 mg/d | 10 [0–14] | 17 [14–20] | 23 [20–29] | 35 [29–42] | 54 [42–320] |
| HR (95% CI) | |||||
| Model 1 | Ref. | 0.68 (0.63, 0.72) | 0.55 (0.50, 0.60) | 0.50 (0.45, 0.56) | 0.49 (0.43, 0.55) |
| Model 1b | Ref. | 0.82 (0.76, 0.88) | 0.72 (0.66, 0.79) | 0.65 (0.58, 0.73) | 0.61 (0.54, 0.69) |
| Model 2 | Ref. | 0.84 (0.78, 0.90) | 0.75 (0.68, 0.82) | 0.68 (0.61, 0.77) | 0.65 (0.57, 0.75) |
| Flavanones | |||||
| Hesperidin | |||||
| Events, n | 512 | 432 | 388 | 360 | 439 |
| Intake,2 mg/d | 2 [0–4] | 6 [4–9] | 12 [9–18] | 24 [18–38] | 54 [38–449] |
| HR (95% CI) | |||||
| Model 1 | Ref. | 0.83 (0.76, 0.90) | 0.72 (0.64, 0.81) | 0.71 (0.64, 0.80) | 0.77 (0.68, 0.87) |
| Model 1b | Ref. | 0.92 (0.85, 1.00) | 0.87 (0.77, 0.98) | 0.86 (0.77, 0.96) | 0.91 (0.80, 1.02) |
| Model 2 | Ref. | 0.94 (0.87, 1.02) | 0.90 (0.80, 1.02) | 0.89 (0.80, 1.01) | 0.96 (0.85, 1.09) |
| Flavones | |||||
| Apigenin | |||||
| Events, n | 545 | 443 | 405 | 336 | 402 |
| Intake,2 mg/d | 2 [0–2] | 3 [2–4] | 5 [4–5] | 6 [5–8] | 10 [8–46] |
| HR (95% CI) | |||||
| Model 1 | Ref. | 0.80 (0.74, 0.87) | 0.68 (0.62, 0.75) | 0.62 (0.56, 0.69) | 0.67 (0.60, 0.75) |
| Model 1b | Ref. | 0.91 (0.84, 0.99) | 0.83 (0.76, 0.92) | 0.78 (0.70, 0.87) | 0.84 (0.74, 0.94) |
| Model 2 | Ref. | 0.93 (0.85, 1.01) | 0.87 (0.78, 0.96) | 0.83 (0.74, 0.93) | 0.92 (0.81, 1.05) |
| Anthocyanins | |||||
| Cyanidin | |||||
| Events, n | 552 | 396 | 364 | 384 | 435 |
| Intake,2 mg/d | 1 [0–1] | 1 [1–1] | 2 [1–3] | 4 [3–8] | 17 [8–203] |
| HR (95% CI) | |||||
| Model 1 | Ref. | 0.71 (0.67, 0.76) | 0.58 (0.52, 0.65) | 0.61 (0.55, 0.67) | 0.82 (0.71, 0.94) |
| Model 1b | Ref. | 0.84 (0.79, 0.90) | 0.75 (0.68, 0.84) | 0.77 (0.70, 0.86) | 0.90 (0.79, 1.04) |
| Model 2 | Ref. | 0.87 (0.82, 0.93) | 0.81 (0.72, 0.90) | 0.83 (0.74, 0.92) | 0.96 (0.83, 1.10) |
| Delphinidin | |||||
| Events, n | 561 | 354 | 424 | 350 | 442 |
| Intake,2 mg/d | 0 [0–1] | 1 [1–1] | 2 [1–4] | 5 [4–8] | 18 [8–188] |
| HR (95% CI) | |||||
| Model 1 | Ref. | 0.72 (0.66, 0.78) | 0.60 (0.54, 0.68) | 0.66 (0.59, 0.74) | 0.86 (0.76, 0.98) |
| Model 1b | Ref. | 0.83 (0.76, 0.90) | 0.75 (0.66, 0.85) | 0.78 (0.70, 0.88) | 0.89 (0.78, 1.01) |
| Model 2 | Ref. | 0.84 (0.77, 0.91) | 0.77 (0.68, 0.87) | 0.81 (0.72, 0.91) | 0.93 (0.81, 1.05) |
| Malvidin | |||||
| Events, n | 661 | 567 | 234 | 330 | 339 |
| Intake,2 mg/d | 0 [0–1] | 2 [1–6] | 6 [6–6] | 11 [6–14] | 36 [14–114] |
| HR (95% CI) | |||||
| Model 1 | Ref. | 0.77 (0.73, 0.82) | 0.54 (0.48, 0.61) | 0.54 (0.49, 0.61) | 0.56 (0.48, 0.64) |
| Model 1b | Ref. | 0.86 (0.80, 0.91) | 0.69 (0.61, 0.77) | 0.68 (0.60, 0.76) | 0.61 (0.51, 0.72) |
| Model 2 | Ref. | 0.86 (0.81, 0.92) | 0.70 (0.62, 0.79) | 0.69 (0.61, 0.78) | 0.62 (0.52, 0.74) |
HRs (95% CIs) for PAD hospitalizations during 23 y of follow-up, obtained from restricted cubic splines based on Cox proportional hazards models. Model 1 adjusted for age and sex; Model 1b adjusted for age, sex, BMI, smoking status, physical activity, alcohol intake, and socioeconomic status (income); Model 2 adjusted for all covariates in Model 1b plus energy intake and intakes of fish, red meat, processed food, PUFAs, MUFAs, and SFAs. PAD, peripheral artery disease.
Values are median [range].
FIGURE 4.
HRs based on cubic spline curves to describe the association between major flavonoid compound intakes and total peripheral artery disease hospitalizations among participants of the Danish Diet, Cancer, and Health cohort (n = 55,647). HRs and 95% CIs are based on Cox proportional hazards models adjusted for age, sex, BMI, smoking status, physical activity, socioeconomic status (income), and alcohol intake (Model 1b) and are comparing the specific amount of flavonoid compound intake (horizontal axis) with the median intake for participants in the lowest intake quintile.
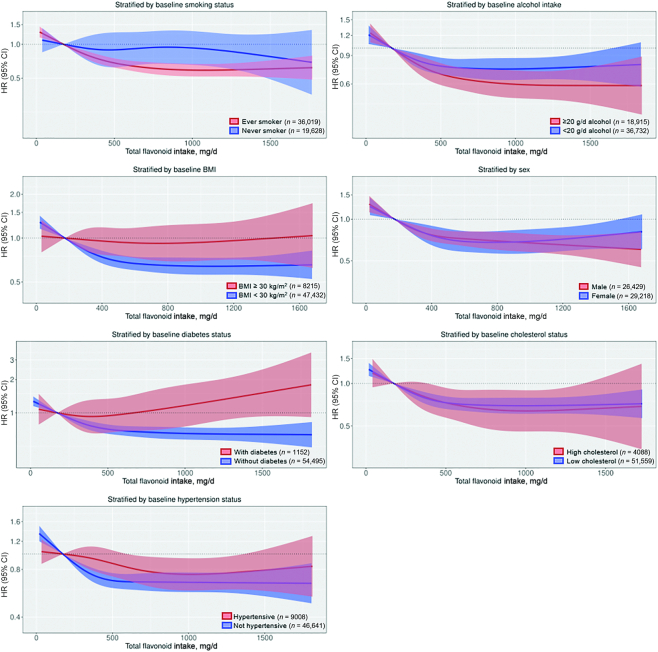
Associations between total flavonoid intakes and PAD hospitalizations stratified by risk factors for PAD
On a relative scale, the association between total flavonoid intake and PAD hospitalizations appeared to differ according to baseline smoking status, BMI, and diabetes status; higher total flavonoid intakes were associated with a significantly lower risk of PAD hospitalizations only in smokers, normal to overweight participants, and those without diabetes at baseline (Figure 5). However, on an absolute scale, the 20-y absolute risk difference between average male or female participants in Q5 and those in Q1 was greatest for current smokers (male: 2.61%; female: 1.55%) and those with diabetes at baseline (male: 2.11%; female: 1.27%); there was no difference based on alcohol intake or BMI (Supplemental Tables 5 and 6). The association did not differ according to sex, cholesterol concentrations, or hypertension status on an absolute or a relative scale (Figure 5, Supplemental Tables 5 and 6). Supplemental Table 7 presents the sample size and number of events for each subgroup.
FIGURE 5.
Multivariable-adjusted association between total flavonoid intake and total peripheral artery disease hospitalizations stratified by baseline smoking status (P-interaction = 0.32), alcohol intake (P-interaction = 0.40), BMI (P-interaction = 0.12), sex (P-interaction = 0.12), diabetes status (P-interaction < 0.01), cholesterol concentrations (P-interaction = 0.31), and hypertension status (P-interaction = 0.16). HRs and 95% CIs are based on Cox proportional hazards models and are comparing the specific amount of flavonoid intake (horizontal axis) with the median intake for participants in the lowest intake quintile (174 mg/d). All analyses were standardized for age, sex, BMI, smoking, physical activity, socioeconomic status (income), and alcohol intake (Model 1b).
Validated case analysis
Using only validated cases, 950 participants were hospitalized for PAD. Compared with a total flavonoid intake of 174 mg/d and after multivariable adjustments (Model 1b), an intake of 1000 mg/d was associated with a 38% lower risk of a PAD hospitalization (HR: 0.62; 95% CI: 0.51, 0.74) (Supplemental Figure 2).
Discussion
A primary prevention approach incorporating nutrition strategies may be advantageous in reducing the global burden and considerable associated morbidity of PAD. In this prospective cohort study, conducted in 55,647 Danish residents free of PAD at baseline, higher intakes of total flavonoids and all flavonoid subclasses were nonlinearly associated with a lower risk of PAD hospitalizations. Furthermore, higher total flavonoid intakes were associated with lower risks of hospitalizations for atherosclerosis, aneurysms, and other PVD (primarily intermittent claudication), and both revascularization or endovascular surgery and lower extremity amputations. On both a relative and an absolute scale, the greatest benefits observed for higher flavonoid intakes were in current or former smokers.
The role that nutrition plays in the primary prevention of PAD is not well understood. Previous observational studies suggested that a diet characterized by low intakes of vitamins, dietary fiber, and PUFAs, and a high intake of red meat, was associated with a higher incidence of PAD (19–21). Owing to the underlying pathogenesis of PAD, a review summarizing current knowledge on nutritional patterns among patients with PAD strongly advocated for a diet rich in foods with anti-inflammatory and antioxidant properties (22). One such diet, the Mediterranean-style diet, is characterized by high intakes of extra virgin olive oil, vegetables, fruits, nuts and pulses, and legumes, and a moderate intake of red wine, all of which are rich sources of flavonoids (23). Evidence for the beneficial effects of the Mediterranean-style diet in the primary prevention of PAD comes from a post hoc analysis of the PREDIMED trial (24). However, there is a lack of information on the potential benefits of a flavonoid-rich diet in mitigating PAD. We have demonstrated that flavonoid intake is inversely associated with atherosclerotic cardiovascular disease, most strongly for PAD (9). In a previous case-control study (n = 200), a higher flavonoid intake was associated with lower odds of peripheral arterial occlusive disease, where the OR (95% CI) for a 1-SD higher total flavonoid intake was 0.46 (0.28, 0.77) (8). Furthermore, the strongest associations were observed for the flavonol, flavone, and flavan-3-ols subclasses. In the present study, inverse associations appeared to be the strongest for the flavonol and flavanol oligo + polymers subclasses. It is likely that tea, chocolate, wine, apples, and pears were the main food sources of these subclasses in the present cohort (25). Although caution must be taken when interpreting these findings because they are on a relative scale, both true biological variability and greater precision in the estimation of intakes of these flavonoid subclasses may explain these stronger associations. The inverse associations observed between flavonoid intake and both revascularization or endovascular surgery and major amputation procedures suggest that flavonoids may play a role not only in the development of PAD, but also in the progression of PAD.
The main risk factors for PAD—age, smoking, and diabetes mellitus—can cause an increase in inflammation, arterial stiffness, and oxidative stress, and a decrease in NO bioavailability with a concomitant decrease in vasodilation (7). Flavonoids may impede the initiation and progression of PAD by restoring endothelial homeostasis through the augmentation of NO bioavailability and by attenuating inflammation through upregulation of the anti-inflammatory nuclear factor erythroid 2-related factor 2 (Nrf2) pathway and downregulation of the proinflammatory NF-κβ pathway (6). The present study found that in current and former smokers, a higher flavonoid intake was strongly associated with a lower risk of PAD, supporting the hypothesis that flavonoids may play a role in counteracting inflammation and oxidative stress in these participants. Setting a precedent for this finding, an inverse association between fruit and vegetable intake and PAD incidence was shown to be present only in current or former smokers (26). It may be that flavonoids only exhibit detectable PAD-preventing effects in settings of significant inflammation and oxidative stress, such as that induced by smoking. That no significant association was observed in nonsmokers, and statistical tests for interaction were not significant, may be due to the very low number of events in this subgroup. Importantly, although these findings suggest that flavonoids may afford more protection against PAD to smokers, smokers with a high flavonoid intake were still at a higher risk of PAD than nonsmokers with a low flavonoid intake. Although the HR of PAD was lower for high alcohol consumers than for low alcohol consumers with a total flavonoid intake >500 mg/d, the absolute risk difference between high and low flavonoid intake for these 2 groups was comparable. Similarly, although there was evidence that the associations differed according to baseline BMI on a relative scale, the absolute risk difference between high and low flavonoid intake was the same for those who were normal or overweight and those who were obese. Conversely, although on a relative scale there was evidence that flavonoid intake was not associated with PAD in persons with diabetes, on an absolute scale, the absolute risk difference between high and low flavonoid intake for an average male participant with diabetes was notable (2.11%). These results should be interpreted with caution and warrant further investigation because few participants had prevalent diabetes at baseline (n = 1152). These findings suggest that the mechanisms by which flavonoids ameliorate atherosclerosis may interact with the mechanisms by which smoking and diabetes lead to atherosclerosis.
Our results emphasize the importance of a flavonoid-rich diet in mitigating PAD risk. Despite the positive findings of the present study, evidence from randomized controlled trials is urgently needed. Future interventional studies of flavonoid intake should include patients at risk of PAD and investigate early markers of PAD such as the ankle-brachial index.
Limitations
Owing to the observational nature of the study we were not able to infer causality or rule out residual confounding. We acknowledge common FFQ limitations, in particular that not all flavonoid-rich foods, for example berries, were included in the questionnaire. We acknowledge that flavonoid intake may have changed over the 23 y of follow-up; however, this would have likely attenuated any associations. Furthermore, the associations observed cannot be attributed to flavonoid intake alone, because participants with higher flavonoid intakes tended to have a healthier underlying dietary pattern, eating more fruits and vegetables and less red and processed meat. However, flavonoid intake was strongly associated with PAD after adjustments were made for a number of potential dietary confounders. We were unable to determine the association between flavonoid intake and outpatient visits for PAD, owing to the low validity of outpatient diagnoses. Misclassification bias is always present when using registry-based outcomes; however, our validated cases analysis gave similar results meaning that bias was limited. Using clinical measurements specific for PAD such as the ankle-brachial index could have provided additional valuable information on PAD severity and flavonoid intake.
Conclusion
A diet rich in flavonoids was strongly associated with a lower risk of a hospitalization for PAD, revascularizations or endovascular surgery, and lower extremity amputations. Therefore, ensuring an adequate intake of flavonoid-rich foods may be helpful in mitigating PAD risk.
Supplementary Material
ACKNOWLEDGEMENTS
The authors’ responsibilities were as follows—NPB, FD, KM, AC, and JMH: designed the research (project conception, development of the overall research plan, and study oversight); AT: conducted the original cohort study; AS and CK: calculated flavonoid intake from the FFQ data; NPB, KM, and FD: analyzed the data; NPB and FD: wrote the paper and had primary responsibility for the final content; KM, AC, CPB, JRL, KDC, CK, GG, CT-P, AS, AT, and JMH: assisted with interpretation of the results and critically reviewed the manuscript; and all authors: read and approved the final manuscript. The authors report no conflicts of interest.
Notes
Supported by Danish Heart Foundation grant 17-R115-A7443-22062 (to FD), Gangstedfonden grant A35136 (to FD), National Health and Medical Research Council Early Career Fellowship grant APP1159914 (to NPB), National Health and Medical Research Council Career Development Fellowship grant APP1107474 (to JRL), and National Health and Medical Research Council Senior Research Fellowship grant APP1116937 (to JMH). The Danish Diet, Cancer, and Health Study was funded by the Danish Cancer Society, Denmark.
Where authors are identified as personnel of the International Agency for Research on Cancer/WHO, the authors alone are responsible for the views expressed in this article and they do not necessarily represent the decisions, policy, or views of the International Agency for Research on Cancer/WHO.
Supplemental Figures 1 and 2 and Supplemental Tables 1–7 are available from the “Supplementary data” link in the online posting of the article and from the same link in the online table of contents at https://academic.oup.com/ajcn/.
Abbreviations used: COPD, chronic obstructive pulmonary disease; ICD, International Classification of Diseases; PAD, peripheral artery disease; PVD, peripheral vascular disease.
Contributor Information
Nicola P Bondonno, School of Medical and Health Sciences, Edith Cowan University, Perth, Western Australia, Australia; School of Biomedical Sciences, University of Western Australia, Perth, Western Australia, Australia; Institute for Global Food Security, Queen's University Belfast, Belfast, United Kingdom.
Kevin Murray, School of Population and Global Health, University of Western Australia, Perth, Western Australia, Australia.
Aedin Cassidy, Institute for Global Food Security, Queen's University Belfast, Belfast, United Kingdom.
Catherine P Bondonno, School of Medical and Health Sciences, Edith Cowan University, Perth, Western Australia, Australia; School of Biomedical Sciences, University of Western Australia, Perth, Western Australia, Australia.
Joshua R Lewis, School of Medical and Health Sciences, Edith Cowan University, Perth, Western Australia, Australia; School of Biomedical Sciences, University of Western Australia, Perth, Western Australia, Australia.
Kevin D Croft, School of Biomedical Sciences, University of Western Australia, Perth, Western Australia, Australia.
Cecilie Kyrø, The Danish Cancer Society Research Centre, Copenhagen, Denmark.
Gunnar Gislason, Department of Cardiology, Herlev & Gentofte University Hospital, Copenhagen, Denmark; The National Institute of Public Health, University of Southern Denmark, Odense, Denmark; The Danish Heart Foundation, Copenhagen, Denmark.
Christian Torp-Pedersen, Department of Clinical Investigation and Cardiology, Nordsjælland Hospital, Hillerød, Denmark.
Augustin Scalbert, International Agency for Research on Cancer, Lyon, France.
Anne Tjønneland, The Danish Cancer Society Research Centre, Copenhagen, Denmark; Department of Public Health, Faculty of Health and Medical Sciences, University of Copenhagen, Copenhagen, Denmark.
Jonathan M Hodgson, School of Medical and Health Sciences, Edith Cowan University, Perth, Western Australia, Australia; School of Biomedical Sciences, University of Western Australia, Perth, Western Australia, Australia.
Frederik Dalgaard, Department of Cardiology, Herlev & Gentofte University Hospital, Copenhagen, Denmark.
Data Availability
Data described in the article, code book, and analytic code will not be made available owing to restrictions related to Danish law and the protection of patient privacy. The combined set of data as used in this study can only be made available through a trusted third party, Statistics Denmark. This state organization holds the data used for this study.
References
- 1. Hiatt WR, Goldstone J, Smith SC Jr, McDermott M, Moneta G, Oka R, Newman AB, Pearce WH, Writing Group 1 . Atherosclerotic peripheral vascular disease symposium II: nomenclature for vascular diseases. Circulation. 2008;118(25):2826–9. [DOI] [PubMed] [Google Scholar]
- 2. Fowkes FGR, Rudan D, Rudan I, Aboyans V, Denenberg JO, McDermott MM, Norman PE, Sampson UK, Williams LJ, Mensah GA et al. Comparison of global estimates of prevalence and risk factors for peripheral artery disease in 2000 and 2010: a systematic review and analysis. Lancet. 2013;382(9901):1329–40. [DOI] [PubMed] [Google Scholar]
- 3. Bhatt DL, Steg PG, Ohman EM, Hirsch AT, Ikeda Y, Mas J-L, Goto S, Liau C-S, Richard AJ, Röther J et al. International prevalence, recognition, and treatment of cardiovascular risk factors in outpatients with atherothrombosis. JAMA. 2006;295(2):180–9. [DOI] [PubMed] [Google Scholar]
- 4. Criqui MH, Denenberg JO, Bird CE, Fronek A, Klauber MR, Langer RD. The correlation between symptoms and non-invasive test results in patients referred for peripheral arterial disease testing. Vasc Med. 1996;1(1):65–71. [DOI] [PubMed] [Google Scholar]
- 5. US Department of Agriculture USDA Database for the Flavonoid Content of Selected Foods: release 3.1. [Internet] Beltsville, MD: Beltsville Human Nutrition Research Center, USDA Agricultural Research Service; 2014; [cited 18 August, 2016] Available from: https://www.ars.usda.gov/ARSUserFiles/80400525/Data/Flav/Flav_R03-1.pdf. [Google Scholar]
- 6. Williamson G, Kay CD, Crozier A. The bioavailability, transport, and bioactivity of dietary flavonoids: a review from a historical perspective. Compr Rev Food Sci Food Saf. 2018;17(5):1054–112. [DOI] [PubMed] [Google Scholar]
- 7. Hamburg NM, Creager MA. Pathophysiology of intermittent claudication in peripheral artery disease. Circ J. 2017;81(3):281–9. [DOI] [PubMed] [Google Scholar]
- 8. Lagiou P, Samoli E, Lagiou A, Skalkidis Y, Katsouyanni K, Petridou E, Trichopoulos D. Flavonoid classes and risk of peripheral arterial occlusive disease: a case–control study in Greece. Eur J Clin Nutr. 2006;60(2):214–19. [DOI] [PubMed] [Google Scholar]
- 9. Dalgaard F, Bondonno NP, Murray K, Bondonno CP, Lewis JR, Croft KD, Kyrø C, Gislason G, Scalbert A, Cassidy A et al. Associations between habitual flavonoid intake and hospital admissions for atherosclerotic cardiovascular disease: a prospective cohort study. Lancet Planet Health. 2019;3:E450–9. [DOI] [PubMed] [Google Scholar]
- 10. Lynge E, Sandegaard JL, Rebolj M. The Danish National Patient Register. Scand J Public Health. 2011;39(7_suppl):30–3. [DOI] [PubMed] [Google Scholar]
- 11. Bondonno NP, Dalgaard F, Kyrø C, Murray K, Bondonno CP, Lewis JR, Croft KD, Gislason G, Scalbert A, Cassidy A et al. Flavonoid intake is associated with lower mortality in the Danish Diet Cancer and Health Cohort. Nat Commun. 2019;10(1):3651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Neveu V, Perez-Jiménez J, Vos F, Crespy V, Du Chaffaut L, Mennen L, Knox C, Eisner R, Cruz J, Wishart D et al. Phenol-Explorer: an online comprehensive database on polyphenol contents in foods. Database. 2010:bap024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lasota A, Overvad K, Eriksen H, Tjønneland A, Schmidt E, Grønholdt M-L. Validity of peripheral arterial disease diagnoses in the Danish National Patient Registry. Eur J Vasc Endovasc Surg. 2017;53(5):679–85. [DOI] [PubMed] [Google Scholar]
- 14. Schmidt M, Schmidt SAJ, Sandegaard JL, Ehrenstein V, Pedersen L, Sørensen HT. The Danish National Patient Registry: a review of content, data quality, and research potential. Clin Epidemiol. 2015;7:449–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Gauthier J, Wu QV, Gooley TA. Cubic splines to model relationships between continuous variables and outcomes: a guide for clinicians. Bone Marrow Transplant. 2020;55(4):675–80. [DOI] [PubMed] [Google Scholar]
- 16. Noordzij M, Leffondré K, van Stralen KJ, Zoccali C, Dekker FW, Jager KJ. When do we need competing risks methods for survival analysis in nephrology?. Nephrol Dial Transplant. 2013;28(11):2670–7. [DOI] [PubMed] [Google Scholar]
- 17. Criqui MH, Aboyans V. Epidemiology of peripheral artery disease. Circ Res. 2015;116(9):1509–26. [DOI] [PubMed] [Google Scholar]
- 18. R Core Team R: A language and environment for statistical computing [Internet]. Vienna, Austria: R Foundation for Statistical Computing; 2019; [accessed 1 October, 2019]. Available from: https://www.R-project.org/. [Google Scholar]
- 19. Mazidi M, Wong ND, Katsiki N, Mikhailidis DP, Banach M. Dietary patterns, plasma vitamins and Trans fatty acids are associated with peripheral artery disease. Lipids Health Dis. 2017;16(1):254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ogilvie RP, Lutsey PL, Heiss G, Folsom AR, Steffen LM. Dietary intake and peripheral arterial disease incidence in middle-aged adults: the Atherosclerosis Risk in Communities (ARIC) Study. Am J Clin Nutr. 2017;105(3):651–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Donnan PT, Thomson M, Fowkes F, Prescott RJ, Housley E. Diet as a risk factor for peripheral arterial disease in the general population: the Edinburgh Artery Study. Am J Clin Nutr. 1993;57(6):917–21. [DOI] [PubMed] [Google Scholar]
- 22. Nosova EV, Conte MS, Grenon SM. Advancing beyond the “heart-healthy diet” for peripheral arterial disease. J Vasc Surg. 2015;61(1):265–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Vasilopoulou E, Georga K, Joergensen MB, Naska A, Trichopoulou A. The antioxidant properties of Greek foods and the flavonoid content of the Mediterranean menu. Curr Med Chem Immunol Endocr Metab. 2005;5(1):33–45. [Google Scholar]
- 24. Ruiz-Canela M, Martínez-González MA. Lifestyle and dietary risk factors for peripheral artery disease. Circ J. 2014;78(3):553–9. [DOI] [PubMed] [Google Scholar]
- 25. Zamora-Ros R, Knaze V, Rothwell JA, Hémon B, Moskal A, Overvad K, Tjønneland A, Kyrø C, Fagherazzi G, Boutron-Ruault M-C et al. Dietary polyphenol intake in Europe: the European Prospective Investigation into Cancer and Nutrition (EPIC) study. Eur J Nutr. 2016;55(4):1359–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Heffron SP, Rockman CB, Adelman MA, Gianos E, Guo Y, Xu JF, Berger JS. Greater frequency of fruit and vegetable consumption is associated with lower prevalence of peripheral artery disease. Arterioscler Thromb Vasc Biol. 2017;37(6):1234–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data described in the article, code book, and analytic code will not be made available owing to restrictions related to Danish law and the protection of patient privacy. The combined set of data as used in this study can only be made available through a trusted third party, Statistics Denmark. This state organization holds the data used for this study.