Abstract
Radionuclide tritium is widely used in the nuclear energy production industry and creates a threat to human health through radiation exposure. Herein, the radioactive elimination and radioprotective effect of hydrogen-rich water (HRW), a potential antioxidant with various medical applications, on tritiated water (HTO) exposure, was studied in vitro and in vivo. Results showed that intragastric administration of HRW effectively promoted the elimination of urinary tritium, decreased the level of serum tritium and tissue-bound tritium (OBT), and attenuated the genetic damage of blood cells in mice exposed to HTO (18.5 MBq/kg). Pretreatment with HRW effectively reduces tritium accumulation in HTO-treated human blood B lymphocyte AHH-1 cells. In addition, the anti-oxidative properties of HRW could attenuate the increased intracellular ROS (such as O2•-, •OH and ONOO−), resulting in reversing the exhaustion of cellular endogenous antioxidants (reduced GSH and SOD), decreasing lipid peroxidation (MDA), relieving DNA oxidative damage, and depressing cell apoptosis and cytotoxicity induced by HTO exposure. In conclusion, HRW is expected to be an effective radioactive elimination agent through the competition effect of isotope exchange or a radioprotective agent by scavenging free radicals induced by HTO exposure.
Keywords: tritium exposure, hydrogen-rich water, radioactive elimination, free radical, antioxidation
INTRODUCTION
Tritium (3H) is a radioisotope of hydrogen and emits β-particles with a mean energy of 5.72 keV. It can easily bind to oxygen and another H to form tritiated water (HTO, similar to H2O), which is an important by-product of the nuclear energy production industry [1]. The biological effect of tritium and the potential toxicity to human health were studied in the past decades [2]. It is indicated that tritium can be easily ingested by organisms and trapped into biological tissues to form organically bound tritium (OBT) or exchange organic tritium (EOT), leading to long-term radiation-induced oxidative damage to cells [3–6]. Despite the inconsistent evidence on the radiotoxicity, it is conceivable that the radioactive damage induced by β-particles in organisms is dependent on the level and duration of tritium exposure [7–9]. Therefore, excretive promotion and antioxidative treatment in the early stages of tritium exposure appears useful to protect tissue from radiation-induced oxidative damage. However, there is no effective expelling or detoxifying agent for tritium poisoning as yet. Considering the similar physical and chemical properties of tritium and hydrogen, we hypothesize that hydrogen with high concentration may compete with tritium and exchange out the EOT during HTO exposure [10, 11].
Molecular hydrogen has been demonstrated to exhibit excellent antioxidant properties because of its ability to clear free radicals, such as hydroxyl radicals (•OH) and peroxynitrite (ONOO−), which are harmful free radicals overexpressed in various oxidative stress diseases [12–14]. It has attracted much attentions as a potential therapeutic gas, as a high concentration of hydrogen can attenuate the injury induced by various diseases such as ischemia–reperfusion (I/R), inflammation and allograft [15–17]. Considering the multiple advantages of molecular hydrogen, such as small molecular weight which can facility diffusion into organelles, excellent oxidation resistance and biocompatibility, good tolerance and low cost, it has served as a novel radioprotective agent in recent years [18–20]. Therefore, molecular hydrogen is hypothesized to attenuate the oxidative damage induced by tritium exposure.
In this study, the excretive promotion and radioprotective effect of hydrogen-rich water (HRW), a liquid with supersaturated hydrogen gas, were investigated in mice and cultured cells exposed to HTO, and it’s role as a novel agent to protect against tritium poisoning was explored.
MATERIALS AND METHODS
Materials
HRW (1 mM) stored at 4 °C in an aluminum bag was purchased from Beijing Huoliqingyuan Biotechnology (Beijing, China). Standardized HTO (37 MBq/mL) and scintillation cocktail were purchased from PerkinElmer (USA). The cell counting kit-8 (CCK-8) and apoptosis kit (FITC-Annexin V/PI) were purchased from Dojindo (Japan). Dihydroethidium (DHE) was purchased from Invitrogen (USA). Hydroxyphenyl fluorescein (HPF) and cytochalasin B were purchased from Sigma (USA). The 8-Hydroxy-2-deoxyguanosine (8-OHdG) ELISA kit was purchased from Abcam (UK). Malondialdehyde (MDA), superoxide dismutase (SOD) and reduced glutathione (GSH) assay kits were purchased from Nanjing Jiancheng (China). 2′,7′-Dichlorodihydrofluorescein diacetate (DCFH-DA) and the lactate dehydrogenase (LDH) release assay kit were purchased from Beyotime (Shanghai, China).
Mice and treatments
Male BALB/c mice (8 weeks old, 22–25 g) were obtained from our University Animal Center under protocols approved by the Animal Ethics Committee of the our university. Mice were randomized into the following groups: (i) control (n = 20): mice were intraperitoneally injected with 100 μL of purified water, followed by normal feeding; (ii) HTO (n = 20): mice were treated with 3.7 MBq/mL of HTO (18.5 MBq/kg) through intraperitoneal injection; (iii) H2O + HTO (n = 20): mice were additionally administered with 0.4 mL of H2O once-daily via the gastrointestinal tract 14 days after HTO administration; (iv) HRW + HTO (n = 20): the treatments were similar to the H2O + HTO group, except the H2O was instead administered via 1 mM of HRW. The urine excretion volume of each mouse was monitored at the same time each morning after HTO administration using a metabolic cage (Tecniplast, Milan, Italy). At 2, 4, 7 and 14 days after HTO administration, the tail vein blood was collected 8 h after daily administration of HRW or H2O for routine blood examination using a blood cell analyzer (Sysmex, Japan). A random 5 mice in each group were executed through cervical dislocation at the above-mentioned time points, and femoral artery blood was collected and centrifuged to detect the radioactivity in serum through a liquid-scintillation counting method. The organs (testis, kidney, spleen, small intestine and liver) of the mice were harvested and weighed 7 days after HTO injection for OBT detection.
In vitro experiments
Human blood B lymphocyte (AHH-1) cells were purchased from the cell bank of the Chinese Academy of Sciences (Beijing, China) and maintained in RPMI 1640 (Gibco) medium containing 10% fetal bovine serum (FBS; Gibco). For experiments, the medium of cells in logarithmic growth phase was replaced with H2O- or HRW- (1 mM) dissolved dehydrated medium and maintained for 10 min to adapt. Then, different concentrations of HTO were added to the cells and incubated for different times until testing.
Cell viability was measured at 48 h after HTO administration by the CCK-8 method using a microplate reader (Spectra Max M4, Molecular Devices, USA) at an absorbance of 450 nm. LDH leakage assay was also carried out to evaluate the cytotoxicity according to the manufacturer’s protocol and was detected at an absorbance of 490 nm. Apoptosis was analyzed at 48 h through incubation with Annexin V-FITC/PI stain after cell harvesting and was measured using a flow cytometer (BD FACS Calibur, East Rutherford, NJ).
To determine the production of total ROS in the cells subjected to HTO/HRW, cells were incubated with 10 μM DCFH-DA for 30 min at 24 h after HTO administration. For •OH and ONOO− detection, 5 μM HPF, a fluorescent probe which could be specifically oxidized by •OH and ONOO− to exhibit bright green fluorescence (Ex/Em = 490/515 nm), was added to the cells treated with HTO/HRW for 0.5, 2, 8 and 24 h, and incubated for 40 min at 37°C. In addition, 10 μM superoxide indicator DHE, which exhibits blue fluorescence in the cytosol until oxidized, was added to the cells treated with HTO/HRW for 0.5, 2, 8 and 24 h and incubated for 60 min to detect intracellular O2•-. The fluorescence was analyzed by the multifunctional microplate reader. 8-OHdG levels in cells were determined at 24 h after HTO administration [21]. Briefly, cells were collected and lysed, followed by extraction of purified DNA using a DNA extractor kit. Then the isolated DNA was digested using nuclease P1 following the manufacturer’s instructions. The 8-OHdG levels of samples were measured by ELISA through adding the samples to an anti-8-OHdG antibody pre-coated plate and incubating with biotinylated IgG. For other biochemical estimations, SOD, GSH and MDA levels in cells were assayed at 24 h after HTO administration following the manufacturer’s protocol based on color reaction and were quantitated using know standards using a microplate reader.
The radioactivity of tritium in urine, serum, tissues or cultured cells
The radioactivity of tritium in harvested urine, serum, tissues, cell lysates and medium was detected through a liquid-scintillation counting method [22]. Typically, for OBT detection, tissues were rinsed repeatedly with distilled water to remove superficial tritium, followed by weighing and freeze drying to remove the free tritium in tissues. The dried tissues were re-hydrated with distilled water and digested in 2 mL of nitric acid using a microwave digestion system (PerkinElmer Inc., USA). The prepared digested samples, urine, serum, cell lysates or medium were mixed with a scintillation cocktail using the volume ratio 1:2 and stored for 24 h in a dark place. The radioactivity of β-rays in samples was measured using a liquid-scintillation counter (Model 1220 Quantulus, PerkinElmer Inc., USA).
The absorbed dose (Gy) of β-rays emitted from HTO for tissues was calculated according to a previous report [23]. Briefly, the absorbed dose rate per day (dβ) is directly proportional to the production of tritium radioactivity per kilogram of wet tissue (Q Bq/kg) and the average energy (Eβ = 5.7 ke V) released per disintegration can be calculated using the following formula: dβ (Gy/day) = 7.89 × 10−11 × Q (Bq/kg), where 7.89 × 10−11 is a conversion factor and Q (Bq/kg) is the mean tritium concentration in the tissue at time (t). The total dose (Dt) was calculated by the following formula: 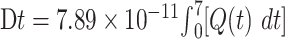 ,where Q(t) is the mean tritium concentration in the tissue at time t [24]. For culture cells, the absorbed dose-rate D (Gy/h) was calculated according to the following equation: D = 3.29 × 10−3 × C × W, where 3.29 × 10−3 is the conversion factor, W is the water content of cells (0.80 mL/g) and C is the specific radioactivity of tritium (MBq/mL); the density of cells is ~3.2 × 107/ml.
,where Q(t) is the mean tritium concentration in the tissue at time t [24]. For culture cells, the absorbed dose-rate D (Gy/h) was calculated according to the following equation: D = 3.29 × 10−3 × C × W, where 3.29 × 10−3 is the conversion factor, W is the water content of cells (0.80 mL/g) and C is the specific radioactivity of tritium (MBq/mL); the density of cells is ~3.2 × 107/ml.
Micronucleus assay
To analyze the micronucleus in polychromatic erythrocytes (MnPCEs), the bilateral femurs of experimental mice were collected and the marrows were obtained through flushing with saline [25]. After centrifugation, the marrows were suspended in 50 μL of FBS and smeared on slides. Then, the slides were dried in air and fixed with absolute methanol. For the micronucleus analysis of cultured cells, AHH-1 cells were incubated with 4.5 μg/mL of cytochalasin B, an agent that inhibits nuclear division, at 24 h before harvest. Then, cells were hypotonically treated for 2 min with 0.075 M potassium chloride, followed by fixing with methanol and acetic acid (volume ratio 3:1). The suspension was dropped on the slide and dried. All the prepared slides were stained with 4% Giemsa for 20 min at room temperature and observed using a microscope, and 1000 PCEs or binuclear cells were counted for the presence of micronucleus.
Comet assay
The comet assay was performed as previous describe [26]. Typically, 10 μL of blood or cell suspension was mixed with 240 μL of low-melting-point agarose (1.7%) and dropped onto a normal melting-point agarose (1%) pre-covered slide. The samples were lysed in the dark at 4°C for 1 h using an erythrocyte lysate (pH 10.0) with 1% Triton X-100 and 10% DMSO. Then, DNA was denatured by immersing the slides into the precooled electrophoresis buffer with 1 mM EDTA and 300 mM NaOH (pH 10.0). Electrophoresis was applied at 25 V/300 mA for 25 min. Subsequently, the slides were neutralized with phosphate buffer saline, 0.01mM (PBS) (pH 7.2) and stained with 100 μL of ethyl bromide (2.5 μg/mL), followed by observation using a fluorescence microscope (Olympus DP80, Shinjuku-ku, Tokyo, Japan). The extent of DNA damage was analyzed using CASP software and evaluated as the percentage of DNA in the tail of the comet (tail DNA%).
Statistical analysis
All the data presented in the study were derived from at least three independent experiments. We used SPSS for Windows Version 18.0 (SPSS Inc., Chicago, IL, USA) to analyze the data. Statistical analysis was performed using one way analysis of variance (ANOVA) and variance between groups was determined using the least significant difference test (LSD). P < 0.05 was considered to be statistically significant.
RESULTS
HRW facilitates radioactive elimination in HTO-exposed mice
To study whether HRW could affect radioactive elimination in an organism, mice were intraperitoneally injected with HTO followed by once-daily H2O or HRW intragastric administration. The levels of tritium in the urine, serum and tissues in mice were measured at different times. As shown in Fig. 1A, due to the additional intragastric administration of H2O or HRW, the daily urine excretion volumes (1–7 days) in H2O + HTO and HRW + HTO groups were higher than that in mice treated with HTO alone (P < 0.05). The radioactivity of urine in mice was rapidly increased within 24 h after HTO administration, followed by a gradual decrease. However, mice treated with additional HRW (HRW + HTO groups) exhibited significant higher urinary radioactivity compared to other groups within 3 days after HTO administration (P < 0.05) (Fig. 1B). Furthermore, the total tritium elimination through urine within 7 days after HTO administration in HRW-treated mice (~0.31 × 106 Bq) was also higher than in other groups (P < 0.05) (Fig. 1C). In addition, the total dose of beta-rays emitted from tritium in urine within 7 days was calculated, and the Dt (0–7 day) in control, HTO, H2O + HTO and HRW + HTO groups were 5.01 × 10−6, 4.19 × 10−3, 5.06 × 10−3 and 6.31 × 10−3 Gy, respectively. The results indicate that HRW could promote the excretion of urine tritium even more effectively than H2O.
Fig. 1.
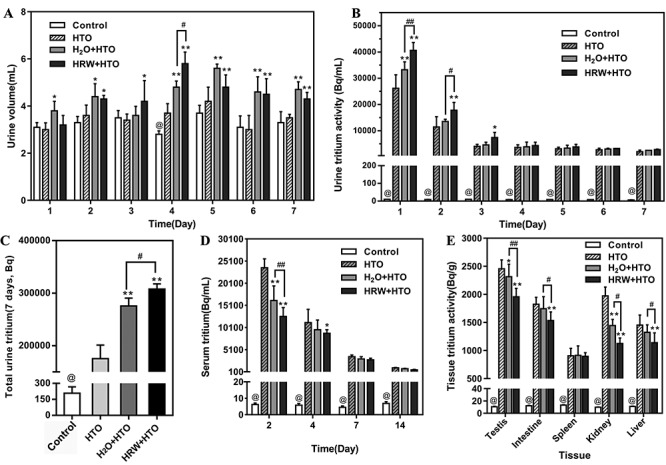
Effect of HRW on the levels of tritium in urine, serum and tissues in mice exposed to HTO. The urine excretion (A), radioactivity of urine (B), total tritium elimination through urine in the first 7 days (C), radioactivity of serum (D) and tissues (E) at day 7 in the mice treated with HTO (18.5 MBq/kg) or purified water, followed by once-daily administration of HRW or H2O. @P < 0.01, compared to other groups; *P < 0.05, **P < 0.01, compared to mice treated with HTO only; #P < 0.01, ##P < 0.01.
Similarly, additional H2O or HRW intragastric administration could also decrease the radioactivity of tritium in serum, especially within 4 days after HTO administration (P < 0.05). However, the levels of serum radioactivity in the HRW + HTO group were significant lower than in mice in the H2O + HTO group by the second day after HTO administration (P < 0.01) (Fig. 1D). Furthermore, the radioactivity of OBT in organs, especially in testis, kidney, small intestine and liver, was significantly increased by the seventh day in HTO-exposed mice. However, the radioactivity in these organs was significantly lower in the HRW + HTO group compared to H2O + HTO and HTO groups (P < 0.01) (Fig. 1E). The absorbed dose rates (dβ) in serum and tissues were also calculated 7 days after HTO administration (Table 1), and the results suggested an apparent difference between groups. The results indicated that extra HRW could eliminate radioactivity in tissues and blood in HTO-exposed mice, and exhibited a stronger evacuation effect than H2O.
Table 1.
The absorbed dose rate per day (dβ) in tissues 7 days after HTO administration (×10−11Gy/day)
| Tissue | Control | HTO | H2O | HRW |
|---|---|---|---|---|
| Serum | 0.035 ± 0.006 | 27.615 ± 3.156 | 23.670 ± 3.945 | 22.092 ± 2.840 |
| Testis | 0.082 ± 0.006 | 19.377 ± 1.246 | 18.257 ± 1.751 | 15.448 ± 1.176 |
| Intestine | 0.097 ± 0.005 | 14.391 ± 0.978 | 13.768 ± 1.696 | 12.111 ± 1.207 |
| Spleen | 0.105 ± 0.005 | 7.148 ± 1.049 | 7.203 ± 1.333 | 7.061 ± 0.521 |
| Kidney | 0.077 ± 0.004 | 15.574 ± 1.230 | 11.401 ± 0.868 | 8.876 ± 0.773 |
| Liver | 0.088 ± 0.002 | 11.472 ± 1.404 | 10.446 ± 1.057 | 8.994 ± 1.372 |
HRW attenuates the genetic toxicity of the blood system induced by HTO in mice
Subsequently, the effect of HRW on the genetic damage induced by HTO exposure in mice was investigated. First, a micronucleus assay in PCEs, a class of red blood cells before maturation in marrow, was performed. As shown in Fig. 2A, HTO significantly increased the appearance of MnPCEs and showed a peak at day 4 (about 17‰). However, the rates of MnPCEs in the HRW + HTO group were significant lower than that in the H2O + HTO group at 2, 4 and 7 days after HTO administration (P < 0.05), although lower MnPCEs rates were observed in both of these two groups compared with the mice treated with HTO alone. The DNA damage of blood nucleated cells was also measured by alkaline comet assay. Similar to the micronucleus assay, the tail DNA% was markedly elevated in mice pretreated with HTO, and reached a peak at day 4 after HTO administration (14%). However, additional H2O or HRW administration could effectively decrease the DNA damage induced by HTO in mice, whereas the tail DNA% in the HRW + HTO group (~8%) was significantly lower than that in H2O + HTO group (~11.5%) at day 4 after HTO administration (P < 0.01) (Fig. 2B). In addition, additional HRW administration also reversed the decrease of while blood cell (WBC) and platelet count (PLT) counts induced by HTO at day 2 after HTO administration (P < 0.05) (Figs 2C and D). These results indicate that HRW exhibited more effective attenuation of the genetic damage in the blood system induced by HTO in mice compared to H2O.
Fig. 2.
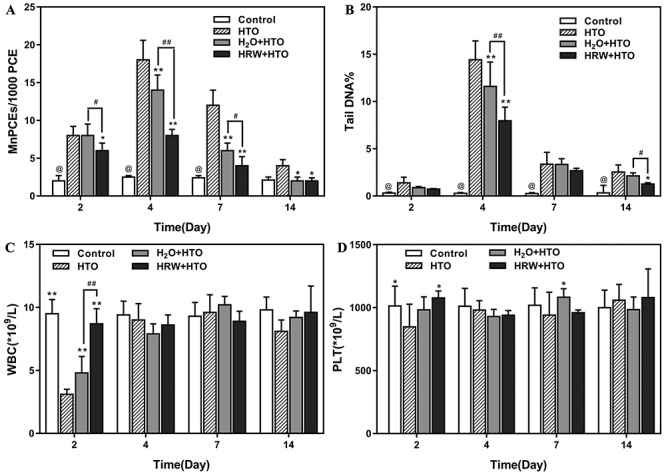
Effect of HRW on the damage to the blood system induced by HTO. The appearance of MnPCEs of marrow as indicated by micronucleus analysis (A), tail DNA% of blood nucleated cells performed by comet assay (B), WBC count (C) and PLT (D) of blood in the mice treated with HTO (18.5 MBq/kg) or purified water, followed by once-daily administration of HRW or H2O. @P < 0.01, compared to other groups; *P < 0.05, **P < 0.01, compared to mice treated with HTO only; #P < 0.01, ##P < 0.01.
HRW protects cultured cells from injury induced by HTO
To study the protective effect of HRW on cells exposed to HTO, AHH-1 cells were maintained in HRW- or H2O-dissolved dehydrated medium and treated with different concentrations of HTO and the cell viability was investigated by CCK-8 test. An apparent cytotoxicity of HTO to the cells was observed when the radioactivity was >9.25 × 105 Bq/mL, with 72.1% survival in cells pretreated with 3.7 × 106 Bq/mL of HTO (P < 0.05). However, the viability of cells maintained in 1 mM HRW medium was significantly higher than for cells treated with HTO alone (Fig. 3A). Besides, the LDH leakage of AHH-1 cells induced by HTO was also decreased by HRW administration (Fig. 3B). Furthermore, HRW could attenuate the cell apoptosis induced by 3.7 × 106 Bq/mL of HTO (total apoptosis rate: 15.31 vs 21.02%) as indicated by flow cytometry (Figs 3C and D). This suggested that HRW could protect cultured cells from injury induced by HTO.
Fig. 3.
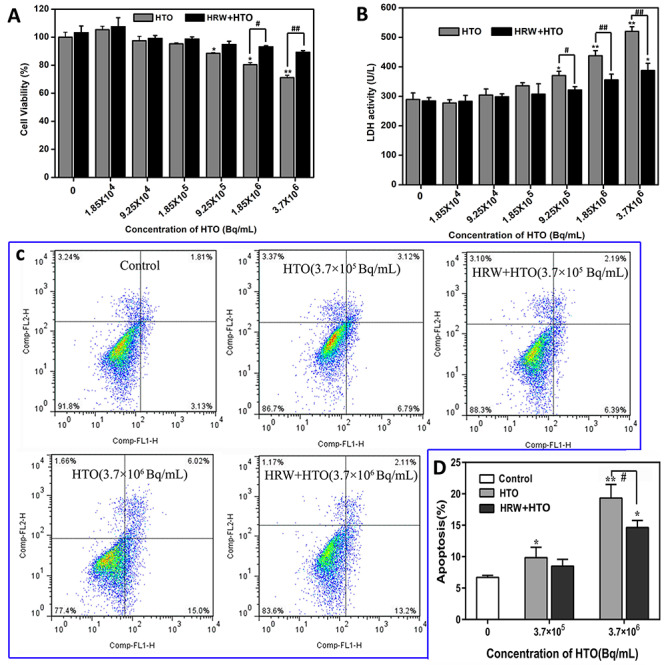
Effect of HRW on the injury of AHH-1 cells as a result of HTO exposure. The cell viability (A), cellular LDH leakage (B), apoptosis analyzed by flow cytometry (C), and total apoptosis rate (D) of AHH-1 cells incubated in H2O- or HRW-dissolved dehydrated medium with or without different doses of HTO. *P < 0.05, **P < 0.01, compared to control group; #P < 0.01, ##P < 0.01.
HRW attenuates the genetic toxicity of cultured cells induced by HTO
Micronucleus and comet assays were performed to investigate the effect of HRW on the genetic toxicity in AHH-1 cells induced by HTO. The rates of micronucleus in binuclear lymphocytes increased along with the elevated radioactivity of HTO (52‰ for 3.7 × 105 Bq/mL and 90‰ for 3.7 × 106 Bq/mL). However, 1 mM of HRW could reduce the micronucleus rates at both HTO doses (45‰ for 3.7 × 105 Bq/mL and 53‰ for 3.7 × 106 Bq/mL, P < 0.05) (Figs 4A and B). Similarly, HRW management decreased the tail DNA% induced by 3.7 × 106 Bq/mL of HTO (9.2 vs 15.8%, P < 0.01), as characterized by comet assays (Figs 4C and D). Furthermore, the levels of 8-OHdG, which is one of the markers of DNA oxidative damage caused by free radicals induced by HTO, were also decreased in the HRW + HTO group compare to the cells treated with 3.7 × 106 Bq/mL of HTO alone (4.11 ± 0.19 vs 6.83 ± 0.24 ng/mL, P < 0.01) (Fig. 4E). The results indicated that the chromosomal and DNA damage in cultured cells induced by HTO could be attenuated by a certain concentration of HRW.
Fig. 4.
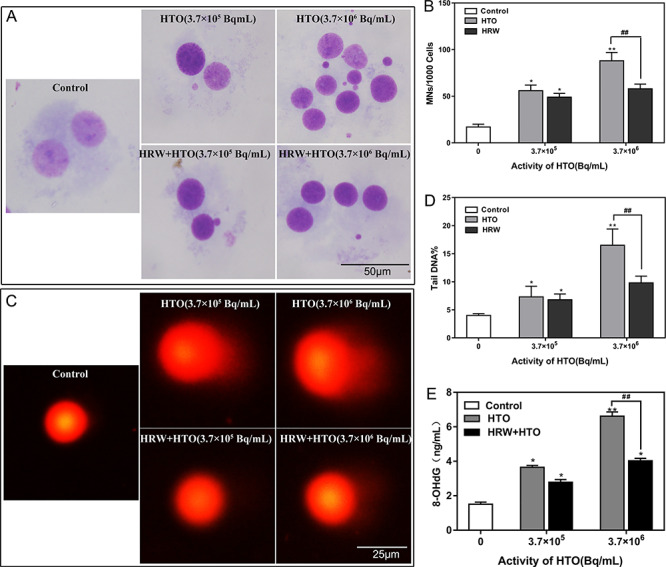
Effect of HRW on the genetic toxicity of AHH-1 cells induced by HTO. The micronucleus analysis images (A), statistics of the rates of micronucleus per 1000 binuclear lymphocytes (B), comet assays images (C), average tail DNA% of 100 cells (D), and 8-OHdG levels performed by ELISA method (E) in cells incubated in H2O- or HRW-dissolved dehydrated medium with or without different doses of HTO. *P < 0.05, **P < 0.01, compared to control group; ##P < 0.01.
HRW suppresses oxidative stress in cells exposed to HTO
As HRW has shown excellent antioxidant properties and free radical scavenging activity in various diseases [27], we hypothesized that HRW could reduce the ROS production induced by HTO. As showed in Fig. 5A, a definitive increase in total intracellular ROS production was observed at 24 h in cells exposed to HTO (P < 0.01). However, the intracellular ROS levels induced by 3.7 × 106 Bq/mL of HTO in the cells maintained in HRW medium were significantly lower than in cells maintained in H2O-dissolved medium (P < 0.01) (Fig. 5A). In addition, the levels of O2•- (indicated by the fluorescence of DHE), which is the main oxidation substrate and product of biomolecules, significantly increased with increasing doses and incubation times with HTO. However, the DHE fluorescence levels in HRW + HTO groups were much lower than in cells exposed to 3.7 × 106 Bq/mL of HTO alone at all the incubation times (P < 0.05) (Fig. 5B). Furthermore, •OH was produced by radiolysis of H2O in cells and is considered to be one of the most harmful free radicals to organisms. Contrarily, HRW could also attenuate the increase in intracellular •OH and ONOO− induced by HTO, as indicated by HPF fluorescence (P < 0.05) (Fig. 5C). These findings suggest that the attenuation of ROS by HRW, including the clearance of O2•-, •OH and ONOO−, contributes to its protective effect on HTO exposure.
Fig. 5.
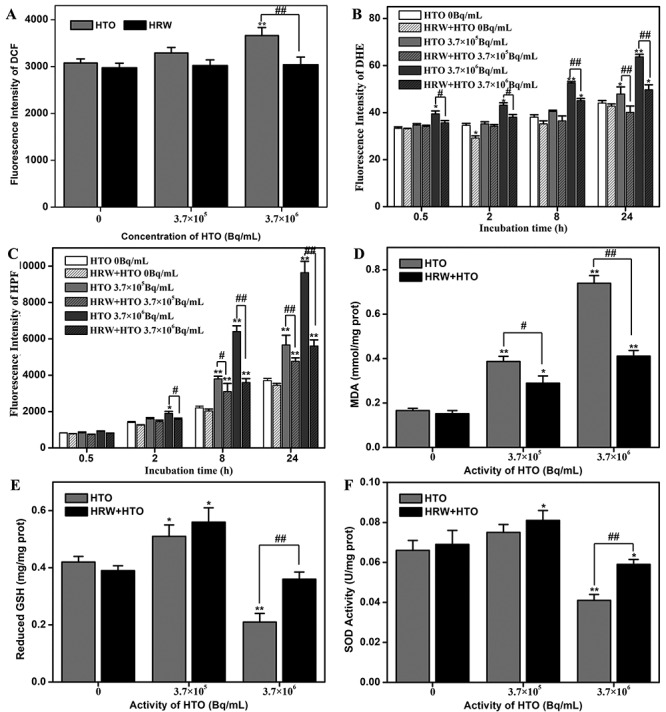
Effect of HRW on oxidative stress in cells exposed to HTO. The total ROS at 24 h after HTO administration indicated by DCF fluorescence (A), intracellular O2•- indicated by DHE fluorescence (B) and •OH indicated by HPF fluorescence (C) at different incubation times (0.5–24 h) after HTO administration. The intracellular MDA (D), reduced GSH (E), and SOD (F) levels at 24 h after HTO administration. *P < 0.05, **P < 0.01, compared to control group at the same incubation time; #P < 0.01, ##P < 0.01.
Excess ROS can induce cellular oxidative stress and peroxidation of biomolecules. However, the increased levels of intracellular MDA, a product of lipid peroxidation induced by increasing HTO exposure, were effectively suppressed by 1 mM HRW (P < 0.05) (Fig. 5D). In addition, cellular endogenous antioxidants, such as reduced GSH and SOD, will take part in the antioxidant reaction against ROS, and oxidative damage will occur as a result of the imbalance between antioxidant and oxidative stress. Interestingly, a slight increase in intracellular reduced GSH and SOD was observed in cells exposed to 3.7 × 105 Bq/mL of HTO (Figs 5E and F), which might be ascribed to the low-dose excitatory effect of ionizing radiation [28], and this phenomenon would explain the imperceptible cytotoxicity of HTO at low radioactivity. However, intracellular reduced GSH and SOD were visibly exhausted in cells exposed to 3.7 × 106 Bq/mL of HTO, whereas this attenuation could be reversed by 1 mM HRW effectively (P < 0.01) (Figs 5E and F). The results indicated that HRW could protect cells from oxidative damage induced by HTO through free radical scavenging and reversing the exhaustion of cellular endogenous antioxidants.
HRW promotes the excretion of 3H
Promoting the intracellular exchange of 1H and 3H might also contribute to the protective effect of HRW [29]. To certify this hypothesis, cells were cultured in different concentrations of HRW- (0.2, 0.5 and 1.0 mM) dissolved dehydrated medium and were incubated with a series of doses of HTO for 24 h, and the radioactivity of tritium in cell lysates and medium was detected using liquid scintillation counting. As a result, intracellular radioactivity was significantly evacuated by 1 mM HRW and partly evacuated by 0.5 mM HRW at a series of doses of HTO (3.7 × 104 to ×106 Bq/mL, P < 0.05) (Fig. 6A). The evacuation of intracellular 3H resulted in a certain elevation of radioactivity in the medium (P < 0.05) (Fig. 6B). In addition, the dose (Gy) of β-rays emitted from tritium in cultured cells was estimated according to a formula provided in the literature [24] (Table 2). The results suggest that promotion of intercellular tritium exchange or evacuation of 3H might also be responsible for the protective effect of HRW.
Fig. 6.
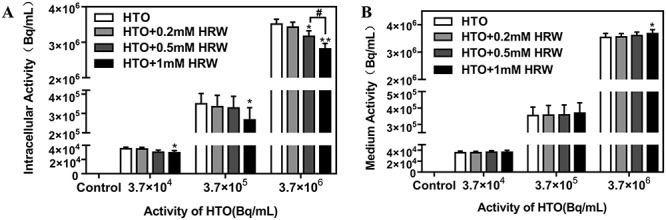
Effect of HRW on tritium exchange or evacuation. The radioactivity of tritium in cell lysates (A) and medium (B) in AHH-1 cells incubated in different concentrations (0.2, 0.5 and 1 mM) of HRW-dissolved dehydrated medium with different doses of HTO at 24 h. *P < 0.05, **P < 0.01, compared to HTO group; #P < 0.01.
Table 2.
The dose of β-rays emitted from tritium in cultured cells (μGy/h)
| HTO (Bq/mL) | HRW | |||
|---|---|---|---|---|
| 0 mM | 0.2 mM | 0.5 mM | 1 mM | |
| 0 | 0.08 ± 0.02 | 0.07 ± 0.02 | 0.08 ± 0.01 | 0.09 ± 0.02 |
| 3.7 × 104 | 92.45 ± 5.94 | 91.68 ± 6.35 | 79.54 ± 7.93 | 77.53 ± 8.22 |
| 3.7 × 105 | 920.70 ± 140.14 | 881.78 ± 153.75 | 864.96 ± 158.48 | 727.86 ± 166.14 |
| 3.7 × 106 | 9248.04 ± 356.23 | 9011.05 ± 371.75 | 8332.72 ± 411.45 | 7408.11 ± 404.18 |
DISCUSSION
As a main waste product of nuclear energy production, tritium can be discharged into the environment directly and eventually ingested by organisms, resulting in long-term internal radiation exposure [30]. It is suggested that the occupational exposure of the population to tritium expands gradually, and the levels of tritium exposure in radiation workers trends to increase annually [31, 32]. In the past decades, the physical and chemical properties, as well as the biological effect of tritium have been widely studied [33–35]. It is reported that 99% of tritium is present in the environment in the form of HTO, which is easily absorbed by the human body through the respiratory tract, digestive tract or skin [36]. The absorbed tritium can quickly enter into the blood circulation and participate in the metabolism of water, leading to an accumulation in organs with plenty of water to form OBT, as clarified in the present study [37, 38]. The metabolism of HTO in mice was visualized as described in this study. It was shown that most of the tritium was excreted within 24 h through urine, and the retention of tritium in the body could damage the blood system and tissues after 2 days of HTO administration (compared with control mice). Generally, the tritium bound to oxygen, nitrogen, sulfur or phosphorus in organic molecules can be easily replaced by isotope exchange or enzymatic reaction, and the excretive promotion effect of HRW may mainly be directed to such tritium in this study, whereas tritium bounded to carbon is difficult to exchange [39, 40].
The β-rays of tritium mainly cause the ionization and excitation of biological molecules, resulting in radiation-induced oxidative damage to organisms through generating an abundance of free radicals. It has been demonstrated that chronic intake of HTO at low dose can increase the presence of leukemia or malignant tumors, and he reproductive and genetic toxicity have been also confirmed following HTO exposure [3, 33, 41]. In the present study, the subacute toxicity of HTO was clarified, and genetic material was damaged within a short time after HTO administration, as indicated by micronucleus and comet assay. However, the cumulative radiation effect of OBT may play an important role in toxicity, which is the main cause of chronic radiation diseases induced by HTO. It is indicated that the ingested HTO will be rapidly distributed throughout the body, just like the metabolism of water, and a small fraction of tritium will eventually become incorporated into organic molecules to form OBT [42]. In our view, HRW facilitates the radioactive elimination in the early stages of HTO exposure through isotope exchange and reduces the residence of OBT in tissues. This view was also clarified by the decorporation effect of HRW on the HTO-exposed AHH-1 cells in our study. As a result, the stochastic toxicity effect of tritium may be modified by HRW. Therefore, the effect of regular oral HRW on long-term low-dose HTO exposure should be further studied in the future.
Free radicals are the most important mechanism of radiation injury induced by the β-rays of tritium [43]. It is indicated that peroxidation of lipids, proteins and DNA induced by ROS in cells is significantly correlated with the radiotoxicity of HTO [4]. Fortunately, it has been demonstrated that high concentrations of hydrogen can act as an antioxidant through clearance of the excess free radicals in cells, and can be applied in various disease therapies [16, 44]. In addition, it has been reported that HRW can effectively neutralize the free radicals produced by X- or γ-rays irradiation, and play an important anti-apoptosis and anti-inflammatory role, resulting in a radiation protection effect on mice and cells [45]. In the present study, ROS production, such as O2•-, •OH and ONOO−, in AHH-1 cells was elevated by HTO exposure, whereas it was effectively neutralized by HRW. The reduced intracellular ROS resulted in a lower degree of lipid peroxidation and replenishment of the exhausted cellular endogenous antioxidants, leading to the relief of oxidative damage in cells. The anti-apoptotic effect of HRW in AHH-1 cells was also clarified through flow cytometry analysis in this study. Based on our results, we hypothesize that the mechanism of radiation protection of HRW is as follows (Fig. 7): on one hand, the high concentration of hydrogen in HRW competes with tritium to facilitate the free or weakly bonded tritium diffusing out of cells, and inhibits the formation of OBT through isotope exchange [10]. On the other hand, the antioxidant activity of HRW and the neutralization of free radicals result in reduced ROS production, suppressed oxidative stress, alleviation of DNA and chromosomal damage and reduction of cell apoptosis induced by HTO exposure. However, long-term toxicity tests should be undertaken to further investigate the radiation protection of HRW against HTO.
Fig. 7.
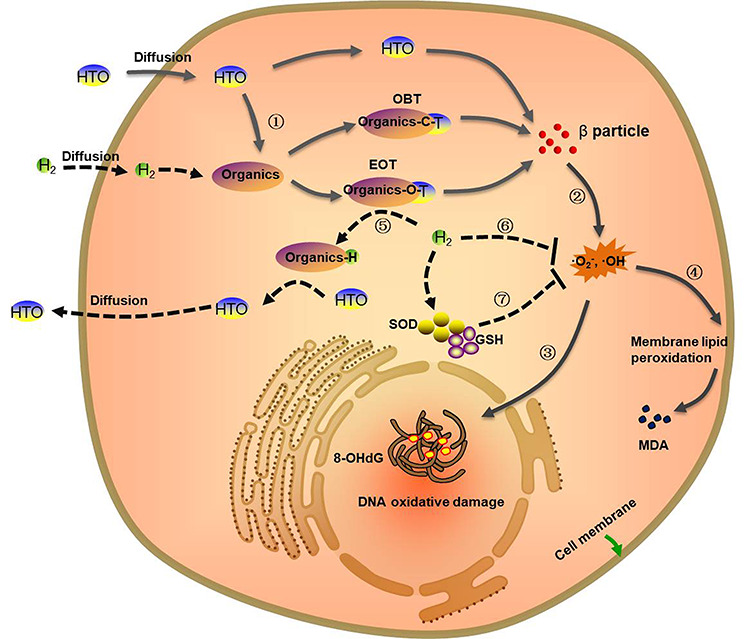
Mechanism of HRW in the attenuation of the radiotoxicity induced by tritium exposure. The solid arrow represents the damage mechanism of HTO in cells. The dotted arrow represents the mechanism of HRW for tritium excretion and treatment. ①The HTO entering the organism is bound to organic molecules to form OBT and EOT. ②The beta particles emitted by OBT, EOT and free HTO induce an increase in intracellular ROS, such as •OH and O2•-, through radiolysis of H2O and oxidation of biomolecules. ③The excess ROS results in DNA oxidative damage and products 8-OHdG. ④The free radicals also lead to membrane lipid peroxidation to destroy the biofilm structure and products MDA. ⑤HRW with a high concentration of hydrogen competitively binds to the binding sites of tritium in organics to facilitate the free or weakly bonded tritium diffusing out of cells through isotope exchange. ⑥The antioxidant activity of HRW can neutralize excess free radicals (•OH, O2•-). ⑦The exhaustion of cellular endogenous antioxidants (GSH and SOD) stimulated by ROS can also be reversed by HRW, resulting in the relief of oxidative stress injury.
CONCLUSIONS
Radiation protection is more important than treatment, especially for radiation workers. In this paper, we explore HRW as an eccritic and protective agent for HTO exposure in mice and cells. We demonstrated that HRW with a high concentration of molecular hydrogen could facilitate the evacuation of HTO and reduce the residual radiation. HRW might thus contribute to the competition effect of hydrogen to exchange EOT after HTO administration. Further study indicated that HRW could neutralize intracellular excess ROS, decrease damage to DNA and chromosomes, and protect mice and cells from injuries induced by HTO.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (No. 81472914, 81773364, 11375124, 81803173, AWS13J002), the National Key Research and Development Project (2017YFC0113904), the Chongqing Basic and Frontier Research Project (cstc2015jcyjys10001).
Contributor Information
Hong Li, Centre for Diseases Prevention and Control of Eastern Theater, Nanjing 210002, China; State Key Laboratory of Trauma Burns and Combined Injury, Institute of Combined Injury, Chongqing Engineering Research Center for Nanomedicine, College of Preventive Medicine, Army Medical University, Chongqing 400038, China.
Yaru Yin, State Key Laboratory of Trauma Burns and Combined Injury, Institute of Combined Injury, Chongqing Engineering Research Center for Nanomedicine, College of Preventive Medicine, Army Medical University, Chongqing 400038, China.
Jing Liu, State Key Laboratory of Trauma Burns and Combined Injury, Institute of Combined Injury, Chongqing Engineering Research Center for Nanomedicine, College of Preventive Medicine, Army Medical University, Chongqing 400038, China.
Binghui Lu, State Key Laboratory of Trauma Burns and Combined Injury, Institute of Combined Injury, Chongqing Engineering Research Center for Nanomedicine, College of Preventive Medicine, Army Medical University, Chongqing 400038, China.
Huimin Wan, State Key Laboratory of Trauma Burns and Combined Injury, Institute of Combined Injury, Chongqing Engineering Research Center for Nanomedicine, College of Preventive Medicine, Army Medical University, Chongqing 400038, China.
Luxun Yang, State Key Laboratory of Trauma Burns and Combined Injury, Institute of Combined Injury, Chongqing Engineering Research Center for Nanomedicine, College of Preventive Medicine, Army Medical University, Chongqing 400038, China.
Weidong Wang, Department of Radiation Oncology, Sichuan Cancer Hospital, Chengdu 610041, China.
Rong Li, State Key Laboratory of Trauma Burns and Combined Injury, Institute of Combined Injury, Chongqing Engineering Research Center for Nanomedicine, College of Preventive Medicine, Army Medical University, Chongqing 400038, China.
AUTHOR CONTRIBUTIONS
H.L., Y.Y., J.L. and B.L. performed the research and wrote the paper. H.W. and L.Y. collated and analyzed the data. W.W. and R.L. designed the study and wrote the paper. All authors have read and approved the final submitted manuscript.
CONFLICT OF INTEREST
None declared.
Data availability statement
The datasets generated and/or analysed during the current study are available from the corresponding author on reasonable request.
REFERENCES
- 1. Jeffers RS, Parker GT. Development, description and validation of a tritium environmental release model (TERM). J Environ Radioact 2014;127:95–104. [DOI] [PubMed] [Google Scholar]
- 2. Arcanjo C, Adam-Guillermin C, Murat El Houdigui S et al. Effects of tritiated water on locomotion of zebrafish larvae: A new insight in tritium toxic effects on a vertebrate model species. Aquat Toxicol 2020;2019:105384. [DOI] [PubMed] [Google Scholar]
- 3. Gagnaire B, Arcanjo C, Cavalie I et al. Tritiated water exposure in zebrafish (danio rerio): Effects on the early-life stages. Environ Toxicol Chem 2020;39:648–58. [DOI] [PubMed] [Google Scholar]
- 4. Rozhko TV, Badun GA, Razzhivina IA et al. On the mechanism of biological activation by tritium. J Environ Radioact 2016;157:131–5. [DOI] [PubMed] [Google Scholar]
- 5. Jaeschke BC, Millward GE, Moody AJ et al. Tissue-specific incorporation and genotoxicity of different forms of tritium in the marine mussel, Mytilus edulis. Environ Pollut 2011;159:274–80. [DOI] [PubMed] [Google Scholar]
- 6. Hunter N, Muirhead CR. Review of relative biological effectiveness dependence on linear energy transfer for low-LET radiations. J Radiol Prot 2009;29:5–21. [DOI] [PubMed] [Google Scholar]
- 7. Flegal M, Blimkie M, Roch-Lefevre S et al. The lack of cytotoxic effect and radioadaptive response in splenocytes of mice exposed to low level internal beta-particle irradiation through tritiated drinking water in vivo. Int J Mol Sci 2013;14:23791–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Trabalka JR, Kocher DC. Energy dependence of dose and dose-rate effectiveness factor for low-let radiations: Potential importance to estimation of cancer risks and relationship to biological effectiveness. Health Phys 2007;93:17–27. [DOI] [PubMed] [Google Scholar]
- 9. Quan Y, Zhou C, Deng B et al. The low dose effects of human mammary epithelial cells induced by internal exposure to low radioactive tritiated water. Toxicol In Vitro 2019;61:104608–9. [DOI] [PubMed] [Google Scholar]
- 10. Valero M, Bouzouita D, Palazzolo A et al. NHC-stabilized iridium nanoparticles as catalysts in hydrogen isotope exchange reactions of anilines. Angew Chem Int Ed Engl 2020;59:3517–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Pieters G, Pfeifer V, Certiat M et al. Hydrogen isotope exchange catalyzed by ru nanocatalysts: Labelling of complex molecules containing N-heterocycles and reaction mechanism insights. Chem Eur J 2020;26:4988–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Chen T, Tian M-M, Han Y. Hydrogen sulfide: A multi-tasking signal molecule in the regulation of oxidative stress responses. J Exp Bot 2020;71:2862–9. [DOI] [PubMed] [Google Scholar]
- 13. Ohsawa I, Ishikawa M, Takahashi K et al. Hydrogen acts as a therapeutic antioxidant by selectively reducing cytotoxic oxygen radicals. Nat Med 2007;13:688–94. [DOI] [PubMed] [Google Scholar]
- 14. Liu Z-N, Wang X-F, Li L et al. Hydrogen sulfide protects against paraquat-induced acute liver injury in rats by regulating oxidative stress, mitochondrial function, and inflammation. Oxid Med Cell Longev 2020;2020:1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hayashida K, Sano M, Ohsawa I et al. Inhalation of hydrogen gas reduces infarct size in the rat model of myocardial ischemia-reperfusion injury. Biochem Biophys Res Commun 2008;373:30–5. [DOI] [PubMed] [Google Scholar]
- 16. Hong Y-C, Sun L, Sun R-Q et al. Combination therapy of molecular hydrogen and hyperoxia improves survival rate and organ damage in a zymosan-induced generalized inflammation model. Exp Ther Med 2016;11:2590–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Iketani M, Ohsawa I. Molecular hydrogen as a neuroprotective agent. Curr Neuropharmacol 2017;15:324–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Qian L, Shen J-L, Chuai Y-H et al. Hydrogen as a new class of radioprotective agent. Int J Biol Sci 2013;9:887–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Baeeri M, Mohammadi-Nejad S, Rahimifard M et al. Molecular and biochemical evidence on the protective role of ellagic acid and silybin against oxidative stress-induced cellular aging. Mol Cell Biochem 2018;441:21–33. [DOI] [PubMed] [Google Scholar]
- 20. Zhou P, Lin B, Wang P et al. The healing effect of hydrogen-rich water on acute radiation-induced skin injury in rats. J Radiat Res 2019;60:17–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Vorilhon S, Brugnon F, Kocer A et al. Accuracy of human sperm DNA oxidation quantification and threshold determination using an 8-OHdG immuno-detection assay. Hum Reprod 2018;33:553–62. [DOI] [PubMed] [Google Scholar]
- 22. Ko YG, Kim CJ, Cho YH et al. Combustion/absorption process for the separation of (14)C and (3)H in radwastes released from nuclear power plants and their analysis. J Hazard Mater 2017;331:13–20. [DOI] [PubMed] [Google Scholar]
- 23. Kunugita N, Dohi S, Yamamoto H et al. Biological assessment of the enhancement of tritium excretion by administration of diuretics and excessive water in mice. J Radiat Res 1990;31:361–74. [DOI] [PubMed] [Google Scholar]
- 24. Yamaguchi T, Muraiso C, Furuno-Fukushi I et al. Water content in cultured mammalian cells for dosimetry of beta-rays from tritiated water. J Radiat Res 1990;31:333–9. [DOI] [PubMed] [Google Scholar]
- 25. De Morais CR, Pereira BB, Almeida Sousa PC et al. Evaluation of the genotoxicity of neurotoxic insecticides using the micronucleus test in Tradescantia pallida. Chemosphere 2019;227:371–80. [DOI] [PubMed] [Google Scholar]
- 26. Singh NP. The comet assay: Reflections on its development, evolution and applications. Mutat Res Rev Mutat Res 2016;767:23–30. [DOI] [PubMed] [Google Scholar]
- 27. Kura B, Kalocayova B, LeBaron TW et al. Regulation of microRNAs by molecular hydrogen contributes to the prevention of radiation-induced damage in the rat myocardium. Mol Cell Biochem 2019;457:61–72. [DOI] [PubMed] [Google Scholar]
- 28. Hurem S, Martin LM, Brede DA et al. Dose-dependent effects of gamma radiation on the early zebrafish development and gene expression. PLoS One 2017;12:e0179259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Takeda M, Miyanoiri Y, Terauchi T et al. Use of H/D isotope effects to gather information about hydrogen bonding and hydrogen exchange rates. J Magn Reson 2014;241:148–54. [DOI] [PubMed] [Google Scholar]
- 30. Straume T, Carsten AL. Tritium radiobiology and relative biological effectiveness. Health Phys 1993;65:657–72. [DOI] [PubMed] [Google Scholar]
- 31. Little MP, Wakeford R. Systematic review of epidemiological studies of exposure to tritium. J Radiol Prot 2008;28:9–32. [DOI] [PubMed] [Google Scholar]
- 32. Wu X, Liu Y, Kearfott K et al. Evaluation of public dose from FHR tritium release with consideration of meteorological uncertainties. Sci Total Environ 2020;709:136085–13. [DOI] [PubMed] [Google Scholar]
- 33. Adam-Guillermin C, Pereira S, Della-Vedova C et al. Genotoxic and reprotoxic effects of tritium and external gamma irradiation on aquatic animals. Rev Environ Contam Toxicol 2012;220:67–103. [DOI] [PubMed] [Google Scholar]
- 34. Ota M, Kwamena NA, Mihok S et al. Role of soil-to-leaf tritium transfer in controlling leaf tritium dynamics: Comparison of experimental garden and tritium-transfer model results. J Environ Radioact 2017;178-179:212–31. [DOI] [PubMed] [Google Scholar]
- 35. Eyrolle-Boyer F, Boyer P, Claval D et al. Apparent enrichment of organically bound tritium in rivers explained by the heritage of our past. J Environ Radioact 2014;136:162–8. [DOI] [PubMed] [Google Scholar]
- 36. Gueguen Y, Priest ND, Dublineau I et al. In vivo animal studies help achieve international consensus on standards and guidelines for health risk estimates for chronic exposure to low levels of tritium in drinking water. Environ Mol Mutagen 2018;59:586–94. [DOI] [PubMed] [Google Scholar]
- 37. Priest ND, Blimkie MS, Wyatt H et al. Tritium ( 3 H) retention in mice: Administered as HTO, DTO or as 3 H-Labeled amino-acids. Health Phys 2017;112:439–44. [DOI] [PubMed] [Google Scholar]
- 38. Galeriu D, Melintescu A. Retention of tritium in reference persons: A metabolic model. Derivation of parameters and application of the model to the general public and to workers. J Radiol Prot 2010;30:445–68. [DOI] [PubMed] [Google Scholar]
- 39. Pointurier F, Baglan N, Alanic G et al. Determination of organically bound tritium background level in biological samples from a wide area in the south-west of France. J Environ Radioact 2003;68:171–89. [DOI] [PubMed] [Google Scholar]
- 40. Etnier EL, Travis CC, Hetrick DM. Metabolism of organically bound tritium in man. Radiat Res 1984;100:487–502. [PubMed] [Google Scholar]
- 41. Roch-Lefevre S, Gregoire E, Martin-Bodiot C et al. Cytogenetic damage analysis in mice chronically exposed to low-dose internal tritium beta-particle radiation. Oncotarget 2018;9:27397–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Hill RL, Johnson JR. Metabolism and dosimetry of tritium. Health phys 1993;65:628–47. [DOI] [PubMed] [Google Scholar]
- 43. Welsh JS. Beta radiation. Oncologist. 2006;11:181–3. [DOI] [PubMed] [Google Scholar]
- 44. Xin H-G, Zhang B-B, Wu Z-Q et al. Consumption of hydrogen-rich water alleviates renal injury in spontaneous hypertensive rats. Mol Cell Biochem 2014;392:117–24. [DOI] [PubMed] [Google Scholar]
- 45. Chuai Y-H, Gao F, Li B-L et al. Hydrogen-rich saline attenuates radiation-induced male germ cell loss in mice through reducing hydroxyl radicals. Biochem J 2012;442:49–56. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated and/or analysed during the current study are available from the corresponding author on reasonable request.


