Abstract
Aims/Introduction
To appraise guidelines on the antiplatelet strategy of prevention of cardiovascular disease (CVD) in patients with type 2 diabetes mellitus, and highlight the consensuses and controversies to aid clinician decision‐making.
Materials and Methods
A systematic search was carried out for guidelines regarding CVD prevention or focusing on type 2 diabetes patients. Appraisal of Guidelines for Research and Evaluation II instrument was utilized to appraise the quality of included guidelines.
Results
Of the 15 guidelines with discrepant Appraisal of Guidelines for Research and Evaluation II scores (66%; interquartile range 51–71%), 10 were defined as “strongly recommended” guidelines. For secondary prevention, >60% of guidelines advocated that the dual antiplatelet therapy was used within 12 months when the type 2 diabetes patients experienced acute coronary syndrome and/or post‐percutaneous coronary intervention or coronary artery bypass grafting, with subsequent long‐term aspirin use. For primary prevention, 80% of guidelines supported that aspirin should not be routinely used by patients with type 2 diabetes. No consensus on whether to prolong dual antiplatelet therapy in secondary prevention, and whether to use aspirin in type 2 diabetes patients with high CVD risk exists in current guidelines.
Conclusions
Physicians should use the recommendations from “strongly recommended” guidelines to make informed decisions and know the consensuses of current guidelines. Dual antiplatelet therapy should be used within 12 months when type 2 diabetes patients experience acute coronary syndrome and/or percutaneous coronary intervention/coronary artery bypass grafting, with subsequent long‐term aspirin use. In primary prevention, aspirin should not be routinely used by individuals with type 2 diabetes, but might be considered for those with high CVD risk.
Keywords: Antiplatelet strategy, Cardiovascular disease, Type 2 diabetes mellitus
The study appraised the current guidelines on the antiplatelet strategy of prevention of cardiovascular disease in patients with type 2 diabetes mellitus utilizing the Appraisal of Guidelines for Research and Evaluation II instrument. In conclusion, we found that current guidelines regarding the antiplatelet strategy of cardiovascular disease prevention for patients with type 2 diabetes still have considerable discrepancies, but the consensuses could help clinicians to make wise decisions in choosing an antiplatelet strategy for type 2 diabetes patients.
INTRODUCTION
Cardiovascular disease (CVD) remains a leading cause of mortality and morbidity among type 2 diabetes patients 1 . Persons with type 2 diabetes are at increased risk for CVD, and worse outcomes when CVD is present 2 , 3 . Prevention is key for type 2 diabetes patients with established CVD and those at risk of future CVD events 4 , 5 . As already described in the literature, antiplatelet therapy in diabetes was a well‐recognized effective strategy and the cornerstone of pharmacological treatment for preventing CVD 5 , 6 , so the guideline‐recommended antiplatelet strategy should be used for patients with type 2 diabetes. However, there are still some physicians who did not use the clinical guideline due to the wide choice available, lack of awareness of guidelines and inconsistency in published recommendations 7 . Therefore, assessing the quality of guidelines and summarizing the recommendations regarding antiplatelet strategies for CVD prevention in type 2 diabetes patients might help physicians to better use guidelines in clinical work.
The purpose of this study was to appraise the guidelines systematically utilizing the Appraisal of Guidelines for Research and Evaluation II (AGREE II) instrument 8 and provide summary recommendations about antiplatelet strategies of CVD prevention in type 2 diabetes patients for clinicians.
METHODS
Data sources and searches
We carried out a systematic literature search to retrieve guidelines for the antiplatelet therapy of type 2 diabetes in PubMed, Web of Science, EMBASE and the Cochrane Library databases with the keywords “antiplatelet therapy,” “guideline,” “diabetes mellitus” and “coronary artery disease” from January 2009 to December 2019. The detailed search strategies of each database are listed in Table S1 (Supplementary Materials). All relevant guideline websites were also searched (Website address in Table S2 in Supplementary Materials).
Study selection
References that met the Institute of Medicine definition for clinical practice guidelines were included9. Guidelines were included if they focused on the management of type 2 diabetes or CVD, and contained the recommendations on the antiplatelet therapy for CVD prevention in type 2 diabetes patients. In addition, only guidelines in English and the latest version were included.
Titles and abstracts were assessed by two independent reviewers, and then full‐text screening. Disagreements were resolved by face‐to‐face discussion, or in the case of persistent disagreement, by consultation with a third researcher. The third researcher also checked the final selection of guidelines.
Appraisal of clinical guidelines
We assessed the quality of development for each included guideline using the latest 23‐item AGREE II instrument 8 (the details of 6 domains in Table S3 in Supplementary Materials). Each item was independently rated on a 7‐point Likert scale by two reviewers, and then the rigor scores of each domain were calculated according to the formula from AGREE II 8 . The average AGREE II scores of each guideline were obtained by expressing the sum of individual item scores as a percentage of the maximum possible score. The reproducibility of average AGREE II scores of two reviewers was great, with an intraclass correlation coefficient of 0.82. Finally, the guideline was defined as “strongly recommended” if most domains (>3 domains) scored >60%; the guideline was “recommended” if most domains scored between 30% and 60%, and if most domain scores were <30%, the guideline was “not recommended” 10 . Furthermore, editorial independence from the funding body and conflicts of interest (COI) were further assessed by two reviewers using four items from the Reporting Item for Practice Guidelines in Healthcare checklist 11 (the details in Table S4 in Supplementary Materials). The relationships between the proportion of guideline panel members with reported industry relationships and AGREE II scores were also assessed 12 .
Data extraction and synthesis
The characteristics of guidelines and all relevant recommendations were extracted in accordance with a predesigned step by two independent reviewers. Any disagreement on data abstracting was resolved by a group discussion to reach consensus. Two tables for the description of guideline characteristics and the comparison of the recommendations from the selected guidelines were constructed, respectively.
Statistical analysis
Continuous variables that were normally distributed were presented as the mean and standard deviation (mean ± SD). Otherwise, these data were presented as medians and interquartile ranges (25–75 percentiles). Reproducibility between reviewers on AGREE II scores was assessed using the intraclass correlation coefficient by the two‐way random model. The correlation between the proportion of guideline panel members with reported industry relationships and the AGREE II score was examined using Pearson’s correlation analysis, of which five guidelines that had no explicit disclosure on COI were excluded from the analyses. Additionally, all included guidelines were divided into two groups with or without explicit disclosure on COI to evaluate the effect of COI on the rigor of the development of guidelines. The differences of overall AGREE II scores and every domain score between two groups were examined using the independent samples t‐test or Mann–Whitney U‐test. Given the limited number of guidelines, these were explorative quantitative analyses. A P‐value <0.05 was accepted as statistical significance. All analyses were carried out using IBM SPSS 20.0 (SPSS, Chicago, IL, USA) and GraphPad Prism 8.0 (GraphPad Software, La Jolla, CA, USA) software.
RESULTS
Our systematic search retrieved 7,140 titles, of which 6,397 were identified as potentially eligible after duplicates removed. Many were excluded on the basis of the title (n = 6,151), abstract (n = 190) and on review of the full report (n = 41). Finally, 15 guidelines relevant to antiplatelet strategies of CVD prevention in type 2 diabetes patients were included (Figure 1). Table 1 summarizes the characteristics of selected guidelines, along with the AGREE II scores and COI. The guideline references are listed in Table S5 (Supplementary Materials).
Figure 1.
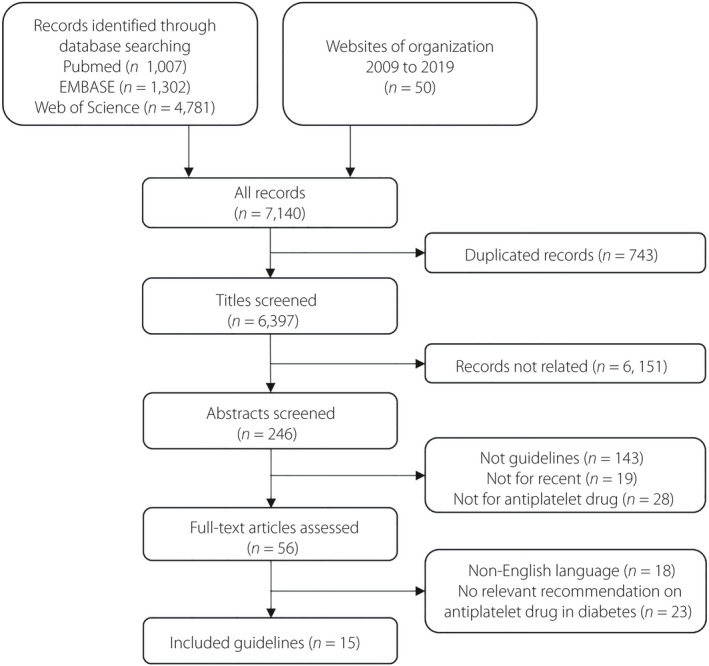
Flow chart showing the clinical practice guideline selection process.
Table 1.
General characteristics of the included 15 guidelines‡‡
| Guidelines identifier, Year † |
Organization(s) responsible for guidelines development |
Region | Target population | COI appraisal (RIGHT) ‡ | Proportion of panel members§ | AGREE II score (%) | Guideline status |
|---|---|---|---|---|---|---|---|
| ADA, 2019 | American Diabetes Association | USA | DM | DADG, DCEM, DTCG, DRFG | 14/23 | 69 | Strongly recommended |
| AACE, 2015 | American Association of Clinical Endocrinologists | USA. | DM | DADG, DCEM, DTCG | 30/35 | 63 | Strongly recommended |
| ESC/EASD, 2019 | European Society of Cardiology and European Association for the Study of Diabetes | Europe | DM and pre‐DM | DADG, DCEM, DTCG | 128/150 | 75 | Strongly recommended |
| ESC, 2017 | European Society of Cardiology | Europe | CAD | DADG, DCEM, DTCG | 121/140 | 68 | Strongly recommended |
| ESC, 2016 | European Society of Cardiology | Europe | General population | DADG, DCEM, DTCG | 85/124 | 71 | Strongly recommended |
| ESC, 2015 | European Society of Cardiology | Europe | NSTE‐ACS | DADG, DCEM, DTCG | 100/119 | 66 | Recommended |
| NICE, 2015 | National Institute for Health and Clinical Excellence | UK | T2DM | DADG, DCEM, DTCG | 9/14 | 73 | Strongly recommended |
| SIGN, 2010 | Scottish Intercollegiate Guidelines Network | UK | DM | – | – | 63 | Strongly recommended |
| CDA, 2018 | Canadian Diabetes Association | Canada | DM | DADG, DCEM, DTCG | 80/168 | 73 | Strongly recommended |
| CCS, 2011 | Canadian Cardiovascular Society | Canada | ASCVD | DADG, DTCG | 12/12 | 69 | Strongly recommended |
| RACGP, 2016 | Royal Australian College of General Practitioners | Australia | T2DM | – | – | 48 | Recommended |
| Baker IDI, 2015 | Baker Heart and Diabetes Institute | Australia | T2DM | DADG, DCEM | 50/83 | 64 | Strongly recommended |
| JDS, 2016 | Japan Diabetes Society | Japan | DM | – | – | 42 | Recommended |
| KDA, 2015 | Korean Diabetes Association | Korea | DM | – | – | 36 | Recommended |
| IDF, 2012 | International Diabetes Federation | International | T2DM | – | – | 57 | Recommended |
Proportion of panel members who reported industry relationships.
ASCVD, arteriosclerotic cardiovascular disease; DADG, disclosure of how to access the declarations on the guideline; CAD, coronary artery disease; DCEM, describe how conflicts of interest were evaluated and managed; DM, diabetes mellitus; DRFG, disclosure the role of funder(s) in the different stages of guideline development; DTCG, disclosure the types of conflicts of interest (financial and nonfinancial) that are relevant to the guidelines development; NSTE‐ACS, non‐ST‐elevation acute coronary syndrome; T2DM, type 2 diabetes mellitus.
The guideline references are listed in Table S5 (Supplementary Materials).
The conflicts of interest (COI) of guidelines was evaluated using the Reporting Item for Practice
Guidelines in Healthcare (RIGHT) checklist.
The included 15 guidelines were appraised utilizing AGREE II, and the average scores were 66% (interquartile range 51‐71%), of which 10 guidelines were “strongly recommended” (Table 1). The AGREE II scores of six domains of the included 15 guidelines are listed in Figure S1 (Supplementary Materials), and we found the generally low scores in D5 (applicability; 38±16%) and the enormous heterogeneity of D6 (editorial independence) scores (92% (interquartile range 54–92%; Figure S2 in Supplementary Materials). The heterogeneity of D6 caused by five guidelines that did not provide a statement about COI of group members or report that they were developed independently from funding organizations. The remaining 10 guidelines with explicit disclosure of COI described at least one item from the Reporting Item for Practice Guidelines in Healthcare checklist (Table 1). In these limited 10 guidelines, no correlation between the proportion of panel members with an industry relationship and the AGREE II score was observed (Pearson’s correlation r = –0.226; P = 0.531).
Difference of guidelines with or without explicit disclosure of COI
To further assess the effects of editorial independence on formulating the recommendations in the guidelines, guidelines were categorized according to with or without explicit disclosure of COI. The rigor scores of each domain of these two groups are minutely described in Figure 2a,b and showed that the average AGREE II scores of guidelines with explicit COI disclosure were significantly higher compared with the guidelines without explicit COI disclosure (P = 0.013; Figure 2c). Similar results were also found in D3 (rigor of development; P = 0.003), D5 (P = 0.002) and D6 (P = 0.001) when comparing these two groups in the average AGREE II scores and six domains scores (Figure 2c).
Figure 2.
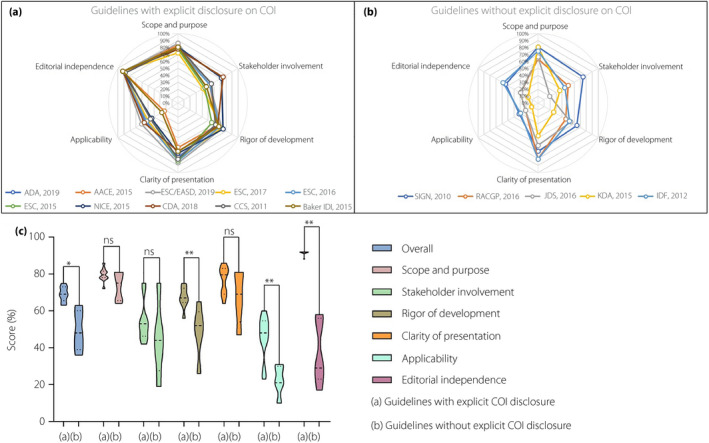
The final Appraisal of Guidelines for Research and Evaluation II (AGREE II) scores of 15 included guidelines. AGREE II scores are plotted for each guideline for comparison. Higher scores are plotted toward the outside of the graph. (a) AGREE II scores of guidelines with explicit disclosure on conflicts of interest (COI). (b) AGREE II scores of guidelines without explicit disclosure on COI. (c) The comparison of the overall and six domain scores between two groups with or without explicit disclosure on COI.
Secondary prevention of CVD
Recommendations for antiplatelet strategies of CVD prevention in type 2 diabetes patients extracted from 15 included guidelines are shown in Table 2. In the included 15 guidelines, there was consensus on the use of antiplatelet agents in type 2 diabetes patients for CVD secondary prevention. For the type 2 diabetes patients with acute coronary syndrome (ACS) and/or post‐percutaneous coronary intervention (PCI) or coronary artery bypass grafting (CABG) <12 months, the dual antiplatelet therapy (DAPT) for a year was advocated in both 10 of 15 guidelines (67%) and seven of 10 “strongly recommended” guidelines (70%; Table 2; Figure 3), and shortening duration of DAPT was suggested by only one guideline (from the Scottish Intercollegiate Guidelines Network published in 2010). Of course, the DAPT within 12 months after ACS and/or PCI or CABG in type 2 diabetes patients has been widely recognized by cardiologists and applied in the clinical work.
Table 2.
Recommendations on antiplatelet therapy of secondary and primary prevention for cardiovascular disease in patients with type 2 diabetes
| Guidelines identifier, Year † | Antiplatelet strategy of secondary prevention (class of recommendation) ‡ | Antiplatelet strategy of primary prevention (class of recommendation) ‡ | |||
|---|---|---|---|---|---|
| T2DM with ACS and/or post PCI/CABG <12 months | T2DM with CCS | T2DM with high ASCVD risk | T2DM with moderate/low ASCVD risk | ||
| Prolongation of DAPT | Long‐term antiplatelet agent | ||||
| ADA, 2019 | DAPT for 1 year (A) | Prolongation may have benefits (B) | ASA (A) | ASA may be considered after a discussion on the benefits (C) | Not recommended |
| AACE, 2015 | – | – | ASA (A) | ASA may be considered (D) | Not recommended |
| ESC/EASD, 2019 | DAPT for 1 year (IA) | Prolongation up to 3 years may be considered (IIb) | ASA (A) | ASA may be considered (IIb) | Not recommended (IIIb) |
| ESC, 2017 | DAPT for 1 year (IA) | Prolongation up to 3 years may be considered (IIb) | – | – | – |
| ESC, 2016 | DAPT for 1 year (IA) | Prolongation may be considered after careful assessment of the benefits (IIb) | ASA (A) | Not recommended (IIIa) | Not recommended (IIIa) |
| ESC, 2015 | DAPT for 1 year (IA) | Prolongation may be considered after careful assessment of the benefits (IIb) | – | – | – |
| NICE, 2015 | DAPT for 1 year (IA) | – | ASA (A) | Not recommended | Not recommended |
| SIGN, 2010 | STE‐ACS: ASA + C of 1 months; NSTE‐ACS: ASA + C of 3 months | – | ASA (A) | Not recommended | Not recommended |
| CDA, 2018 | DAPT for 1 year (A) | – | ASA (B) | ASA may be used (D) | ASA not be used routinely (A) |
| CCS, 2011 | – | – | ASA (IA) | ASA may be considered while aged >40 years and at low risk for major bleeding (IIb) | ASA not be used routinely (IIIA) |
| RACGP, 2016 | DAPT for 1 year (B) | – | ASA (A) or C (A) or ASA + dipyridamole (B) | Not recommended | Not recommended |
| Baker IDI, 2015 | DAPT for 1 year (B) | – | ASA (A) or C (A) or ASA + dipyridamole (B) | – | – |
| JDS, 2016 | – | – | Antiplatelet agents (A) | Not recommended (A) | Not recommended (A) |
| KDA, 2015 | DAPT for 1 year (B) | – | ASA (IA) | 10‐year risk >10%: ASA may be considered (E); 10‐year risk 5–10%: ASA may be considered based on clinical judgment (E) | 10‐year risk < 5%: Not recommended (C) |
| IDF, 2012 | – | – | ASA or C | Not recommended | Not recommended |
ACS, acute coronary syndrome; ASA, acetylsalicylic acid; ASCVD, arteriosclerotic cardiovascular disease; C, clopidogrel; CABG, coronary artery bypass grafting; CCS, chronic coronary syndrome; CVD, cardiovascular disease; DAPT, dual antiplatelet therapy (ASA + P2Y12 inhibitor); NSTEACS, non‐ST‐segment elevation acute coronary syndrome; PCI, percutaneous coronary intervention; STE‐ACS, ST‐segment elevation acute coronary syndrome; T2DM, type 2 diabetes mellitus.
The guideline references are listed in Table S5 (Supplementary Materials).
The level of evidence on each recommendation was adopted from respective guidelines.
Figure 3.
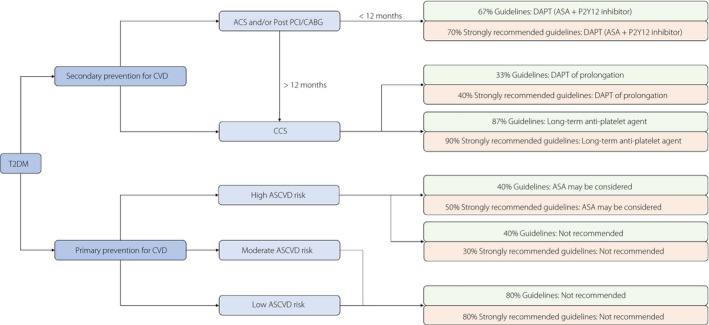
The summary of recommendations regarding antiplatelet strategies in type 2 diabetes patients for cardiovascular disease (CVD) prevention. ACS, acute coronary syndrome; ASA, acetylsalicylic acid; ASCVD, arteriosclerotic cardiovascular disease; CABG, coronary artery bypass grafting; CCS, chronic coronary syndrome; DAPT, dual antiplatelet therapy; PCI, percutaneous coronary intervention; T2DM, type 2 diabetes mellitus.
However, there was no consensus on whether to prolong the DAPT and the duration of prolongation. Five of 15 guidelines (33%) advocated that prolongation of DAPT beyond 12 months should be considered in type 2 diabetes patients with ACS and/or PCI/CABG, of which four guidelines (40%) were “strongly recommended” (Table 2; Figure 3). Two “strongly recommended” guidelines (European Society of Cardiology [ESC]/European Association for the Study of Diabetes 2019; ESC 2017) supported the prolongation of DAPT up to 3 years, but three other guidelines did not describe the duration of DAPT prolongation. Most of these guidelines were published by the ESC, and might represent a common, positive European viewpoint for the prolongation of DAPT. Furthermore, the class of these recommendations was “class II” or “class B” in their guideline, and the remaining 10 guidelines did not provide the recommendation regarding the prolongation of DAPT.
A total of 13 of 15 included guidelines (87%) advocated low‐dose aspirin as a long‐term secondary prevention strategy for those with diabetes and a history of CVD, of which nine guidelines (90%) were “strongly recommended” (Table 2; Figure 3). The consensus came from the high‐class recommendations (class A or class IA) in their guideline. The remaining two guidelines did not provide the relevant recommendation because of their different concerns (ESC 2017 focused on DAPT in coronary artery disease; ESC 2015 focused on patients with non‐ST‐elevation ACS).
Primary prevention of CVD
The use of antiplatelet agents in the primary prevention of CVD in type 2 diabetes patients remains controversial and should be different for those with different CVD risks. For patients with type 2 diabetes and high CVD risk, the use of low‐dose aspirin was advocated by six guidelines (40%), including five “strongly recommended” guidelines (50%), but another six guidelines (40%) did not suggest offering antiplatelet therapy (Table 2; Figure 3). The class of these recommendations was lower (class C, D, E or class IIb). Therefore, the use of antiplatelet agents in those with type 2 diabetes and high CVD risk remains confusing for physicians in clinical work.
There was a consensus that antiplatelet therapy was not recommended for individuals with type 2 diabetes and moderate/low CVD risk. Of 15 guidelines, 12 (80%) suggested not offering antiplatelet therapy for adults with type 2 diabetes and moderate/low CVD risk, of which eight guidelines (80%) were “strongly recommended” (Table 2; Figure 3), and no guideline recommended using antiplatelet therapy for these patients.
DISCUSSION
We identified 15 guidelines, of which 10 were “strongly recommended” guidelines, involving antiplatelet therapy that could be carried out in type 2 diabetes patients for the prevention of CVD. A great variation in the rigor of development was found among the guidelines, particularly in transparency about COI. The guidelines with explicit disclosure of COI might achieve higher quality, and the recommendations from those guidelines might also be a more important reference for clinical practice. This systematic review aimed to provide a summary of current guidelines regarding the use of antiplatelet therapy in type 2 diabetes patients available to physicians in the form of a quick reference, which allows for easy comparison. There were some consensuses that DAPT should be used within 12 months when the type 2 diabetes patients experienced ACS and/or PCI/CABG, with subsequent long‐term low‐dose aspirin use for CVD secondary prevention, and aspirin should not be routinely used by patients with type 2 diabetes and moderate/low CVD risk for primary prevention. Guidelines on whether to prolong the DAPT after 12 months for type 2 diabetes patients with ACS and/or PCI/CABG, the duration of DAPT prolongation, and whether to use antiplatelet agents in individuals with type 2 diabetes and high CVD risk differ.
The current systematic review, unlike the previously published reviews that summarized the antiplatelet strategy of CVD prevention in diabetes in current guidelines and studies 13 or that compared the diabetes guidelines 14 or the DAPT guidelines 15 , carried out a broad search and used a validated instrument to appraise the quality of included guidelines. All of the included guidelines in this review were published in the past 10 years and represent the most recent recommendations. This article can therefore be of additional value to the development of the subsequent guidelines and the clinical decisions on antiplatelet strategy in type 2 diabetes patients.
Guidelines generally recommend a management decision that DAPT was used within 12 months when the type 2 diabetes patients experienced ACS and/or PCI/CABG, with subsequent long‐term low‐dose aspirin use. However, they differ with regard to whether to prolong the DAPT and the prolonged duration. This is partly because there was insufficient evidence to confirm the benefit of DAPT prolongation after 12 months in the type 2 diabetes patients with ACS and/or PCI/CABG. The DAPT study 16 investigated the effect of DAPT for 30 months versus 12 months post‐stenting in 9,961 patients, and showed that DAPT beyond 12 months after PCI, as compared with aspirin therapy, significantly reduced the risks of CVD and stent thrombosis. The subgroup analysis of the 3,391 DM patients in the DAPT study also showed that continued DAPT beyond 1 year after coronary stenting is associated with a reduced risk of myocardial infarction (MI) in diabetes patients, although this benefit is attenuated in comparison with patients without diabetes 17 . Furthermore, the benefit from DAPT prolongation in patients with MI was also confirmed by the Prevention of Cardiovascular Events in Patients with Prior Heart Attack Using Ticagrelor Compared to Placebo on a Background of Aspirin‐Thrombolysis in Myocardial Infarction 54 (PEGASUS‐TIMI 54) study 18 . This evidence propelled the formation of recommendation (DAPT prolongation after 12 months in the type 2 diabetes patients with ACS and/or PCI/CABG) in ESC guidelines. However, a high‐quality meta‐analysis published in Lancet 19 showed that although treatment with DAPT beyond 12 months after PCI reduced the risks of stent thrombosis and MI, it was associated with increased mortality because of an increased risk of non‐cardiovascular mortality not offset by a reduction in cardiac mortality. These studies resulted in the controversies of current guidelines regarding whether to prolong the DAPT after 12 months for type 2 diabetes patients with ACS and/or PCI/CABG, but it was worth noting that these studies did not focus on diabetes patients.
In the past few months, several findings from the large Effect of Ticagrelor on Health Outcomes in Diabetes Mellitus Patients Intervention Study (THEMIS trial), that focused on diabetes patients with proven coronary artery disease but without a history of MI or stroke, were published and showed the long‐term therapy with ticagrelor plus aspirin had a lower incidence of ischemic cardiovascular events in patients with diabetes, stable coronary artery disease and previous PCI 20 , 21 . These findings from the THEMIS trial have reference value for the formation of the recommendation of subsequent guidelines about CVD secondary prevention in patients with type 2 diabetes, and reduce the controversy among current guidelines. For the patient with diabetes and stable coronary artery disease, clinicians might be able to prolong the DAPT (ticagrelor plus aspirin) after 12 months in PCI. Typical secondary prevention trials in diabetes patients with a history of MI or stroke, designed to evaluate, for example, the optimal dosing and bioavailability of P2Y12 receptor or aspirin, the effectiveness of inhibiting other platelet receptors, the use of oral anticoagulants, or the using duration of DAPT, are highly desirable in future.
In CVD primary prevention for patients with type 2 diabetes, antiplatelet therapy is not recommended for those with moderate/low CVD risk, but whether to use it in those with high CVD risk is controversial in current guidelines. Despite some controversies, these recommendations were more reliable than before. Just over a decade ago, international guidelines were advocating the routine use of aspirin for CVD primary prevention in diabetes patients, a recommendation based on rather weak and historical data 22 , 23 . The beneficial effect of aspirin when used for primary prevention in patients with diabetes was challenged in subsequent studies 24 , 25 , 26 , but these analyses included underpowered studies and used people from different periods with varying degrees of vascular protective therapies, thus questioning the contemporary applications of the findings. More recently, the landmark A Study of Cardiovascular Events in Diabetes (ASCEND study) data were published in N Engl J Med 27 , and showed that aspirin use prevented cardiovascular events in patients with diabetes, but it also caused major bleeding events, and the absolute benefits were largely counterbalanced by the bleeding hazard. Superficially, the benefits from the use of aspirin for primary prevention in diabetes were conclusively negated. However, it was noteworthy that most bleeding episodes were upper gastrointestinal bleeding that could have been prevented by additional gastric protective therapies. Therefore, guideline‐recommended care that aspirin should not be routinely used for primary prevention in patients with type 2 diabetes should be well known by clinicians, but low‐dose aspirin plus proton pump inhibitor might be beneficial for patients with type 2 diabetes and high CVD risk. In addition, the ASCEND study did not reveal whether patients with poor diabetes control would have shown more benefit, so further studies focusing on this issue are required.
Several possible limitations of this review should be taken into consideration. First, although the quality of the guideline was comprehensively assessed by a validated instrument (AGREE II tool), that only considered the reported information related to the development of the guideline. It did not rule out the possibility that the detailed information was not reported in some guidelines, causing a lower AGREE II score. Second, the AGREE II tool considers the whole guideline and is not intended for individual recommendations. Despite the quality of development across the whole guideline having a high potential to influence the quality of individual recommendations 28 , 29 , 30 , a solid recommendation might also be created within a poorly developed guideline and vice versa. Third, the AGREE II tool was unable to evaluate the quality of the content of guidelines and the quality of the evidence underpinning the recommendations. Even if the quality of the development methods correlates with the quality of the content 30 , it might be possible to create a solid guideline with a poor process. Fourth, although several means and tools were used to appraise the influence of COI, the true degree of influence by industry relationships was difficult to identify. Fifth, despite using comprehensive search strategies, we might still have missed some eligible guidelines.
Current guidelines regarding the antiplatelet strategy of CVD prevention in patients with type 2 diabetes still have considerable discrepancies. We encourage physicians to use the guidelines with higher AGREE II rigor scores and explicit disclosure of COI for deciding on the antiplatelet strategy for type 2 diabetes patients, and fully understand the consensus of current guidelines. For secondary prevention, DAPT should be used within 12 months when type 2 diabetes patients experience ACS and/or PCI/CABG, with subsequent long‐term use of low‐dose aspirin. For primary prevention, aspirin should not be routinely used in individuals with type 2 diabetes, but might be considered for those with high CVD risk.
DISCLOSURE
The authors declare no conflict of interest.
Supporting information
Figure S1 The AGREE II scores of six domainsofthe included15 guidelines.
Figure S2 The distribution of AGREE IIscores in the overall and six domainsof15 included guidelines.
Table S1 The detailed search strategy of databases.
Table S2 Website of guideline organizations and professional societies.
Table S3 Clinical guideline quality domains used in the Appraisal ofGuidelines for Research andEvaluation II (AGREE II) tool.
Table S4 Four items from the RIGHT (Reporting Item for Practice Guidelines in Healthcare) checklist to assess the conflicts of interest (COI).
Table S5 References of 15 included guidelines.
Acknowledgment
We thank all participants and researchers of eligible guidelines. This study had no funding support.
J Diabetes Investig 2021; 12: 99–108
References
- 1. Paneni F, Beckman JA, Creager MA, et al Diabetes and vascular disease: pathophysiology, clinical consequences, and medical therapy: part I. Eur Heart J 2013; 34: 2436–2443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. De Bacquer D, De Backer G, Ostor E, et al Predictive value of classical risk factors and their control in coronary patients: a follow‐up of the EUROASPIRE I cohort. Eur J Cardiovasc Prev Rehabil 2003; 10: 289–295. [DOI] [PubMed] [Google Scholar]
- 3. Pennells L, Kaptoge S, Wood A, et al Equalization of four cardiovascular risk algorithms after systematic recalibration: individual‐participant meta‐analysis of 86 prospective studies. Eur Heart J 2019; 40: 621–631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Pagidipati NJ, Navar AM, Pieper KS, et al Secondary Prevention of Cardiovascular Disease in Patients With Type 2 Diabetes Mellitus: International Insights From the TECOS Trial (Trial Evaluating Cardiovascular Outcomes With Sitagliptin). Circulation 2017; 136: 1193–1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bowman L, Mafham M, Wallendszus K, et al Effects of Aspirin for Primary Prevention in Persons with Diabetes Mellitus. N Engl J Med 2018; 379: 1529–1539. [DOI] [PubMed] [Google Scholar]
- 6. Sharma AN, Deyell JS, Sharma SN, et al Role of and Recent Evidence for Antiplatelet Therapy in Prevention of Cardiovascular Disease in Diabetes. Curr Cardiol Rep 2019; 21: 78. [DOI] [PubMed] [Google Scholar]
- 7. Dallongeville J, Banegas JR, Tubach F, et al Survey of physicians’ practices in the control of cardiovascular risk factors: the EURIKA study. Eur J Prev Cardiol 2012; 19: 541–550. [DOI] [PubMed] [Google Scholar]
- 8. Dans AL, Dans LF. Appraising a tool for guideline appraisal (the AGREE II instrument). J Clin Epidemiol 2010; 63: 1281–1282. [DOI] [PubMed] [Google Scholar]
- 9. Trustworthy IOMU, Guidelines CP. Clinical Practice Guidelines We Can Trust. Washington (DC): National Academies Press; (US) 2011. [Google Scholar]
- 10. Ou Y, Goldberg I, Migdal C, et al A critical appraisal and comparison of the quality and recommendations of glaucoma clinical practice guidelines. Ophthalmology 2011; 118: 1017–1023. [DOI] [PubMed] [Google Scholar]
- 11. Chen Y, Yang K, Marusic A, et al A Reporting Tool for Practice Guidelines in Health Care: The RIGHT Statement. Ann Intern Med 2017; 166: 128–132. [DOI] [PubMed] [Google Scholar]
- 12. Ferket BS, Genders TS, Colkesen EB, et al Systematic review of guidelines on imaging of asymptomatic coronary artery disease. J Am Coll Cardiol 2011; 57: 1591–1600. [DOI] [PubMed] [Google Scholar]
- 13. Sharma AN, Deyell JS, Sharma SN, et al Role of and Recent Evidence for Antiplatelet Therapy in Prevention of Cardiovascular Disease in Diabetes. Curr Cardiol Rep 2019; 21: 78. [DOI] [PubMed] [Google Scholar]
- 14. Cornell S. Comparison of the diabetes guidelines from the ADA/EASD and the AACE/ACE. J Am Pharm Assoc (2003) 2017; 57: 261–265. [DOI] [PubMed] [Google Scholar]
- 15. Capodanno D, Alfonso F, Levine GN, et al. ACC/AHA Versus ESC Guidelines on Dual Antiplatelet Therapy: JACC Guideline Comparison. J Am Coll Cardiol 2018;72(23 Pt A):2915–2931. [DOI] [PubMed] [Google Scholar]
- 16. Mauri L, Kereiakes DJ, Yeh RW, et al Twelve or 30 months of dual antiplatelet therapy after drug‐eluting stents. N Engl J Med 2014; 371: 2155–2166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Meredith IT, Tanguay JF, Kereiakes DJ, et al Diabetes Mellitus and Prevention of Late Myocardial Infarction After Coronary Stenting in the Randomized Dual Antiplatelet Therapy Study. Circulation 2016; 133: 1772–1782. [DOI] [PubMed] [Google Scholar]
- 18. Bonaca MP, Bhatt DL, Cohen M, et al Long‐term use of ticagrelor in patients with prior myocardial infarction. N Engl J Med 2015; 372: 1791–1800. [DOI] [PubMed] [Google Scholar]
- 19. Palmerini T, Benedetto U, Bacchi‐Reggiani L, et al Mortality in patients treated with extended duration dual antiplatelet therapy after drug‐eluting stent implantation: a pairwise and Bayesian network meta‐analysis of randomised trials. Lancet 2015; 385: 2371–2382. [DOI] [PubMed] [Google Scholar]
- 20. Steg PG, Bhatt DL, Simon T, et al Ticagrelor in patients with stable coronary disease and diabetes. N Engl J Med 2019; 381: 1309–1320. [DOI] [PubMed] [Google Scholar]
- 21. Bhatt DL, Steg PG, Mehta SR, et al Ticagrelor in patients with diabetes and stable coronary artery disease with a history of previous percutaneous coronary intervention (THEMIS‐PCI): a phase 3, placebo‐controlled, randomised trial. Lancet 2019; 394: 1169–1180. [DOI] [PubMed] [Google Scholar]
- 22. Final report on the aspirin component of the ongoing Physicians’ Health Study. N Engl J Med 1989; 321: 129–135. [DOI] [PubMed] [Google Scholar]
- 23. Aspirin effects on mortality and morbidity in patients with diabetes mellitus. Early Treatment Diabetic Retinopathy Study report 14. ETDRS Investigators. JAMA 1992; 268: 1292–1300. [DOI] [PubMed] [Google Scholar]
- 24. Sacco M, Pellegrini F, Roncaglioni MC, et al Primary prevention of cardiovascular events with low‐dose aspirin and vitamin E in type 2 diabetic patients: results of the Primary Prevention Project (PPP) trial. Diabetes Care 2003; 26: 3264–3272. [DOI] [PubMed] [Google Scholar]
- 25. Belch J, MacCuish A, Campbell I, et al The prevention of progression of arterial disease and diabetes (POPADAD) trial: factorial randomised placebo controlled trial of aspirin and antioxidants in patients with diabetes and asymptomatic peripheral arterial disease. BMJ 2008; 337: a1840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Mahmoud AN, Gad MM, Elgendy AY, et al Efficacy and safety of aspirin for primary prevention of cardiovascular events: a meta‐analysis and trial sequential analysis of randomized controlled trials. Eur Heart J 2019; 40: 607–617. [DOI] [PubMed] [Google Scholar]
- 27. Bowman L, Mafham M, Wallendszus K, et al Effects of Aspirin for Primary Prevention in Persons with Diabetes Mellitus. N Engl J Med 2018; 379: 1529–1539. [DOI] [PubMed] [Google Scholar]
- 28. Grol R. Successes and failures in the implementation of evidence‐based guidelines for clinical practice. Med Care 2001; 39(8 Suppl 2): I46–I54. [DOI] [PubMed] [Google Scholar]
- 29. Davis DA, Taylor‐Vaisey A. Translating guidelines into practice. A systematic review of theoretic concepts, practical experience and research evidence in the adoption of clinical practice guidelines. CMAJ 1997; 157: 408–416. [PMC free article] [PubMed] [Google Scholar]
- 30. Grimshaw JM, Russell IT. Effect of clinical guidelines on medical practice: a systematic review of rigorous evaluations. Lancet 1993; 342: 1317–1322. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1 The AGREE II scores of six domainsofthe included15 guidelines.
Figure S2 The distribution of AGREE IIscores in the overall and six domainsof15 included guidelines.
Table S1 The detailed search strategy of databases.
Table S2 Website of guideline organizations and professional societies.
Table S3 Clinical guideline quality domains used in the Appraisal ofGuidelines for Research andEvaluation II (AGREE II) tool.
Table S4 Four items from the RIGHT (Reporting Item for Practice Guidelines in Healthcare) checklist to assess the conflicts of interest (COI).
Table S5 References of 15 included guidelines.


