Summary
Immune checkpoint inhibitors (ICIs) have reshaped cancer therapy. ICIs enhance T cell activation through various mechanisms and may help reverse the exhausted phenotype of tumour-infiltrating lymphocytes. However, disrupting the key role that checkpoint molecules play in immune homeostasis may result in autoimmune complications. A broad range of immune-related adverse events (irAEs) involve almost every organ but mostly affect the skin, digestive system, lung, endocrine glands, nervous system, kidney, blood cells, and musculoskeletal system. They are usually manageable but can be life-threatening. The incidence of irAEs is not very different in patients with hepatocellular carcinoma (HCC) compared to other tumour types, although there is a trend towards a higher incidence of hepatic irAEs. HCC usually develops on a background of cirrhosis with associated systemic manifestations. Extrahepatic organ dysfunction in cirrhosis may cause signs and symptoms that overlap with irAEs or increase their severity. Available guidelines for the management of irAEs have not specifically considered the assessment of toxicities in the context of patients with liver cancer and cirrhosis. This review addresses the toxicity profile of ICIs in patients with HCC, focusing on the challenges that the underlying liver disease poses to their diagnosis and management. Challenges include late recognition, inadequate work-up and delayed treatment, overdiagnosis and inappropriate interruption of ICIs, complications caused by immunosuppressive therapy, and increased cost. A specific algorithm for the management of hepatic irAEs is provided.
Keywords: Immunotherapy, Nivolumab, Pembrolizumab, Tremelimumab, Durvalumab, Ipilimumab, Hepatotoxicity
Introduction
Immune checkpoint molecules regulate the immune response, preventing inappropriate immune activation and allowing self-tolerance.1 However, they also provide a mechanism by which tumours can evade immune surveillance, and inhibitory checkpoint molecules have become major targets for anticancer therapy. Antibodies against cytotoxic T-lymphocyte antigen-4 (CTLA-4) and programmed cell death-protein 1 (PD-1) or its ligand (PD-L1) block their negative signals and enhance T cell activation. These immune checkpoint inhibitors (ICIs) have transformed the treatment of several cancer types. CTLA-4 is the target of ipilimumab and tremelimumab, while PD-1 is the target of nivolumab and pembrolizumab, and PD-L1 is the target of durvalumab, atezolizumab and avelumab.
Key point.
Because immune checkpoint molecules have a fundamental role in maintaining immune homeostasis, their modulation can result in a wide range of immune-related adverse events, involving almost every organ.
ICIs have shown signs of activity against HCC. They result in objective remissions that are durable and associated with prolonged survival in 15–20% of patients. Based on results from single-arm phase II trials,2,3 nivolumab and pembrolizumab have been approved by US and other regulatory agencies to treat patients who progress or are intolerant to sorafenib. Phase III trials comparing pembrolizumab versus best supportive care in the second-line setting4 and nivolumab versus sorafenib as first-line systemic therapy5 have nevertheless failed to demonstrate superiority in terms of overall survival, despite clear trends for improved outcomes in both trials. Based on these and other data, a number of phase III trials testing combinations of ICIs with tyrosine-kinase inhibitors (TKIs), antiangiogenic antibodies or other ICIs are now underway.6 Recently, the combination of atezolizumab and bevacizumab has shown better progression free and overall survival compared with sorafenib in the first-line setting. This will likely establish checkpoint inhibition as a global standard of care in the management of advanced HCC.
Because of their fundamental role in maintaining immune homeostasis, blockade of inhibitory checkpoint molecules results in a broad range of immune-related adverse events (irAEs), resulting from impaired self-tolerance which may involve almost every organ. irAEs are frequent, although usually manageable, when ICIs are used as single agents, but they can be life-threatening. With PD-1 or PD-L1 [PD-(L)1] inhibitors, the development of irAEs is unrelated to the dose, with an incidence of 27% for all grades and 6% for grade 3 or higher.7 With CTLA-4 inhibitors, the overall incidence of irAEs fluctuates according to the dose and is higher at 72% for all grades and 24% for grade 3 or higher.8 Although direct comparisons are not available, meta-analyses indicate that rash and colitis are significantly more frequent during CTLA-4 blockade, and suggest that there are no major differences between agents targeting each checkpoint.7–9 Median time to onset is typically shorter for CTLA-4 than for PD-(L)1 inhibitors.10 In a recent meta-analysis, 42 fatal irAEs were recorded among 6,528 patients treated with an ICI (0.64%) and ipilimumab-induced colitis was the most common cause of fatal irAE.9 None of these meta-analyses, however, included patients with liver cancer. Although T cell infiltration and activation are assumed to be the primary events, the mechanisms of irAEs are largely unknown. This is partly because empiric therapy is often started, and tissue biopsies are seldom obtained. It is interesting to note, however, that among patients with autoimmune diseases treated with a PD-(L)1 inhibitor, exacerbation of the autoimmune condition occurred rarely and the incidence of irAEs was similar to clinical trials where patients with autoimmune diseases were excluded.11
Hepatocellular carcinoma (HCC) usually develops on a background of chronic liver disease12 which itself may give rise to systemic manifestations. Multiple organs may show signs of dysfunction13 and cause signs and symptoms that can overlap with irAEs and may increase their severity. Scientific societies such as SITC, ASCO and ESMO have provided general guidelines for the management of irAEs.14–16 However, they have not specifically considered the assessment of toxicities in the context of patients with liver cancer and cirrhosis, where careful interpretation is required to differentiate irAEs from liver-related events. In this review, we will address the toxicity profile of ICIs in patients with HCC focusing on the challenges that the underlying liver disease poses to their diagnosis and management.
The challenges of immune-related toxicities in patients with HCC
Cirrhosis is a disease with multiple causes characterised by diffuse fibrosis, disruption of the intrahepatic venous flow, portal hypertension and liver failure.
If the cause is not successfully treated, it progresses in a non-linear course leading to hepatic and extrahepatic complications. A relatively long period of silent disease (compensated cirrhosis) leads to a second period of frequent complications (decompensated cirrhosis) including ascites, variceal haemorrhage or hepatic encephalopathy, which carries a poor prognosis.17 In parallel with the progressive deterioration of liver structure and function, other organs frequently develop secondary dysfunction. Rather than following a gradual worsening of their general condition towards liver insufficiency, patients suffer from acute complications frequently triggered by a precipitating event. Additionally, cirrhosis disturbs the liver’s homeostatic immune function. The term cirrhosis-associated immune dysfunction defines an acquired alteration of innate and acquired immunity that leads to both systemic inflammation and immunodeficiency,18 and results in increased mortality. Systemic inflammation results from persistent immune cell stimulation and enhanced production of proinflammatory cytokines while immunodeficiency is due to derangement of local immunity within the liver and of systemic immune cells.
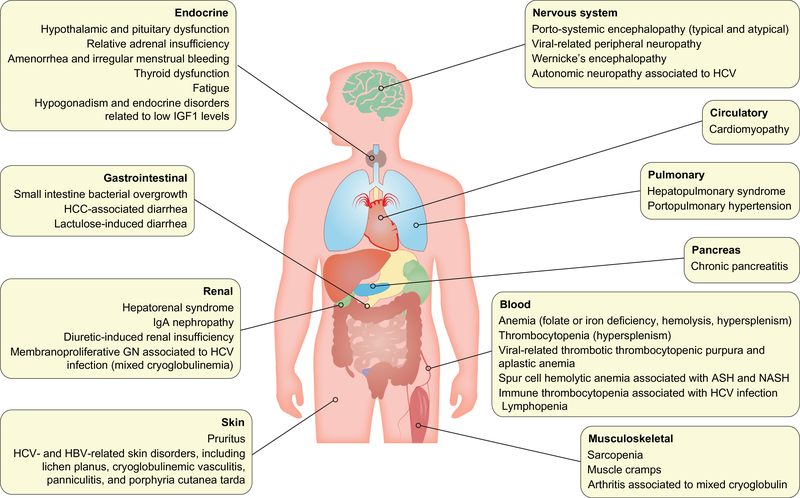
Many extrahepatic disorders associated with cirrhosis cause symptoms that may not be easy to distinguish from irAEs and can synergise to worsen organ function. The most important of which are summarised in Fig. 1. Similarly, almost any organ can be affected by irAEs. In general, the incidence of irAEs is not very different in patients with HCC than in patients with other tumour types like melanoma, as shown in Table 1. Overall, the reported incidence of irAEs for the PD-1 inhibitors nivolumab and pembrolizumab is 10–20% in HCC and above 30% in other tumours. Individually, there is a trend towards a higher incidence of hepatic irAEs and a lower incidence of pneumonitis in HCC.
Fig. 1. Most common organ comorbidities associated with chronic liver diseases.
*ASH: alcoholic steatohepatitis; HCC: hepatocellular carcinoma; NASH: non-alcoholic steatohepatitis.
Table 1.
Immune-related adverse events in patients with HCC (incidence is presented as percentage of patients).
| CTLA-4 |
PD-1 |
PD-(L)1 & CTLA-4 |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Agent(s) | Tremelimumab |
Nivolumab |
Pembrolizumab | Nivolumab + ipilimumab |
Durvalumab + tremelimumab | ||||
| Dose | 15 mg/kg q3m25 | 3.5 & 10 mg/kg q4w122 | Various doses123 | 240 mg q2w19 | 200 mg/kg q3w3,4 | Nivolumab 1 mg/kg + ipilimumab 3 mg/kg q3w53 | Nivolumab 3 mg/kg + ipilimumab 1 mg/kg q3w53 | Nivolumab 3 mg/kg q2w + ipilimumab 1 mg/kg q6w53 | Durvalumab 20 mg/kg + tremelimumab 1 mg/kg q4w26 |
| n | 21 | 32 | 262 | 49** | 104 & 279 | 50 | 49 | 49 | 40 |
| Discontinuation due to toxicity | 0 | 13 | 3.4 | 4 | 5.0–17.2 | n.r. | n.r. | n.r. | 7.5 |
| Treatment-related AE | |||||||||
| All grades | n.r. | n.r. | 77.0 | 51 | 60.9–73 | 94 | 71 | 79 | 60 |
| Grade ≥3 | n.r. | n.r. | 18 | n.r. | 18.6–26 | 53 | 29 | 31 | 20 |
| irAE | |||||||||
| All grades | n.r. § | n.r. | 18 | n.r. | 10–18.3 | 29 | 22 | 17 | n.r. |
| Grade ≥3 | n.r. § | n.r. | 1 | n.r. | 0–7.2 | 4 | 4 | 0 | n.r. |
| Rash | |||||||||
| All grades | 65 | 16* | 18 | n.r. | 8–10 | 29 | 22 | 17 | 12.5 |
| Grade ≥3 | 15 | 3 | 1 | n.r. | 0–1 | 4 | 4 | 0 | 0 |
| Pruritus | |||||||||
| All grades | n.r. | 9* | 20 | 12 | 12–18 | 45 | 33 | 29 | 22.5 |
| Grade ≥3 | n.r. | 3 | 1 | 0 | 0–0 | 4 | 0 | 0 | 0 |
| Diarrhoea | |||||||||
| All grades | 30 | n.r. | 14 | n.r. | 11 | 24 | 12 | 17 | 12.5 |
| Grade ≥3 | 5 | n.r. | 1 | n.r. | 0 | 4 | 2 | 2 | 2.5 |
| Colitis | |||||||||
| All grades | n.r. | 6* | n.r. | n.r. | 1.4 | 10 | 2 | 2 | 2.5 |
| Grade ≥3 | n.r. | 0 | n.r. | n.r. | 0.8 | 6 | 2 | 2 | 2.5 |
| AST | |||||||||
| All grades | 70 | 34* | 9.9 | 10 | 14 | 20 | 20 | 13 | 17.5 |
| Grade ≥3 | 45 | 22 | 5.3 | 6 | 7 | 16 | 8 | 4 | 10 |
| ALT | |||||||||
| All grades | 55 | 19* | 9.5 | 10 | 9 | 16 | 14 | 8 | 20 |
| Grade ≥3 | 25 | 9 | 3.4 | 4 | 4 | 8 | 6 | 0 | 5 |
| Hepatitis | |||||||||
| All grades | n.r. | n.r. | 0.6* | n.r. | 1.8–3 | 20 | 12 | 6 | n.r. |
| Grade ≥3 | n.r. | n.r. | 0.6* | n.r. | 1.4 | 20 | 10 | 6 | n.r. |
| Pneumonitis | |||||||||
| All grades | n.r. | n.r. | 1.3* | n.r. | 2.2 | 10 | 0 | 0 | 2.5 |
| Grade ≥3 | n.r. | n.r. | 0.6* | n.r. | 0.4 | 6 | 0 | 0 | 2.5 |
| Adrenal insufficiency | |||||||||
| All grades | n.r. | 3* | n.r. | n.r. | 0.6–3 | 18 | 6 | 6 | n.r. |
| Grade ≥3 | n.r. | 3 | n.r. | n.r. | 0–2 | 4 | 0 | 0 | n.r. |
| Hypothyroidism | |||||||||
| All grades | n.r. | 6* | n.r. | n.r. | 5–6 | 0 | 0 | 2 | n.r. |
| Grade ≥3 | n.r. | 3 | n.r. | n.r. | 0–0.6 | 0 | 0 | 0 | n.r. |
| Hypophysitis | |||||||||
| All grades | n.r. | n.r. | n.r. | n.r. | 0.4 | n.r. | n.r. | n.r. | n.r. |
| Grade ≥3 | n.r. | n.r. | n.r. | n.r. | 0 | n.r. | n.r. | n.r. | n.r. |
AEs, adverse events; ALT, alanine aminotransferase; AST, aspartate aminotransferase; CTLA-4, cytotoxic T-lymphocyte antigen-4; HCC, hepatocellular carcinoma; ICI, immune checkpoint inhibitors; irAE, immune-related adverse event; PD-1, programmed cell death-protein 1; PD-L1, PD-1, programmed cell death 1 ligand 1.
Only AEs grade ≥3 were reported
no patient received steroids.
Patients with Child-Pugh B status.
irAEs vary in clinical significance and impact on patient safety and survival. Clinical impact depends on the organ involved, the severity of the toxicity, and response to treatment. For those that may be life-threatening, early recognition and treatment is key to a successful outcome. In the cirrhotic patient, this may be a difficult task when symptoms of a given irAE overlap with those of a cirrhosis-related disorder. Baseline evaluation should take this into account (Box 1). On the one hand, late recognition may delay treatment and worsen prognosis. On the other, overdiagnosis of irAEs may result in inappropriate interruption of effective anticancer therapy, complications caused by immunosuppressive therapy, unnecessary interventions and increased cost. This is particularly true if the treating physician is not familiar with the management of cirrhosis. Corticosteroids as immunosuppressors may have more relevant consequences in patients with cirrhosis than in others, although this issue has not been adequately confirmed. Importantly, the use of the PD-1 inhibitor nivolumab in patients with moderate liver dysfunction (Child-Pugh class B) is not associated with an increased rate of irAEs.19
Box 1. Pre-treatment evaluation recommended for patients with hepatocellular carcinoma before starting immune checkpoint inhibitors.
aThese tests should not be repeated if available within a reasonable time. ACTH, adrenocorticotropic hormone; ALT, alanine aminotransferase; AST, aspartate aminotransferase; BMP, N-terminal pro B-type natriuretic peptide; CMV, cytomegalovirus; ECG, electrocardiogram; GGT, gamma glutamyltransferase; HbA1c, glycated haemoglobin; INR, international normalised ratio; TSH, thyroid-stimulating hormone; T4, thyroxine.
History
Detailed questioning for prior decompensations of cirrhosis (ascites, edema, GI hemorrhage, jaundice or encephalopathy), including date of last gastroscopy
Detailed questioning for any co-morbidities, in particular those of autoimmune origin or associated with cirrhosis
History of base line bowel habits (frequency of bowel movements, usual stool consistency)
Detailed questioning for past or current use of alcohol, recreational drugs, medicines, and homeopathic or herbal medicinal products
Physical examination
Height and weight. Heart and respiratory rates. Blood pressure. Oxygen saturation on room air at rest and after 1-min or 6-min ambulation
Regular physical examination including skin and mucosal visual exam, thyroid palpation and search for signs of ascites, edema and encephalopathy
Blood and urine tests
Complete blood cell count
Glucose and lipid profile (HbA1c if abnormal glucose)
Liver enzymes (AST, ALT, alkaline phosphatase, GGT)
Liver function tests (total bilirubin, albumin, prothrombine activity/INR)
Creatinine, urea, urinalysis
Sodium, potassium, calcium
TSH (free T3 and T4 if abnormal TSH)
Total creatine kinase
HBsAg and HBcAb. If positive, HBsAb, HBeAg, HBeAb, HBV-DNA and HDV antibodiesa
HCVAb. If positive, HCV-RNA unless known eradication of HCV infectiona
HIV and CMV antibodiesa
Troponin
Alpha-fetoprotein
Other tests
ECG
Chest and abdominal CT or MRI scan
Additional tests recommended in patients with suspected co-existing organ disease
BNP and troponin if any ascites, edema or dyspnea or cardiac enlargement on chest imaging
Pulmonary function tests and oxygen saturation on room air at rest and after 6-min ambulation if dyspnea or lung abnormalities on chest imaging
Key point.
Cirrhosis has a variety of hepatic and extrahepatic manifestations that may overlap with toxicities from immune checkpoint inhibitors - Differential diagnosis is mandatory.
The most important questions that should be answered when dealing with a potential or established irAE are how frequently to monitor, when to hospitalise, when to withhold or permanently discontinue ICIs, when to initiate and how to escalate immunosuppression. General recommendations for the management of irAEs in patients with HCC are provided in Table 2, with potential coexisting disorders that must be considered in the diagnostic work-up listed in Table 3. It is very important to keep in mind that early engagement with other specialties according to target organ is recommended. Resuming ICIs after an irAE is a difficult decision, particularly if the patient is free from tumour progression. Among 93 patients with different tumour types who experienced moderate to severe irAEs (46% grade 2, 39% grade 3, 15% grade 4) including hepatitis (18%), skin events (15%), pneumonitis (14%), and colitis (12%), 40 were re-challenged with the same anti-PD-(L)1 agent and the same or a different irAE occurred in 22 patients (55%).20 A shorter time to the initial irAE was linked to the occurrence of a second irAE, but the second irAEs were not found to be more severe than the first.
Table 2.
General management of non-liver immune-related toxicities in patients with hepatocellular carcinoma.
| CTCAE v. 5.0 grade | Grade 1 | Grade 2 | Grade 3 | Grade 4 |
|---|---|---|---|---|
| Definition | Mild | Moderate | Severe | Life-threatening |
| ICI modification | • Continue ICI. • Consider withholding ICI for suspected pneumonitis or myocarditis during diagnostic work-up. |
• Withhold ICI until ≤grade 1 (except for hypothyroidism, adrenalitis, limited rash or sensory neuropathy). • ICI can be resumed after completion of steroid taper. • Consider permanent discontinuation for pneumonitis, myocarditis, or peripheral neuromotor syndromes based on clinical judgment. |
• Permanently discontinue CTLA-4 inhibitors in any event. • Permanently discontinue PD-(L)1 inhibitors except for hypothyroidism, adrenal insufficiency, nephritis. or rash that resolve within 30 days. |
• Permanently discontinue any ICI. |
| Monitoring | • Monitor within 2 weeks or more frequently depending on irAE and clinical judgment. | • Refer to the specialist • Monitor within 1 week or more frequently depending on irAE and clinical judgment. |
• Monitor every 2–3 days or more frequently depending on irAE and clinical judgment. • Refer to the specialist |
• Continuous monitoring during hospitalisation. |
| Medical therapy | • Not needed | • Initiate steroids (prednisone at 0.5–1 mg/kg/day or equivalent PO or i.v.). • Decision to start steroids can be differed a few days for nephritis. •If it worsens, treat as grade 3. |
• Initiate steroids immediately (prednisone at 1–2 mg/kg/day or equivalent i.v.). Intravenous route for pneumonitis, diarrhoea, and others based on clinical judgment. • If no improvement, consider infliximab, particularly for pneumonitis and colitis. |
• Manage as grade 3. |
ICI, immune checkpoint inhibitor; irAE, immune-related adverse event.
Table 3.
Potential coexisting disorders that must be considered in the diagnostic work-up of immune-related adverse events in patients with hepatocellular carcinoma.
| Organ | iRAE | Chronic liver disease | Cancer | Others |
|---|---|---|---|---|
| Skin | • Pruritus • Rash • Erythema multiforme, psoriasis, urticaria and rosacea. • Severe cutaneous adverse reactions, including Steven-Johnson Syndrome, toxic epidermal necrolysis, and DRESS |
• Pruritus • HCV- and HBV-related skin disorders, including lichen planus, polyarteritis nodosa, cryoglobulinaemic vasculitis, and porphyria cutanea tarda. |
• Biliary tract obstruction due to liver nodules or hilar lymphadenopathies. | • Cutaneous toxicity from other medications |
| GI tract | • Diarrhoea • Colitis |
• Small intestine bacterial overgrowth • Chronic pancreatitis |
• HCC-associated diarrhoea | • Clostridium difficile • Antibiotic-induced dysbacteriosis • Lactulose-induced diarrhoea |
| Liver | • Hepatitis • AST/ALT elevation |
• Flares or viral infection | • Tumour progression in the liver. | • Hepatotoxicity from other medications • Benign biliary obstruction |
| Lung | • Pneumonitis | • Hepatopulmonary syndrome • Porto-pulmonary hypertension |
• Tumour progression in the lung. | • Pneumonia |
| Thyroid | • Hypothyroidism • Hyperthyroidism • Graves’ disease |
• Reduced peripheral conversion of T4 to T3. • Thyroid dysfunction. |
||
| Adrenal glands and hypophysis | • Adrenal insufficiency • Hypophysitis |
• Hypogonadism • Hypothalamic-pituitary dysfunction • Relative adrenal insufficiency • Hypogonadism |
• Bilateral adrenal metastasis. | • Hyponatremia induced by diuretics |
| Kidney | • Nephritis | • Hepatorenal syndrome • HCV-related glomerulonephritis (mixed cryoglobulinemia) • HBV-related nephropathy • IgA nephropathy |
• Iodinated contrast agents • Renal dysfunction induced by diuretics |
|
| Nervous system | • Encephalitis • Aseptic meningitis • Peripheral neuropathy • Myasthenia gravis • Guillain-Barre syndrome • Autonomic neuropathy • Transverse myelitis |
• Porto-systemic encephalopathy (typical and atypical) • Viral-related peripheral neuropathy • Wernicke’s encephalopathy • Autonomic neuropathy associated to HCV infection |
• Tumour progression in the brain or bone (spine). • Carcinomatous meningitis • Paraneoplastic hypercalcemia. |
• Opiates, psychotropic drugs |
| Blood and bone marrow | • Cytopenias • Haemolytic anaemia • Red cell aplasia • Bone marrow failure • Haemophilia A • Hemophagocytic lymphohistiocytosis • Macrophage activation syndrome |
• Hypersplenism and bone marrow depression • Anaemia due to folate or iron deficiency • Haemolytic anaemia • Viral-related thrombotic thrombocytopenic purpura and aplastic anaemia. • Immune thrombocytopenia associated with HCV infection. • Lymphopenia related to HCC therapies such as internal or external radiation |
• Tumour bleeding • Bone marrow involvement |
• Heparin-induced thrombocytopenia |
DRESS (drug rash with eosinophilia and systemic symptoms). ALT, alanine aminotransferase; AST, aspartate aminotransferase; GI, gastrointestinal; T3, triiodothyronine; T4, thyroxine.
Toxicities by target organ
In a large meta-analysis in which no HCC trial was included, the most frequent target organs for irAEs during CTLA-4 inhibition were the skin (44%) and the gastrointestinal tract (35%). Endocrine glands and the liver were involved in 6% and 5%, respectively. Other events, including the nervous and musculoskeletal systems, blood and eyes were rarely involved.8 Skin, endocrine, and hepatic irAEs were high grade in less than 5%, compared to 11% for gastrointestinal events. In another meta-analysis including nearly 3,000 treated patients, the most frequent target organs for irAEs during PD-(L)1 inhibition were the skin (pruritus 10%, rash 11%), the gastrointestinal tract (diarrhoea 11%) and the thyroid (hypothyroidism 7%).7 The liver was involved less frequently (increased aspartate aminotransferase [AST] or alanine aminotransferase [ALT] 4%, hepatitis 1%). Overall grade 3 or 4 irAEs occurred in less than 2% of patients.
In the following sections we will describe in detail the most important irAEs, the disorders affecting the same organ that are associated with chronic liver disease, and the diagnostic clues specific to patients with HCC. We will provide specific recommendations for the management of such irAEs in patients with HCC, which largely mirror the management of irAEs in patients with other malignancies. irAEs are evaluated for severity and graded according to the National Cancer Institute’s Common Terminology Criteria for Adverse Events (CTCAE v. 5.0).21 Grading in this review will refer to these criteria unless otherwise specified.
Skin rash, inflammatory dermatitis and other cutaneous adverse reactions
Cutaneous AEs are the most common AEs reported in clinical trials of ICIs and can occur within 2 weeks of starting therapy, or as late as a year. There are a wide range of clinical manifestations of skin toxicity, but rash and pruritus are the most common. In non-HCC patients, rash occurs in 20–30% of those receiving CTLA-4 inhibitors, 10–20% those receiving PD-1 inhibitors and 30–40% of those receiving a combination of both.7,22–24 Similarly, pruritus occurs in 30–35% of those receiving CTLA-4 inhibitors, around 20% those receiving PD-1 inhibitors and 35% of those receiving a combination. Less common AEs include dry skin, erythema, alopecia and vitiligo, although the latter has mainly been reported in those treated for melanoma. Erythema multiforme, psoriasis, urticaria and rosacea occur in less than 1% and more serious and potentially life-threatening side effects such as toxic epidermal necrolysis, Stevens-Johnson syndrome and DRESS (drug rash with eosinophilia and systemic symptoms) are rare with an incidence of less than 0.1%. Skin irAEs are grade 3 or higher in less than 2% of patients.
To date, data regarding skin toxicity from ICIs in HCC is largely consistent with that seen in other tumour types (Table 1). In the dose escalation cohort of single-agent nivolumab, there was no clear dose-response relationship for rash or pruritus.2 Rash occurred in 15–30% of those receiving nivolumab, 8–10% of those receiving pembrolizumab and 17–29% of those receiving a combination of nivolumab and ipilimumab. Similarly, pruritus occurred in 20–27% of those receiving nivolumab, 12–18% of those receiving pembrolizumab and 30–45% of those receiving a combination. Skin irAEs were grade 3 or higher in less than 1% of patients with monotherapies and 4% with the combination. Data from single-agent tremelimumab is more limited but grade 1 or 2 rash was reported in 65%, and grade 3 rash in 5% of those treated within a small phase II trial. This incidence is higher than seen in other tumour types but caution is warranted in view of the small numbers of patients included.25 Among 40 patients included in the phase I trial combining durvalumab and tremelimumab, pruritus and rash were reported in 22.5% and 12.5% but none had grade 3–4 skin toxicity.26
Skin disorders associated with chronic liver disease
There is wide spectrum of cutaneous manifestations of chronic liver disease.27 Pruritus is a common (>15%) complaint in patients with HCV infection even in the absence of overt cholestasis.28 Bile salts possibly mediate pruritus by interacting with other pruritogens. Polyarteritis nodosa and cryoglobulinemia are associated with both HBV and HCV infection, while porphyria cutanea tarda has been observed in patients with hepatitis C, alcohol-related liver disease and haemochromatosis. More than 40% of patients with HCV produce cryoglobulins and 15% develop cryoglobulinemia vasculitis.28 Lichen planus is also seen in a range of chronic liver diseases but is particularly associated with HCV infection. As an autoimmune condition, primary biliary cirrhosis can occur in the context of other autoimmune conditions including Sjogren’s syndrome, CREST syndrome, morphea and lichen planus. All these conditions can cause dermatologic symptoms or lesions.
General management
In the absence of a pre-existing skin disorder, any dermatologic problem should be considered an irAE. However, when evaluating skin conditions in patients with HCC treated with ICIs, it is important to bear in mind the many cutaneous manifestations of chronic liver disease and engage with the dermatology team to ensure appropriate management. Alternative causes of skin conditions should also be considered including infection, other drug reactions, and underlying systemic disease. The interpretation of skin toxicity is becoming more challenging as combinations of PD1 inhibitors and TKIs emerge. TKIs used for HCC include sorafenib, lenvatinib, regorafenib and cabozantinib, all of which are associated with skin toxicity. However, the type of adverse effect and time course can help distinguish between ICI- and TKI-related events. For TKIs, the onset of rash is usually within weeks of starting and palmar-plantar erythema is the most common AE, reported in 52% and 27% of patients receiving sorafenib and lenvatinib, respectively.29 This compares with around 2% for PD1 inhibitors. Additionally, the relatively short half-life of TKIs results in rapid resolution of skin toxicity over the course of days, which contrasts with the weeks or months that may be required for ICI-related toxicity to resolve.
A full evaluation of the extent of cutaneous involvement and systemic effects should be documented. Topical emollients and topical mild steroids (i.e., triamcinolone 0.1%) along with antihistamines are the mainstay of treatment of mild grade 1 or 2 reactions; in such cases ICIs can be continued. For more symptomatic grade 2 or 3 reactions, a biopsy should be considered. In addition to topical therapy, oral prednisolone at a dose of 0.5 to 1 mg/kg should be initiated or, for more serious symptoms, methyl prednisolone 1–2 mg/kg should be used. ICIs can be resumed on resolution to grade 1 if appropriate. For grade 4 or potentially life threating cutaneous toxicity, ICIs should be permanently discontinued and the patient admitted under close supervision of a dermatologist. Methyl prednisolone 1–2 mg/kg should be started and weaned slowly according to response.16,14
Diarrhoea and colitis
Diarrhoea is one of the more common adverse events associated with ICIs and may occur with or without underlying colitis. Diarrhoea is defined as an increase in stool frequency above the patient’s baseline, whereas colitis is characterised by a constellation of diarrhoea, abdominal pain, and radiographic or endoscopic findings of colonic inflammation; colitis may also manifest with rectal bleeding.30 The most frequent histopathologic finding is acute colitis with crypt abscesses and frequent epithelial apoptotic bodies, while the remaining cases resemble lymphocytic colitis.31 Severe colitis may result in life-threatening colonic perforation and peritonitis.32 Diarrhoea occurs in 30–50% of patients receiving CTLA-4 inhibitors, 10–25% of those receiving PD-(L)1 inhibitors and 43% of those receiving a combination.7,22–24 Colitis occurs in 10–12% of patients receiving CTLA-4 inhibitors, 1–2% of those receiving PD-(L)1 inhibitors and 14% of those receiving a combination. Grade 3 or higher events are reported in 7–8% of patients under CTLA-4 blockade, 0.5–1.5% of those under PD-(L)1 blockade and 8–9% of those under dual blockade.
Key point.
Diarrhoea and colitis are potentially serious adverse events associated with ICIs, as well as being common symptoms in cirrhotics, so patients should be evaluated regularly for symptoms of these conditions.
The incidence of diarrhoea with anti-PD-1 agents in HCC is consistent with that reported in other tumour types. Diarrhoea occurs in 11% of patients receiving pembrolizumab, 14% of those receiving nivolumab, 12–24% of those under a combination of nivolumab and ipilimumab, and 12% of those under a combination of durvalumab and tremelimumab (Table 1). As expected, the incidence was higher with tremelimumab, with a 30% rate of grade 1 and 2 diarrhoea and a 5% rate of grade 3 diarrhoea.25 Grade 3 or higher events are reported in 1% of those under PD-(L)1 blockade and 2–4% of those under dual blockade. Similar trends are observed for the more restrictive definition of colitis, which is mostly grade 3, occurring in 1% of patients on PD-(L)1 inhibitors and 2–6% under dual CTLA-4 and PD-(L)1 blockade.
Diarrhoea associated with chronic liver disease
Patients with chronic liver disease and cirrhosis are at risk of multiple forms of intestinal dysfunction that may manifest in the form of altered bowel habits and diarrhea.33 Some of these conditions may be overlooked or mistaken for immune therapy-induced colitis. The first such condition is small intestinal bacterial overgrowth syndrome (SIBO), which is characterised by an increase in the number or alterations in the type of bacteria in the upper gastrointestinal tract. The gold standard for diagnosis is proximal jejunal aspiration revealing ≥105 bacteria (i.e. colony-forming units) per ml.34 However, hydrogen glucose breath test is preferred in clinical practice due to its lower cost, non-invasiveness and adequate sensitivity and specificity (62% and 78%, respectively). Manifestations of SIBO can mirror irritable bowel syndrome with a variety of symptoms that include abdominal bloating, abdominal pain, and diarrhoea. SIBO is present in as many as 50–60% of patients with cirrhosis.35,36 One of the main reasons for the development of SIBO in cirrhotic patients is intestinal dysmotility.37 Concurrent medical problems such as diabetes mellitus and autonomic neuropathy contribute to intestinal dysmotility and the development of SIBO.38,39
HCC-associated diarrhoea is a vague entity that is seen in clinical practice but is poorly studied. In a small cohort series, 47.8% of patients with HCC had at least 1 episode of diarrhoea in the 3 months prior to diagnosis versus 8.7% of controls.40 The diarrhoea appeared to vary significantly in severity and chronicity, but the patients with HCC and diarrhoea had worse liver function than those with HCC and no diarrhoea. In another series, diarrhoea was reported in 21% of 211 patients with HCC reviewed retrospectively.41 Diarrhoea in the setting of HCC is likely to be multifactorial. Case reports have described paraneoplastic diarrhoea related to VIP and prostaglandin production.42,43
Chronic pancreatitis may ultimately cause exocrine pancreatic dysfunction with steatorrhea.44 Clinical studies have reported a range of 6–16% for the co-incidence of chronic pancreatitis in patients with liver cirrhosis.45 Coexistence of both conditions is most likely in the setting of alcohol-related liver disease.
General management
Patients should be evaluated for symptoms of diarrhoea and colitis regularly while receiving ICIs. It is critical that a good history be obtained prior to initiation of checkpoint inhibitor therapy to establish an accurate baseline and to be able to determine whether patients diarrhoea has worsened on treatment. If patients report new onset diarrhoea or worsening of existing diarrhoea, a work-up to rule out non-immune causes should be performed and include infectious aetiologies as well as non-colitis immune-mediated toxicities such as hyperthyroidism. Furthermore, a careful review of medications that could contribute to diarrhoea should be conducted, especially for patients who have recently been started on lactulose and may need a dose adjustment.
In the absence of alternative aetiologies, the assumption should be that the patient has immune-mediated diarrhoea and/or colitis. The diagnosis is frequently made on the basis of clinical signs and symptoms, but colonoscopy remains the gold standard and is helpful for the assessment of severity, prognostication and confirmation of diagnosis.46 Endoscopic evaluation should not delay the initiation of appropriate therapy as discussed below, and is more critical in cases that do not respond to therapy, or before infliximab initiation.
For grade 1 diarrhoea (<4 stools/day over baseline), treatment continuation is reasonable with close monitoring; if the diarrhoea persists or worsens, interruption of ICIs is warranted until resolution of diarrhoea. For grade 2 diarrhoea or colitis, treatment with the ICIs should be withheld; if the symptoms persist for about 3 days or longer, oral corticosteroids should be initiated at 0.5 to 1 mg/kg. For grade 3 symptoms, hospitalisation should be considered to maintain hydration and expedite work-up; checkpoint inhibitor therapy should be withheld and corticosteroids initiated at a dose of 1–2 mg/kg, preferably intravenously. If there is no improvement within 3 days, additional immunosuppressive therapy with drugs such as infliximab is indicated. For grade 4 events, in addition to the aforementioned therapy, ICIs should be discontinued permanently. In a recently published retrospective review, the earlier introduction of selective immunosuppressive therapy such as infliximab was associated with fewer hospitalisations, less frequent steroid failure, and a shorter duration of symptoms.47
Limited data are available regarding the resumption of ICIs after an episode of immune-mediated diarrhoea and/or colitis. Guidelines indicate that ICIs can be restarted in the case of grade 1 to 3 toxicity after resolution of symptoms to grade 0–1 and once the dose of steroids is equivalent to 10 mg of prednisone or less per day. In a large multicentre retrospective review, it was noted that 30% of patients who had experienced immune-mediated diarrhoea/colitis were treated again with an ICI; upon repeat exposure to ICI therapy, 34% of patients experienced immune-mediated diarrhoea/colitis with the majority of events being grade 2 diarrhoea or grade 1 colitis. On multivariate analysis, prior treatment with anti-PD-(L)1 antibody, higher grade of diarrhoea at the initial diagnosis of immune-mediated diarrhoea/colitis, the need for immunosuppressive therapy, and longer duration of symptoms were all associated with a higher risk of recurrence of immune-mediated diarrhoea/colitis upon repeat exposure48.
Hepatitis
The term hepatitis or immune-related hepatitis is commonly used to describe any alteration in liver tests induced by ICIs. However, a histopathologic correlate is only available for the more intense grade ≥3 cases. Both the laboratory profile and the pathological features are different according to the ICI. PD-(L)1 inhibitors typically only cause elevations in aminotransferases (AST/ALT) while CTLA-4 inhibitors may also result in cholestasis with increased alkaline phosphatase, gamma-glutamyltransferase or bilirubin, and mixed patterns may be observed with combinations. The histology related to anti-CTLA-4 agents is characterised by granulomatous hepatitis, including fibrin ring granulomas and central vein endotheliitis, while PD-(L)1 inhibitors are associated with lobular hepatitis.49
Hepatitis tends to be asymptomatic even in severe cases, so regular examination of liver tests is warranted in all treated patients. It usually appears 4 to 12 weeks after initiation of therapy but may appear at later stages, too. CTLA-4 and PD-1 blockade may in exceptional circumstances cause rapidly progressing hepatic failure.50,51 In non-HCC patients, hepatitis of any grade and grade ≥3 has been reported to occur in 4–6% and 1–2%, respectively, of patients treated with CTLA-4 or PD-1 inhibitors, and in 15–20% and 5–15% of patients treated with the combination of both.7,22–24
The incidence of hepatitis is slightly higher in patients with HCC than in other tumour types. Increased AST occurred in 9–10% of patients receiving nivolumab, 9–14% of those receiving pembrolizumab, 13–20% of those receiving nivolumab plus ipilimumab and 17.5% of those receiving durvalumab plus tremelimumab. This difference can be explained at least in part by baseline alterations in liver function tests and the fact that tumour progression inside the liver may also induce changes in laboratory values that can be regarded as hepatitis. In a phase II trial with pembrolizumab, immune-related hepatitis was reported if one of the following changes in liver tests appeared and other possible aetiologies were excluded, i) AST or ALT values change from <2x the upper limit of normal (ULN) at baseline to ≥5x ULN; or from ≥2x ULN at baseline to >3x the baseline level; or reach >500 U/L regardless of baseline level; or ii) total bilirubin values change from <1.5 mg/dl at baseline to >2.0 mg/dl; or from ≥1.5 mg/dl to ≥2x the baseline level; or reach >3.0 mg/dl regardless of baseline level.3 With these strict criteria, the incidence of immune-related hepatitis was 3%. In line with this observation, nivolumab at doses 1–10 mg/kg caused hepatitis in only 1 out of 30 patients with chronic hepatitis C and no HCC.52 Hepatitis grade ≥3 occurred in less than 2% of patients under anti-PD-(L)1 agents but in up to 20% of those receiving the highest dose of ipilimumab in combination with nivolumab.53
Hepatitis associated with chronic liver disease
HCC is usually the result of a chronic liver disease caused by chronic HCV or HBV infection, alcoholic or non-alcoholic steatohepatitis, etc. Therefore, most cases of HCC develop on a background of chronic inflammation and progressive liver fibrosis.12 Additionally, patients with chronic HBC or HCV infection show T cell responses against viral antigens.54 Actually, T cell exhaustion is one of the main mechanisms resulting in chronic viral infection.55 Reinvigoration of antiviral immunity is associated with hepatitis flares in patients chronically infected with HBV and HCV.56 This is particularly true for hepatitis B and every clinical trial has excluded patients who were not under effective antiviral therapy with direct antiviral agents. In early trials with PD-1 agents, a cut-off value of <100 IU/ml of HBV-DNA was applied, while in more recent trials this cut-off value has been raised to <500 IU/ml. No cases of clinically relevant hepatitis flares have been reported. Whether patients with viral loads higher than 500 UI/ml can be treated safely is unknown. Therefore, the recommendation is not to treat under these circumstances until evidence of safety is provided. Some clinical trials have proposed that patients negative for HBsAg but positive for anti-core antibodies (anti-HBc) should be treated with antiviral agents preemptively, although it is highly unlikely that this would be needed based on the information available at this point. Unlike for HBV, patients with HCV infection have not been excluded from clinical trials based on their circulating HCV viral load. Indeed, transient decreases in HCV-RNA have been reported with nivolumab2 and significant decreases were observed with tremelimumab.25
General management
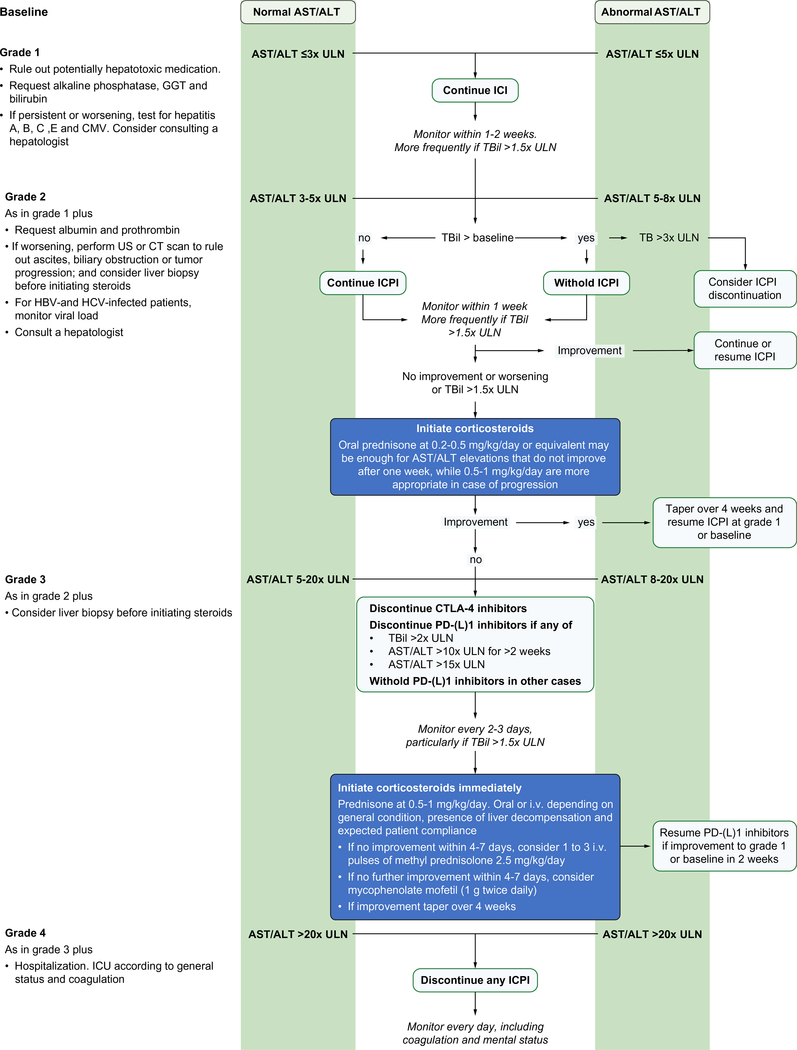
Besides being chronically inflamed, the liver is most frequently involved in tumour spread at the advanced stage because it is also the most frequent site of recurrence after resection or percutaneous ablation. Therefore, patients with HCC generally have altered liver tests that may confound the diagnosis of ICI-induced hepatitis. Exceptions are patients with low liver tumour burden and eradicated HCV infection, effectively treated HBV infection, or a healthy liver. Subsequent increases in liver tests should raise the suspicion of an irAE but may also be due to spontaneous fluctuations, tumour progression inside the liver or other causes. In fact, in controlled trials in the second line setting, the incidence of any grade increased AST among placebo-treated patients ranged from 10–20%.57–59 Therefore, timing and tumour response are important to determine causality. To avoid premature interruption of ICIs, stopping rules were adapted accordingly in clinical trials. Any physician using ICIs in patients with HCC has to be familiar with these rules and modified grading of liver AEs. A set of specific recommendations is summarised in Fig. 2.
Fig. 2. Management of hepatitis induced by ICI in patients with hepatocellular carcinoma.
ALT, alanine aminotransferase; AST, aspartate aminotransferase; CMV, cytomegalovirus; GGT, gamma glutamyltransferase; ICI, immune checkpoint inhibitor; Tbil, total bilirubin; ULN, upper limit of normal.
Key point.
The diagnosis of hepatitis can be confounded by the underlying liver disease in patients with HCC - Starting steroid therapy immediately after any elevation in aminotransferases should be avoided
Flares may occur in patients with HCV or HBV infection. In HBV infection, they are associated with an increase in viral load and HBV-DNA should be measured if moderate to severe hepatitis occurs in patients positive for HBsAg or anti-HBc. The role of pre-existing cytomegalovirus infection has not been studied but cytomegalovirus reactivation has been reported as a cause of hepatitis in patients receiving ipilimumab.60 Newly acquired viral hepatitis may also appear. Other aetiologies should be ruled out as in non-HCC patients, in particular drug toxicities.
Besides monitoring aminotransferases, it is important to measure alkaline phosphatase as well as bilirubin, albumin and prothrombin to evaluate synthetic liver function. If hepatitis becomes more severe, an ultrasound or CT scan is mandatory to rule out biliary obstruction (that may raise aminotransferases before bilirubin) and tumour progression (particularly in the form of portal or hepatic vein invasion), and to detect ascites early. Cirrhosis at any stage is associated with portal hypertension and reduced liver functional reserve. The consequences of ICI-related liver toxicities may consequently be more severe in patients with HCC. Consultation with a hepatologist is strongly recommended for any hepatitis grade 2 or higher.
Hepatitis does not always warrant immediate steroid therapy. Aminotransferases often return to baseline levels whether ICIs are continued or dosing is delayed.49,25 Repeated testing within 1 week and ruling out exposure to hepatotoxic drugs or substances is recommended for grade 1/2 elevations. The decision to start steroids can be based on progressive elevation or when elevated aminotransferase levels are associated with increased bilirubin (direct bilirubin predominantly) or liver decompensation. When aminotransferases decline or return to baseline levels, hepatitis may not necessarily recur if ICIs are reintroduced.49,25 The dose of steroids can be adjusted individually. Lower doses of oral prednisone may be enough for grade 2 to 3 elevations in aminotransferases that do not progress after 1 week, while higher doses are more appropriate in case of further increases. Intravenous pulses of corticosteroids or the addition of a second immunosuppressive drug like mycophenolate mofetil (MMF) (1 g twice daily) should be reserved for refractory cases. Anti-thymocyte globulin therapy was used in 1 case of ipilimumab-associated steroid-resistant hepatitis leading to hepatic failure, with a favourable outcome.50
Although HBV hepatitis flares have not been reported with the current restrictions to therapy based on viral load, if a viral flare is observed or suspected, the decision to start steroids, change the antiviral agent, or do both has to be taken individually after consultation with a hepatologist. If a delay in obtaining viral serologies, viral load or liver ultrasound results is expected, steroids should be started pending confirmation of moderate hepatitis, or if hepatitis is associated with liver decompensation. Whether obtaining a liver biopsy to confirm diagnosis is useful is unknown. A standardised histologic evaluation may identify hallmarks that differentiate autoimmune hepatitis from drug-induced liver injury.61 Whether this may be helpful in patients receiving ICIs is something that deserves to be explored prospectively. When steroids are given in doses equal to or higher than 1 mg/kg/day, prescribing antibiotics to prevent opportunistic infections should be considered. Infliximab is contraindicated because of potential hepatotoxicity.
Pneumonitis
Pneumonitis is a rare but potentially serious toxicity of ICIs. The clinical presentation is variable, with initial symptoms including cough, fever and shortness of breath. Acute respiratory failure may ensue rapidly. The time from ICI initiation to pneumonitis is unpredictable, ranging from 9 days to over 1 year.62,63 Over 75% of patients show mild to moderate pneumonitis. However, pneumonitis worsens in a subset of patients despite steroids and immunosuppressors, resulting in death from pneumonitis or complications of infection.62,64. In retrospective series, chronic smoking is associated with a slightly higher risk of deterioration.62 In the overall cancer population, it occurs at a rate of 2–3% with PD-(L)1 inhibitors, and the incidence increases to 7% in combination with CTLA-4 inhibitors.7,22–24 The incidence of grade ≥3 pneumonitis is below 2%. A histological correlate for the imaging signs of pneumonitis is lacking.
The incidence of pneumonitis is extremely low in patients with HCC receiving single-agent ICIs; around 1% in patients receiving nivolumab and pembrolizumab with virtually no grade 3 or higher severity (Table 1). For combinations of CTLA-4 and PD-(L)1 inhibitors, pneumonitis occurred in 10% of patients receiving nivolumab plus ipilimumab (and only in the high ipilimumab combination) and 2.5% of those receiving durvalumab plus tremelimumab.26,53
Lung disorders associated with chronic liver disease
Two syndromes with different pathophysiology may mimic symptoms of immune-mediated pneumonitis: the hepatopulmonary syndrome and porto-pulmonary hypertension.65 The former is the most common cause of respiratory insufficiency in patients with cirrhosis and is caused by intrapulmonary vascular dilatations.66 Abnormal oxygenation as defined by an alveolar-arterial oxygen gradient >15 mmHg (>20 mmHg in older patients) is the mainstay of hepatopulmonary syndrome. It should be ruled out in any patient with HCC and cirrhosis who reports dyspnoea prior to or during ICI therapy. Pulse oximetry may identify all patients with PaO2 <70 mmHg using an oxygen saturation cut-off <96%.67 Arterial blood gas analysis should follow the detection of low oxygen saturation. Orthodeoxia (oxygen desaturation while lying) and platypnoea may be additional complaints or findings.68 Spirometry is typically normal while the diffusion capacity of carbon dioxide is frequently decreased. Transthoracic contrast-enhanced echocardiography confirms the diagnosis. Lung perfusion scanning cannot differentiate intracardiac from intrapulmonary shunting. Chronic obstructive pulmonary disease, asthma, ascites, hepatic hydrothorax or more rarely idiopathic pulmonary fibrosis, can also cause or exacerbate dyspnoea in cirrhotics.
Porto-pulmonary hypertension is characterised by increased pulmonary vascular resistance in patients with portal hypertension in the absence of any other causative factor, such as mitral stenosis or left ventricular failure.65 The pathophysiology is poorly understood but genetic factors and a dysregulation of vasoactive and inflammatory mediators are likely involved.69 It is not related to the degree of portal hypertension or liver dysfunction. Increased pulmonary arterial pressure can be suspected on transthoracic echocardiography and confirmed by right heart catheterisation
General management
Early detection is the key to improving the prognosis of pneumonitis. Hence, clinicians should have a low threshold of suspicion and be ready to arrange further investigations in patients with symptoms of pneumonitis. Pulse oximetry at rest and after 1 min of walking can be useful for screening minimally symptomatic patients. Chest x-ray is usually the first investigation if pulse oximetry is not available but a high-resolution CT scan should be ordered if the suspicion is high even when x-ray results are normal. Radiological findings include ground glass opacities, organising pneumonia-like appearance and interstitial pneumonitis.62,70 An abnormal chest x-ray or CT excludes the hepatopulmonary syndrome and may diagnose concurrent chronic respiratory conditions such as emphysema or bronchiectasis. Lung function tests are not specific but may help in ruling out other disorders.
Early multidisciplinary input from respiratory and/or infectious disease specialists is recommended to help in the diagnostic work-up and to rule out infection by bronchoscopy. Additional investigations such as work-up for virus, mycoplasma or legionella may be considered in the context of clinical picture and local epidemiology. Biopsy of involved lung tissue is not necessary unless there is diagnostic doubt. With the criteria mentioned, immune pneumonitis can usually be distinguished from other disorders.
Management depends on the severity of symptoms. For asymptomatic patients with radiographic changes only (grade 1), ICIs should be delayed and patients should be monitored closely for early symptoms every 2–3 days. For grade 2 pneumonitis with mild to moderate symptoms, hospitalisation and consultation with respiratory and infectious disease physicians is indicated. Urgent investigations should be arranged but oral prednisolone at 1 mg/kg/day and antibiotics should be started immediately. If there is no improvement in the next 2–3 days, patients should be managed as per grade 3 to 4 disease (severe symptoms or evidence of hypoxia/respiratory distress). Intravenous methylprednisolone should be started promptly at 1–2 mg/kg/day, and ICU/respiratory support must be considered. The addition of further immunosuppressive therapy, including infliximab (5 mg/kg which can be repeated after 2 weeks if needed) or MMF (1 g twice daily), may be considered if there is no improvement. Re-administration of ICIs may be considered in patients recovering from grade 1–2 pneumonitis while ICIs should be stopped permanently in case of grade 3–4 pneumonitis.
Thyroiditis
Key point.
Early detection and treatment is the key to improving the prognosis of patients who experience pneumonitis – ICIs should be stopped permanently in more severe cases.
Thyroid irAEs are most often asymptomatic and may present in the form of hyper- or hypothyroidism, or a full-blown thyroiditis. Hypothyroidism preceded or not by hyperthyroidism is the most common event. Signs of hypothyroidism include fatigue, weight gain, bradycardia and slow bowel transit, while those of hyperthyroidism may comprise fatigue, nervousness, weight loss, and palpitations. Diagnosis of ICI-related thyroid dysfunction is based on thyroid hormone levels compared with pre-ICI values.71 The rate of irAEs varies according to the treatment received. The incidence of any thyroid abnormality has been reported as 39% following PD-1 inhibitor treatment, 23.0% following ipilimumab, and 50% following combination treatment for several indications.72 The incidence of hypothyroidism is 5–7% with CTLA-4 inhibitors, 7–11% with PD-(L)1 inhibitors, and 10% with combinations of both.7,22–24 At 5%, the incidence rate of immune-related thyroid dysfunction in patients with HCC is similar to that observed in patients with other tumour types. The pathogenesis of immune-mediated thyroiditis is largely unknown and anti-thyroid antibodies only develop in a minority of patients. Inflammation is frequently identified in patients with thyroid dysfunction by FDG-PET scan or Tc99m-scintigraphy.73
Thyroid disorders associated with chronic liver disease
Alterations in thyroid hormone regulation and metabolism are frequently observed in cirrhosis. Peripheral conversion of thyroxine (T4) to triiodothyronine (T3) is reduced in patients with cirrhosis, since the liver is one of the major sites of conversion.74 Therefore, cirrhotics show higher free T4 and reduced free T3 values than normal individuals, and the decrease in T3 correlates closely with the degree of liver dysfunction. On the other hand, patients with fatty liver disease have higher thyroid-stimulating hormone (TSH) levels and a higher rate of subclinical hypothyroidism than healthy controls.75
General management
The management of immune-related thyroid dysfunction is well established in patients with other tumours. In patients with HCC, the main difference relates to the complexity of establishing a clinical diagnosis when the symptoms may mimic those related to the complications of cirrhosis. Small isolated changes in thyroid hormone levels should also be considered as linked to the underlying liver disease. Signs and symptoms of hypothyroidism in cirrhotics include fatigue, myalgia, muscle cramps, or increased aminotransferases.76 Ascites and jaundice are rarely the first symptoms.
If a patient receiving ICIs is screened regularly for TSH and free T4, thyroid dysfunction can be detected when the patient is asymptomatic. When analysing the results of TSH and T4 it is important to ensure that there is no iodine saturation related to contrast medium injection. Hypothyroidism does not require ICIs to be stopped. In hyperthyroidism, ICIs should only be interrupted in patients who are unwell. When symptoms develop or hormone changes are progressive, consultation with the endocrinologist is recommended. There is no need to start specific treatment when the patient is asymptomatic. For symptomatic hypothyroidism, thyroid replacement therapy can be started at a dose of 25–50 μg. For hyperthyroidism, beta-blockers are indicated if symptomatic (e.g., atenolol 25–50 mg/day in order to maintain a heart rate below 90 bpm. Graves’ disease should be managed per standard guidelines as in patients with no liver disease under the supervision of an endocrinologist.
Hypophysitis and adrenal insufficiency
The most relevant pituitary gland and adrenal gland disorders triggered by immunotherapy are hypophysitis and primary adrenal insufficiency. Hypophysitis appears clinically with headache and fatigue and more rarely with visual changes like diplopia. More often, it is suspected in an asymptomatic patient under ICIs when laboratory tests show central hypothyroidism (a low TSH and low free T4). Central adrenal insufficiency is also observed in most patients and anterior panhypopituitarism (adrenal insufficiency, hypothyroidism and hypogonadism) occur in around 50% of patients with hypophysitis.77 Pituitary enlargement and other radiological features can usually be observed on MRI.
Hypophysitis is exceedingly rare with PD-1 inhibitors and fairly uncommon during CTLA-4 blockade.7,22–24 Incidence with ipilimumab shows a trend to dose dependency, from ≤10% at a dose of 3 mg/kg to 17% at 10 mg/kg. After dual CTLA-4 and PD-1 blockade, the incidence is similar at 13%. The median time from starting ipilimumab to diagnosis of hypophysitis is 8–9 weeks, but this complication may appear at any time during ICI therapy.
Primary adrenal dysfunction presents with fatigue, dizziness, low blood pressure and orthostatic hypotension, muscle aches, nausea or vomiting. Symptoms may develop slowly and insidiously. If left untreated, hypoglycaemia, dehydration, and disorientation may develop. The rates of adrenal dysfunction with tremelimumab, pembrolizumab and nivolumab are 0%,25 2%,3 and 1%,2 respectively. Adrenal dysfunction may occur at any time during or even after ICIs.
Immune-related pituitary and adrenal irAEs have been described at very low frequencies in patients with HCC (Table 1). Hypophysitis was not reported in nivolumab or pembrolizumab trials, while adrenal insufficiency occurred in 0.5% to 3% of patients during pembrolizumab therapy. However, the limited number of patients evaluated does not rule out the risk of developing this potential irAE. Importantly, this irAE can occur several months after discontinuing an ICI.78
Hypopituitarism and adrenal disorders associated with chronic liver disease
The dysfunction of the hypothalamic-pituitary-adrenal axis in patients with liver disease has been extensively described and worsens in parallel with the severity of cirrhosis.79 Relative adrenal insufficiency is well described80 but mainly in decompensated cirrhotics who are not candidates for immunotherapy. Data available for patients with compensated cirrhosis or advanced fibrosis is very scarce. Among patients admitted due to complications, the prevalence was comparable (20% to 30%) in Child-Pugh A, B and C81 and similar in a small group of 10 ambulatory patients. The impact of liver diseases on sex hormone production is also very well established. The prevalence of hypogonadism in cirrhotics ranges from 20% to 65%.82 Following gonadotropin-releasing hormone stimulation, the response by luteinizing hormone is only altered in Child-Pugh B and C patients.
General management
As mentioned, some symptoms of cirrhosis overlap with those of thyroid dysfunction or adrenal insufficiency. For this reason, the main recommendation in patients with HCC is to assume an active role in its early identification. Patient under ICIs can be screened regularly for TSH and free T4 (v.g. every 4–6 weeks). The finding of central hypothyroidism necessitates complete hormone testing including adrenocorticotropic hormone (ACTH), corticotropin-releasing hormone, cortisol, luteinizing hormone, follicle-stimulating hormone, testosterone, and prolactin. For the initial screening of a symptomatic patient in whom hypophysitis or adrenal insufficiency is suspected, at least serum levels of TSH, free T4, ACTH, cortisol, sodium and potassium should be measured. An ACTH stimulation test is warranted if ACTH or cortisol levels are abnormal or the suspicion is strong. If hypopituitarism is detected, a pituitary MRI is recommended. When interpreting the results of the tests, the peculiarities of the cirrhotic patients should be considered.
There are no specific recommendations for management in cirrhotic patients. For patients with very mild or no symptoms, intervention is not needed but early consultation with the endocrinologist is highly recommended. In symptomatic patients, when hypophysitis is confirmed by hormones and MRI, corticosteroids should be started (prednisone 1–2 mg/kg/day or equivalent) as well as hormone replacement (central adrenal insufficiency: hydrocortisone 100 mg i.v. as a starting dose; central hypothyroidism: levothyroxine 1 mg/kg). Patients with severe symptoms should be admitted for evaluation and work-up. Patients with suspicion of an adrenal crisis (severe dehydration, hypotension, shock) should be managed in the intensive care unit. Sepsis must be ruled out and the crisis be managed per standard guidelines.
Nephritis
Renal dysfunction during ICI therapy was initially considered a rare event occurring in <1% of patients.83 Later it became clear that the incidence of renal toxicity might be higher at 9.9–29%,84 particularly when ipilimumab and nivolumab are used in combination or sequentially.85 In a system-based review of 139 case reports or series, acute kidney injury (AKI) occurred in 16 cases (11.5%).86 In the first clinical trial of CTLA-4 blockade with tremelimumab in patients with HCC and chronic HCV infection, acute renal failure was reported in 3 out of 15 patients and was considered to be related to underlying cirrhosis, not to ICIs.25 AKI was reported in 1 out of 262 patients with HCC treated with nivolumab.2 Although the incidence of AKI appears to be lower in HCC than in other tumours, it should be pointed out that the rate of kidney-related AEs in other tumour types was initially underestimated and then increased as data became more mature. Similar underreporting is possible in patients with HCC, in whom the attribution of causality may be challenging. Underlying kidney disease of a non-immune cause is not a reason to exclude patients from ICIs, which appear to be safe even in patients with baseline renal function impairment.
Key point.
Underlying kidney disease of a non-immune cause is not a reason to exclude patients from ICIs, which appear to be safe in such patients.
AKI occurs earlier with CTLA-4 inhibitors (after 2–3 months) than with PD-1 inhibitors (after 3–10 months).84 In a series of 13 biopsy-proven cases, AKI developed 21 to 245 days after ICI start (median 91 days), median peak serum creatinine was 4.5 mg/dl (interquartile range, 3.6–7.3), and 4 patients required haemodialysis.87 Acute tubulo-interstitial nephritis was the histopathological lesion in 12 patients, sometimes with granulomatous features. Ten out of these 12 patients were treated with corticosteroids with complete or partial improvement in renal function in 2 and 7 patients, respectively. In contrast, renal function did not improve in the 2 patients not given corticosteroids. Lupus-like immune complex glomerulonephritis has been described in a patient with nephrotic syndrome and preserved renal function under treatment with ipilimumab.88
Renal disorders associated with chronic liver disease
Impaired kidney function is frequently observed during the natural course of chronic liver disease and needs to be distinguished from ICI-related toxicity.89 Viral hepatitis may produce renal disorders. Epidemiological studies have shown a relationship between HBV infection and the development of proteinuria and impaired kidney function. The presence of immune complexes in the kidney suggests an immune-complex basis for the disease90 while histology may show membranous nephropathy. Meanwhile, extrahepatic manifestations of HCV infection include various types of renal diseases. The most common are essential mixed cryoglobulinemia leading to membranoproliferative glomerulonephritis, membranoproliferative glomerulonephritis without cryoglobulinemia, and membranous glomerulonephritis.91
Disturbances in circulatory function frequently result in renal failure in cirrhotics. The presumed mechanism is increased production or activity of vasodilators triggered by portal hypertension.89 The decline in renal perfusion reduces glomerular filtration rate and sodium excretion. Diagnosis of hepatorenal failure is suggested by a progressive rise in serum creatinine, a very low rate of sodium excretion (urine sodium concentration <10 mEq/L) and oliguria. Urine sediment is often normal and there is no or minimal proteinuria. Precipitating factors include bacterial infection or gastrointestinal bleeding. Fluid depletion by large volume paracentesis or diuretics may also contribute to renal dysfunction.
General management
Any increase in creatinine levels during ICI therapy should be considered a potential irAE. However, all other causes of renal failure have to be ruled out before assuming it is an irAE. Early renal consultation is recommended and a kidney biopsy should be performed if ICI-related nephritis is considered the most likely event. The risk of post-interventional bleeding needs to be addressed in patients with poor liver function and coagulopathy. If biopsy shows podocytopathy or acute interstitial nephritis, ICIs should be discontinued and a course of corticosteroids installed. Prednisone 1 mg/kg tapered over a period of 1–2 months is a possible option. Close monitoring of serum creatinine is recommended. Resumption of ICIs can be considered once nephritis has resolved. Potentially nephrotoxic drugs such as non-steroidal anti-inflammatory drugs should always be avoided.84
Key point.
Cytopenias are common in chronic liver diseases. Toxicity from ICIs should be suspected if progressive or intense.
Neurological toxicity
Clinical manifestations of neurological toxicity include a broad spectrum that involves the central as well as peripheral nervous systems, including myasthenia gravis, peripheral neuropathy, Guillain-Barre syndrome, autonomic neuropathy, aseptic meningitis, encephalitis and transverse myelitis.92–94 The overall incidence of neurological irAEs is 3.8% and 6.1% among patients treated with CTLA-4 and PD-1 inhibitors, respectively.95 The incidence increases to 12% in patients receiving a combination of CTLA-4 and PD-1 inhibitors.95 The severity of most neurological irAEs is mild to moderate, with grade 3 or above events observed in less than 1% of patients.95 In HCC, grade 3 or higher encephalopathy occurred in 3 out of 20 patients during treatment with tremelimumab but all events were attributed to underlying cirrhosis.25 For PD-1 inhibitors, grade ≥3 events ranged from 0–1.9%.2,3
Neurological disorders associated with chronic liver disease
Signs and symptoms of central and peripheral nervous system disorders are common in patients with cirrhosis. Hepatic encephalopathy is the most frequent disorder. Its clinical expression comprises a variety of signs and symptoms including cognitive defects (from mild inattentiveness to dementia), altered conscious state (from sleepiness to coma) or impaired neuromuscular function (asterixis and hyperreflexia).96 Up to 2% of patients with cirrhosis develop chronic acquired hepatocerebral degeneration, that includes neuropsychiatric changes and movement disorders, such as tremor, dysarthria, ataxia, parkinsonism.97 Cognitive dysfunction is the rule, but it may be difficult to identify. Both entities are typically associated with large portocollateral shunting and may occur in a patient with HCC and normal liver function. Superimposed mechanisms include brain accumulation of toxic compounds such as ammonia, lactate or manganese; altered permeability of the blood-brain barrier; or inflammation due to circulating cytokines. Some other neurological complications are linked exclusively to specific disorders, like alcoholism and Wernicke encephalopathy. Finally, impaired autonomic function is often found in individuals with chronic HCV infection, even in the absence of cirrhosis.98
General management
Corresponding investigations vary according to the clinical picture. In general, it is necessary to exclude alternative aetiologies for related neurological symptoms. For example, an MRI scan of the brain is indicated to rule out brain or leptomeningeal metastases in patients with central nervous system symptoms. In patients with hepatic tumours who present with encephalopathy, detailed history on drinking and diet could help rule out differential diagnoses of Wernicke encephalopathy and porto-systemic encephalopathy, and electroencephalography may also be required to rule out subclinical seizure activity. Serum ammonia levels should also be measured to exclude hepatic encephalopathy. Other less common possibilities such as autoimmune encephalitis or paraneoplastic syndrome may also be ruled out by clarifying the point of onset of confusion or headache in relation to malignancy and immunotherapy, as well as by checking paraneoplastic autoantibodies.93,94 For patients with peripheral sensory or motor neuropathy, nerve conduction studies and/or electromyography are required for work-up of peripheral sensory or motor neuropathy. After ruling out space-occupying lesions, obtaining cerebral spinal fluid is frequently an important work-up procedure for evaluation of the protein, glucose, cell count, cytology, bacterial and herpes simplex viral infection. Autonomic neuropathy, apart from being ICI-related, could also be related to HCV infection. HCV-RNA, liver tests (especially ALT and AST) and serum cryoglobulins could help distinguish between the 2 diagnoses, and it has been reported that antiviral therapy may potentially reverse the autonomic neuropathy.98,99 In all neurological toxicities, consultation with neurologists is recommended especially when there is grade 2 or higher neurological toxicity.
In general, the threshold for withholding ICIs is low when there are symptoms suggestive of Guillain-Barre syndrome or myasthenia gravis due to potentially life-threatening respiratory compromise. In patients with mild but progressive symptoms, steroid treatment and additional measures such as intravenous immunoglobulin or plasma plasmapheresis should be considered as early as possible, when appropriate. Although steroids are generally not indicated in idiopathic Guillain-Barre syndrome, they may still be considered in addition to intravenous immunoglobulin. For encephalitis and aseptic meningitis, steroids should be administered early, with concurrent antivirals and antibiotics considered for herpes simplex virus infection and bacterial infection, respectively. Hepatic function and ammonia levels should always be monitored even in patients with established neurological diagnoses. There are currently no data to guide the resumption of ICIs following recovery from neurological toxicity. In general, for grade 3–4 neurological complications, ICIs should be discontinued permanently, and even for patients who recover from mild grade 1–2 toxicity, extreme caution should be exercised before resumption of ICIs, with the benefits and risks discussed in detail with patients.
Blood and bone marrow toxicity
Haematologic irAEs are uncommon and include haemolytic anaemia, red cell aplasia, bone marrow failure, haemophilia A, hemophagocytic lymphohistiocytosis, macrophage activation syndrome, and haematological cytopenias affecting one or more haematological cell lines.100 In addition, non-clinically relevant increases in any white blood cells (neutrophils, lymphocytes, monocytes and eosinophils) may also be observed during treatment with ICIs. Data on the incidence of haematologic irAEs in clinical trials of patients with HCC is scarce. Anaemia was reported in 4% of patients receiving pembrolizumab3 and 8% of those receiving nivolumab,2 and it was grade 3 in 2% of patients. As it occurs with other irAEs, the pathogenesis of haematologic irAEs is largely unknown, although autoantibody production may be an underlying mechanism as platelet-associated autoantibodies are frequently detected in patients with thrombocytopenia.101
Haematologic disorders associated with chronic liver disease
Cytopenias are common in chronic liver diseases. Portal hypertension may produce congestive splenomegaly with increased intrasplenic sequestration and destruction of blood cells, particularly when associated with portal vein thrombosis or tumour invasion by HCC. Alcohol can have a direct toxic effect on blood cell precursors in the bone marrow. In other cases, the mechanisms are particular to each cytopenia.
Thrombocytopenia is the most frequent disorder, occurring in almost 78% of patients with compensated cirrhosis.102 Spontaneous bleeding is very rare but a platelet count ≤60,000/μl is associated with a 5% rate of bleeding events after liver biopsy.103 Besides hypersplenism and alcohol toxicity, decreased activity of thrombopoietin and antiplatelet antibodies may contribute to the development of thrombocytopenia in chronic liver disease.104 Anaemia of diverse aetiology occurs in almost 75% of patients with chronic liver disease.105 Asymptomatic cirrhotics with gastric or oesophageal varices or portal hypertensive gastropathy may have a slow chronic loss of blood into the gut and develop anaemia due to iron deficiency, while vitamin B12 and folate deficiency due to reduced dietary intake or intestinal malabsorption emerge commonly in cirrhosis.
General management
If cytopenias are progressive or reach a clinically significant level during ICI therapy, then specific investigations should be performed to check for autoimmune causes.
Relative changes in cell count are more important than absolute levels for raising the suspicion of an ongoing irAE, while the need for specific therapies like transfusions or growth factors set the time for in-depth testing. Causal attribution is complicated by the fact that both cirrhosis and malignant disease may also cause cytopenias. A high proportion of around 40% of patients with cancer have anaemia at diagnosis and an additional 30% develop anaemia as the disease progresses.106 Different mechanisms may lead to anaemia in patients with cancer. They include impaired proliferative response of the bone marrow to erythropoietin stimulation due to overproduction of inflammatory cytokines, inappropriately low production of erythropoietin by the kidney, shortened erythrocyte survival, and impaired iron utilization.107 Extensive tumour burden and bone marrow metastasis may also cause microangiopathic haemolytic anaemia and leucoerythroblastosis.108
Key point.
ICIs must be permanently discontinued in patients that experience a cardiac complication related to ICIs.
The development or worsening of pre-existing anaemia should trigger an evaluation for common causes; a peripheral smear, reticulocyte count, ferritin, iron and transferrin saturation index, haptoglobin and Coombs test should be ordered. If occult gastrointestinal bleeding is suspected, upper endoscopy could be considered. If anaemia requires transfusion and the source cannot be identified, a bone marrow biopsy may be indicated to rule out red cell aplasia. Likewise, in the absence of a precipitating factor such as non-malignant portal vein thrombosis or progression of malignant portal vein thrombus, the development or worsening of pre-existing thrombocytopenia warrants the search for platelet-bound IgG. If platelet count falls below 60,000/μl or is associated with any bleeding event, a bone marrow biopsy may be indicated to rule out myelodysplasia or bone marrow metastasis. Any patient with an unexplained severe or rapidly progressing cytopenia should be referred to a haematologist for evaluation.
Musculoskeletal toxicity
Musculoskeletal symptoms occur in up to 40% patients and seem to be more common with PD-(L)1 inhibitors than CTLA-4 inhibitors. The most common adverse events are arthropathies, polymyalgia-like syndrome and myositis, but vasculitis and temporal arteritis have also been reported. Arthritis can present as a symmetrical polyarthritis associated with synovitis, or oligoarthritis limited to a few large joints. Reactive arthritis with conjunctivitis and uveitis has also been described. Grades 1/2 and 3 myalgia were reported in 6% and 1% of patients with HCC treated with pembrolizumab3 in a phase II trial, but no cases were reported in the phase III trial.4 The incidence of grade 1/2 arthralgia in these trials was 5% and 2.5%, respectively, and myasthenia syndrome and myositis occurred in 0.5% and 1% in the phase II trial. One of 21 patients treated with tremelimumab had grade 2 arthralgia, but no musculoskeletal events have been reported for nivolumab,2 nor to date from the combination trials of ipilimumab plus nivolumab53 or durvalumab plus tremelimumab.26 Currently, there is no evidence to suggest that patients with HCC have higher rates of musculoskeletal toxicity and if anything, the incidence is lower than expected.
Musculoskeletal disorders in chronic liver disease
Up to 50% of patients with autoimmune hepatitis have other autoimmune conditions such as rheumatoid arthritis, but this condition is generally regarded as a contraindication to ICIs. Both hepatitis B and C infection are associated with extrahepatic manifestations including arthritis, sicca syndrome and polyarteritis nodosa.109 Arthritis during acute HBV infection characteristically presents with symmetric polyarthritis and is associated with immune complexes containing the HBV antigens and antibodies in the serum and synovial fluid.110 HCV has also been linked to the development of symmetrical small joint polyarthritis although it is typically not erosive or deforming. Although 85% are positive for rheumatoid factor, anti-cyclic citrullinated peptide (CCP) is found in less than 6%.111 The incidence of HCV-related arthritis seems to have been overestimated in the past and is in the region of 1%.112 Alcohol is associated with both acute and chronic myopathy, with acute myopathy presenting in up to 2% of alcoholics.113 Acute alcohol myopathy is characterised by rhabdomyolysis and results in a symmetrical proximal polymyopathy. Elevated serum creatine kinase (CK) and myoglobin may be found. Chronic alcoholic myopathy typically manifests with progressive weakness over weeks or months and is present in up to 50% patients with cirrhosis.114
General management
Investigations should be conducted to rule out other differential diagnoses, such as rheumatoid arthritis, gout, pseudogout and septic arthritis. Blood tests including erythrocyte sedimentation rate (ESR), C reactive protein (CRP), antinuclear antibody (ANA), rheumatoid factor (RF), anti-CCP should be performed, along with X-rays of the effected joints. HLA B27 should be tested if there is spinal involvement and expert rheumatology advice sought in the presence of synovitis. Polymyalgia-like syndrome is associated with muscle pain and stiffness and needs to be distinguished from fibromyalgia and statin-induced myopathy. ESR and CRP will be elevated but CK is not significantly raised. By contrast, myositis is rare but potentially fatal; it is characterised by weakness rather than pain and is associated with markedly elevated CK. Additional investigations should include ESR, CRP, ANA, RF, anti-CCP. Troponin, electrocardiography and echocardiography should also be considered since myocarditis can occur concurrently. Electromyogram and muscle biopsy may be indicated in selected cases. To date, there is no evidence that ICIs cause reactivation of HCV or HBV. New onset arthritis in a patient with chronic HBV or HCV who is being treated with an ICI should be assumed to be drug-related in the first instance.
Grade 1 inflammatory arthritis or polymyalgia-like syndrome can be managed symptomatically with paracetamol or non-steroidal anti-inflammatory drugs, and ICI can be continued. If activities of daily living are limited by muscle pain or stiffness, or by joint inflammation (grade 2), ICI should be withheld and systemic corticosteroids should be started at a dose equivalent to 10–20 mg/day of prednisolone. Severe functional limitation or joint destruction (grade 3/4) warrants a higher dose of oral prednisolone 0.5–1 mg/kg, again with slow taper. If symptoms worsen or fail to improve, or the dose of prednisolone cannot be reduced to ≤10 mg/day after 3 months, disease-modifying antirheumatic drugs such as methotrexate, TNF-α or IL-6 inhibitors may be added.
For myositis, mild weakness without elevated CK can be managed with simple painkillers and ICI therapy can be continued. If the CK is elevated or function is limited, prednisolone should be started and ICI withheld until the CK is normal and the dose of prednisolone is less than 10 mg/day. Severe weakness that limits self-care (grade 3–4) warrants a higher dose of steroid equivalent to 1–2 mg/kg of prednisolone, and ICIs should be discontinued permanently if there is evidence of cardiac involvement. Hospital admission and referral to rheumatology or neurology may be needed and the addition of further immunosuppressive agents such as methotrexate, azathioprine and MMF may be needed if CK fails to return to normal. Plasmapharesis or intravenous immunoglobulin can be considered.
Cardiac toxicity
Cardiovascular complications from ICI include myocarditis, pericarditis, myocardial fibrosis, cardiomyopathy, heart failure, dysrhythmias and cardiac arrest. Histological examination has revealed lymphocytic infiltration of the myocardium and conduction system.115 Adverse cardiac events can be life-threatening and require early recognition and prompt intervention. Time to onset ranges from 2 to 32 weeks, with a median of 10 weeks.116 Adverse cardiac events are associated with a range of symptoms including chest pain, palpitations, breathlessness, oedema, effusions, fatigue, malaise or weakness. Cardiac complications are estimated to occur in less than 0.1% of patients but are more common in patients receiving combination (PD-1/CTLA-4) therapy (0.27%) compared with single-agent ICI (0.06%).16 To date the number of patients with HCC reported to have experienced cardiac toxicity is very low. One out of 104 patients treated with pembrolizumab3 was reported to have grade 3 cardiac failure but no other cardiac events have so far been reported among 262 patients treated with single-agent nivolumab,2 148 patients treated with combination nivolumab and ipilimumab,53 nor 40 patients treated with durvalumab and tremelimumab.26 Outside of clinical trials, there has been 1 case report of a man with HCC who developed bradychardia due to sick sinus syndrome. This was found to be secondary to immune-mediated adrenal insufficiency rather than myositis and resolved with glucocorticoid supplementation.117
Cardiac complications of chronic liver disease
Chronic liver disease is associated with a range of cardiac complications. Cirrhotic cardiomyopathy has been recognised as a distinct entity in which there is diastolic dysfunction, decreased cardiac output and prolonged QT interval which can predispose patients to dysrhythmias.118 Patients with liver disease secondary to fatty liver disease and diabetes have pre-existing risk factors for cardiac disease including coronary heart disease and ventricular failure, and high levels of alcohol intake are associated with a higher risk of atrial fibrillation.119 Hepatitis B infection has also been associated with myocarditis.120 Because of the prevalence of coincident cardiac disease in patients with chronic liver disease, it is prudent to perform baseline investigations including chest x-ray, electrocardiography and troponin, along with brain natriuretic peptide and echocardiography in selected patients.
General management
Investigations should include electrocardiography, chest x-ray, troponin, CK, brain natriuretic peptide and echocardiogram. Troponin is elevated in over 94% cases of myocarditis and a third will have electrocardiographic changes.121 Early involvement of cardiology is recommended and selected patients may require cardiac catheterisation or cardiac MRI. ICIs should be permanently discontinued in the event of an adverse cardiac event of any grade. The equivalent of 1–2 mg/kg prednisolone should be administered and the patient should be admitted for monitoring and cardiology review. In the absence of a rapid response, the dose of steroid should be escalated to 1 g per day and the addition of MMF, tacrolimus or antithymocyte globulin should be considered in the absence of a response to steroids. Standard cardiac medications may be required for management of ventricular dysfunction or dysrhythmia. Infliximab is contraindicated at high doses in patients with moderate-severe heart failure and should be avoided under these circumstances.14
Conclusions
ICIs have reshaped cancer therapy, and indications for their use are likely to continue growing. While ICIs have demonstrated clinical benefit in a number of cancers, including HCC, they are also associated with a broad range of irAEs, which can affect almost every organ. Herein, we reviewed the toxicity profile of ICIs in patients with HCC, focusing on the challenges that the underlying liver disease poses to their diagnosis and management, and providing a specific algorithm for the management of hepatic irAEs.
Supplementary Material
Acknowledgements
Our gratitude to Lucia Sangro for her help editing the manuscript.
Financial support
TM is part funded by the NIHR University College London Hospitals Biomedical Research Centre, London, UK.
Conflicts of interest
BS has received consultancy fees from Adaptimmune, Astra Zeneca, Bayer, BMS, BTG, Eli Lilly, Ipsen, Novartis, Merck, Roche, Sirtex Medical, Terumo; and research grants from BMS and Sirtex Medical. SLC has received research grants from MSD. TM has received consultancy fees from Astra Zeneca, Bayer, Beigene, BMS, BTG, Eisai, andMSD; and research grants from Bayer and BTG. MR has received consultancy fees from AstraZeneca, Bayer, BMS, Ipsen, Lilly and Roche; lecture fees from Bayer, BMS, Gilead, and Lilly; and research grants from Bayer and Ipsen. AEK has received consultancy fees from, Bayer, BMSCelgene, CytomX, Eisai, Exelixis, Novartis and Roche, and; and research grants from AstraZeneca and Astex. PG has received consultancy fees from Bayer, BMS, MSD, AstraZeneca, SIRTEX, Merck, Lilly, Adaptimmune, Eisai, Roche, Ipsen; and research grants from Bayer.
Abbreviations
- ACTH
adrenocorticotropic hormone
- AKI
acute kidney injury
- ALT
alanine aminotransferase
- ANA
antinuclear antibody
- ASH
alcoholic steatohepatitis
- AST
aspartate aminotransferase
- BMP
N-terminal pro B-type natriuretic peptide
- CCP
cyclic citrullinated peptide
- CK
creatine kinase
- CMV
cytomegalovirus
- CRP
C reactive protein
- CTLA-4
cytotoxic T-lymphocyte antigen-4
- ECG
electrocardiogram
- ESR
erythrocyte sedimentation rate
- GGT
gamma glutamyltransferase
- HbA1c
glycated haemoglobin
- HCC
hepatocellular carcinoma
- ICI
immune checkpoint inhibitors
- irAE
immune-related adverse event
- NASH
non-alcoholic steatohepatitis
- PD-1
programmed cell death-protein 1
- PD-L1
PD-1, programmed cell death 1 ligand 1
- RF
rheumatoid factor
- SIBO
small intestinal bacterial overgrowth
- T3
triiodothyronine
- T4
thyroxine
- Tbil
total bilirubin
- TKI
tyrosine-kinase inhibitors
- TSH
thyroid-stimulating hormone
- ULN
upper limit of normal
Footnotes
Please refer to the accompanying ICMJE disclosure forms for further details.
Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jhep.2019.10.021.
References
Author names in bold designate shared co-first authorship
- [1].Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer 2012;12:252–264. 10.1038/nrc3239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].El-Khoueiry AB, Sangro B, Yau T, Crocenzi TS, Kudo M, Hsu C, et al. Nivolumab in patients with advanced hepatocellular carcinoma (CheckMate 040): an open-label, non-comparative, phase 1/2 dose escalation and expansion trial. Lancet 2017;389:2492–2502. 10.1016/S0140-6736(17)31046-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Zhu AX, Finn RS, Edeline J, Cattan S, Ogasawara S, Palmer D, et al. Pembrolizumab in patients with advanced hepatocellular carcinoma previously treated with sorafenib (KEYNOTE-224): a non-randomised, open-label phase 2 trial. Lancet Oncol 2018;19:940–952. 10.1016/S1470-2045(18)30351-6. [DOI] [PubMed] [Google Scholar]
- [4].Finn RS, Ryoo BY, Merle P, Kudo M, Bouattour M, Lim HY, et al. Results of KEYNOTE-240: phase 3 study of pembrolizumab (Pembro) vs best supportive care (BSC) for second line therapy in advanced hepatocellular carcinoma (HCC). J Clin Oncol 2019;37, abstr 4004. [Google Scholar]
- [5].Yau T, Park JW, Finn RS, Cheng A-L, Mathurin P, Edeline J, et al. LBA38_PRCheckMate 459: a randomized, multi-center phase III study of nivolumab (NIVO) vs sorafenib (SOR) as first-line (1L) treatment in patients (pts) with advanced hepatocellular carcinoma (aHCC). Ann Oncol 2019. 10.1093/annonc/mdz394.029. [DOI] [Google Scholar]
- [6].Greten TF, Sangro B. Targets for immunotherapy of liver cancer. J Hepatol 2017;68:157–166. 10.1016/j.jhep.2017.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Wang P-F, Chen Y, Song S-Y, Wang T-J, Ji W-J, Li S-W, et al. Immune-related adverse events associated with anti-PD-1/PD-L1 treatment for malignancies: a meta-analysis. Front Pharmacol 2017;8 10.3389/fphar.2017.00730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Bertrand A, Kostine M, Barnetche T, Truchetet M-E, Schaeverbeke T. Immune related adverse events associated with anti-CTLA-4 antibodies: systematic review and meta-analysis. BMC Med 2015;13:211 10.1186/s12916-015-0455-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].De Velasco G, Je Y, Bossé D, Awad MM, Ott PA, Moreira RB, et al. Comprehensive meta-analysis of key immune-related adverse events from CTLA-4 and PD-1/PD-L1 inhibitors in cancer patients. Cancer Immunol Res 2017;5:312–318. 10.1158/2326-6066.CIR-16-0237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Larkin J, Chiarion-Sileni V, Gonzalez R, Grob JJ, Cowey CL, Lao CD, et al. Efficacy and safety in key patient subgroups of nivolumab (NIVO) alone or combined with ipilimumab (IPI) versus IPI alone in treatment-naïve patients with advanced melanoma (MEL) (CheckMate 067). Eur J Cancer 2016;51:S664–S665. 10.1016/s0959-8049(16)31822-6. [DOI] [Google Scholar]
- [11].Leonardi GC, Gainor JF, Altan M, Kravets S, Dahlberg SE, Gedmintas L, et al. Safety of programmed death–1 pathway inhibitors among patients with non–small-cell lung cancer and preexisting autoimmune disorders. J Clin Oncol 2018;36:1905–1912. 10.1200/JCO.2017.77.0305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Llovet JM, Zucman-Rossi J, Pikarsky E, Sangro B, Schwartz M, Sherman M, et al. Hepatocellular carcinoma. Nat Rev Dis Prim 2016;2:16018 10.1038/nrdp.2016.18. [DOI] [PubMed] [Google Scholar]
- [13].Minemura M, Tajiri K, Shimizu Y. Systemic abnormalities in liver disease. World J Gastroenterol 2009;15:2960 10.3748/wjg.15.2960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Brahmer JR, Lacchetti C, Schneider BJ, Atkins MB, Brassil KJ, Caterino JM, et al. Management of immune-related adverse events in patients treated with immune checkpoint inhibitor therapy: american society of clinical oncology clinical practice guideline. J Clin Oncol 2018;36:1714–1768. 10.1200/JCO.2017.77.6385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Puzanov I, Diab A, Abdallah K, Bingham CO, Brogdon C, Dadu R, et al. Managing toxicities associated with immune checkpoint inhibitors: consensus recommendations from the Society for Immunotherapy of Cancer (SITC) Toxicity Management Working Group. J Immunother Cancer 2017;5:95 10.1186/s40425-017-0300-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Haanen JBAG, Carbonnel F, Robert C, Kerr KM, Peters S, Larkin J, et al. Management of toxicities from immunotherapy: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-upy. Ann Oncol 2017;28, iv119–42. doi: 10.1093/annonc/mdx225. [DOI] [PubMed] [Google Scholar]
- [17].D’Amico G, Garcia-Tsao G, Pagliaro L. Natural history and prognostic indicators of survival in cirrhosis: a systematic review of 118 studies. J Hepatol 2006;44:217–231. 10.1016/j.jhep.2005.10.013. [DOI] [PubMed] [Google Scholar]
- [18].Albillos A, Lario M, Álvarez-Mon M. Cirrhosis-associated immune dysfunction: distinctive features and clinical relevance. J Hepatol 2014;61:1385–1396. 10.1016/j.jhep.2014.08.010. [DOI] [PubMed] [Google Scholar]
- [19].Kudo M, Matilla A, Santoro A, Melero I, Gracian AC, Acosta-Rivera M, et al. Checkmate-040: Nivolumab (NIVO) in patients (pts) with advanced hepatocellular carcinoma (aHCC) and Child-Pugh B (CPB) status. J Clin Oncol 2019;37 10.1200/JCO.2019.37.4_-suppl.327, 327–327. [DOI] [Google Scholar]
- [20].Simonaggio A, Michot JM, Voisin AL, Le Pavec J, Collins M, Lallart A, et al. Evaluation of readministration of immune checkpoint inhibitors after immune-related adverse events in patients with cancer. JAMA Oncol 2019. 10.1001/jamaoncol.2019.1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Cancer Institute N. Common Terminology Criteria for Adverse Events (CTCAE) Common Terminology Criteria for Adverse Events (CTCAE) v5.0. 2017. [Google Scholar]
- [22].Ribas A, Kefford R, Marshall MA, Punt CJA, Haanen JB, Marmol M, et al. Phase III randomized clinical trial comparing tremelimumab with standard-of-care chemotherapy in patients with advanced melanoma. J Clin Oncol 2013;31:616–622. 10.1200/JCO.2012.44.6112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Weber J, Mandala M, Del Vecchio M, Gogas HJ, Arance AM, Cowey CL, et al. Adjuvant nivolumab versus ipilimumab in resected stage III or IV melanoma. N Engl J Med 2017;377:1824–1835. 10.1056/NEJMoa1709030. [DOI] [PubMed] [Google Scholar]
- [24].Hassel JC, Heinzerling L, Aberle J, Bähr O, Eigentler TK, Grimm M-O, et al. Combined immune checkpoint blockade (anti-PD-1/anti-CTLA-4): evaluation and management of adverse drug reactions. Cancer Treat Rev 2017;57:36–49. 10.1016/j.ctrv.2017.05.003. [DOI] [PubMed] [Google Scholar]
- [25].Sangro B, Gomez-Martin C, De La Mata M, Iñarrairaegui M, Garralda E, Barrera P, et al. A clinical trial of CTLA-4 blockade with tremelimumab in patients with hepatocellular carcinoma and chronic hepatitis C. J Hepatol 2013;59:81–88. 10.1016/j.jhep.2013.02.022. [DOI] [PubMed] [Google Scholar]
- [26].Kelley RK, Abou-Alfa GK, Bendell JC, Kim T-Y, Borad MJ, Yong W-P, et al. Phase I/II study of durvalumab and tremelimumab in patients with unresectable hepatocellular carcinoma (HCC): Phase I safety and efficacy analyses. J Clin Oncol 2017;35 10.1200/JCO.2017.35.15_suppl.4073, 4073–4073. [DOI] [Google Scholar]
- [27].Dogra S, Jindal R. Cutaneous manifestations of common liver diseases. J Clin Exp Hepatol 2011;1:177–184. 10.1016/S0973-6883(11)60235-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Cacoub P, Poynard T, Ghillani P, Charlotte F, Olivi M, Charles Piette J, et al. Extrahepatic manifestations of chronic hepatitis C. Arthritis Rheum 1999;42:2204–2212, doi:. [DOI] [PubMed] [Google Scholar]
- [29].Kudo M, Finn RS, Qin S, Han K-H, Ikeda K, Piscaglia F, et al. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: a randomised phase 3 non-inferiority trial. Lancet 2018;391:1163–1173. 10.1016/S0140-6736(18)30207-1. [DOI] [PubMed] [Google Scholar]
- [30].Postow MA. Managing immune checkpoint-blocking antibody side effects. Am Soc Clin Oncol Educ B 2015. 10.14694/edbook_am.2015.35.76. [DOI] [PubMed] [Google Scholar]
- [31].Chen JH, Pezhouh MK, Lauwers GY, Masia R. Histopathologic features of colitis due to immunotherapy with anti-PD-1 antibodies. Am J Surg Pathol 2017;41:643–654. 10.1097/PAS.0000000000000829. [DOI] [PubMed] [Google Scholar]
- [32].Mitchell KA, Kluger H, Sznol M, Hartman DJ. Ipilimumab-induced perforating colitis. J Clin Gastroenterol 2013;47:781–785. 10.1097/MCG.0b013e31828f1d51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Fukui H, Wiest R. Changes of intestinal functions in liver cirrhosis. Inflamm Intest Dis 2016;1:24–40. 10.1159/000444436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Khoshini R, Dai S-C, Lezcano S, Pimentel M. A systematic review of diagnostic tests for small intestinal bacterial overgrowth. Dig Dis Sci 2008;53:1443–1454. 10.1007/s10620-007-0065-1. [DOI] [PubMed] [Google Scholar]
- [35].Bauer TM, Steinbruckner B, Brinkmann FE, Ditzen AK, Schwacha H, Aponte JJ, et al. Small intestinal bacterial overgrowth in patients with cirrhosis: prevalence and relation with spontaneous bacterial peritonitis. Am J Gastroenterol 2001;96:2962–2967. 10.1111/j.1572-0241.2001.04668.x. [DOI] [PubMed] [Google Scholar]
- [36].Pande C, Kumar A, Sarin SK. Small-intestinal bacterial overgrowth in cirrhosis is related to the severity of liver disease. Aliment Pharmacol Ther 2009;29:1273–1281. 10.1111/j.1365-2036.2009.03994.x. [DOI] [PubMed] [Google Scholar]
- [37].Chesta J, Defilippi C, Defilippi C. Abnormalities in proximal small bowel motility in patients with cirrhosis. Hepatology 1993;17:828–832. [PubMed] [Google Scholar]
- [38].Dooley CP, el Newihi HM, Zeidler A, Valenzuela JE. Abnormalities of the migrating motor complex in diabetics with autonomic neuropathy and diarrhea. Scand J Gastroenterol 1988;23:217–223. [DOI] [PubMed] [Google Scholar]
- [39].Chaudhry V, Corse AM, O’Brian R, Cornblath DR, Klein AS, Thuluvath PJ. Autonomic and peripheral (sensorimotor) neuropathy in chronic liver disease: a clinical and electrophysiologic study. Hepatology 1999;29:1698–1703. 10.1002/hep.510290630. [DOI] [PubMed] [Google Scholar]
- [40].Bruix J, Castells A, Calvet X, Feu F, Bru C, Sola M, et al. Diarrhea as a presenting symptom of hepatocellular carcinoma. Dig Dis Sci 1990;35:681–685. 10.1007/BF01540166. [DOI] [PubMed] [Google Scholar]
- [41].Lai CL, Lam KC, Wong KP, Wu PC, Todd D. Clinical features of hepatocellular carcinoma: review of 211 patients in Hong Kong. Cancer 1981;47:2746–2755, doi:. [DOI] [PubMed] [Google Scholar]
- [42].Steiner E Hepatocellular carcinoma presenting with intractable diarrhea. Arch Surg 1986;121:849 10.1001/archsurg.1986.01400070119025. [DOI] [PubMed] [Google Scholar]
- [43].Solinas A Hepatocellular carcinoma and the watery diarrhea syndrome. Arch Surg 1988;123:124 10.1001/archsurg.1988.01400250134033. [DOI] [PubMed] [Google Scholar]
- [44].Majumder S, Chari ST. Chronic pancreatitis. Lancet 2016;387:1957–1966. 10.1016/S0140-6736(16)00097-0. [DOI] [PubMed] [Google Scholar]
- [45].Aghdassi AA, Schneider A, Kahl M, Schütte K, Kuliaviene I, Salacone P, et al. Analysis of lifestyle factors in patients with concomitant chronic pancreatitis and liver cirrhosis. Pancreatology 2017;17:698–705. 10.1016/j.pan.2017.07.194. [DOI] [PubMed] [Google Scholar]
- [46].Prieux-Klotz C, Dior M, Damotte D, Dreanic J, Brieau B, Brezault C, et al. Immune checkpoint inhibitor-induced colitis: diagnosis and management. Target Oncol 2017;12:301–308. 10.1007/s11523-017-0495-4. [DOI] [PubMed] [Google Scholar]
- [47].Abu-Sbeih H, Ali FS, Wang X, Mallepally N, Chen E, Altan M, et al. Early introduction of selective immunosuppressive therapy associated with favorable clinical outcomes in patients with immune checkpoint inhibitor-induced colitis. J Immunother Cancer 2019;7:93 10.1186/s40425-019-0577-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Abu-Sbeih H, Ali FS, Naqash AR, Owen DH, Patel S, Otterson GA, et al. Resumption of immune checkpoint inhibitor therapy after immune-mediated colitis. J Clin Oncol 2019;JCO1900320 10.1200/JCO.19.00320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].De Martin E, Michot J-M, Papouin B, Champiat S, Mateus C, Lambotte O, et al. Characterization of liver injury induced by cancer immunotherapy using immune checkpoint inhibitors. J Hepatol 2018;68:1181–1190. 10.1016/j.jhep.2018.01.033. [DOI] [PubMed] [Google Scholar]
- [50].Chmiel KD, Suan D, Liddle C, Nankivell B, Ibrahim R, Bautista C, et al. Resolution of severe ipilimumab-induced hepatitis after antithymocyte globulin therapy. J Clin Oncol 2011;29:e237–e240. 10.1200/JCO.2010.32.2206. [DOI] [PubMed] [Google Scholar]
- [51].Wu Z, Lai L, Li M, Zhang L, Zhang W. Acute liver failure caused by pembrolizumab in a patient with pulmonary metastatic liver cancer: a case report. Medicine (Baltimore) 2017;96 10.1097/MD.0000000000009431, e9431–e9431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Gardiner D, Lalezari J, Lawitz E, DiMicco M, Ghalib R, Reddy KR, et al. A randomized, double-blind, placebo-controlled assessment of BMS-936558, a fully human monoclonal antibody to programmed death-1 (PD-1), in patients with chronic hepatitis C virus infection. PLoS One 2013;8 10.1371/journal.pone.0063818, e63818–e63818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Yau T, Kang YK, TY K, El-Khoueiry AB, A S, Sangro B, et al. Nivolumab (NIVO) + ipilimumab (IPI) combination therapy in patients (pts) with advanced hepatocellular carcinoma (aHCC): results from CheckMate 040. J Clin Oncol 2019;37:abstr 4012. [Google Scholar]
- [54].Flecken T, Schmidt N, Hild S, Gostick E, Drognitz O, Zeiser R, et al. Immunodominance and functional alterations of tumor-associated antigen-specific CD8 + T-cell responses in hepatocellular carcinoma. Hepatology 2014;59:1415–1426. 10.1002/hep.26731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Boni C, Fisicaro P, Valdatta C, Amadei B, Di Vincenzo P, Giuberti T, et al. Characterization of hepatitis B virus (HBV)-specific T-cell dysfunction in chronic HBV infection. J Virol 2007;81:4215–4225. 10.1128/JVI.02844-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Tsai SL, Chen PJ, Lai MY, Yang PM, Sung JL, Huang JH, et al. Acute exacerbations of chronic type B hepatitis are accompanied by increased T cell responses to hepatitis B core and e antigens. Implications for hepatitis B e antigen seroconversion. J Clin Invest 1992;89:87–96. 10.1172/JCI115590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Bruix J, Qin S, Merle P, Granito A, Huang Y-H, Bodoky G, et al. Regorafenib for patients with hepatocellular carcinoma who progressed on sorafenib treatment (RESORCE): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 2017;389:56–66. 10.1016/S0140-6736(16)32453-9. [DOI] [PubMed] [Google Scholar]
- [58].Abou-Alfa GK, Meyer T, Cheng A-L, El-Khoueiry AB, Rimassa L, Ryoo B-Y, et al. Cabozantinib in patients with advanced and progressing hepatocellular carcinoma. N Engl J Med 2018;379:54–63. 10.1056/NEJMoa1717002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Zhu AX, Park JO, Ryoo B-Y, Yen C-J, Poon R, Pastorelli D, et al. Ramucirumab versus placebo as second-line treatment in patients with advanced hepatocellular carcinoma following first-line therapy with sorafenib (REACH): a randomised, double-blind, multicentre, phase 3 trial. Lancet Oncol 2015;16:859–870. 10.1016/S1470-2045(15)00050-9. [DOI] [PubMed] [Google Scholar]
- [60].Uslu U, Agaimy A, Hundorfean G, Harrer T, Schuler G, Heinzerling L. Autoimmune colitis and subsequent CMV-induced hepatitis after treatment with ipilimumab. J Immunother 2015;38:212–215. 10.1097/CJI.0000000000000081. [DOI] [PubMed] [Google Scholar]
- [61].Suzuki A, Brunt EM, Kleiner DE, Miquel R, Smyrk TC, Andrade RJ, et al. The use of liver biopsy evaluation in discrimination of idiopathic autoimmune hepatitis versus drug-induced liver injury. Hepatology 2011;54:931–939. 10.1002/hep.24481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Naidoo J, Wang X, Woo KM, Iyriboz T, Halpenny D, Cunningham J, et al. Pneumonitis in patients treated with anti-programmed death-1/programmed death ligand 1 therapy. J Clin Oncol 2017;35:709–717. 10.1200/JCO.2016.68.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Chuzi S, Tavora F, Cruz M, Costa R, Chae YK, Carneiro BA, et al. Clinical features, diagnostic challenges, and management strategies in checkpoint inhibitor-related pneumonitis. Cancer Manag Res 2017;9:207–213. 10.2147/CMAR.S136818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Friedman CF, Proverbs-Singh TA, Postow MA. Treatment of the immune-related adverse effects of immune checkpoint inhibitors. JAMA Oncol 2016;2:1346 10.1001/jamaoncol.2016.1051. [DOI] [PubMed] [Google Scholar]
- [65].Porres-Aguilar M, Altamirano JT, Torre-Delgadillo A, Charlton MR, Duarte-Rojo A. Portopulmonary hypertension and hepatopulmonary syndrome: a clinician-oriented overview. Eur Respir Rev 2012;21:223–233. 10.1183/09059180.00007211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Rodríguez-Roisin R, Krowka MJ. Hepatopulmonary syndrome–a liver-induced lung vascular disorder. N Engl J Med 2008;358:2378–2387. 10.1056/NEJMra0707185. [DOI] [PubMed] [Google Scholar]
- [67].Arguedas MR, Singh H, Faulk DK, Fallon MB. Utility of pulse oximetry screening for hepatopulmonary syndrome. Clin Gastroenterol Hepatol 2007;5 10.1016/j.cgh.2006.12.003, 749–754.e1. [DOI] [PubMed] [Google Scholar]
- [68].Gómez FP, Martínez-Pallí G, Barberà JA, Roca J, Navasa M, Rodríguez-Roisin R. Gas exchange mechanism of orthodeoxia in hepatopulmonary syndrome. Hepatology 2004;40:660–666. 10.1002/hep.20358. [DOI] [PubMed] [Google Scholar]
- [69].Liberal R, Grant CR, Baptista R, Macedo G. Porto-pulmonary hypertension: a comprehensive review. Clin Res Hepatol Gastroenterol 2015;39:157–167. 10.1016/j.clinre.2014.12.011. [DOI] [PubMed] [Google Scholar]
- [70].Tirumani SH, Ramaiya NH, Keraliya A, Bailey ND, Ott PA, Hodi FS, et al. Radiographic profiling of immune-related adverse events in advanced melanoma patients treated with ipilimumab. Cancer Immunol Res 2015;3:1185–1192. 10.1158/2326-6066.CIR-15-0102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Illouz F, Drui D, Caron P, Do Cao C. Expert opinion on thyroid complications in immunotherapy. Ann Endocrinol (Paris) 2018;79:555–561. 10.1016/j.ando.2018.07.007. [DOI] [PubMed] [Google Scholar]
- [72].Morganstein DL, Lai Z, Spain L, Diem S, Levine D, Mace C, et al. Thyroid abnormalities following the use of cytotoxic T-lymphocyte antigen-4 and programmed death receptor protein-1 inhibitors in the treatment of melanoma. Clin Endocrinol (Oxf) 2017;86:614–620. 10.1111/cen.13297. [DOI] [PubMed] [Google Scholar]
- [73].de Filette J, Jansen Y, Schreuer M, Everaert H, Velkeniers B, Neyns B, et al. Incidence of thyroid-related adverse events in melanoma patients treated with pembrolizumab. J Clin Endocrinol Metab 2016;101:4431–4439. 10.1210/jc.2016-2300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Nomura S, Pittman CS, Chambers JB, Buck MW, Shimizu T. Reduced peripheral conversion of thyroxine to triiodothyronine in patients with hepatic cirrhosis. J Clin Invest 1975;56:643–652. 10.1172/JCI108134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Guo Z, Li M, Han B, Qi X. Association of non-alcoholic fatty liver disease with thyroid function: A systematic review and meta-analysis. Dig Liver Dis 2018;50:1153–1162. 10.1016/j.dld.2018.08.012. [DOI] [PubMed] [Google Scholar]
- [76].Huang MJ, Liaw YF. Clinical associations between thyroid and liver diseases. J Gastroenterol Hepatol 1995;10:344–350. 10.1111/j.1440-1746.1995.tb01106.x. [DOI] [PubMed] [Google Scholar]
- [77].Faje A Immunotherapy and hypophysitis: clinical presentation, treatment, and biologic insights. Pituitary 2016;19:82–92. 10.1007/s11102-015-0671-4. [DOI] [PubMed] [Google Scholar]
- [78].Ohara N, Kobayashi M, Ohashi K, Ito R, Ikeda Y, Kawaguchi G, et al. Isolated adrenocorticotropic hormone deficiency and thyroiditis associated with nivolumab therapy in a patient with advanced lung adenocarcinoma: a case report and review of the literature. J Med Case Rep 2019;13:88 10.1186/s13256-019-2002-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Zietz B, Lock G, Plach B, Drobnik W, Grossmann J, Scholmerich J, et al. Dysfunction of the hypothalamic-pituitary-glandular axes and relation to Child-Pugh classification in male patients with alcoholic and virus-related cirrhosis. Eur J Gastroenterol Hepatol 2003;15:495–501. 10.1097/01.meg.0000059115.41030.e0. [DOI] [PubMed] [Google Scholar]
- [80].Karagiannis AK. Adrenal insufficiency in patients with decompensated cirrhosis. World J Hepatol 2015;7:1112–1124. 10.4254/wjh.v7.i8.1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Acevedo J, Fernández J, Prado V, Silva A, Castro M, Pavesi M, et al. Relative adrenal insufficiency in decompensated cirrhosis: relationship to short-term risk of severe sepsis, hepatorenal syndrome, and death. Hepatology 2013;58:1757–1765. 10.1002/hep.26535. [DOI] [PubMed] [Google Scholar]
- [82].Kumar KVSH, Pawah AK, Manrai M. Occult endocrine dysfunction in patients with cirrhosis of liver. J Fam Med Prim Care n.d.;5:576–80. doi: 10.4103/2249-4863.197293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Hofmann L, Forschner A, Loquai C, Goldinger SM, Zimmer L, Ugurel S, et al. Cutaneous, gastrointestinal, hepatic, endocrine, and renal side-effects of anti-PD-1 therapy. Eur J Cancer 2016;60:190–209. 10.1016/j.ejca.2016.02.025. [DOI] [PubMed] [Google Scholar]
- [84].Wanchoo R, Karam S, Uppal NN, Barta VS, Deray G, Devoe C, et al. Adverse renal effects of immune checkpoint inhibitors: a narrative review. Am J Nephrol 2017;45:160–169. 10.1159/000455014. [DOI] [PubMed] [Google Scholar]
- [85].Murakami N, Motwani S, Riella LV. Renal complications of immune checkpoint blockade. Curr Probl Cancer n.d.;41:100–10. doi: 10.1016/j.currproblcancer.2016.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Bajwa R, Cheema A, Khan T, Amirpour A, Paul A, Chaughtai S, et al. Adverse effects of immune checkpoint inhibitors (Programmed death-1 inhibitors and cytotoxic T-lymphocyte-associated protein-4 Inhibitors): results of a retrospective study. J Clin Med Res 2019;11:225–236. 10.14740/jocmr3750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Cortazar FB, Marrone KA, Troxell ML, Ralto KM, Hoenig MP, Brahmer JR, et al. Clinicopathological features of acute kidney injury associated with immune checkpoint inhibitors. Kidney Int 2016;90:638–647. 10.1016/j.kint.2016.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Fadel F, El Karoui K, Knebelmann B. Anti-CTLA4 antibody-induced lupus nephritis. N Engl J Med 2009;361:211–212. 10.1056/NEJMc0904283. [DOI] [PubMed] [Google Scholar]
- [89].Ginès P, Schrier RW. Renal failure in cirrhosis. N Engl J Med 2009;361:1279–1290. 10.1056/NEJMra0809139. [DOI] [PubMed] [Google Scholar]
- [90].Elewa U, Sandri AM, Kim WR, Fervenza FC. Treatment of hepatitis B virus-associated nephropathy. Nephron Clin Pract 2011;119, c41–9; discussion c49. doi: 10.1159/000324652. [DOI] [PubMed] [Google Scholar]
- [91].Latt N, Alachkar N, Gurakar A. Hepatitis C virus and its renal manifestations: a review and update. Gastroenterol Hepatol (N Y) 2012;8:434–445. [PMC free article] [PubMed] [Google Scholar]
- [92].Spain L, Walls G, Julve M, O’Meara K, Schmid T, Kalaitzaki E, et al. Neurotoxicity from immune-checkpoint inhibition in the treatment of melanoma: a single centre experience and review of the literature. Ann Oncol 2017;28:377–385. 10.1093/annonc/mdw558. [DOI] [PubMed] [Google Scholar]
- [93].Williams TJ, Benavides DR, Patrice K-A, Dalmau JO, de Ávila ALR, Le DT, et al. Association of autoimmune encephalitis with combined immune checkpoint inhibitor treatment for metastatic cancer. JAMA Neurol 2016;73:928–933. 10.1001/jamaneurol.2016.1399. [DOI] [PubMed] [Google Scholar]
- [94].Feng S, Coward J, McCaffrey E, Coucher J, Kalokerinos P, O’Byrne K. Pembrolizumab-induced encephalopathy: a review of neurological toxicities with immune checkpoint inhibitors. J Thorac Oncol 2017;12:1626–1635. 10.1016/j.jtho.2017.08.007. [DOI] [PubMed] [Google Scholar]
- [95].Cuzzubbo S, Javeri F, Tissier M, Roumi A, Barlog C, Doridam J, et al. Neurological adverse events associated with immune checkpoint inhibitors: review of the literature. Eur J Cancer 2017;73:1–8. 10.1016/j.ejca.2016.12.001. [DOI] [PubMed] [Google Scholar]
- [96].American Association for the Study of Liver Diseases, European Association for the Study of the Liver. Hepatic encephalopathy in chronic liver disease: 2014 practice guideline by the European Association for the Study of the Liver and the American Association for the Study of Liver Diseases. J Hepatol 2014;61:642–59. doi: 10.1016/j.jhep.2014.05.042. [DOI] [PubMed] [Google Scholar]
- [97].Stracciari A, Mattarozzi K, D’Alessandro R, Baldin E, Guarino M. Cognitive functioning in chronic acquired hepatocerebral degeneration. Metab Brain Dis 2008;23:155–160. 10.1007/s11011-008-9088-3. [DOI] [PubMed] [Google Scholar]
- [98].Osztovits J, Horvath T, Abonyi M, Tóth T, Visnyei Z, Bekö G, et al. Chronic hepatitis C virus infection associated with autonomic dysfunction. Liver Int 2009;29:1473–1478. 10.1111/j.1478-3231.2009.02075.x. [DOI] [PubMed] [Google Scholar]
- [99].Osztovits J, Horvath E, Tax J, Csihi L, Horvath T, Littvay L, et al. Reversible autonomic dysfunction during antiviral treatment in patients with chronic hepatitis C virus infection: anti-HCV therapy and autonomic function. Hepat Mon 2011;11:114–118. [PMC free article] [PubMed] [Google Scholar]
- [100].Calvo R Hematological side effects of immune checkpoint inhibitors: the example of immune-related thrombocytopenia. Front Pharmacol 2019;10:454 10.3389/fphar.2019.00454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Jotatsu T, Oda K, Yamaguchi Y, Noguchi S, Kawanami T, Kido T, et al. Immune-mediated thrombocytopenia and hypothyroidism in a lung cancer patient treated with nivolumab. Immunotherapy 2018;10:85–91. 10.2217/imt-2017-0100. [DOI] [PubMed] [Google Scholar]
- [102].Qamar AA, Grace ND, Groszmann RJ, Garcia-Tsao G, Bosch J, Burroughs AK, et al. Incidence, prevalence, and clinical significance of abnormal hematologic indices in compensated cirrhosis. Clin Gastroenterol Hepatol 2009;7:689–695. 10.1016/j.cgh.2009.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Seeff LB, Everson GT, Morgan TR, Curto TM, Lee WM, Ghany MG, et al. Complication rate of percutaneous liver biopsies among persons with advanced chronic liver disease in the HALT-C trial. Clin Gastroenterol Hepatol 2010;8:877–883. 10.1016/j.cgh.2010.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Peck-Radosavljevic M Thrombocytopenia in chronic liver disease. Liver Int 2017;37:778–793. 10.1111/liv.13317. [DOI] [PubMed] [Google Scholar]
- [105].Gonzalez-Casas R, Jones EA, Moreno-Otero R. Spectrum of anemia associated with chronic liver disease. World J Gastroenterol 2009;15:4653–4658. 10.3748/wjg.15.4653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Ludwig H, Van Belle S, Barrett-Lee P, Birgegård G, Bokemeyer C, Gascón P, et al. The European Cancer Anaemia Survey (ECAS): a large, multinational, prospective survey defining the prevalence, incidence, and treatment of anaemia in cancer patients. Eur J Cancer 2004;40:2293–2306. 10.1016/j.ejca.2004.06.019. [DOI] [PubMed] [Google Scholar]
- [107].Ludwig H, Fritz E. Anemia in cancer patients. Semin Oncol 1998;25:2–6. [PubMed] [Google Scholar]
- [108].Delsol G, Guiu-Godfrin B, Guiu M, Pris J, Corberand J, Fabre J. Leukoerythroblastosis and cancer frequency, prognosis, and physiopathologic significance. Cancer 1979;44:1009–1013, doi:. [DOI] [PubMed] [Google Scholar]
- [109].Cheah JT, Faragon JJ, Marks KM. Management of hepatitis B and C infections in rheumatologic disease. Best Pract Res Clin Rheumatol 2018;32:848–868. 10.1016/j.berh.2019.04.001. [DOI] [PubMed] [Google Scholar]
- [110].Wands JR, Mann E, Alpert E, Isselbacher KJ. The pathogenesis of arthritis associated with acute hepatitis-B surface antigen-positive hepatitis. Complement activation and characterization of circulating immune complexes. J Clin Invest 1975;55:930–936. 10.1172/JCI108022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Sene D, Ghillani-Dalbin P, Limal N, Thibault V, van Boekel T, Piette J-C, et al. Anti-cyclic citrullinated peptide antibodies in hepatitis C virus associated rheumatological manifestations and Sjogren’s syndrome. Ann Rheum Dis 2006;65:394–397. 10.1136/ard.2005.038042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112].Younossi Z, Park H, Henry L, Adeyemi A, Stepanova M. Extrahepatic manifestations of hepatitis C: a meta-analysis of prevalence, quality of life, and economic burden. Gastroenterology 2016;150:1599–1608. 10.1053/j.gastro.2016.02.039. [DOI] [PubMed] [Google Scholar]
- [113].Preedy VR, Ohlendieck K, Adachi J, Koll M, Sneddon A, Hunter R, et al. The importance of alcohol-induced muscle disease. J Muscle Res Cell Motil 2003;24:55–63. [DOI] [PubMed] [Google Scholar]
- [114].Urbano-Marquez A, Fernandez-Sola J. Effects of alcohol on skeletal and cardiac muscle. Muscle Nerve 2004;30:689–707. 10.1002/mus.20168. [DOI] [PubMed] [Google Scholar]
- [115].Johnson DB, Balko JM, Compton ML, Chalkias S, Gorham J, Xu Y, et al. Fulminant myocarditis with combination immune checkpoint blockade. N Engl J Med 2016;375:1749–1755. 10.1056/NEJMoa1609214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [116].Jain V, Bahia J, Mohebtash M, Barac A. Cardiovascular complications associated with novel cancer immunotherapies. Curr Treat Options Cardiovasc Med 2017;19:36 10.1007/s11936-017-0532-8. [DOI] [PubMed] [Google Scholar]
- [117].Hsu C-Y, Su Y-W, Chen S-C. Sick sinus syndrome associated with anti-programmed cell death-1. J Immunother Cancer 2018;6:72 10.1186/s40425-018-0388-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118].Ruiz-del-Arbol L, Serradilla R. Cirrhotic cardiomyopathy. World J Gastroenterol 2015;21:11502–11521. 10.3748/wjg.v21.i41.11502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [119].Gallagher C, Hendriks JML, Elliott AD, Wong CX, Rangnekar G, Middeldorp ME, et al. Alcohol and incident atrial fibrillation – a systematic review and meta-analysis. Int J Cardiol 2017;246:46–52. 10.1016/j.ijcard.2017.05.133. [DOI] [PubMed] [Google Scholar]
- [120].Ursell PC, Habib A, Sharma P, Mesa-Tejada R, Lefkowitch JH, Fenoglio JJJ. Hepatitis B virus and myocarditis. Hum Pathol 1984;15:481–484. 10.1016/s0046-8177(84)80085-4. [DOI] [PubMed] [Google Scholar]
- [121].Pradhan R, Nautiyal A, Singh S. Diagnosis of immune checkpoint inhibitor-associated myocarditis: a systematic review. Int J Cardiol 2019. 10.1016/j.ijcard.2019.07.025. [DOI] [PubMed] [Google Scholar]
- [122].Duffy AG, Ulahannan SV, Makorova-Rusher O, Rahma O, Wedemeyer H, Pratt D, et al. Tremelimumab in combination with ablation in patients with advanced hepatocellular carcinoma. J Hepatol 2017;66:545–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [123].Sangro B, Melero I, Yau T, Hsu C, Kudo M, Kim TY, et al. Nivolumab in sorafenib-naive and -experienced patients with advanced hepatocellular carcinoma: survival, hepatic safety, and biomarker assessments in CheckMate-040. Hepatology A 2017;66:82. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.