Abstract
The origin of isolates routinely used by the community of Aspergillus fumigatus researchers is periodically a matter of intense discussion at our centre, as the construction of recombinant isolates have sometimes followed convoluted routes, the documentation describing their lineages is fragmented, and the nomenclature is confusing. As an aide memoir, not least for our own benefit, we submit the following account and tabulated list of strains (Table 1) in an effort to collate all of the relevant information in a single, easily accessible document. To maximise the accuracy of this record we have consulted widely amongst the community of Medical Mycologists using these strains. All the strains described are currently available from one of these organisations, namely the Fungal Genetics Stock Centre (FGSC), FungiDB, Ensembl Fungi and The National Collection of Pathogenic Fungi (NCPF) at Public Health England. Display items from this manuscript are also featured on FungiDB.
Lay abstract
We present a concise overview on the definition, origin and unique genetic makeup of the Aspergillus fumigatus isolates routinely in use by the fungal research community, to aid researchers to describe past and new strains and the experimental differences observed more accurately.
Keywords: Aspergillus fumigatus, strain, lineage, isolate
Sequenced A. fumigatus isolates
Fully annotated genomic sequences for two A. fumigatus strains Af293 and A1163 (Figs. 1 and 2) are available at FungiDB (https://fungidb.org/fungidb). FungiDB also compiles gene, protein, and chromosome sequence information about these and other Aspergillus species with descriptions and classifications of their biological roles, molecular functions, and subcellular localizations, while also offering tools for analyses and comparison of sequences and links to literature information.
Figure 1.
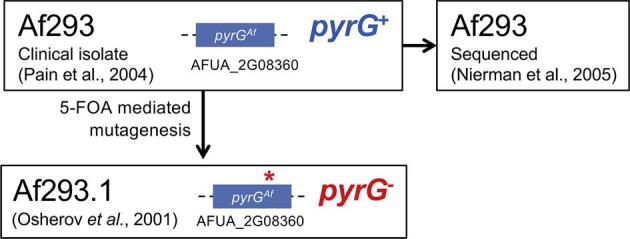
Af293 and derivative thereof. Diagram depicting provenance and scientific use of Af293 strains. The uridine/uracil requirements and the genomic organization of the pyrG locus is indicated for each isolate within this lineage.
Figure 2.
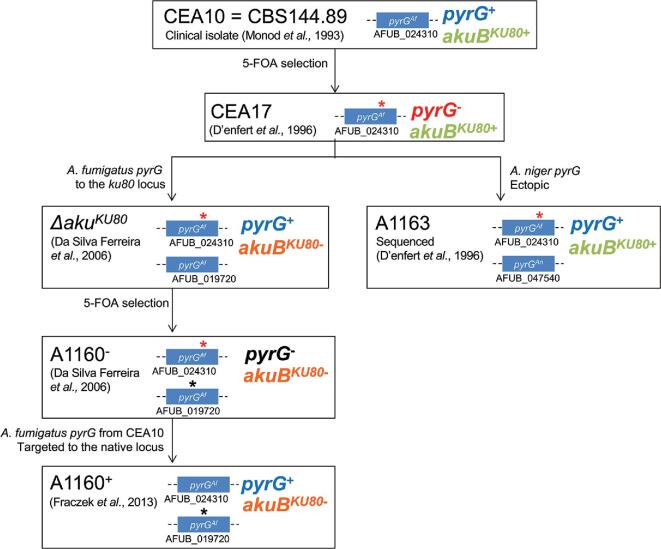
CEA10 and derivatives thereof. Diagram depicting provenance and scientific use of CEA10 strains. The uridine/uracil requirements and the genomic organization of the pyrG and akuBKU80 loci is indicated for each isolate within this lineage.
As of the beginning of 2020, Ensembl Fungi (http://fungi.ensembl.org/index.html) and NCBI (https://www.ncbi.nlm.nih.gov/genome/genomes/18) contain the genomic sequences of 5 and 14 different A. fumigatus isolates, respectively, and genomic data for many hundreds more A. fumigatus isolates have been deposited into genomic databases including the NCBI bioprojects database (https://www.ncbi.nlm.nih.gov/bioproject/), which lists more than 100 entries. Genome sequences for a cohort of 66 isolates were curated and characterized in a recent study of genetic diversity in secondary metabolism gene clusters,1 and for a cohort of 24 clinical and environmental isolates in a recent study of azole resistance.2
Af293 and derivatives
Af293 is a clinically derived strain, which was isolated in 1993 from a lung biopsy taken postautopsy from a neutropenic patient that received treatment for rheumatoid arthritis, developing severe sepsis syndrome. Microscopy of the tissue revealed septate hyphae consistent with aspergillosis. From the biopsy, the Public Health Laboratory at the Royal Shrewsbury Hospital grew a culture yielding A. fumigatus. Samples were sent to Hope Hospital where it was assigned the name Af293. In 1998, the isolate was sent to the National Collection of Pathogenic Fungi (NCPF) and assigned NCPF7367.3 The strain was sequenced in 2005 making it the first A. fumigatus isolate with a publicly available full genome sequence.4 At the present time, the Af293 genome remains the only genome sequence of the species to have been fully physically reconstructed as chromosomes. The same study furnished the community with an oligomer-based microarray technology, which was subsequently used for multiple transcriptomic analyses.5–12
Organized on eight chromosomes, the Af293 genome spans 29.4 Mb. While the sequence of Af293 is over 97% identical to the A1163 genome, significant diversity of genetic content was found in the telomeric and subtelomeric regions.13 Comparative analysis of a larger set of genomes revealed that Af293 belongs to a different clade than the CEA10/A1163 lineage14 (where A1163 is the sequenced derivative isolate of the clinical isolate CEA10, see Fig. 2). When compared to the CEA10/A1163 lineage Af293 was found to exhibit a slower growth rate on solid Aspergillus minimal media, while having a faster growth rate in liquid media and heightened sensitivity to hypoxic conditions as well as being less pathogenic than CEA10 in a triamcinolone murine model of infection.15 Similar attenuation of virulence was reported from a zebrafish model of infection, seemingly due to differences in neutrophil and macrophage mediated killing of A. fumigatus.16 Distinct mechanistic bases for leukocyte recruitment in response to Af293 or CEA10 infection could also be correlated with strain-specific immune responses in vivo.17
To facilitate a genetic screen for itraconazole resistance, a derivative strain Af293.1 was generated by Osherov et al.18 by exposing Af293 to 4-nitroquinoline 1-oxide, followed by selection on uracil and uridine supplemented media containing 5-fluoro-orotic acid (5-FOA) to select for loss of function mutations in the pyrG (AFUA_2G08360) gene (Fig. 1).
CEA10 and derivatives
CEA10 is a clinically derived strain isolated in the early ’90s from a patient with invasive aspergillosis.19,20 Due to the use of CEA10 as a progenitor strain in which non-homologous end joining mutants have been constructed21 strains in the CEA10 lineage (Fig. 2) have been extensively utilized by many research groups. CEA10 is more pathogenic in murine models of infection than the Af293 and ATCC46645 isolates.5,15
In 1996 d'Enfert et al. utilized CEA10 to develop a pyrG-blaster tool with which to elicit iterative gene deletions in a single strain.22 The pyrG-blaster cassette consisted of the Aspergillus niger pyrG gene flanked by a direct repeat that encodes the neomycin phosphotransferase of transposon Tn5. In order to produce a pyrimidine auxotroph with which to select for insertions of the pyrG-blaster, 4-nitroquinoline-N-oxide mutagenesis and 5-FOA selection were implemented yielding a pyrimidine auxotrophic derivative of CEA10, designated CEA17.
CEA17, which was found to harbor a single point mutation in the gene that encodes orotidine-5′-phosphate decarboxylase (pyrG, AFUB_024310) resulting in the generation of a premature stop codon (Fig. 3, C to T transition at + 1413 from the start codon), provided the basis for subsequent development of a first pyrG-dependent homologous transformation system for A. fumigatus.23
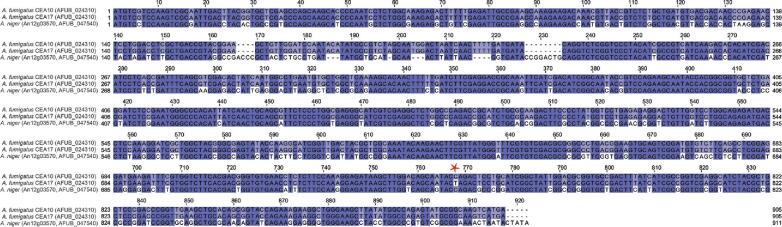
Figure 3.
Alignment of the DNA sequences of A. fumigatus pyrG gene from CEA10 and CEA17 and A. niger pyrG as present in A1163. The asterisk indicates the C nucleotide in position 1413 that has changed into a T in the CEA17 strain, leading to the transition from glutamine to a premature stop codon. This nucleotide change can be used as a diagnostic marker to differentiate strains within the CEA10 lineage. A. fumigatus pyrG and A. niger pyrG share 77.16% identity. As A. niger pyrG is ectopically integrated in the genome of A. fumigatus A1163, the presence of this gene can be used as a diagnostic marker to differentiate A. fumigatus A1163 from the rest of the isolates within the CEA10 lineage.
A CEA17 derivative, harboring a functional copy of the A. niger pyrG (An12g03570, named AFUB_047540 in the annotation of the A1163 genome), and designated A1163 was subsequently sequenced at the J. Craig Venter Institute, in collaboration with Celera Genomics.13 Although the lineage of the A1163 isolate is irrefutably confirmed by genetic analysis, the precise origin of this laboratory isolate remains unclear.
The A1163 genome is 29.2 Mb in size. The genome is believed to be organized onto eight chromosomes but, unlike the Af293 genome, has not been fully physically reconstructed and is reported as 223 contig sequences.
∆akuB KU80 is a CEA17 derivative lacking the ku80 (AFUB_019720) gene, also named akuBKU8024 and was generated to facilitate molecular manipulations of A. fumigatus. The akuBKU80 gene encodes one component of the heterodimeric Ku protein complex, which is an essential mediator of the nonhomologous end joining (NHEJ) DNA repair pathway. The ∆akuBKU80 strain exhibits dramatically heightened rates (80% compared to 4% in wild type strains) of targeted genomic integration of exogenous DNA by homologous recombination.24 The akuBKU80 gene was replaced with a zeo-pyrG cassette, that contains the A. fumigatus pyrG gene (from isolate ATCC46645) amplified from the pCDA21 plasmid25 and the Streptoalloteichus hindustanus Sh ble gene conferring resistance to the antibiotic zeocin26 under the control of the EM7 promoter from plasmid pEM7-zeo (Invitrogen, Thermo Fisher). ∆akuBKU80 therefore has two copies of the pyrG gene, one mutated, nonfunctional copy at the native pyrG locus (d'Enfert et al. 199622) and one functional copy at the akuBKU80 locus. As expected for strains deficient in NHEJ, the ∆akuBKU80 isolate is moderately sensitive24 to the chemical methane methyl sulfonate (MMS).
To render ∆akuBKU80 amenable pyrG-mediated gene replacements, a uridine/uracil auxotroph of ∆akuBKU80 was generated by selection on 5-FOA and deposited at the FGSC as A1160.24 The mutation in the pyrG allele inserted at the akuBKU80 genomic locus has not been so far characterized by sequencing. A1160 was later further manipulated to obtain a prototrophic ∆akuBKU80 strain named A1160 pyrG+, with which to facilitate the study of antifungal drug transporters via gene replacement strategies using a dominant selection marker.27 In order to restore a functional pyrG gene at its native locus, A1160 was converted to uridine/uracil prototrophy via targeted insertion of a functional A. fumigatus pyrG from CEA10 into the pyrG genomic locus.27 In later years the isolate was renamed as MFIG001 and utilized as the progenitor isolate for the genome-wide A. fumigatus knockout library.28 This strain is available via NCPF at Public Health England as part of the transcription factor knockout library.
ATCC46645 and derivatives
The ATCC46645 Fresenius strain (American Type Culture Collection) was isolated from pus removed via a bronchoscopy from the left bronchus of a patient with an acute febrile respiratory infection29 and its first scientific use reported in 1997.30
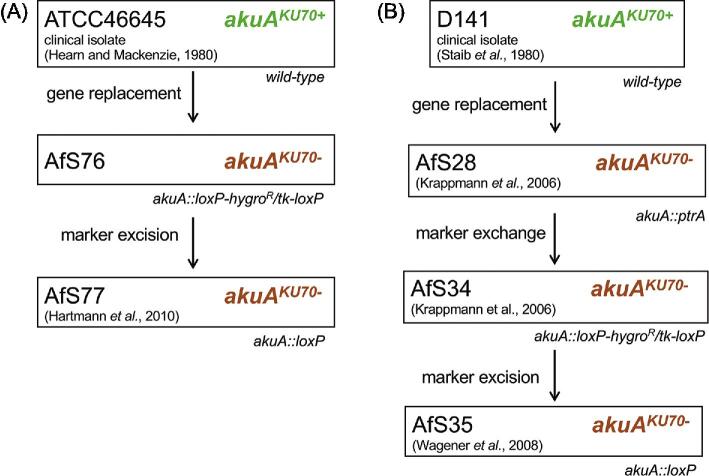
In ATCC46645, the akuAKU70 gene (identified by identity to AFUA_5g07740 in Af293 as ATCC46645 does not have lineage specific identifiers), which is essential for the non-homologous end joining machinery, was deleted by replacing its coding sequence with the loxP-hygR/tk-loxP marker module to yield the intermediate strain AfS76 (Fig. 4A). AfS76 was in turn modified to construct AfS77/A1280, in which the loxP-hygR/tk-loxP cassette was excised via transient Cre expression from pSK215.31
Figure 4.
ATCC46645, D141 and derivatives thereof. Diagram depicting strains originating from ATCC46645 (A) or D141 (B) with respective details on origin and references. The uridine/uracil requirements and the genomic organization of the akuAKU70 locus are indicated for each isolate within these lineages.
Direct comparison of ATCC46645 and CEA10 infectivity in a leukopenic murine model of infection, revealed that a ∼10 times higher inoculum of ATCC46645 was necessary than that required for CEA10 to achieve similar mortality rates.5 Nevertheless, low dose infection models (with 5 × 103 conidia) have been established for ATCC46645.32
Interestingly, macrophage phagocytosis rates of the ATCC46645 strain are lower than those reported for the CEA10 lineage, perhaps caused by differences in growth rate.33
D141 and derivatives
A. fumigatus D141 was isolated by Staib et al. in 1980 from a 45-year old male with an aspergilloma. The patient sample was mainly composed of a white A. fumigatus variant mixed with a typical looking greenish A. fumigatus, which was named D14134 (Fig. 4B). D141 served as a prototrophic, marker/resistance-free wild-type isolate to generate the NHEJ-deficient strain AfS28/A115735 by deleting the akuAKU70 gene and replacing it with the ptrA resistance marker (containing the ptrA gene, AO090003000090, from Aspergillus oryzae under control of its native promoter, which confers resistance to the antibiotic pyrithiamine).36 The genome sequence of AfS28 is available via the JGI genome portal (project ID: 1098580 and 1098483). A derivative of AfS28 is the AfS34/A1158 strain35; this strain was selected for hygromycin resistance and pyrithiamine sensitivity after the exchange of the ptrA marker with the recyclable loxP-hygR/tk-loxP marker cassette.37 In AfS34, the loxP-hygR/tk-loxP marker was excised by transient expression of the Cre recombinase from the autonomously replicating plasmid pSK215 to result in the marker/resistance-free derivative AfS35/A1159 (akuAKU70::loxP).38 This strain was recently sequenced and genomic, metabolomics and infection comparison with the A1163 and CEA10 strains found significant differences in carbon- and nitrogen metabolism, protease secretion, cell wall metabolism and virulence in a triamcinolone murine model of pulmonary aspergillosis.39
Table 1.
Table of isolates.
| Name | Genotype | Other denominations | Origin | Source | Sequence accession number | Phenotype | Reference |
|---|---|---|---|---|---|---|---|
| Af293 | Wild type; MAT1-2 | FGSC A1100, IHEM18963, CBS101355, NCPF7367, MYA-5609 | Lung biopsy specimen of neutropenic IPA patient | David Denning Laboratory | GCA_000002655.1 | Pyr+5-FOAS | 3,4 |
| Af293.1 | pyrG−; MAT1-2 | FGSC A1137 | Af293 | … | … | Pyr−5-FOAR | 18 |
| CEA10 | Wild type; MAT1-1 | CBS 144–89CBS 144.89, AF10 | Patient with IPA | CBS-KNAW Fungal Biodiversity Centre | … | Pyr+5-FOAS | 19,20 |
| CEA17 | pyrG−; MAT1-1 | … | CEA10 | … | … | Pyr−5-FOAR | 22 |
| A1163 | pyrG−, pyrGAn; MAT1-1 | … | CEA17 | … | GCA_000150145.1 | Pyr+5-FOAS | 22 |
| ΔakuB KU80 | ΔakuB ku80::pyrGAf-zeo; MAT1-1 | FGSC A1151 | CEA17 | … | … | Pyr+5-FOASMMSSZeoR | 24 |
| A1160− | ΔakuB ku80::pyrG−-zeo; MAT1-1 | … | ΔakuBKU80 | … | … | Pyr−5-FOARMMSS (*)ZeoR (*) | 24 |
| A1160+ | ΔakuB ku80::pyrG−-zeo, pyrG−::pyrGAf; MAT1-1 | A1160p+, MFIG001 | A1160− | … | … | Pyr+5-FOASMMSS (*)ZeoR (*) | 27 |
| ATCC46645 | Wild type; MAT1-1 | … | Pus from bronchoscopy from the left bronchus of a patient with acute febrile respiratory infection. | … | … | Pyr+5-FOAS | 29 |
| AfS77 | ΔakuA::loxP; MAT1-1 | FGSC A1280 | ATCC46645 | … | … | Pyr+5-FOAS | 31 |
| D141 | Wild type; MAT1-1 | … | Isolated from an aspergilloma | … | … | Pyr+5-FOAS | 34 |
| AfS35 | ΔakuA::loxP; MAT1-1 | FGSC A1159 | D141 | … | … | Pyr+5-FOAS | 38 |
| AfS28 | ΔakuA::ptrA; MAT1-1 | FGSC A1157 | D141 | … | … | Pyr+5-FOASPtrR | 35 |
| AfS34 | ΔakuA::loxP-hygroR/tk-loxP; MAT1-1 | FGSC A1158 | AfS28 | … | … | Pyr+5-FOASHygroR | 35 |
(*) data not available, phenotype inferred from parental isolate of origin. IPA: invasive pulmonary aspergillosis; Pyr+ and Pyr− pyrimidine: prototroph or auxotroph, respectively; S and R superscript: ‘sensitive’ and ‘resistant,’ respectively; 5-FOA: 5-fluoro-orotic acid; MMS: methane methyl sulfonate; Ptr: pyrithiamine; Zeo: zeocin; Hygro: Hygromycin.
Conclusions
An expanding number of studies demonstrate differences between A. fumigatus isolate phenotypes,5,15,39,40 immune responses17,33,41 and virulence in various models of infection.15,39,42–44 The use of different experimental conditions and murine models/strains does not always allow exhaustive cross-comparison of the vast amount of virulence data from A. fumigatus isolates. However, upon parallel challenge with different clinical isolates, significant interstrain variability was observed with respect to the survival of different model organisms, such as flies,43 mice,15,41,42 zebrafish,16 and waxworm.44 Likewise, a wide strain-dependent variation has been documented with regards to macrophage phagocytosis and killing in vitro33,39 and cytokine production by dendritic cells41in vitro and/or in vivo (using immunocompetent C57BL/6 mice). These findings highlight a crucial need to understand the lineage of strains routinely used in the laboratory and, whenever possible going forward, to construct and test A. fumigatus mutants in multiple genetic backgrounds.
Acknowledgments
The authors would like to thank Jorge Amich, Sara Gago, Axel Brakhage, Nir Osherov, William Nierman, Natalie Fedorova, and Hubertus Haas for providing feedback on this letter.
Contributor Information
Margherita Bertuzzi, Manchester Fungal Infection Group, Faculty of Biology, Medicine and Health, The University of Manchester, Manchester Academic Health Science Centre, Core Technology Facility, Manchester, UK; Lydia Becker Institute of Immunology and Inflammation, Biology, Medicine and Health. The University of Manchester, Manchester Academic Health Science Centre, Manchester, UK.
Norman van Rhijn, Manchester Fungal Infection Group, Faculty of Biology, Medicine and Health, The University of Manchester, Manchester Academic Health Science Centre, Core Technology Facility, Manchester, UK; Lydia Becker Institute of Immunology and Inflammation, Biology, Medicine and Health. The University of Manchester, Manchester Academic Health Science Centre, Manchester, UK.
Sven Krappmann, Institute of Clinical Microbiology, Immunology and Hygiene, University Hospital Erlangen, Friedrich-Alexander University Erlangen-Nürnberg, Germany.
Paul Bowyer, Manchester Fungal Infection Group, Faculty of Biology, Medicine and Health, The University of Manchester, Manchester Academic Health Science Centre, Core Technology Facility, Manchester, UK; Lydia Becker Institute of Immunology and Inflammation, Biology, Medicine and Health. The University of Manchester, Manchester Academic Health Science Centre, Manchester, UK.
Michael J Bromley, Manchester Fungal Infection Group, Faculty of Biology, Medicine and Health, The University of Manchester, Manchester Academic Health Science Centre, Core Technology Facility, Manchester, UK; Lydia Becker Institute of Immunology and Inflammation, Biology, Medicine and Health. The University of Manchester, Manchester Academic Health Science Centre, Manchester, UK.
Elaine M Bignell, Manchester Fungal Infection Group, Faculty of Biology, Medicine and Health, The University of Manchester, Manchester Academic Health Science Centre, Core Technology Facility, Manchester, UK; Lydia Becker Institute of Immunology and Inflammation, Biology, Medicine and Health. The University of Manchester, Manchester Academic Health Science Centre, Manchester, UK; MRC Centre for Medical Mycology, University of Exeter, Geoffrey Pope Building, Stocker Road, Exeter EX4 4QD, UK.
Funding
This work was supported by MRC grants MR/S001824/1 and MR/L000822/1 to EMB, and MR/M02010X/1 to EMB, MJB and PB and by the Wellcome Trust grant 208396/Z/17/Z to MJB and PB.
Declaration of interest
M.J.B. is a consultant to Synlab GmbH, the director and shareholder of Syngenics Limited and is a substantive shareholder in PiQ Laboratories Ltd. The remaining authors report no conflicts of interest.
References
- 1. Lind AL, Wisecaver JH, Lameiras C et al. Drivers of genetic diversity in secondary metabolic gene clusters within a fungal species. PLOS Biology. 2017; 15: e2003583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Abdolrasouli A, Rhodes J, Beale MA et al. Genomic context of azole resistance mutations in Aspergillus fumigatus determined using whole-genome sequencing. mBio. 2015; 6: e00536–00515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Pain A, Woodward J, Quail MA et al. Insight into the genome of Aspergillus fumigatus: analysis of a 922 kb region encompassing the nitrate assimilation gene cluster. Fungal Genet Biol. 2004; 41: 443–453. [DOI] [PubMed] [Google Scholar]
- 4. Nierman WC, Pain A, Anderson MJ et al. Genomic sequence of the pathogenic and allergenic filamentous fungus Aspergillus fumigatus. Nature. 2005; 438: 1151–1156. [DOI] [PubMed] [Google Scholar]
- 5. Bertuzzi M, Schrettl M, Alcazar-Fuoli L et al. The pH-responsive PacC transcription factor of Aspergillus fumigatus governs epithelial entry and tissue invasion during pulmonary aspergillosis. PLoS Pathog. 2014; 10: e1004413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. McDonagh A, Fedorova ND, Crabtree J et al. Sub-telomere directed gene expression during initiation of invasive aspergillosis. PLoS Pathog. 2008; 4: e1000154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Loss O, Bertuzzi M, Yan Y et al. Mutual independence of alkaline- and calcium-mediated signalling in Aspergillus fumigatus refutes the existence of a conserved druggable signalling nexus. Mol Microbiol. 2017; 106: 861–875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Liu H, Gravelat FN, Chiang LY et al. Aspergillus fumigatus AcuM regulates both iron acquisition and gluconeogenesis. Mol Microbiol. 2010; 78: 1038–1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Soriani FM, Malavazi I, da Silva Ferreira ME et al. Functional characterization of the Aspergillus fumigatus CRZ1 homologue, CrzA. Mol Microbiol. 2008; 67: 1274–1291. [DOI] [PubMed] [Google Scholar]
- 10. Urb M, Pouliot P, Gravelat FN, Olivier M, Sheppard DC. Aspergillus fumigatus induces immunoglobulin E-independent mast cell degranulation. J Infect Dis. 2009; 200: 464–472. [DOI] [PubMed] [Google Scholar]
- 11. Schrettl M, Beckmann N, Varga J et al. HapX-mediated adaption to iron starvation is crucial for virulence of Aspergillus fumigatus. PLOS Pathogens. 2010; 6: e1001124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Willger SD, Puttikamonkul S, Kim KH et al. A sterol-regulatory element binding protein is required for cell polarity, hypoxia adaptation, azole drug resistance, and virulence in Aspergillus fumigatus. PLoS Pathog. 2008; 4: e1000200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Fedorova ND, Khaldi N, Joardar VS et al. Genomic islands in the pathogenic filamentous fungus Aspergillus fumigatus. PLoS Genet. 2008; 4: e1000046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Garcia-Rubio R, Monzon S, Alcazar-Fuoli L, Cuesta I, Mellado E. Genome-wide comparative analysis of Aspergillus fumigatus strains: the reference genome as a matter of concern. Genes (Basel). 2018; 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kowalski CH, Beattie SR, Fuller KK et al. Heterogeneity among isolates reveals that fitness in low oxygen correlates with Aspergillus fumigatus virulence. mBio. 2016; 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Rosowski EE, Raffa N, Knox BP, Golenberg N, Keller NP, Huttenlocher A. Macrophages inhibit Aspergillus fumigatus germination and neutrophil-mediated fungal killing. PLoS Pathog. 2018; 14: e1007229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Caffrey-Carr AK, Kowalski CH, Beattie SR et al. Interleukin 1α is critical for resistance against highly virulent Aspergillus fumigatus isolates. Infect Immun. 2017; 85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Osherov N, Kontoyiannis DP, Romans A, May GS. Resistance to itraconazole in Aspergillus nidulans and Aspergillus fumigatus is conferred by extra copies of the A. nidulans P-450 14alpha-demethylase gene, pdmA. J Antimicrob Chemother. 2001; 48: 75–81. [DOI] [PubMed] [Google Scholar]
- 19. Girardin H, Latge JP, Srikantha T, Morrow B, Soll DR. Development of DNA probes for fingerprinting Aspergillus fumigatus. J Clin Microbiol. 1993; 31: 1547–1554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Monod M, Paris S, Sarfati J, Jaton-Ogay K, Ave P, Latge JP. Virulence of alkaline protease-deficient mutants of Aspergillus fumigatus. FEMS Microbiol Lett. 1993; 106: 39–46. [DOI] [PubMed] [Google Scholar]
- 21. da Silva Ferreira ME, Kress MR, Savoldi M et al. The akuB(KU80) mutant deficient for nonhomologous end joining is a powerful tool for analyzing pathogenicity in Aspergillus fumigatus. Eukaryot Cell. 2006; 5: 207–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. d'Enfert C. Selection of multiple disruption events in Aspergillus fumigatus using the orotidine-5'-decarboxylase gene, pyrG, as a unique transformation marker. Curr Genet. 1996; 30: 76–82. [DOI] [PubMed] [Google Scholar]
- 23. Weidner G, d'Enfert C, Koch A, Mol PC, Brakhage AA. Development of a homologous transformation system for the human pathogenic fungus Aspergillus fumigatus based on the pyrG gene encoding orotidine 5'-monophosphate decarboxylase. Curr Genet. 1998; 33: 378–385. [DOI] [PubMed] [Google Scholar]
- 24. da Silva Ferreira ME, Kress MR, Savoldi M et al. The akuBKU80 mutant deficient for nonhomologous end joining is a powerful tool for analyzing pathogenicity in Aspergillus fumigatus. Eukaryot Cell. 2006; 5: 207–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Chaveroche MK, Ghigo JM, d'Enfert C. A rapid method for efficient gene replacement in the filamentous fungus Aspergillus nidulans. Nucleic Acids Res. 2000; 28: E97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Gatignol A, Durand H, Tiraby G. Bleomycin resistance conferred by a drug-binding protein. FEBS Lett. 1988; 230: 171–175. [DOI] [PubMed] [Google Scholar]
- 27. Fraczek MG, Bromley M, Buied A et al. The cdr1B efflux transporter is associated with non-cyp51a-mediated itraconazole resistance in Aspergillus fumigatus. J Antimicrob Chemother. 2013; 68: 1486–1496. [DOI] [PubMed] [Google Scholar]
- 28. Furukawa T, van Rhijn N, Fraczek M et al. The negative cofactor 2 complex is a key regulator of drug resistance in Aspergillus fumigatus. Nat Commun. 2020; 11: 427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Hearn VM, Mackenzie DW.. Mycelial antigens from two strains of Aspergillus fumigatus: an analysis by two-dimensional immunoelectrophoresis. Mykosen. 1980; 23: 549–562. [PubMed] [Google Scholar]
- 30. Jahn B, Koch A, Schmidt A et al. Isolation and characterization of a pigmentless-conidium mutant of Aspergillus fumigatus with altered conidial surface and reduced virulence. Infect Immun. 1997; 65: 5110–5117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Hartmann T, Dumig M, Jaber BM et al. Validation of a self-excising marker in the human pathogen Aspergillus fumigatus by employing the beta-rec/six site-specific recombination system. Appl Environ Microbiol. 2010; 76: 6313–6317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Liebmann B, Mühleisen TW, Müller M et al. Deletion of the Aspergillus fumigatus lysine biosynthesis gene lysF encoding homoaconitase leads to attenuated virulence in a low-dose mouse infection model of invasive aspergillosis. Arch Microbiol. 2004; 181: 378–383. [DOI] [PubMed] [Google Scholar]
- 33. Kraibooj K, Schoeler H, Svensson CM, Brakhage AA, Figge MT. Automated quantification of the phagocytosis of Aspergillus fumigatus conidia by a novel image analysis algorithm. Front Microbiol. 2015; 6: 549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Staib F, Mishra SK, Rajendran C et al. A notable Aspergillus from a mortal aspergilloma of the lung: new aspects of the epidemiology, serodiagnosis and taxonomy of Aspergillus fumigatus. Zentralbl Bakteriol A. 1980; 247: 530–536. [PubMed] [Google Scholar]
- 35. Krappmann S, Sasse C, Braus GH. Gene targeting in Aspergillus fumigatus by homologous recombination is facilitated in a nonhomologous end-joining-deficient genetic background. Eukaryotic Cell. 2006; 5: 212–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kubodera T, Yamashita N, Nishimura A. Pyrithiamine resistance gene (ptrA) of Aspergillus oryzae: cloning, characterization and application as a dominant selectable marker for transformation. Biosci Biotechnol Biochem. 2000; 64: 1416–1421. [DOI] [PubMed] [Google Scholar]
- 37. Hartmann T, Cairns TC, Olbermann P, Morschhauser J, Bignell EM, Krappmann S. Oligopeptide transport and regulation of extracellular proteolysis are required for growth of Aspergillus fumigatus on complex substrates but not for virulence. Mol Microbiol. 2011; 82: 917–935. [DOI] [PubMed] [Google Scholar]
- 38. Wagener J, Echtenacher B, Rohde M et al. The putative alpha-1,2-mannosyltransferase AfMnt1 of the opportunistic fungal pathogen Aspergillus fumigatus is required for cell wall stability and full virulence. Eukaryotic Cell. 2008; 7: 1661–1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Ries LNA, Steenwyk JL, de Castro PA et al. Nutritional heterogeneity among Aspergillus fumigatus strains has consequences for virulence in a strain- and host-dependent manner. Front Microbiol. 2019; 10: 854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Gago S, Overton NLD, Ben-Ghazzi N et al. Lung colonization by Aspergillus fumigatus is controlled by ZNF77. Nat Commun. 2018; 9: 3835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Rizzetto L, Giovannini G, Bromley M, Bowyer P, Romani L, Cavalieri D. Strain dependent variation of immune responses to A. fumigatus: definition of pathogenic species. PLoS One. 2013; 8: e56651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Mondon P, De Champs C, Donadille A, Ambroise-Thomas P, Grillot R. Variation in virulence of Aspergillus fumigatus strains in a murine model of invasive pulmonary aspergillosis. J Med Microbiol. 1996; 45: 186–191. [DOI] [PubMed] [Google Scholar]
- 43. Ben-Ami R, Lamaris GA, Lewis RE, Kontoyiannis DP. Interstrain variability in the virulence of Aspergillus fumigatus and Aspergillus terreus in a Toll-deficient Drosophila fly model of invasive aspergillosis. Med Mycol. 2010; 48: 310–317. [DOI] [PubMed] [Google Scholar]
- 44. Cheema MS, Christians JK.. Virulence in an insect model differs between mating types in Aspergillus fumigatus. Med Mycol. 2011; 49: 202–207. [DOI] [PubMed] [Google Scholar]