Abstract
Aims/Introduction
We investigated the effect of FreeStyle LibreTM on glycemic control in Japanese type 2 diabetes patients treated with basal–bolus insulin therapy.
Materials and Methods
This prospective, 90‐day single‐arm study enrolled 94 adults with type 2 diabetes treated with insulin. A 14‐day masked baseline phase was followed by an 11‐week treatment phase during which participants used the device to monitor glucose levels. The primary end‐point was time spent in hypoglycemia (<70 mg/dL) for baseline versus study end (days 76–90). Secondary end‐points included other measures of glycemic control, along with patient satisfaction using the Japanese Diabetes Treatment and Satisfaction Questionnaire.
Results
Time spent in hypoglycemia was low at baseline (0.51 ± 0.93 h/day) and did not significantly decrease at study end (0.47 ± 0.63 h/day, P = 0.6354). Time in range, time in hyperglycemia and estimated A1c all improved versus baseline (by +1.7 ± 3.0 h/day, −1.6 ± .4 h/day and −0.4 ± 0.8%, respectively, P < 0.0001 in each). Finger stick tests fell from 2.9 ± 1.3 to 1.9 ± 1.4/day, and mean scanning frequency during the intervention phase was 11.3/day. The mean treatment satisfaction score increased by 11.8 ± 5.3 (P < 0.0001). Two severe hypoglycemia‐related adverse events were reported; one of which was possibly related to the device. Three participants reported mild device‐related skin trauma, site discomfort or subcutaneous bleeding.
Conclusions
Use of FreeStyle Libre by Japanese type 2 patients diabetes treated with basal–bolus insulin therapy showed a low baseline of hypoglycemia, and enabled improved glycemic control and treatment satisfaction.
Keywords: Intermittently scanned continuous glucose monitoring, Hypoglycemia, Type 2 diabetes
Use of flash glucose monitoring technology in Japanese subjects with type 2 diabetes, treated with multiple daily insulin injections, resulted in increased time in range and decreased time in hyperglycemia without increasing hypoglycemia.
INTRODUCTION
The incidence of diabetes is increasing globally, due to the challenges of an aging population and rising rates of obesity. In Japan, the prevalence of diabetes is currently estimated at 7.7%, driven primarily by a high incidence of type 2 diabetes 1 . Whereas glycated hemoglobin (HbA1c) is a key measure in the treatment of this condition, continuous glucose monitoring (CGM) is also useful to optimize glucose management and thus it is recommended in some circumstances to help achieve glucose targets in type 2 diabetes patients 2 , 3 .
Evidence of the benefit of real‐time CGM or flash glucose monitoring in participants with type 2 diabetes treated with insulin is limited, but growing 4 , 5 , 6 , 7 , 8 . Flash glucose monitoring has been shown to be effective in type 1 and type 2 diabetes patients in the IMPACT 9 and REPLACE 5 clinical trials, respectively. Both studies showed a reduction in time spent in hypoglycemia and an improvement in mean glucose values when the device was introduced to patients treated with basal–bolus insulin therapy 5 , 9 . Like most clinical trials, participants in these studies were typically white 6 , 7 or their ethnicity was not reported 4 , 6 . However, the pathophysiology of type 2 diabetes is different between East Asian and white patients 10 , 11 , with lower rates of hypoglycemia 12 and use of lower doses of insulin in the former. It is thus unknown whether the use of flash glucose monitoring yields similar beneficial effects in the East Asian population. We carried out the current study to evaluate the efficacy of FreeStyle Libre on glycemic control in Japanese adults with type 2 diabetes treated with basal–bolus insulin therapy.
METHODS
Study design and participants
This prospective, open‐label, single arm study (University Hospital Medical Information Network; UMIN000023593) was designed to investigate the effect of FreeStyle Libre, a sensor‐based flash glucose monitoring system (Abbott Diabetes Care, Witney, UK), on glycemic control in Japanese adults with type 2 treated with basal–bolus insulin therapy. The study was carried out over a period of 3 months at five diabetes centers in Japan. Approval for the study was given by an independent ethics committee. All participating centers concurred with the Japanese Ethical Guidelines for Medical and Health Research 13 and the International Council for Harmonization Guidelines for Good Clinical Practice 14 . All participants gave written, informed consent.
Patients were recruited from the general diabetes population at each study site. The study inclusion and exclusion criteria are listed in Table S1. Physical measurements were taken after consent, screening for eligibility and enrolment. For the 14‐day baseline period, all participants used the system in “masked mode” and sensor glucose measurements were not visible to participants or investigators. Participants were asked to scan their sensor when carrying out self‐monitoring of blood glucose (SMBG) fingerstick tests and at least every 8 h. Glucose management was supported by SMBG, as required, utilizing the strip‐port of the FreeStyle Libre reader.
Participants with sensor data for at least 50% of the masked 14‐day wear period (or ≥650 individual sensor readings) continued into the 11‐week open‐label treatment phase, during which they used sensor glucose data for self‐management. Participants were requested to maintain SMBG testing for adjunctive use, in accordance with the device labeling in Japan, and to record insulin doses, food intake and exercise on the reader throughout the study. In line with standard diabetes care, use of current or historical glucose data to self‐manage glucose levels was encouraged, including insulin titration. Training on glucose sensor data interpretation was not provided, and no treatment protocols or insulin titration algorithms were used. At clinic visits on days 15, 30 and 60, sensor glucose data reports (generated using system software 15 ) were reviewed with an attending physician for personalized glucose management.
Outcomes
The primary outcome was the change in time spent in hypoglycemia (sensor glucose <70 mg/dL) utilizing the device and SMBG during the final 14 days of the treatment phase (days 76–90) compared with SMBG use only during the masked baseline phase (days 1–14). Prespecified secondary end‐points were the change from baseline for sensor‐derived glycemic measures of: time in range (70–180 mg/dL), mean glucose level, time and events in hyperglycemia (>180, 240 and 300 mg/dL), estimated A1c, time and events in hypoglycemia (<70, 55 and 45 mg/dL) and area under the curve, as well as glycemic variability measures including standard deviation (SD), low blood glucose index, high blood glucose index, SD of glucose rate of change and continuous overall net glycemic action.
An event was defined as two or more consecutive sensor readings, at 15‐min intervals, outside the predefined glucose range. Event duration was calculated from the first reading outside the range to the first reading returning within the range. Additional prespecified outcomes were: analysis of glycemic outcomes by day (06.00–23.00 hours) and night (23.00–06.00 hours), subgroup analysis by age and duration of diabetes, frequency of glucose fingerstick testing, number of sensor scans carried out, and change in total daily dose of insulin, bodyweight and body mass index. The length of system use (defined as the percentage of data collected, relative to continuous device wear) was also analyzed.
Patient‐reported outcome measures were assessed using the Japanese Diabetes Treatment and Satisfaction Questionnaire (DTSQ) at baseline and study end (day 90) 16 . Questionnaire results for healthcare professionals (sensor glucose report use) were assessed at study end, and for users at baseline (sensor application) and at study end (device use).
Adverse events (AEs) and sensor‐insertion or sensor‐wear symptoms were monitored throughout the study. Any symptomatic hypoglycemic events reported by participants were recorded as an AE. Additionally, any episodes of diabetic ketoacidosis or hyperosmolar hyperglycemic state and severe hypoglycemic events were assessed. A severe hypoglycemic event was defined as requiring assistance of another person to actively administer carbohydrates, glucagon or take other corrective actions 17 . If plasma glucose concentrations were not available during a severe event, neurological recovery after the return of plasma glucose level to normal was considered enough evidence that the event was induced by a low plasma glucose concentration (≤70 mg/dL) 17 .
Statistical analysis
A sample size of 83 was required to provide 80% power to detect a difference of 35% with a significance level of 5% between the baseline and final phases in the primary end‐point of time spent <70 mg/dL. Time in hypoglycemia, other glycemic measures, estimated A1c from sensor glucose data, total daily dose of insulin, bodyweight and body mass index were considered using a paired t‐test. A one‐sample t‐test was used to compare DTSQ scores with zero change. All participants with at least 72 h of masked baseline sensor glucose results were included in the full analysis set. Data analysis was carried out by Abbott Diabetes Care using SAS version 9.2 (SAS Institute, Cary, NC, USA) or higher for all analyses.
RESULTS
A total of 94 participants were enrolled between 1 February and 1 July 2017 across the five centers (Table 1). Two participants were excluded from the full analysis set (n = 92), because <72 h of sensor data were collected during the 14‐day baseline phase (Figure 1). All 94 participants were included in the safety analysis.
Table 1.
Baseline characteristics of study participants
| Characteristic | Participants (n = 92) |
|---|---|
| Male | 52/92 (56.5%) |
| Age (years) | 63.6 ± 12.7 |
| BMI (kg/m2) | 24.4 ± 4.2 |
| Duration of diabetes (years) | 19 ± 11 |
| Duration of insulin use (years) | 11 ± 8 |
| Self‐reported BG frequency per day | 3.1 ± 2.1 |
| Insulin administration by pen | 92/92 (100.0%) |
| Screening HbA1c (%) | 7.47 ± 0.66 |
| Screening HbA1c (mmol/mol) | 58.1 ± 7.2 |
Table shows the full analysis set. Values are the mean ± standard deviation or n/N (%).
BG, blood glucose; BMI, body mass index; HbA1c, glycated hemoglobin.
Figure 1.
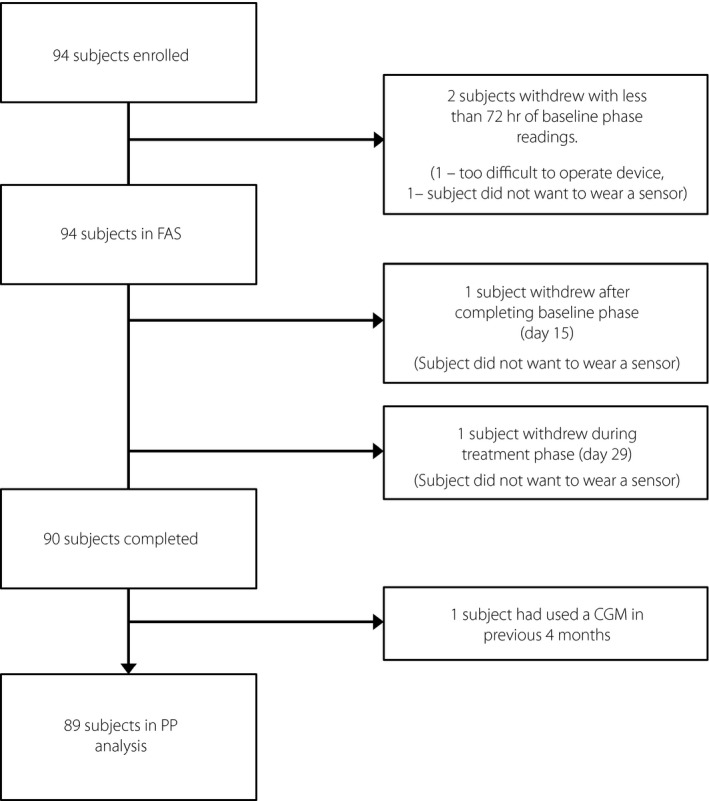
Trial profile. CGM, continuous glucose monitoring; FAS, full analysis set; PP, per protocol.
Time in hypoglycemia (<70 mg/dL) fell from 0.51 ± 0.93 h/day (mean ± SD) at baseline to 0.47 ± 0.63 h/day at study end (days 76–90), a change of −0.04 ± 0.83 h/day (−8.1%), which was not statistically significant (P = 0.6354). Time spent in hypoglycemia <55 mg/dL and <45 mg/dL were both prespecified secondary end‐points that also fell without reaching statistical significance (Table 2). Figure 2 shows the time spent in each glycemic range during the study, 27% of participants (25/92) had 0 h/daytime in hypoglycemia at baseline. Of the 67 participants with non‐zero time in hypoglycemia at baseline, 34 (51%) experienced a ≥30% reduction in time spent in hypoglycemia by study end. There was no change in time in hypoglycemia from baseline by day, night, age or duration of diabetes.
Table 2.
Change in glycemic control measures
| Baseline (days 1–14) | Final phase (days 76–90) | Change | 95% CI For Change | P‐value | |
|---|---|---|---|---|---|
|
Time in range 70–180 mg/dL (h) † |
15.0 ± 4.0 | 16.7 ± 3.7 | 1.7 ± 3.0 | (1.1, 2.3) | <0.0001 |
| Mean glucose (mg/dL) | 167 ± 26 | 156 ± 24 | −11 ± 23 | (−16, −6) | <0.0001 |
| Mean glucose (mmol/L) | 9.3 ± 1.5 | 8.7 ± 1.3 | −0.6 ± 1.3 | (−0.9, −0.3) | <0.0001 |
| eA1c (%) | 7.46 ± 0.91 | 7.07 ± 0.84 | −0.39 ± 0.81 | (−0.56, −0.22) | <0.0001 |
| eA1c (mmol/mol) | 58.0 ± 10.0 | 53.8 ± 9.2 | −4.3 ± 8.9 | (−6.1, −2.4) | <0.0001 |
| Glucose <70 mg/dL | |||||
| Time (h) † | 0.51 ± 0.93 | 0.47 ± 0.63 | −0.04 ± 0.83 | (−0.21, 0.13) | 0.6354 |
| Time (h) in day ‡ | 0.25 ± 0.47 | 0.27 ± 0.41 | 0.01 ± 0.43 | (−0.07, 0.10) | 0.7437 |
| Time (h) in night § | 0.25 ± 0.59 | 0.20 ± 0.31 | −0.06 ± 0.54 | (−0.17, 0.05) | 0.3139 |
| Events † | 0.35 ± 0.47 | 0.37 ± 0.44 | 0.02 ± 0.44 | (−0.07, 0.11) | 0.7023 |
| AUC (h × mg/dL) † | 6.30 ± 15.69 | 4.80 ± 8.53 | −1.50 ± 13.08 | (−4.21, 1.21) | 0.2753 |
| Glucose <45 mg/dL | |||||
| Time (h) † | 0.07 ± 0.30 | 0.04 ± 0.13 | −0.03 ± 0.26 | (−0.08, 0.03) | 0.3118 |
| Events † | 0.05 ± 0.16 | 0.04 ± 0.10 | −0.01 ± 0.14 | (−0.04, 0.01) | 0.3265 |
| AUC (h × mg/dL) † | 0.34 ± 1.50 | 0.19 ± 0.58 | −0.15 ± 1.28 | (−0.42, 0.12) | 0.2628 |
| Glucose <55 mg/dL | |||||
| Time (h) † | 0.16 ± 0.48 | 0.11 ± 0.26 | −0.05 ± 0.39 | (−0.13, 0.03) | 0.2128 |
| Events † | 0.12 ± 0.26 | 0.09 ± 0.18 | −0.04 ± 0.20 | (−0.08, 0.01) | 0.0884 |
| AUC (h × mg/dL) † | 1.43 ± 5.25 | 0.92 ± 2.43 | −0.51 ± 4.46 | (−1.43, 0.41) | 0.2758 |
| Glucose >180 mg/dL | |||||
| Time (h) † | 8.5 ± 4.0 | 6.8 ± 3.7 | −1.6 ± 3.4 | (−2.3, −0.9) | <0.0001 |
| Events † | 2.59 ± 0.87 | 2.36 ± 0.77 | −0.23 ± 0.74 | (−0.38, −0.08) | 0.0037 |
| Glucose >240 mg/dL | |||||
| Time (h) † | 3.2 ± 2.5 | 2.1 ± 2.3 | −1.0 ± 2.1 | (−1.5, −0.6) | <0.0001 |
| Events † | 1.35 ± 0.80 | 0.99 ± 0.81 | −0.35 ± 0.68 | (−0.50, −0.21) | <0.0001 |
| Glucose >300 mg/dL | |||||
| Time (h) † | 0.9 ± 1.2 | 0.6 ± 1.0 | −0.4 ± 1.0 | (−0.6, −0.1) | 0.0013 |
| Events † | 0.48 ± 0.45 | 0.34 ± 0.47 | −0.14 ± 0.40 | (−0.23, −0.06) | 0.0011 |
Total n = 92. Data presented as the mean ± standard deviation.
AUC, area under the curve; CI, confidence interval; eA1c, estimated glycated hemoglobin.
Per 24 h day.
Per 17 h day
Per 7 h night.
Figure 2.
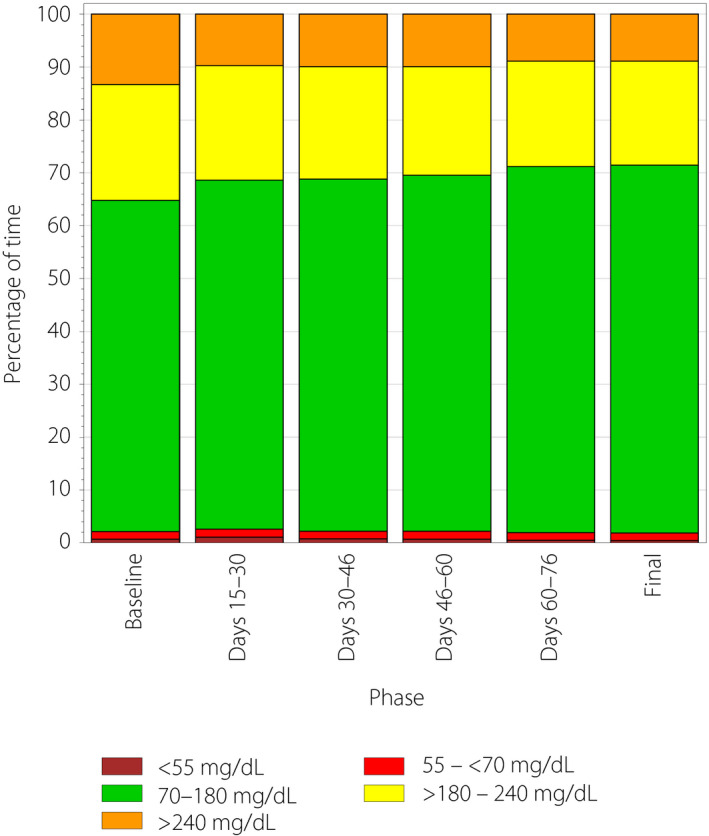
Time spent in each glycemic range by study phase. The colored bars show the average hours per day spent <55 mg/dL (brown), 55 to <70 mg/dL (red), 70–180 mg/dL (green), >180 to 240 mg/dL (yellow) and >240 mg/dL (orange) during each 2‐week phase of the 90‐day study.
The prespecified secondary end‐points are outlined in Table 2. Time in range (70–180 mg/dL) significantly improved by 1.7 ± 3.0 h/day from 15.0 ± 4.0 to 16.7 ± 3.7 h/day (P < 0.0001). Improvements were shown during daytime (06.00–23.00 h; P < 0.0001), at night‐time (P < 0.0001) and for all participants irrespective of age (<65 years and ≥65 years. both P = 0.0005) or duration of diabetes (<20 years, P < 0.0001; ≥20 years, P = 0.0028).
Mean glucose decreased by −11 ± 23 mg/dL during the study period, from 167 ± 26 to 156 ± 24 mg/dL (P < 0.0001). Time and number of events spent in hyperglycemia significantly decreased at all thresholds (Table 2). Time spent >180 mg/dL improved by −1.6 ± 3.4 h/day from 8.5 ± 4.0 to 6.8 ± 3.7 (P < 0.0001), and events per day decreased by 0.23 ± 0.74 from 2.59 ± 0.87 to 2.36 ± 0.77 (P = 0.0037). Time spent >240 mg/dL improved by −1.0 ± 2.1 from 3.2 ± 2.5 to 2.1 ± 2.3 h/day (P < 0.0001), and events decreased by −0.35 ± 0.68 from 1.35 ± 0.80 to 0.99 ± 0.81/day (P < 0.0001). Time spent >300 mg/dL decreased by −0.4 ± 1.0 h/day from 0.9 ± 1.2 to 0.6 ± 1.0 (P = 0.0013), and events improved by −0.14 ± 0.40 from 0.48 ± 0.45 to 0.34 ± 47/day (P = 0.0011). Changes in time in hyperglycemia were not found to be influenced by age, duration of diabetes or by day/night‐time (Table S2–S4, Supporting Information). Figure S1 shows the time spent in each range during the study.
A significant improvement was observed in estimated A1c at study end when compared with baseline (7.07 ± 0.84% vs 7.46 ± 0.91%, a reduction of −0.39 ± 0.81%; P < 0.0001). Estimated A1c improved by ≥ 0.5% for 45% (41/92) of participants (Table 2). This reduction was irrespective of age or duration of diabetes.
Measures of glucose variability were shown to significantly improve, including SD, SD rate of change, high blood glucose index, continuous overall net glycemic action and mean of daily differences (Table 3). The frequency of fingerstick testing was reduced from 2.9 ± 1.3/day (mean ± SD; median 2.9) at baseline to 1.9 ± 1.4/day (median 2.0) during the treatment phase (days 15–90). After the device was unmasked, and sensor data could be utilized by participants, the daily scanning frequency was 11.5 ± 7.1 (median 9.2), which was maintained throughout the treatment phase (11.3 ± 6.7/day; median 9.4).
Table 3.
Change in measures of glycemic variability
| Baseline (days 1–15) |
Final phase (days 76–90) |
Change |
95% CI for change |
P‐value | |
|---|---|---|---|---|---|
| SD glucose (mg/dL) | 57 ± 16 | 52 ± 14 | −5 ± 9 | (−7, −3) | <0.0001 |
| CV glucose (%) | 33.8 ± 7.4 | 33.0 ± 6.8 | −0.8 ± 4.4 | (−1.7, 0.1) | 0.0774 |
| LBGI | 0.58 ± 0.73 | 0.62 ± 0.59 | 0.04 ± 0.69 | (−0.11, 0.18) | 0.6267 |
| HBGI | 8.3 ± 4.3 | 6.5 ± 3.8 | −1.8 ± 3.6 | (−2.5, −1.0) | <0.0001 |
| BGRI | 8.9 ± 4.1 | 7.1 ± 3.7 | −1.8 ± 3.2 | (−2.4, −1.1) | <0.0001 |
| CONGA 1 h (mg/dL) | 38.8 ± 9.0 | 36.2 ± 8.5 | −2.6 ± 4.8 | (−3.5, −1.6) | <0.0001 |
| CONGA 2 h (mg/dL) | 58.4 ± 14.1 | 54.1 ± 13.3 | −4.4 ± 8.0 | (−6.0, −2.7) | <0.0001 |
| CONGA 4 h (mg/dL) | 76.1 ± 19.1 | 69.3 ± 18.2 | −6.8 ± 11.6 | (−9.2, −4.4) | <0.0001 |
| CONGA 6 h (mg/dL) | 79.7 ± 21.8 | 72.3 ± 20.2 | −7.4 ± 13.7 | (−10.2, −4.5) | <0.0001 |
| MODD (mg/dL) | 51.1 ± 15.4 | 47.1 ± 13.5 | −4.1 ± 10.1 | (−6.2, −2.0) | 0.0002 |
| SD rate of change (mg/dL/min) | 0.86 ± 0.19 | 0.81 ± 0.18 | −0.05 ± 0.09 | (−0.07, −0.03) | <0.0001 |
Total n = 92. Data presented as the mean ± standard deviation.
BGRI, blood glucose risk index; CI, confidence interval; CONGA, continuous overall net glycemic action; CV, coefficient of variation; HBGI, high blood glucose index; LBGI, low blood glucose index; MODD, mean of daily differences; SD, standard deviation.
The total daily dose of insulin did not change throughout the study period (28.7 ± 18.7 units [mean ± SD] at baseline to 28.7 ± 20.2 units by study end [day 90; P = 0.9836]). Total basal insulin decreased from 11.9 ± 9.0 (mean ± SD) units at baseline to 11.2 ± 9.3 units by study end (P = 0.0040), and bolus insulin tended to rise by 0.7 ± 4.4 units from 16.9 ± 12.2 to 17.5 ± 13.3 (P = 0.1500).
Scores for the DTSQ significantly improved from baseline to study end (day 90), including treatment satisfaction (P < 0.0001; Figure S2). Participants’ perception of episodes of both hypoglycemia and hyperglycemia also improved (P = 0.0062 and P = 0.0310, respectively; Figure S2). User questionnaire results were equally positive for participants (sensor application and wear) and healthcare professionals (use of glucose reports).
A total of 273 AEs were experienced by 60 (63.8%; n = 94) participants, including five serious AEs reported for five (5.3%) participants (two severe, three moderate). Of these, 257 AEs were related to symptomatic hypoglycemia, including 254 mild AEs for 52 (55.3%) participants and one moderate AE for one participant. Two participants reported two hypoglycemia‐related serious AEs; one was reported as possibly device‐related (further investigation concluded the device was functioning as intended), and the second was reported as not device‐related. Both participants received glucose treatment; hospital admission was not required. No episodes of diabetic ketoacidosis or hyperosmolar hyperglycemic state were reported during the study. None of the AEs or serious AEs led to participant withdrawal.
There were 68 mild sensor insertion/wear symptoms experienced by 29 (30.9%) participants. These included bleeding (n = 18), erythema (n = 18), bruising (n = 12), itching (n = 8), pain (n = 7), edema (n = 3), induration (n = 1) and rash (n = 1); all resolved quickly. In addition, three mild sensor wear‐related AEs were experienced by three participants (skin trauma, minor discomfort at site, subcutaneous bleeding at site), all of which resolved without treatment.
DISCUSSION
This trial is the first to show that flash glucose monitoring is effective at improving glycemic control in Japanese type 2 diabetes treated with insulin. Although the primary end‐point of change in time in hypoglycemia did not reach statistical significance, other indices of glycemic control, including time in range, mean glucose and time in hyperglycemia, were significantly improved. Time in range improved by 1.7 ± 3.0 h/day (7.1%) as a result of reduced hyperglycemia at all thresholds >180 mg/dL. Overall, glucose levels were managed effectively to achieve benefits in glucose control without an increase in hypoglycemia, with a clinically relevant decrease of ≥30% time in hypoglycemia being observed in 51% of participants. In addition, both patient and healthcare professional satisfaction with the use of this technology was also found to be high.
Information on the frequency of hypoglycemia during treatment of diabetes in non‐Western countries is limited 18 , 19 , 20 , and hypoglycemia is a great concern for patients using insulin in Japan 21 . In the present study, a low level of hypoglycemia was observed at baseline, and 27% of participants did not experience glucose levels <70 mg/dL. The minimal time spent in hypoglycemia contrasts to that reported for white people with type 2 diabetes, using either flash glucose monitoring 5 or real‐time CGM 6 . The difference in pathophysiology and treatment regimens of the condition might explain the different frequency of hypoglycemia in the two populations. While the frequency of insulin use for management of type 2 diabetes is comparable between Japan and Western countries 22 , type 2 diabetes in Japanese individuals is characterized by less insulin resistance than white individuals, such that lower doses of insulin are required to achieve glycemic control 10 , 11 . Mean total daily insulin doses of 70–90 units are reported in trials with CGM for white people with type 2 diabetes 6 , 7 , which is approximately threefold greater than those observed in the present study. Another explanation for the low baseline level of hypoglycemia might be the investigators’ high awareness of hypoglycemia as an adverse effect of insulin 20 and its associated risks 21 , 23 , particularly in an aging population 24 . Furthermore, the concerns and fears of the patients for hypoglycemia are high in Japan 25 . Standard care during insulin therapy for type 2 diabetes patients in Japan thus might emphasize a low level of hypoglycemia. Nevertheless, the low hypoglycemia at baseline was maintained while reducing hyperglycemia in the current study, which likely shows the usefulness of the device.
Estimated A1c from CGM data is recognized as a useful measurement for clinical management 26 . In the present study, baseline mean estimated A1c measurements fulfilled the international HbA1c goal for this population 27 , reflecting well‐controlled glycemia, which is characteristic of type 2 diabetes patients in Japan and is in contrast to Western populations 28 , 29 . At study end, the mean estimated A1c had improved further, irrespective of age or duration of diabetes, and was aligned with the more stringent HbA1c target recommended by the Japan Diabetes Society 30 . Furthermore, in 45% of participants, estimated A1c improved by ≥0.5%. The reduction in estimated A1c without an increase in hypoglycemia in a relatively elderly population with existing good control is a key strength of this trial. Improvement of HbA1c without increased hypoglycemia was also observed with other flash glucose monitoring studies in white people 5 , 7 . The current results, along with existing data, show the benefits of utilizing flash glucose monitoring technology 5 , 7 , 8 , 31 , 32 , 33 , 34 , 35 or real‐time CGM in type 2 diabetes patients treated with insulin 4 , 6 .
In Japan, insulin dose titration for type 2 diabetes is generally physician‐led, and poor patient concordance with insulin regimens is acknowledged as an issue 20 . Most participants (92%) in the current study used fixed insulin doses; 17% dose‐adjusted for the current glucose level and 14% for their meals. The mean total daily dose of insulin was unchanged at study end, although the total basal insulin dose slightly decreased and the bolus insulin dose tended to increase. Other small dose adjustments might not have been detected, however. Given that insulin doses were almost unchanged, it is likely that improvements in glycemia in the study were largely due to behavior modifications by participants. The data generated from sensor use would have assisted discussions between healthcare professionals and the study participants, facilitating decisions about the clinical management. Furthermore, visualization of current glycemia and glycemic fluctuation might have enabled participants to better understand the relationship between the glucose control and changes to their prescribed insulin regimen and dietary intake, as well as other behaviors.
Sensor scanning rates and SMBG fingerstick testing were similar to those reported for a study population of white people with type 2 diabetes 5 . During the treatment phase (when sensor results were visible), the average daily scanning frequency was more than threefold greater than the self‐reported SMBG frequency at baseline. The unprompted reduction in SMBG testing during device use was maintained alongside a mean system utilization rate of 93% (median 96%), whereas the reduction of SMBG was contrary to the current labeling in Japan for adjunctive use for the device. The improvement in DTSQ scores with treatment using the device is consistent with previous findings for Europeans with diabetes 5 , 9 , and those reported for short‐term use of flash glucose monitoring in Japan 36 . These findings and the positive user questionnaire responses from both participants and healthcare professionals indicate good acceptance of the system.
Sensor wear‐related events and symptoms were similar to those reported for comparable study populations 5 , 35 . Two participants reported a severe hypoglycemic event, with one participant requiring an ambulance for hospital attendance. The devices used by both patients were functioning normally at the time of the incidents. which were determined to be either unrelated or possibly related to the device.
The single‐arm design was a limitation of this study. Furthermore, change in time spent in hypoglycemia as the primary outcome might not have been the ideal choice in this cohort with a low frequency of hypoglycemia. In Japan, individuals with diabetes have different cultural sensitivities about insulin therapy and hypoglycemia 37 , and there is a tendency to recommend lifestyle changes before implementing pharmacological interventions to improve glycemic control 29 , which might affect the applicability of these findings to other ethnic populations. As all the attending physicians are specialists for diabetes care, it is unknown whether the present results are applicable to care by general practitioners. Further studies are required to assess the effectiveness of this technology in younger and older age groups from different healthcare environments, as well as studies of a longer duration, to determine if results mirror those seen in a largely white population 34 .
In summary, use of flash glucose monitoring technology in Japanese participants with type 2 diabetes, treated with multiple daily insulin injections, results in increased time in range, improvement in glucose variability, a reduction in estimated A1c without increasing hypoglycemia, and is seen as beneficial by this patient population and their carers.
DISCLOSURE
WO received endowment, research funding and honorarium from Astellas Pharma, Abbott Japan and Mitsubishi Tanabe Pharma Corporation; endowment and honorarium from Nippon Boehringer Ingelheim Co., Novartis Pharma, MSD, Sanofi K.K, Sumitomo Dainippon Pharma Co. and Takeda Pharmaceutical Co.; endowment and grants from Eli Lilly Japan; endowment from Novo Nordisk Pharma Ltd., Ono Pharmaceutical Co., Daiichi Sankyo Co.; and research funding from Kyorin Pharmaceutical Co., AstraZeneca and Boehringer Ingelheim Pharma GmbH & Co. KG.
YH received honorarium for lectures from Eli Lilly Japan, AstraZeneca and Takeda Pharmaceutical Co.
TO received fees, research support and/or speaker bureaus from Astellas Pharma, Mitsubishi Tanabe Pharma Corporation, Novo Nordisk Pharma, Ono Pharmaceutical Co., Sanwa Kagaku Kenkyusho Co., and Takeda Pharmaceutical Co.; and research support from Abbott Japan Co., AbbVie GK, Bayer Yakuhin, Daiichi Sankyo Co., Eli Lilly Japan, Japan Tobacco, Kowa Company, Nippon Boehringer Ingelheim Co., Sanofi and Taisho Pharmaceutical Co.
TT received honorarium from Eli Lilly Japan, Sumitomo Dainippon Pharma Co, Astellas Pharma, MSD and Takeda Pharmaceutical Co.; and research funding from Daiichi Sankyo Co. and Sanofi.
YK received research funding from Astellas Pharma, Sumitomo Dainippon Pharma Co., Daiichi Sankyo Co., Eli Lilly Japan, MSD (and honorarium), Mitsubishi Tanabe Pharma Corporation, Sanofi, Pfizer Japan, Novartis Pharma, Takeda Pharmaceutical Co, Japan Tobacco, Novo Nordisk Pharma and Ono Pharmaceutical Co.
KU received research funding or grants from Taisho Toyama Pharmaceutical Co., Nippon Boehringer Ingelheim Co., Sumitomo Dainippon Pharma Co., Terumo Corporation, Kyowa Hakko Kirin Co., Ono Pharmaceutical Co. and Novo Nordisk Pharma.
RN received speaker honoraria from Astellas Pharma Nippon Boehringer Ingelheim Co., Eli Lilly Japan, Kissei Pharmaceutical Co., Medtronic Japan Co., MSD, Novartis Pharma, Novo Nordisk Pharma, Sanofi and Takeda Pharmaceutical Co.; and collaborative research funding with the Japan Diabetes Foundation.
JN received honorarium and endowments/grants from MSD K.K., Ono Pharmaceutical Co., Sanofi, Takeda Pharmaceutical Co. and Novo Nordisk Pharma; honorarium from Mitsubishi Tanabe Pharma Corporation, Terumo Corporation and Nippon Boehringer Ingelheim Co.; research funding from Kissei Pharmaceutical Co; and endowment or grants from Astellas Pharma, Daiichi Sankyo Co., Taisho Toyama Pharmaceutical Co., Sumitomo Dainippon Pharma Co., Eli Lilly Japan, Japan Tobacco and Novartis Pharma.
Supporting information
Table S1 | Study inclusion and exclusion criteria.
Table S2 | Change in hyperglycemia by age.
Table S3 | Change in hyperglycemia by duration of diabetes.
Table S4 | Change in hyperglycemia by day and night.
Figure S1 | Time spent in each glycemic range by study phase.
Figure S2 | Japanese Diabetes Treatment and Satisfaction Questionnaire (change version) treatment satisfaction scale scores (questions 1 and 4–8), and perceived frequency of hypoglycemia and hyperglycemia (questions 2 and 3).
Acknowledgments
The authors thank all the participants in this study and the many individuals involved in the collection of data at the study sites. We thank Zoe Welsh and Katie Cranfield for statistical support (Statistics, Abbott Diabetes Care), and Amanda Cartmale for assistance with manuscript preparation (Scientific Affairs, Abbott Diabetes Care). This study was supported by Abbott Diabetes Care, who provided all study materials, was involved in data analysis and reporting, but was not part of the authors’ interpretation of study results.
J Diabetes Investig 2021; 12: 82–90
Clinical Trial Registry
University Hospital Medical Information Network Clinical Trials Registry
UMIN000023593
References
- 1. International Diabetes Federation . IDF Diabetes Atlas, 8th edn. Brussels, Belgium: International Diabetes Federation, 2017. Japan Country report 2017. Available at: http://reports.instantatlas.com/report/view/846e76122b5f476fa6ef09471965aedd/JPN?clear=true. Last accessed December 2019.
- 2. American Diabetes Association . Standards of medical care in diabetes‐2019. Diabetes Care 2017; 42(Suppl 1): S1–S193. [Google Scholar]
- 3. Inzucchi SE, Bergenstal RM, Buse JB, et al Management of hyperglycemia in type 2 diabetes, 2015: a patient centred approach. Update to a position statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care 2015; 38: 140–149. [DOI] [PubMed] [Google Scholar]
- 4. Vigersky RA, Fonda SJ, Chellapa M, et al Short and long‐term effects of real‐time continuous glucose monitoring in patients with Type 2 diabetes. Diabetes Care 2012; 35: 32–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Haak T, Hanaire H, Ajjan RA, et al Flash glucose‐sensing technology as a replacement for blood glucose monitoring for the management of insulin‐treated type 2 diabetes: a multicenter, open‐label randomized controlled trial. Diabetes Ther 2017; 8: 55–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Beck RW, Riddlesworth TD, Ruedy K, et al Continuous glucose monitoring versus usual care in patients with Type 2 diabetes receiving multiple daily insulin injections. A randomized trial. Ann Intern Med 2017; 167: 365–374. [DOI] [PubMed] [Google Scholar]
- 7. Yaron M, Roitman E, Aharon‐Hananel G, et al Effect of flash glucose monitoring technology on glycemic control and treatment satisfaction in patients with Type 2 diabetes. Diabetes Care 2019; 42: 1178–1184. [DOI] [PubMed] [Google Scholar]
- 8. Evans M, Welsh Z, Ells S, et al The impact of flash glucose monitoring on glycaemic control as measured by HbA1c: a meta‐analysis of clinical trials and real‐world observational studies. Diabetes Therapy 2020; 11: 83–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bolinder J, Antuna R, Geelhoed‐Duijvestijn P, et al Novel glucose‐sensing technology and hypoglycemia in Type 1 diabetes: a multicentre, non‐masked, randomised controlled trial. Lancet 2016; 388: 2254–2263. [DOI] [PubMed] [Google Scholar]
- 10. Møller JB, Pedersen M, Tanaka H, et al Body composition is the main determinant for the difference in Type 2 diabetes pathophysiology between Japanese and Caucasians. Diabetes Care 2014; 37: 796–804. [DOI] [PubMed] [Google Scholar]
- 11. Fukushima M, Usami M, Ikeda M, et al Insulin secretion and insulin sensitivity at different stages of glucose tolerance: a cross‐sectional study of Japanese Type 2 diabetes. Metabolism 2004; 53: 831–835. [DOI] [PubMed] [Google Scholar]
- 12. Khunti K, Alsifri S, Aronson R, et al HAT Investigator Group. Impact of hypoglycaemia on patient‐reported outcomes from a global, 24‐country study of 27,585 people with Type 1 and insulin‐treated Type 2 diabetes. Diabetes Res Clin Pract 2017; 130: 121–129. [DOI] [PubMed] [Google Scholar]
- 13. Japanese Ethical Guidelines for Medical and Health Research Involving Human Participants, March 2015 provisional translation. Available at: http://www.mhlw.go.jp/file/06‐Seisakujouhou‐10600000‐Daijinkanboukouseikagakuka/0000080278.pdf. Last accessed December 2019 .
- 14. International Council for Harmonisation (ICH) Guideline for Good Clinical Practice E6(R2) ., 2016. Available at: https://database.ich.org/sites/default/files/E6_R2_Addendum.pdf. Last accessed December 2019 .
- 15. Abbott Diabetes Care FreeStyle Libre Software . Available at: https://www.FreeStyleLibre.com. Last accessed December 2019.
- 16. Ishii H, Bradley C, Riazi A, et al The Japanese version of the Diabetes Treatment Satisfaction Questionnaire (DTSQ): Translation and clinical evaluation. Int J Clin Exp Med 2000; 192: 809–814. [Google Scholar]
- 17. Seaquist ER, Anderson J, Childs B, et al Hypoglycemia and Diabetes: A Report of a Workgroup of the American Diabetes Association and The Endocrine Society. Diabetes Care 2013; 36: 1384–1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Sako A, Yasunaga H, Matsui H, et al Hospitalization for hypoglycemia in Japanese diabetic patients: a retrospective study using a national inpatient database, 2008–2012. Medicine (Baltimore). 2015; 94: e1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Namba M, Iwakura T, Nishimura R, et al Japan Diabetes Society (JDS) Committee for Surveys on Severe Hypoglycemia. The current status of treatment‐related severe hypoglycemia in Japanese patients with diabetes mellitus: a report from the committee on a survey of severe hypoglycemia in the Japan Diabetes. Society. Diabetol Int 2018; 9: 84–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Matsuoka A, Hirota Y, Takeda A, et al Relationship between glycated hemoglobin level and duration of hypoglycemia in type 2 diabetes patients treated with sulfonylureas: a multicenter cross‐sectional study. J Diabetes Investig 2020; 11: 417–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Harashima S, Nishimura A, Inagaki N. Attitudes of patients and physicians to insulin therapy in Japan: an analysis of the Global Attitude of Patients and Physicians in Insulin Therapy study. Expert Opin Pharmacother 2017; 18: 5–11. [DOI] [PubMed] [Google Scholar]
- 22. Arai K, Takai M, Hirao K, et al Present status of insulin therapy for Type 2 diabetes treated by general practitioners and diabetes specialists in Japan: Third report of a cross‐sectional survey of 15,652 patients. J Diabetes Investig 2012; 3: 396–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Goto A, Goto M, Terauchi Y, et al Association between severe hypoglycemia and cardiovascular disease risk in Japanese patients with type 2 diabetes. J Am Heart Assoc 2016; 5: e002875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Diabetes Japan. Society (JDS)/Japan Geriatrics Society (JGS) Joint Committee on Improving Care for Elderly Patients with Diabetes, Haneda M, Ito H. Glycemic targets for elderly patients with diabetes. Diabetol Int 2016; 7: 331–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Yoshioka N. Psychological reluctance to insulin therapy: as an illness side of diabetes mellitus. Diabetol Int 2018; 9: 82–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Danne T, Nimri R, Battelino T, et al International consensus on the use of continuous glucose monitoring. Diabetes Care 2017; 40: 1631–1640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. American Diabetes Association . Standards of medical care in diabetes 2018. Diabetes Care 2018; 42(Suppl 1): S61–S70. [DOI] [PubMed] [Google Scholar]
- 28. Yokoyama H, Oishi M, Takamura H, et al Large‐scale survey of rates of achieving targets for blood glucose, blood pressure, and lipids and prevalence of complications in type 2 diabetes (JDDM 40). BMJ Open Diabetes Res Care 2016; 4: e000294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Neville SE, Boye KS, Montgomery WS, et al Diabetes in Japan: a review of disease burden and approaches to treatment. Diabetes Metab Res Rev 2009; 25: 705–716. [DOI] [PubMed] [Google Scholar]
- 30. Haneda M, Noda M, Origasa H, et al Japanese Clinical Practice Guideline for Diabetes 2016. Diabetol Int 2018; 9: 1–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ajjan RA, Jackson N, Thomson SA. Reduction in HbA1c using professional flash glucose monitoring in insulin‐treated Type 2 diabetes patients managed in primary and secondary care settings: A pilot, multicentre, randomised controlled trial. Diab Vasc Dis Res 2019; 16: 385–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Hanaire H, Benhamou P, Bures C, et al Real‐world, chart review study to determine the effectiveness of FreeStyle Libre Flash Glucose monitoring system, in adults with Type 2 diabetes in France. Diabetes 2019; 68(Suppl 1): 92‐LB. [Google Scholar]
- 33. Fasching P, Brath H, Ebenbichler C, et al Effectiveness of FreeStyle Libre flash glucose monitoring system observed in real‐world, chart review study in Austria, in adults with type 2 diabetes. Diabetes 2019;68(Suppl 1): 96‐LB. [Google Scholar]
- 34. Kröger J, Aigner U, Maxeiner S, et al Effectiveness of FreeStyle Libre flash glucose monitoring system observed in real‐world, chart review study in adults with Type 2 diabetes. Diabetologie und Stoffwechsel 2019; 14(S01): S37. [Google Scholar]
- 35. Haak T, Hanaire H, Ajjan R, et al Use of flash glucose‐sensing technology for 12 months as a replacement for blood glucose monitoring in insulin‐treated Type 2 diabetes. Diabetes Ther 2017; 8: 573–586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Mitsuishi S, Nishimura R, Harashima SI, et al The effect of novel glucose monitoring system (flash glucose monitoring) on mental well‐being and treatment satisfaction in Japanese people with diabetes. Adv Ther 2018; 35: 72–80. [DOI] [PubMed] [Google Scholar]
- 37. Rubin RR, Peyror M, Siminerio LM. Healthcare and patient‐reported outcomes: results of the cross‐national Diabetes Attitudes, Wishes and Needs (DAWN) study. Diabetes Care 2006; 29: 1249–1255. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1 | Study inclusion and exclusion criteria.
Table S2 | Change in hyperglycemia by age.
Table S3 | Change in hyperglycemia by duration of diabetes.
Table S4 | Change in hyperglycemia by day and night.
Figure S1 | Time spent in each glycemic range by study phase.
Figure S2 | Japanese Diabetes Treatment and Satisfaction Questionnaire (change version) treatment satisfaction scale scores (questions 1 and 4–8), and perceived frequency of hypoglycemia and hyperglycemia (questions 2 and 3).


