Abstract
Purpose
The present study aimed to evaluate the long-term results of definitive chemoradiotherapy (CRT) for unresectable locally advanced esophageal squamous cell carcinoma (LA-ESCC).
Materials and methods
We analyzed eighty patients with unresectable LA-ESCC, who underwent definitive CRT between 2001 and 2014. The 5-year overall survival (OS), cause-specific survival (CSS), and progression-free survival (PFS) rates were calculated, and we investigated the prognostic factors and adverse events.
Results
The median age was 66 years (range, 41–83 years). Histologically, all patients had squamous cell carcinoma. The most common tumor site was the middle thoracic esophagus in 43 (54%) patients. According to the eighth edition of the Union for International Cancer Control TNM classification, sixty-six patients (83%) had T4 disease, 59 (74%) had regional lymph node (LN) metastases, and 35 (44%) had distant LN metastases beyond the regional LN (M1 LYM) disease. Forty-five (56%) and 35 (44%) patients belong to clinical stages IVA and IVB, respectively. The median follow-up period for survivors was 86 months. The 5-year OS, CSS, and PFS rates were 20.2%, 25.7%, and 18.4%, respectively. On univariate analysis, only the performance status score was significantly associated with better overall survival (p = 0.026). Grade 3 or higher late adverse events were observed in 12 (15%) patients, and these included cardiopulmonary adverse events in 6 (8%) patients. Treatment-related death occurred in 3 (4%) patients.
Conclusion
We showed the long-term results of definitive CRT for unresectable LA-ESCC. The survivals are still poor and new treatment strategies need to be developed.
Keywords: unresectable locally advanced esophageal cancer, chemoradiation therapy, the long-term results, squamous cell carcinoma
INTRODUCTION
Over the years, many clinical trials have been conducted with an aim to improve the treatment outcomes in patients with resectable locally advanced esophageal squamous cell carcinoma (LA-ESCC) [1–6]. As a result, neoadjuvant chemotherapy or neoadjuvant chemoradiotherapy (CRT) followed by esophagectomy is currently the standard of care for resectable LA-ESCC [1–3]. The efficacy of definitive CRT as a nonsurgical treatment for resectable LA-ESCC has been demonstrated [4–6], and it has become the standard of care for medically inoperable patients and those who refuse surgery.
Unresectable LA-ESCC has been defined as T4 disease and/or lymph node (LN) metastases beyond the regional LN, such as supraclavicular LN or abdominal LN (M1 LYM) in several prospective trials [7–9]. Currently, the standard of care for unresectable LA-ESCC is definitive CRT based on the evidence for resectable LA-ESCC described above as well as the results of several clinical trials for unresectable LA-ESCC [7–9]. However, most prospective and retrospective studies on unresectable LA-ESCC have evaluated survival and safety over a relatively short term of 2–3 years, and few studies have evaluated the long-term outcomes over a sufficient follow-up period [10]. Recently, a favorable outcome of multidisciplinary treatment with induction chemotherapy combined with CRT and conversion surgery has been reported and is expected to improve the prognosis of unresectable LA-ESCC [11, 12]. In addition, the introduction of immunotherapy is now expected to prolong the survival of patients with far advanced and recurrent esophageal cancer. In this context, we consider that it has become important to demonstrate the long-term outcomes of conventional treatment. In this study, we retrospectively examined the long-term results of definitive CRT for unresectable LA-ESCC.
MATERIALS AND METHODS
Eligibility
The inclusion criteria for this study were as follows: undergoing definitive CRT between 2001 and 2014, histopathologically proven esophageal cancer, T4 and/or M1 LYM according to the eighth edition of the Union for International Cancer Control (UICC) TNM classification, and no active double cancer at the time of diagnosis of esophageal cancer.
Radiotherapy
Three-dimensional radiotherapy (RT) planning was performed. The gross tumor volumes of the primary lesion and metastatic LN were delineated as GTVp and GTVn, respectively. The clinical target volumes of the primary lesion, metastatic LNs, and subclinical LN area were delineated as CTVp, CTVn, and CTVsub, respectively. CTVp was GTVp with a 2 cm margin in the longitudinal direction and a 3 to 5 mm margin in the anteroposterior and lateral directions. CTVn was GTVn with a 3 to 5 mm margin. CTVsub was determined according to the primary sites: cervical, supraclavicular, and upper mediastinal LN area for cervical tumors; supraclavicular, upper mediastinal, and subcarinal LN area for upper thoracic tumors; upper to lower mediastinal and perigastric LN area for middle to lower thoracic tumors; and middle to lower mediastinal, perigastric, and celiac trunk LN area for esophagogastric junction tumors. The planning target volumes for initial irradiation and boost irradiation were delineated as PTVinitial and PTVboost, respectively. PTVinitial included all CTVs with a 5 to 8 mm margin. PTVboost was CTVp and CTVn with a 5 to 8 mm margin. The PTV margin in the craniocaudal direction in the abdomen was set at 10–12 mm to account for respiratory motion. PTVinitial was irradiated using the anteroposterior opposed fields or multiple fields, and the standard prescription dose was 40 Gy at 2 Gy per fraction. PTVboost was irradiated using oblique opposed fields or multiple fields, and the standard prescription dose was 20–26 Gy at 2 Gy per fraction. For boost planning, re-treatment planning CT was performed. An irradiation field of a representative case treated with CRT is shown in Fig. 1.
Fig. 1.
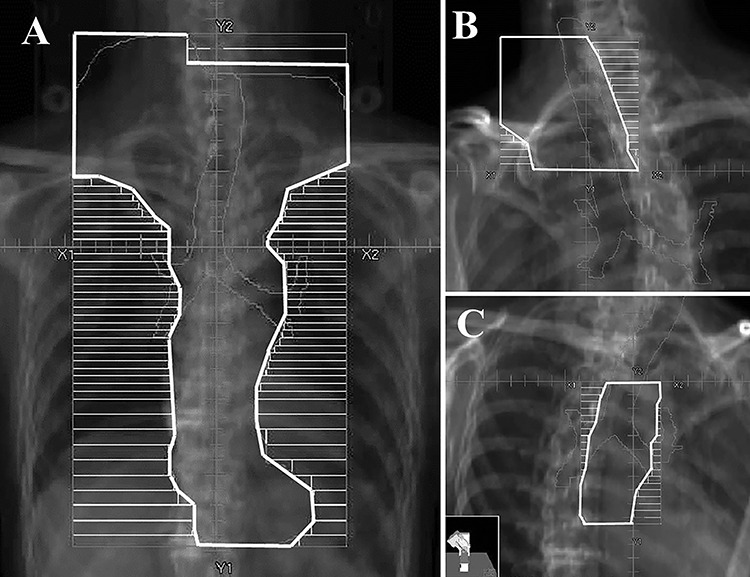
The representative irradiation fields. A: Initial field of anteroposterior opposing beams, B: Boost upper field of oblique opposing beams using a half field technique, C: Boost lower field.
Chemotherapy
The following regimens were mainly used in this study. The first was cisplatin plus 5-fluorouracil (5-FU). Two cycles of cisplatin (70 mg/m2) on day 1 and 5-FU (700 mg/m2) on day 1–4, at an interval of 4 weeks were performed. As for maintenance chemotherapy, from approximately 4 weeks after CRT, two cycles of cisplatin (80 mg/m2) on day 1 and 5-FU (800 mg/m2) on day 1–5, at an interval of 4 weeks were performed. The second was nedaplatin plus 5-FU. The dosage and administration schedule of this regimen were the same as for cisplatin plus 5-FU. The third was docetaxel plus 5-FU. We administered docetaxel (7.5 mg/m2) intravenously on day 1, 8, 22, and 29 and continuous infusion of 5-FU (250 mg/m2) on day 1–5, 8–12, 15–19, 22–26, 29–33, 36–40 and 43–45.
Follow-up
Initial tumor response was assessed by endoscopic biopsy and by enhanced CT from the neck to upper abdomen, according to the Response Evaluation Criteria in Solid Tumors [13], approximately one month after treatment was completed. As the post-treatment evaluation, we performed a physical examination, enhanced CT, and upper gastrointestinal endoscopy, every 4 months in the first year and every 6 months thereafter.
Analysis
Overall survival (OS) was defined as the time from the initiation of RT to death from any cause. Cause-specific survival (CSS) was defined as the time from the initiation of RT to death from esophageal cancer. Progression-free survival (PFS) was defined as the time from the initiation of RT to the first documentation of disease progression or death. The OS, CSS, and PFS rates were calculated using the Kaplan–Meier method. Univariate analysis was performed using the log-rank test. Statistical significance was defined as p < 0.05. All analyses were performed using R version 3.6.2 (The R Foundation for Statistical Computing, Vienna, Austria). Toxicities were assessed using the Common Terminology Criteria for Adverse Events version 4.0. We defined acute adverse events as those occurring within 90 days of treatment initiation, and late adverse events as those occurring after 90 days. The Human Ethics Review Committee in our institution approved the study.
RESULTS
Patient characteristics
Eighty patients met the eligibility criteria. The characteristics of these patients are summarized in Table 1. Their median age was 66 years (range, 41–83 years). The median tumor length was 7 cm (range, 2–12 cm). The most common tumor location was the middle esophagus in 43 (54%) patients. Among the patients, 66 patients (83%) had T4 disease, 59 (74%) had LN metastases, and 35 (44%) had M1 LYM disease. According to the eighth edition of the UICC TNM classification, 45 (56%) patients and 35 (44%) patients belong to clinical stages IVA and IVB, respectively.
Table 1.
Patient characteristics.
| Characteristics | ||
|---|---|---|
| Age (years) | ||
| Median (range) | 66 | (41–83) |
| Sex | ||
| Male | 66 | (83%) |
| Female | 14 | (17%) |
| Performance status | ||
| 0 | 50 | (63%) |
| 1 | 21 | (26%) |
| 2 | 9 | (11%) |
| Tumor length (cm) | ||
| Median (range) | 7 | (2–12) |
| Tumor main location | ||
| Upper | 26 | (33%) |
| Middle | 43 | (54%) |
| Lower/EGJ | 11 | (13%) |
| Histology | ||
| Squamous cell carcinoma | 80 | (100%) |
| T-classification | ||
| T1 | 1 | (1%) |
| T2 | 2 | (2%) |
| T3 | 11 | (14%) |
| T4 | 66 | (83%) |
| N-classification | ||
| N0 | 21 | (26%) |
| N1 | 21 | (26%) |
| N2 | 26 | (33%) |
| N3 | 12 | (15%) |
| M-classification | ||
| M0 | 45 | (56%) |
| M1 | 35 | (44%) |
| Clinical stage | ||
| IVA | 45 | (56%) |
| IVB | 35 | (44%) |
| Irradiation dose (Gy) | ||
| Median (range) | 66 | (58–70) |
| Chemotherapeutic regimen | ||
| Cisplatin +5-FU | 44 | (55%) |
| Nedaplatin +5-FU | 15 | (19%) |
| Docetaxel +5-FU | 21 | (26%) |
Abbreviation: EGJ = esophagogastric junction, FU = fluorouracil.
Treatment
Regarding RT, 77 patients received the conventional fractionated RT with an actual dose of 58–70 Gy. Three patients underwent altered fractionated RT: one patient received accelerated hyper-fractionated RT with a dose of 60 Gy/38 fractions/33 days; two patients received late course accelerated hyper-fractionated RT with doses of 67 Gy/37 fractions/45 days and 62 Gy/34 fractions/39 days. The median total duration of RT was 47 days (range, 33–74 days), and the median actual total dose was 66 Gy (range, 58–70 Gy). Two patients terminated RT at 58 Gy due to toxicity, one with Grade 5 pneumonitis and one with Grade 3 mucositis. No other patient had RT discontinued early after the start of treatment.
The chemotherapeutic regimens of cisplatin plus 5-FU, nedaplatin plus 5-FU, and docetaxel plus 5-FU were used in 44 (55%), 15 (19%), and 21 (26%) patients, respectively.
Outcomes
Data analysis was performed in June 2019. The median follow-up times for survivors and all patients were 86 months (range, 23–137) and 18 months (range, 3–137), respectively. Two patients were lost to follow-up within 60 months, and their follow-up periods were 23 and 42 months. Clinical tumor responses included complete responses in 25 (31%) patients, partial responses in 43 (54%), stable disease in 3 (4%), and progressive disease in 7 (9%). The tumor responses could not be evaluated in 2 patients who died before evaluation. Recurrence occurred in 46 patients (58%). The initial recurrence sites were local in 27 (34%) patients, regional in 8 (10%), and distant in 23 (29%). The major distant metastatic sites were the lungs in 11 (14%) patients and liver in 5 (6%). Of the patients who had recurrences, 28 received chemotherapy, 8 CRT, 2 salvage surgery, and 8 the best supportive care. At the final follow-up, 13 (16%) patients were alive without disease, one (1%) was alive with disease, and 66 (82%) had died. The causes of death were as follows: esophageal cancer in 54 (68%) patients, including 2 with tumor hemorrhage; other diseases in 9 (11%), including 4 with other cancers, 3 with pneumonia and one each with cerebral infarction and senility; and treatment-related causes in 3 (4%), as described later in the Adverse events section. Survival curves are shown in Fig. 2. The 3- and 5-year OS rates were 27.0% and 20.2%, respectively. The 3- and 5-year CSS rates were 30.6% and 25.7%, respectively. The 3- and 5-year PFS rates were 18.4% and 18.4%, respectively. On univariate analysis (Table 2), only the performance status score was significantly associated with OS (hazard ratio [HR], 1.753; 95% confidence interval [CI], 1.066–2.883; p = 0.026). Adverse events.
Fig. 2.
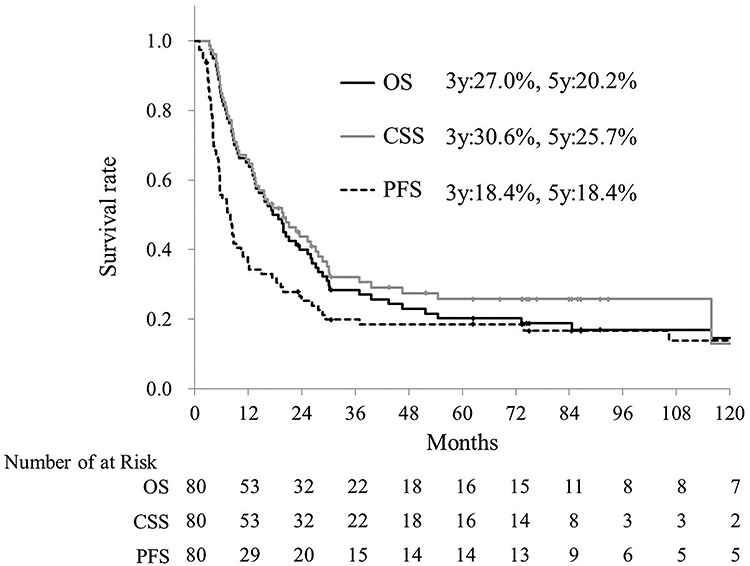
Overall survival (OS, black line), cause-specific survival (CSS, grey line) and progression-free survival (PFS, dot line) curves were shown.
Table 2.
Prognostic factor
| Overall survival | Cause specific survival | Progression free survival | |||||
|---|---|---|---|---|---|---|---|
| Factors | HR (95% CI) | P value | HR (95% CI) | P value | HR (95% CI) | P value | |
| Age (years) | ≥66 vs. < 66 | 0.979 (0.612–1.613) | 0.979 | 1.008 (0.669–1.766) | 0.734 | 0.979 (0.582–1.649) | 0.938 |
| Sex | Male vs. Female | 0.863 (0.461–1.617) | 0.646 | 1.277 (0.695–2.346) | 0.43 | 0.855 (0.432–1.698) | 0.655 |
| Performance status | 0 vs. 1–2 | 1.753 (1.066–2.883) | 0.026 | 1.639 (0.995–2.698) | 0.052 | 1.489 (0.867–2.556) | 0.148 |
| Tumor length (cm) | ≥7 vs. < 7 | 1.096 (0.675–1.779) | 0.71 | 1.192 (0.736–1.928) | 0.475 | 1.129 (0.669–1.901) | 0.649 |
| T-classification | 1–3 vs. 4 | 0.959 (0.513–1.796) | 0.898 | 0.857 (0.465–1.581) | 0.622 | 0.958 (0.483–1.900) | 0.903 |
| N-classification | 0 vs. 1–3 | 1.022 (0.593–1.762) | 0.937 | 1.023 (0.571–1.830) | 0.938 | 0.933 (0.529–1.645) | 0.811 |
| M-classification | 0 vs. 1 | 0.761 (0.463–1.251) | 0.282 | 0.931 (0.549–1.578) | 0.791 | 0.997 (0.594–1.674) | 0.991 |
| Chemotherapy | Platinum based vs. DOC based | 1.288 (0.748–2.219) | 0.36 | 1.156 (0.678–1.969) | 0.593 | 1.365 (0.763–2.442) | 0.293 |
| Irradiation dose (Gy) | ≥66 vs. < 66 | 1.362 (0.817–2.269) | 0.236 | 1.438 (0.834–2.478) | 0.19 | 1.532 (0.894–2.622) | 0.119 |
Abbreviation: HR = hazard ratioo, CI = confidence interval, DOC = docetaxel.
Table 3 shows the acute and late adverse events. Grade 3 or higher acute adverse events included leukopenia in 23 (29%) patients, thrombocytopenia in 8 (10%), esophagitis in 15 (19%), anorexia in 8 (10%), dermatitis in 3 (4%), and pneumonitis associated with chemotherapy in one (1%). There were 13 events of Grade 3 or higher late adverse events in 12 (15%) patients and included 2 events of radiation pneumonitis (3%), 4 of esophageal stricture (5%), 3 of esophageal bronchial fistula (4%), 2 of pleural effusion (3%), one of pericardial effusion (1%), and one of myocardial infarction (1%). The strictures were managed via esophageal dilatation. The fistulas were managed by stenting, bypass surgery, or enterostomy. The occurrence ratio of Grade 3 or higher late cardiopulmonary adverse events was 8% (6 patients). Treatment related death was observed in 3 (4%) patients, including acute pneumonitis associated with chemotherapy, late radiation pneumonitis, and late myocardial infarction in one patient each.
Table 3.
Adverse events
| Grade 2 | Grade 3 | Grade 4 | Grade 5 | |||||
|---|---|---|---|---|---|---|---|---|
| Acute | n | % | n | % | n | % | n | % |
| Leukocytopenia | 35 | (43.8) | 23 | (28.8) | 0 | (0) | 0 | (0) |
| Thrombocytopenia | 8 | (10) | 7 | (8.8) | 1 | (1.3) | 0 | (0) |
| Esophagitis | 42 | (52.5) | 15 | (18.8) | 0 | (0) | 0 | (0) |
| Anorexia | 6 | (7.5) | 8 | (10) | 0 | (0) | 0 | (0) |
| Dermatitis | 12 | (15) | 3 | (3.8) | 0 | (0) | 0 | (0) |
| Pneumonitis* | 1 | (1.3) | 0 | (0) | 0 | (0) | 1 | (1.3) |
| Late | n | % | n | % | n | % | n | % |
| Pneumonitis** | 2 | (2.5) | 1 | (1.3) | 0 | (0) | 1 | (1.3) |
| Pleural effusion | 1 | (1.3) | 2 | (2.5) | 0 | (0) | 0 | (0) |
| Esophagotracheal fistula | 0 | (0) | 3 | (3.8) | 0 | (0) | 0 | (0) |
| Esophageal stenosis | 9 | (11.3) | 4 | (5) | 0 | (0) | 0 | (0) |
| Pericardial effusion | 24 | (30) | 0 | (0) | 1 | (1.3) | 0 | (0) |
| Myocardial infarction | 0 | (0) | 0 | (0) | 0 | (0) | 1 | (1.3) |
*Pneumonitis = pneumonitis associated with chemotherapy.
**Pneumonitis = radiation pneumonitis.
DISCUSSION
We retrospectively examined the long-term results of definitive CRT for unresectable LA-ESCC. The definitive 5-year OS, CSS, and PFS rates were 20.2%, 25.7%, and 18.4%, respectively, with the median follow-up period of 86 months. The toxicities were acceptable.
Several clinical trials have been conducted on definitive CRT for unresectable LA-ESCC, mainly in Japan. The Japan Clinical Oncology Group (JCOG) phase II study JCOG9516 that examined the efficacy of CRT with cisplatin and 5-FU reported that the 2-year OS rate was 31.5% [7]. The phase I/II study JCOG9908, which used nedaplatin and 5-FU with RT, showed the 2-year OS rate of 31% [8]. The randomized study JCOG0303, on low-dose versus standard-dose cisplatin and 5-FU with RT showed the 3-year OS rate of 25.9% [9]. The 3-year OS rate in our study was 27.0%, and this short-term survival rate was almost equivalent to these trials.
Regarding the long-term prognosis of definitive CRT for unresectable LA-ESCC, few prospective and retrospective studies that have evaluated with a sufficient number of patients and sufficient follow-up period [10, 14–15]. Fujita et al. reported that the 5-year OS rate for radical CRT in 23 patients was 13%, with a median follow-up of 51 months [14]. Jingu et al. reported that the 4-year OS rate for radical CRT in 128 patients were 24.4%, with a median follow-up of 46.3 months [15]. In our study, 80 patients were included, and the 5-year OS rate was 20.2%, with a median follow-up of 86 months.
Although outcomes for unresectable LA-ESCC have been unsatisfactory, the development of new therapies for this patient population has long been stalled. Nishimura et al. examined the outcomes of patients with esophageal cancer at different treatment periods (1999–2003 and 2004–2008) and found that stage I and II/III patients showed improved survival but not in patients with unresectable LA-ESCC [16, 17]. Therefore, the development of new therapeutic strategies for unresectable LA-ESCC is warranted.
In Western countries, a standard dose of 50.4 Gy is used for advanced esophageal cancer based on the results of the clinical trial RTOG9405 [18]. Several ongoing clinical trials are evaluating dose-escalation strategies using state-of-the-art RT technology to improve locoregional control, including the SCOPE2 trial (50 Gy versus 60 Gy, NCT02741856), CONCORDE trial (50 Gy versus 66 Gy, NCT01348217), and Art-Deco trial (50.4 Gy versus 61.6 Gy, NTR3532). On the other hand, in Japan, an irradiation dose of 60 Gy, which was adopted in the JCOG clinical trials for unresectable LA-ESCC [7–9], have been commonly used. In this study, we used a median dose of 66 Gy in 33 fractions. There was no statistical difference in survival rates according to the irradiation dose (66 Gy or higher versus < 66 Gy). Further dose escalation is unlikely to be beneficial for patients with unresectable LA-ESCC, as they may lead to an exacerbation of adverse events.
Induction chemotherapy is a promising new strategy that has been developed in recent years. Yokota et al. conducted a phase II trial (COSMOS trial) combining induction chemotherapy using docetaxel plus cisplatin and 5FU (DCF), radical CRT, and conversion surgery (if converted to resectable), and reported promising results [11]. Recently, the latest data from this trial were published and showed a favorable 3-year OS of 46.6% at a median follow-up of 39.3 months [12]. This 3-year OS rate was considerably better than previous reports and our result. Based on this result, the JCOG has conducted a phase III trial (JCOG1510) investigating the efficacy of induction chemotherapy using DCF followed by conversion surgery and/or radical CRT in patients with unresectable LA-ESCC [19]. In addition, immunotherapy has recently been shown to be effective against various malignancies. Kato et al. reported the results of a phase III trial comparing nivolumab with standard chemotherapy (docetaxel or paclitaxel) in patients with unresectable ESCC or esophageal adenosquamous cell carcinoma who were resistant or intolerant to first-line platinum-based chemotherapy. The study showed that nivolumab showed a significant overall survival benefit compared to either paclitaxel or docetaxel, indicating that nivolumab may become the new standard of care for second-line chemotherapy for ESCC that is refractory to first-line therapy [20]. Besides, the KEYNOTE-590 trial showed that pembrolizumab added to chemotherapy is a promising first-line systemic therapy for patients with metastatic squamous cell carcinoma or adenocarcinoma of the esophagus who have PD-L1 combined positive score > 10 tumors [21]. Thus, medical advances have been made that may prolong the prognosis of patients with advanced esophageal cancer, who have had a poor prognosis in the past. We believe that our study regarding long-term prognosis in comparison to newer therapies will be important in the future.
The present study found Grade 3 or higher late adverse events in 15% of patients including cardiopulmonary toxicity in 8% of patients and treatment-related deaths in 4% (2 with pneumonitis and one with myocardial infarction). We consider that the toxicity observed in this study is within an acceptable range. However, late toxicity may be underestimated, as many patients died of cancer early after treatment.
Our study was limited by its retrospective design and the use of multiple types of chemotherapeutic regimens. Due to the poor prognosis of the disease, the evaluation of toxicity in long-term survivors may not be sufficient. Nevertheless, few previous studies have evaluated the long-term results of definitive CRT for unresectable LA-ESCC with a sufficient number of patients, and this fact increases the significance of the results of the present study.
In conclusion, we presented the long-term results of radical CRT for unresectable LA-ESCC. As the survival rate is still poor, the development of new multidisciplinary therapies, such as a combination of induction chemotherapy, CRT, and conversion surgery, is needed.
CONFLICT OF INTEREST
The authors have no conflicts of interest to disclose.
Contributor Information
Masanori Ochi, Department of Radiation Oncology, Hiroshima Red Cross Hospital & Atomic-bomb Survivors Hospital, Hiroshima, Japan.
Yuji Murakami, Department of Radiation Oncology, Hiroshima University Hospital, Hiroshima, Japan.
Ikuno Nishibuchi, Department of Radiation Oncology, Hiroshima University Hospital, Hiroshima, Japan.
Katsumaro Kubo, Department of Radiation Oncology, Hiroshima University Hospital, Hiroshima, Japan.
Nobuki Imano, Department of Radiation Oncology, Hiroshima University Hospital, Hiroshima, Japan.
Yuki Takeuchi, Department of Radiation Oncology, Hiroshima University Hospital, Hiroshima, Japan.
Tomoki Kimura, Department of Radiation Oncology, Hiroshima University Hospital, Hiroshima, Japan.
Yoichi Hamai, Department of Surgical Oncology, Hiroshima University Hospital, Hiroshima, Japan.
Manabu Emi, Department of Surgical Oncology, Hiroshima University Hospital, Hiroshima, Japan.
Morihito Okada, Department of Surgical Oncology, Hiroshima University Hospital, Hiroshima, Japan.
Yasushi Nagata, Department of Radiation Oncology, Hiroshima University Hospital, Hiroshima, Japan.
References
- 1. Ando N, Iizuka T, Ide H et al. Surgery plus chemotherapy compared with surgery alone for localized squamous cell carcinoma of the thoracic esophagus: A Japan clinical oncology group study - JCOG9204. J Clin Oncol 2003;21:4592–6. [DOI] [PubMed] [Google Scholar]
- 2. Ando N, Kato H, Igaki H et al. A randomized trial comparing postoperative adjuvant chemotherapy with cisplatin and 5-fluorouracil versus preoperative chemotherapy for localized advanced squamous cell carcinoma of the thoracic esophagus (JCOG9907). Ann Surg Oncol 2012;19:68–74. [DOI] [PubMed] [Google Scholar]
- 3. Shapiro J, van Lanschot JJB, Hulshof MCCM et al. Neoadjuvant chemoradiotherapy plus surgery versus surgery alone for oesophageal or junctional cancer (CROSS): Long-term results of a randomised controlled trial. Lancet Oncol 2015;16:1090–8. [DOI] [PubMed] [Google Scholar]
- 4. Herskovic A, Martz K, al-Sarraf M et al. Combined chemotherapy and radiotherapy compared with radiotherapy alone in patients with cancer of the esophagus. N Engl J Med 1992;26:1593–8. [DOI] [PubMed] [Google Scholar]
- 5. Cooper JS, Guo MD, Herskovic A et al. Chemoradiotherapy of locally advanced Esophageal cancer long-term follow-up of a prospective randomized trial (RTOG 85-01). JAMA 1999;281:1623–7. [DOI] [PubMed] [Google Scholar]
- 6. Kato K, Muro K, Minashi K et al. Phase II study of chemoradiotherapy with 5-fluorouracil and cisplatin for stage II-III esophageal squamous cell carcinoma: JCOG trial (JCOG 9906). Int J Radiat Oncol Biol Phys 2011;81:684–90. [DOI] [PubMed] [Google Scholar]
- 7. Ishida K, Ando N, Yamamoto S et al. Phase II study of cisplatin and 5-fluorouracil with concurrent radiotherapy in advanced squamous cell carcinoma of the esophagus: A Japan esophageal oncology group (JEOG)/Japan clinical oncology group trial (JCOG9516). Jpn J Clin Oncol 2004;34:615–9. [DOI] [PubMed] [Google Scholar]
- 8. Ishikura S, Ohtsu A, Shirao K et al. A phase I/II study of nedaplatin and 5-fluorouracil with concurrent radiotherapy in patients with T4 esophageal cancer: Japan clinical oncology group trial (JCOG 9908). Esophagus 2005;2:133–7. [Google Scholar]
- 9. Shinoda M, Ando N, Kato K et al. Randomized study of low-dose versus standard-dose chemoradiotherapy for unresectable esophageal squamous cell carcinoma (JCOG0303). Cancer Sci 2015;106:407–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Makino T, Yamasaki M, Tanaka K et al. Treatment and clinical outcome of clinical T4 esophageal cancer: A systematic review. Ann Gastroenterol Surg 2019;3:169–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Yokota T, Kato K, Hamamoto Y et al. Phase II study of chemoselection with docetaxel plus cisplatin and 5-fluorouracil induction chemotherapy and subsequent conversion surgery for locally advanced unresectable oesophageal cancer. Br J Cancer 2016;115:1328–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Yokota T, Kato K, Hamamoto Y et al. A 3-year overall survival update from a phase 2 study of chemoselection with DCF and subsequent conversion surgery for locally advanced unresectable esophageal cancer. Ann Surg Oncol 2020;27:460–7. [DOI] [PubMed] [Google Scholar]
- 13. Eisenhauer EA, Therasse P, Bogaerts J et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1). Eur J Cancer 2009;45:228–47. [DOI] [PubMed] [Google Scholar]
- 14. Fujita H, Sueyoshi S, Tanaka T et al. Is it necessary after chemoradiotherapy for a locally advanced T4 esophageal cancer? Prospective nonrandomized trial comparing chemoradiotherapy with surgery versus without surgery. World J Surg 2005;29:25–30. [DOI] [PubMed] [Google Scholar]
- 15. Jingu K, Umezawa R, Matsushita H et al. Chemoradiotherapy for T4 and/or M1 lymph node esophageal cancer: Experience since 2000 at a high-volume center in Japan. Int J Clin Oncol 2016;21:276–82. [DOI] [PubMed] [Google Scholar]
- 16. Nishimura Y, Koike R, Ogawa K et al. Clinical practice and outcome of radiotherapy for esophageal cancer between 1999 and 2003: The Japanese radiation oncology study group (JROSG) survey. Int J Clin Oncol 2012;17:48–54. [DOI] [PubMed] [Google Scholar]
- 17. Nishimura Y, Jingu K, Itasaka S et al. Clinical outcomes of radiotherapy for esophageal cancer between 2004 and 2008: The second survey of the Japanese radiation oncology study group (JROSG). Int J Clin Oncol 2016;21:88–94. [DOI] [PubMed] [Google Scholar]
- 18. Minsky BD, Pajak TF, Ginsberg RJ et al. INT 0123 (radiation therapy oncology group 94-05) phase III trial of combined-modality therapy for esophageal cancer: High-dose versus standard-dose radiation therapy. J Clin Oncol 2002;20:1167–74. [DOI] [PubMed] [Google Scholar]
- 19. Terada M, Hara H, Daiko H et al. Phase III study of tri-modality combination therapy with induction docetaxel plus cisplatin and 5-fluorouracil versus definitive chemoradiotherapy for locally advanced unresectable squamous-cell carcinoma of the thoracic esophagus (JCOG1510: TRIANgLE). Jpn J Clin Oncol 2019;49:1055–60. [DOI] [PubMed] [Google Scholar]
- 20. Kato K, Cho BC, Takahashi M et al. Nivolumab versus chemotherapy in patients with advanced oesophageal squamous cell carcinoma refractory or intolerant to one prior chemotherapy (ATTRACTION-3): A randomised, openlabel, phase 3 trial. Lancet Oncol 2019;20:1506–17. [DOI] [PubMed] [Google Scholar]
- 21. Kato K, Sun JM, Shar MA et al. LBA8_PR Pembrolizumab plus chemotherapy versus chemotherapy as first-line therapy in patients with advanced esophageal cancer: The phase 3 KEYNOTE-590 study. Ann Oncol 2020;31:S1192. [Google Scholar]


